Abstract
Background: Depression, anxiety, and sleep disturbance are common problems that greatly affect quality of life for many myeloproliferative neoplasm (MPN) patients. App-based mindfulness meditation is a feasible nonpharmacologic approach for managing symptoms. However, previous research has not considered how patients’ overall mental health may influence their responsiveness to these interventions. Objective: The purpose of this study was to conduct an exploratory, secondary analysis of the effects of a smartphone meditation app, Calm, on depression, anxiety, and sleep disturbance in MPN patients based on patients’ baseline levels of Global Mental Health (GMH). Methods: Participants (N = 80) were a subset of MPN patients from a larger feasibility study. Patients were enrolled into an intervention (use Calm for 10 minutes daily for 4 weeks) or educational control group. Results: In multilevel models, there were significant 3-way interactions between time, group, and baseline GMH for depression and anxiety symptoms, with participants in the meditation intervention who reported the poorest baseline GMH experiencing the greatest reduction in symptoms over time. For both intervention and control participants, poorer initial GMH was associated with increases in sleep disturbance symptoms over time. Conclusions: Mindfulness meditation apps, such as Calm, may be effective in reducing depression and anxiety symptoms in MPN patients, particularly for those experiencing mental health difficulties. Given the need for accessible tools to self-manage chronic cancer–related symptoms, especially strong negative emotions, these findings warrant larger efficacy studies to determine the effects of app-based meditation for alleviating depression and anxiety in cancer populations.
Keywords: mental health, depression, anxiety, sleep, meditation, mHealth, mobile app
Introduction
Myeloproliferative neoplasms (MPNs) are a form of rare blood cancers. MPN cancers have a prevalence of 350 000 cases in the United States and an incidence rate of 6/100 000 per year.1,2 These cancers are chronic, and even with optimal medical therapy, individuals diagnosed with MPN often suffer from severe cancer-related symptoms.3 Among MPN patients, mental health difficulties associated with cancer diagnosis, symptoms, and treatment are particularly concerning. Depression and anxiety are 2 of the more prevalent mental health conditions among MPN cancer patients. In a recent sample of 2029 MPN patients, over 25% reported either depression or anxiety symptoms,4 and other studies have reported even higher rates.5,6 Moreover, MPN patients report higher rates of anxiety and depression than do other cancer populations.7 Mental health difficulties associated with cancer such as anxiety and depression can greatly affect MPN patients’ overall quality of life and impair their ability to function normally during day-to-day activities.8,9 The mental health difficulties also affect patients’ outlook toward the future, aspirations, travel plans, and long-term planning.10
Sleep disturbance is also prevalent among MPN patients. Over 50% of MPN patients have difficulty falling and/or staying asleep, and over 30% report a diagnosis of insomnia.11 Not only does sleep disturbance have direct impacts on MPN patients (eg, fatigue), inadequate sleep also exacerbates symptoms of depression and anxiety, such that sleep disturbance also indirectly contributes to total symptom burden.12,13 Moreover, depression or anxiety can impair sleep, creating a positive feedback cycle that perpetuates both sleep disturbance and mental health difficulties associated with cancer.
Despite the prevalence of sleep disturbance and mental health difficulties associated with cancer, one study found that only 63% of MPN patients were willing to accept psychiatric medication.14 Many people are reluctant to accept psychiatric medications due to stigma, concerns about side effects, and skepticism about their effectiveness.15 Furthermore, research suggests that the when used long term, some psychiatric medications confer risk for dependency.16 To address the unmet need for managing sleep disturbance and mental health difficulties in MPN patients, alternative interventions are warranted. Research is turning to nonpharmacologic approaches to treat sleep disturbance and mental health difficulties in MPN patients. Nonpharmacologic approaches, unlike medications, yield fewer side effects and have no concerns for dependence.
Mindfulness meditation is one nonpharmacologic approach for managing sleep disturbance and mental health difficulties associated with cancer in MPN patients that has recently received attention. Mindfulness meditation includes a focus on the present moment, without judgement of thoughts or feelings, in order to increase awareness of the “here and now.”17 Given that becoming caught up in mental distractions is often a component of depression, anxiety, and sleep disturbance (eg, habitual negative thoughts, unproductive worry, rumination),18 mindfulness meditation may be well suited to address these difficulties. Research shows that mindfulness meditation has positive effects on depression and anxiety in populations outside of cancer19,20 and may also positively affect some aspects of sleep.21,22 Mindfulness meditation has also been shown to improve psychological well-being in cancer populations.23 Mobile application (ie, app)-based interventions alleviate the need for patients to attend additional appointments, which may be beneficial for MPN patients, as many must travel to receive specialized care and many experience symptoms that make in-person appointments difficult (eg, fatigue).
A prior study conducted in a convenience sample of MPN patients showed that an app-based meditation intervention was feasible in this population and provided preliminary evidence of improvement in sleep disturbance, depression, and anxiety.24 However, this study did not consider how the severity and variability in patients’ mental health may influence the impact of the intervention. There is a need to understand the responsiveness of app-based meditation in MPN patients based on initial mental health status.
The purpose of this study was to conduct an exploratory, secondary analysis of the effects of a smartphone meditation app, Calm, on mental health difficulties associated with cancer (ie, depression and anxiety) and sleep disturbance in MPN patients based on patients’ baseline mental health, as measured using the Global Mental Health (GMH) scale. Specifically, we assessed whether scores on a baseline measure of GMH modified the relationship between using Calm (or participating in the control intervention) and reported changes in mental health difficulties and sleep disturbance. To do this, we examined the relationship between GMH and changes in depression, anxiety, and sleep disturbance among MPN patients receiving either a mindfulness-meditation app intervention (ie, 4 weeks of using Calm) or a control intervention (ie, educational handout about MPN symptom management). We hypothesized that baseline GMH would modify the relationship between intervention condition and changes in depression, anxiety, and sleep disturbance over time. Specifically, we predicted that the effects of using Calm would be stronger among individuals with poor GMH, while the effects of the control intervention would not depend on GMH.
Methods
Participants
Participants (N = 80) were a subset of MPN patients from a larger study that tested the feasibility and preliminary effects of 2 mindfulness-based apps on severe cancer-related symptoms.24 The current study included participants who either used Calm, a mindfulness meditation smartphone app (n = 29/80; 36%), or received the control intervention during the first 4 weeks of the parent feasibility study (n = 51/80; 64%). Current analyses excluded participants who used Calm during the second 4 weeks of the feasibility study, after having used another app. Participants were recruited online through MPN organizational partners. MPN patients interested in the study completed a web-based eligibility questionnaire. Inclusion criteria were (1) a diagnosis of essential thrombocythemia, polycythemia vera, or myelofibrosis; (2) able to read and understand English; (3) at least 18 years old; (4) reside in the United States; and (5) willing to be randomized. Exclusion criteria were (1) having engaged in meditation or yoga in the prior 6 months (not ≥10 min/day of meditation on ≥5 days/week, or ≥60 min/week of yoga), and (2) having previous experience with either of the 2 meditation apps used in the study. There were no exclusion criteria related to mental health status or diagnoses.
Procedures
In the parent study, patients were randomized into 1 of 4 study groups: one group received Calm for the first 4 weeks of the intervention, another used another meditation app for the first 4 weeks, and the third and fourth (control) groups received educational information related to cancer (and began using 1 of the 2 meditation apps after the first 4 weeks of the study).24 Given higher satisfaction and engagement with the Calm app, the current analyses compare those who used Calm during the first 4 weeks of the intervention to those in the educational control condition (ie, participants who used the other meditation app were not included in analyses due to lower satisfaction and engagement with the app). Participants in the Calm intervention group were asked to download and use Calm for 10 minutes every day for 4 weeks; those in the control group were given a 7-page educational handout containing information about evidence-based MPN fatigue management.
Measures
At baseline and postintervention (ie, 4 weeks), participants were given the National Institutes of Health Patient-Reported Outcomes Measurement Information System (NIH PROMIS)25 8-item short-form measures of Depression, Anxiety, and Sleep disturbance. The PROMIS also includes a 10-item scale measuring Global Health (GH), which can be separated into Mental Health (GMH) and Physical Health (GPH) scales which reflect overall evaluations of respondents’ mental and physical health.26 Items of the GMH and GPH scales do not overlap with items on individual symptom scales. Items on all PROMIS measures are rated using 5-point Likert-type scales. Raw scores are computed by summing the items in that scale, with higher scores reflecting higher levels of the construct being measured. As such, higher scores on the Depression, Anxiety, and Sleep Disturbance scales indicate more severe symptoms, while higher scores on the GH scales indicate better health. The PROMIS measures are reliable and valid assessment tools in diverse populations, including individuals with chronic illness.27-29 Additionally, raw scores of each scale can be converted to t scores that correspond to norms in a representative sample of US adults, such that respondents’ scores can be meaningfully interpreted by comparing them with average scores in the reference population.30
Data Analysis
All analyses were conducted using IBM SPSS 25.0. Participants were characterized by demographic information and baseline PROMIS t scores from the Depression, Anxiety, Sleep Disturbance, and GMH scales. PROMIS scores were compared using t tests to ensure that there were no between-group differences at baseline.
Factorial multilevel models (ie, mixed GLM analyses) were used to compare changes in PROMIS Depression, Anxiety, and Sleep Disturbance t scores between the pre- to postintervention periods. Multilevel modeling is appropriate for these data in that it can account for the nesting of repeated measures within the same individual. Multilevel models can determine how participants responded to the different intervention conditions (ie, Calm vs educational control), and the extent to which the magnitude of those changes is associated with GMH. Given that results showing the positive effects of Calm have been described elsewhere,24 present analyses focused specifically on how individuals’ GMH affected these effects.
All models were estimated using restricted maximum likelihood estimation, default convergence criteria, and a compound symmetrical covariance structure. As an example, the model for changes in depression is described below. Models for anxiety and sleep disturbance were identical.
In the above-mentioned equation, time point i is nested within person j. Outcome variables (γij) consisted of changes in PROMIS t scores on the scales for Depression, Anxiety, and Sleep Disturbance. All outcomes were examined via independent models. Group assignment was effect coded so control = −1 and Calm = 1, time was coded so preintervention = 0 and postintervention = 1, and GMH scores were grand-mean centered, such that the intercept (γ00) reflects the average t score in the overall sample at baseline. The primary effect of interest is the 3-way interaction between time, group, and GMH (γ13). A significant 3-way interaction would suggest that the extent to which participants showed changes in mental health difficulties and/or sleep disturbance simultaneously (1) differed across intervention groups and (2) depended on GMH. Three-way interactions were decomposed using the calculation tool developed by Preacher et al,31 and simple slopes were used to describe the direction and magnitude of these differences. Effect sizes (d) for multilevel models were estimated by dividing the unstandardized regression coefficient by the standard deviation of the outcome variable across the entire sample, derived from level-1 data, including calculated variables that reflected cross-level interactions.32 Effect sizes for simple slopes were calculated as dividing the differences in pre- and postintervention means by the standard deviation of the differences.
Results
Demographic and Clinical Characteristics
Participants predominately identified as female (76%; 61/80), White (95%; 76/80), and non-Hispanic (97%; 75/79; see Table 1). Most participants (71%; 57|80) were college graduates (ie, had received an Associate, Bachelors, or graduate-level degree), and most reported an annual household income of more than $60 000 (66%; 52/79).
Table 1.
Demographic Characteristics of the Sample.
| Category | n (%) |
|---|---|
| Gender (N = 80) | |
| Female | 61 (76.3) |
| Male | 19 (23.8) |
| Race (N = 80) | |
| White | 76 (95.0) |
| Asian | 2 (2.5) |
| American Indian | 1 (1.3) |
| Other | 1 (1.3) |
| Ethnicity (N = 77) | |
| Non-Hispanic | 75 (97.4) |
| Hispanic | 2 (2.6) |
| Education (N = 80) | |
| Did not complete high school | 1 (1.3) |
| High school | 6 (7.5) |
| Some college | 16 (20) |
| Associate degree | 6 (7.5) |
| Bachelor’s degree | 22 (27.5) |
| Graduate degree or higher | 29 (36.3) |
| Annual income (N = 79) | |
| <$20 000 | 5 (6.3) |
| $21 000-40 000 | 6 (7.6) |
| $41 000-60 000 | 16 (20.3) |
| >$61 000 | 52 (65.8) |
Baseline means for PROMIS scale t scores in each group are presented in Table 2. As shown, there were no significant differences between Calm and control groups with regard to baseline clinical characteristics.
Table 2.
Baseline Clinical Characteristics in the Sample, by Group (N = 80).
| PROMIS scale | Mean (SD) |
t (df) | P | |
|---|---|---|---|---|
| Control | Calm | |||
| Depression | 50.58 (7.54) | 50.48 (10.09) | 0.05 (78) | .959 |
| Anxiety | 54.77 (7.89) | 51.14 (10.75) | 1.72 (77) | .090 |
| Sleep Disturbance | 50.73 (8.03) | 53.58 (7.72) | −1.55 (78) | .126 |
| GMH | 45.59 (8.22) | 44.64 (7.61) | 0.51 (78) | .609 |
| GPH | 44.56 (9.09) | 43.67 (9.22) | 0.42 (78) | .674 |
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; df, degrees of freedom; GMH, Global Mental Health; GPH, Global Physical Health.
Changes in Mental Health Difficulties Associated With Cancer and Sleep Disturbance
Depression
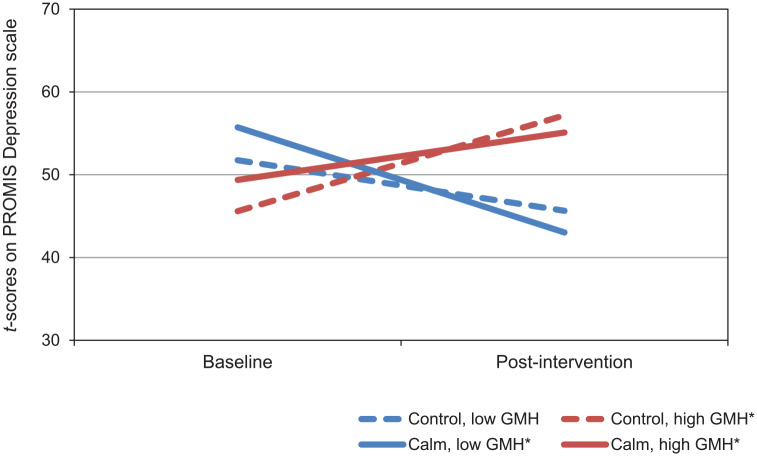
Coefficients for the model of change in PROMIS Depression scores are presented in Table 3. There was a significant main effect of GMH in which lower GMH baseline scores at (ie, those reporting the poorer GMH) were associated with higher levels of depression at baseline. There was also a significant 2-way interaction between time and GMH, such that across both groups, those with lower GMH scores at baseline reported larger reductions in depression over time. The 3-way interaction between time, intervention group, and GMH was statistically significant, although the effect size was small. As shown in Figure 1, those with lower GMH scores at baseline generally had greater reductions in depression than those with higher baseline GMH scores. Participants with poor GMH in the Calm group had the largest decreases in depression (see Table 4).
Table 3.
Fixed Effects From Multilevel Model of Changes in PROMIS Depression t Scores as a Function of Intervention Group and PROMIS GMH Scores.
| Coefficient (SE) | t (df) | P | d | |
|---|---|---|---|---|
| Intercept | 49.36 (5.19) | 9.51 (152) | <.001 | — |
| Time | 0.63 (1.91) | 0.33 (76) | .745 | 0.07 |
| Group | 1.2 (1.6) | 0.75 (150) | .455 | 0.14 |
| GMH | 0.53 (0.17) | 3.11 (150) | .002 | 0.06 |
| Time × Group | −0.31 (2.4) | −0.13 (76) | .896 | −0.04 |
| Time × GMH | −1.52 (0.26) | −5.97 (76) | <.001 | −0.18 |
| Group × GMH | −0.39 (0.21) | −1.86 (150) | .064 | −0.05 |
| Time × Group × GMH | 0.74 (0.31) | 2.4 (76) | .019 | 0.09 |
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; GMH, Global Mental Health; SE, standard error; df, degrees of freedom.
Figure 1.
Simple slopes for model of changes in Patient-Reported Outcomes Measurement Information System (PROMIS) Depression t scores by intervention group in participants with high and low PROMIS Global Mental Health (GMH) scores.
Table 4.
Simple Slopes for Model Changes in PROMIS Depression t Scores by Intervention Group in Participants With High and Low PROMIS GMH Scoresa.
| Slope (SE) | t (df) | P | d | |
|---|---|---|---|---|
| Control | ||||
| Low GMH | −6.10 (1.99) | −3.07 (76) | .073 | −0.48 |
| High GMH | 11.58 (5.87) | 3.33 (76) | .001 | 0.92 |
| Calm | ||||
| Low GMH | −12.70 (6.19) | −2.86 (76) | .006 | −1.01 |
| High GMH | 6.72 (2.10) | 3.20 (76) | .002 | 0.45 |
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; GMH, Global Mental Health; SE, standard error; df, degrees of freedom.
Low and high GMH reflect PROMIS GMH t scores at baseline that are one standard deviation below and above the overall sample mean, respectively.
Anxiety
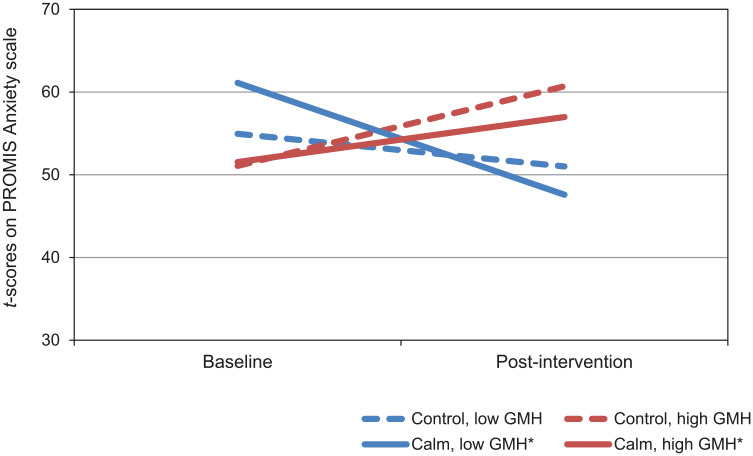
Coefficients for the model of change in PROMIS Anxiety scores are presented in Table 5. There were significant main effects of time and GMH, indicating that across all participants, anxiety decreased over time. There was also a significant main effect of GMH, in which those with lower baseline GMH scores reported higher baseline levels of anxiety. There was a significant 2-way interaction between time and group, which showed that regardless of baseline GMH, participants in the Calm group reported larger reductions in anxiety over time. The 2-way interaction between time and GMH was also significant, such that across groups, participants with lower GMH scores at baseline reported larger reductions in anxiety over time. The interaction between time, group, and mental health was statistically significant, although the effect size was small. Figure 2 shows that poor GMH was associated with larger decreases in anxiety, and that participants with poor GMH who used Calm reported the largest decreases in anxiety (see Table 6).
Table 5.
Fixed Effects From Multilevel Model of Changes in PROMIS Anxiety t Scores as a Function of Intervention Group and PROMIS GMH Scores.
| Coefficient (SE) | t (df) | P | d | |
|---|---|---|---|---|
| Intercept | 54.67 (5.58) | 9.81 (150) | <.001 | — |
| Time | −4.6 (2.07) | −2.22 (75) | .029 | −0.51 |
| Group | −1.43 (1.75) | −0.82 (149) | .416 | −0.16 |
| GMH | 0.41 (0.19) | 2.14 (149) | .034 | 0.05 |
| Time × Group | 6.35 (2.57) | 2.47 (75) | .016 | 0.70 |
| Time × GMH | −1.6 (0.28) | −5.67 (75) | <.001 | −0.18 |
| Group × GMH | −0.2 (0.23) | −0.88 (149) | .383 | −0.02 |
| Time × Group × GMH | 0.9 (0.34) | 2.68 (75) | .009 | 0.10 |
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; GMH, Global Mental Health; SE, standard error; df, degrees of freedom.
Figure 2.
Simple slopes for model of changes in Patient-Reported Outcomes Measurement Information System (PROMIS) Anxiety t scores by intervention group in participants with high and low PROMIS Global Mental Health (GMH) scores.
Table 6.
Simple Slopes for Model Changes in PROMIS Anxiety t Scores by Intervention Group in Participants With High and Low PROMIS GMH Scoresa.
| Slope (SE) | t (df) | P | d | |
|---|---|---|---|---|
| Control | ||||
| Low GMH | −3.95 (2.10) | −1.88 (75) | .064 | −0.30 |
| High GMH | 9.62 (6.23) | 1.54 (75) | .127 | 0.42 |
| Calm | ||||
| Low GMH | −13.53 (6.90) | −4.57 (75) | <.001 | −1.04 |
| High GMH | 5.45 (2.23) | 3.35 (75) | .021 | 0.74 |
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; GMH, Global Mental Health; SE, standard error; df, degrees of freedom.
Low and high GMH reflect PROMIS GMH t scores at baseline that are one standard deviation below and above the overall sample mean, respectively.
Sleep Disturbance
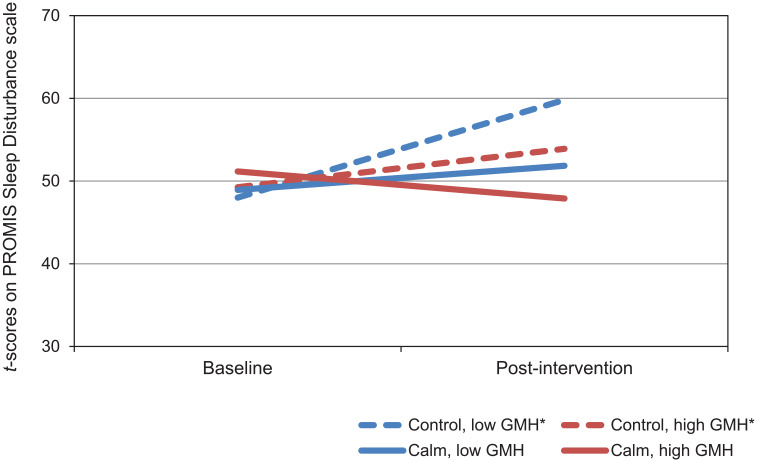
Coefficients for the model of changes in PROMIS Sleep Disturbance scores are presented in Table 7. There was a significant main effect of time, which showed that across all participants, sleep disturbance increased over time. There was also a significant 2-way interaction between time and GMH, such that those with lower GMH scores at baseline reported larger increases in sleep disturbance over time. The 3-way interaction between time, group, and GMH was not significant. Simple slopes are presented in Figure 3 and Table 8.
Table 7.
Fixed Effects From Multilevel Model of Changes in PROMIS Sleep Disturbance t Scores as a Function of Intervention Group and PROMIS GMH Scores.
| Coefficient (SE) | t (df) | P | d | |
|---|---|---|---|---|
| Intercept | 49.33 (5.58) | 8.85 (152) | <.001 | — |
| Time | 4.03 (1.78) | 2.26 (76) | .027 | 0.50 |
| Group | 0.87 (1.73) | 0.51 (148) | .614 | 0.11 |
| GMH | 0.09 (0.18) | 0.47 (148) | .640 | 0.01 |
| Time × Group | −3.34 (2.23) | −1.5 (76) | .139 | −0.42 |
| Time × GMH | −0.51 (0.24) | −2.15 (76) | .035 | −0.06 |
| Group × GMH | 0.03 (0.22) | 0.13 (148) | .895 | 0.00 |
| Time × Group × GMH | 0.03 (0.29) | 0.10 (76) | .918 | 0.00 |
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; GMH, Global Mental Health; SE, standard error; df, degrees of freedom.
Figure 3.
Simple slopes for model of changes in Patient-Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance t scores by intervention group in participants with high and low PROMIS Global Mental Health (GMH) scores.
Table 8.
Simple Slopes for Model Changes in PROMIS Sleep Disturbance t Scores by Intervention Group in Participants with High and Low PROMIS GMH Scoresa.
| Slope (SE) | t (df) | P | d | |
|---|---|---|---|---|
| Control | ||||
| Low GMH | 11.83 (5.49) | 2.16 (76) | .034 | 1.11 |
| High GMH | 4.65 (1.96) | 2.37 (76) | .020 | 0.44 |
| Calm | ||||
| Low GMH | 2.91 (5.78) | 0.51 (76) | .615 | 0.27 |
| High GMH | −3.27 (1.85) | −1.77 (76) | .081 | −0.31 |
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; GMH, Global Mental Health; SE, standard error; df, degrees of freedom.
Low and high GMH reflect PROMIS GMH t scores at baseline that are one standard deviation below and above the overall sample mean, respectively.
Discussion
The purpose of this study was to conduct an exploratory, secondary analysis of the effects of a smartphone meditation app, Calm, on mental health difficulties associated with cancer (ie, depression and anxiety) and sleep disturbance in MPN patients based on patients’ GMH. Specifically, we assessed whether scores on a baseline measure of GMH modified the relationship between using Calm or a control condition and reporting changes in depression, anxiety, and sleep disturbance.
The largest decreases in depression and anxiety were those in the Calm group who reported the poorest baseline GMH. Poorer GMH was associated with increases in sleep disturbance; however, changes in sleep disturbance were not related to being in the Calm group versus the educational control group.
It is not surprising that participants with poorer GMH at baseline reported larger decreases in depression and anxiety than those with better baseline GMH. This is expected given that those with poorer mental health at the start of the study also had more severe depression and anxiety, allowing for a greater level of improvement. Thus, the largest decreases in depression and anxiety were found in those who had poor GMH and who used Calm suggests that app-based meditation is particularly helpful for those with poor mental health.
Calm meditations are developed using a combination of techniques drawn from Mindfulness-Based Stress Reduction and Cognitive Behavioral Therapy, encouraging users to practice moment-to-moment awareness without judgement and to develop awareness of their thoughts, interpretations, and emotional and physiological responses in order to alter their perception or create new, more balanced thoughts. This practice of nonjudgmental present-moment awareness has been demonstrated to reduce stress,33 increase self-efficacy,34 and improve emotion regulation35 all of which have been associated with reductions in anxiety and depression among cancer patients/survivors.36-38 Khoury and colleagues39 suggest that mindfulness-based therapies may be most effective in those with more severe mental illness (ie, depression). In a meta-analytic review, they found across multiple studies (n = 6) that those reporting a more severe level of baseline depression improved to a greater extent from pretreatment to posttreatment as compared with those reporting more mild or moderate depression at baseline. Given the growing body of evidence supporting the benefits of meditation for depression and anxiety in clinical populations, including cancer,40,41 recognizing that the impact may be greater for those in most need is important.
Our findings related to greater reductions in depression and anxiety in those with poor GMH and who used Calm provide evidence that the benefits of in-person mindfulness-based interventions for those with more severe mental health difficulties may extend to app-based interventions. There is a general belief among mental health providers that individuals with more severe mental health symptoms require more intensive treatment.42 Moreover, the severity of mental health symptoms is often a primary differentiator between who is immediately placed into high-intensity interventions (eg, 12-16 in-person, therapist-led sessions of cognitive behavioral therapy),43 even when lower intensity interventions (ie, cost-effective interventions with low demands on “specialist therapist time,” including web- or smartphone-based interventions) have shown comparable if not greater benefits for individuals with more severe symptoms.43,44 More research is needed to understand required dosage and the long-term sustainability of improvements in app-based meditation interventions. However, the current findings suggest that a meditation mobile app such as Calm may be feasible to reduce more severe depression and anxiety symptoms, warranting larger efficacy studies in the future that are fully powered to compare effects to those observed with in-person treatments.
Recent research indicates that emotional distress is prevalent among cancer patients/survivors. Nearly half of cancer patients experience clinically significant depression and anxiety symptoms during treatment,45,46 and for more than 20%, these symptoms are maintained as long-term survivors (ie, ≥5 years after diagnosis).47 More specifically, MPN patients have a different disease course than most tumor-based cancers.48 MPN patients experience long-term and persistent mental health difficulties associated with their cancer diagnosis, symptoms, or treatment, despite the fact that overall improvement in survival is typically better among MPN patients compared with other cancers.10 Thus, MPN patients are faced with managing depression and anxiety that may be more likely to persist or reemerge over time. Meditation apps, such as Calm, may be helpful in the long-term management of more severe depression and anxiety that require consistent intervention. Thus, in general, symptoms of anxiety and depression among cancer patients appear to be well documented, and early identification and effective treatment should be considered essential for comprehensive cancer care.
Poor baseline GMH was associated with increases in sleep disturbance over time; however, there were no differences in the changes in sleep disturbance between the Calm and the control groups. Given recent studies supporting the positive effects of mindfulness meditation interventions on sleep quality,21 the lack of benefits for sleep disturbance was unexpected. A previous review comparing studies testing the effects of mindfulness meditation on sleep found that across studies, there was substantial variability in the size of effects and the aspects of sleep that were affected (ie, not just sleep disturbance, but quantity or quality of sleep).49 Additionally, studies on the effects of mindfulness meditation and sleep have varied in length (ie, 2-16 weeks), but the extent to which duration of meditation practice contributes to sleep benefits remains unclear.21 The current study assessed changes after only 4 weeks of meditation, which may not have been long enough to see effects on sleep disturbance.
Limitations
This study provides a glimpse of the potential for a meditation app, such as Calm, to be effective for those with poor GMH. However, the current study has several limitations. First, the sample was small, predominately female, White, and had above-average education and annual household income. Additionally, mean PROMIS t scores were all within one SD of the general population mean (t = 50), indicating that, on average, the severity of depression, anxiety, and sleep disturbance was within the normal range. This likely reduced the effect sizes for the 3-way interactions, which were small (for depression, d = 0.09; for anxiety, d = 0.10), and may result in analyses that are insufficiently powered to detect effects of the intervention. Given this, the generalizability of the results is unclear, particularly among populations with more severe symptoms. Future research should use larger, more demographically diverse samples of MPN patients with a larger range of symptom severity. Anxiety and depression increased among Calm and control participants with better baseline GMH. This finding was unexpected, and it is unclear whether these changes were related to study participation (eg, if reporting on mental health symptoms made them more salient) or natural variability over time. Future studies should measure the effects of using Calm in longer interventions, with measurements taken at multiple time points.
Future Research
A smartphone-based, mindfulness meditation app, Calm, was effective in reducing depression and anxiety in MPN patients, particularly for those who reported poor GMH; however, the intervention did not significantly affect MPN patients’ sleep disturbance. This information may inform future research. Since the current study suggests that Calm is effective in reducing anxiety and depression among MPN patients, future studies may also seek to train providers to integrate low-intensity nonpharmacological interventions (eg, mindfulness meditation apps, such as Calm) into their standard cancer care as an option to manage mental health difficulties associated with cancer, particularly for patients experiencing severe symptoms that may be otherwise difficult to manage. However, given the unique course of MPN, future studies are needed to determine how app-based meditation may benefit other cancer patient/survivor populations. Additionally, more research is needed to determine whether smartphone-based meditation apps may positively affect sleep disturbance in cancer patients/survivors.
Future studies should consider the mechanisms by which meditation apps benefit cancer patients/survivors. It may be that different content or different components within these apps provide unique effects on anxiety, depression, and sleep disturbance. It is also likely that the characteristics of individual cancer patients/survivors (eg, mental health, symptom severity) affect their response to different app content, components, or smartphone-based meditation in general, indicating the need to better understand which patient/survivor populations may most benefit from these interventions. These unanswered questions underscore the need for large-scale randomized controlled trials of app-based meditation interventions for cancer patient/survivor populations that are sufficiently powered to detect not only the effects of these interventions, but also the mediators and moderators that explain and change those effects in context. Specifically, these studies should recruit MPN patients suffering from significant, clinical levels of anxiety, depression, and sleep disturbance. Ideally, longer follow-up to detect sustained changes over time would better inform the effectiveness of Calm for MPN cancer patients.
Conclusion
These findings contribute to a growing body of evidence supporting the benefits of meditation for clinical populations, including cancer, and that delivering meditation through a smartphone app can help make these benefits more available to patients. Given the prevalence of depression and anxiety among cancer patients, there is an urgent need for easily accessible tools for self-managing mental health difficulties associated with cancer. This study demonstrates that meditation apps, such as Calm, may be effective tools to help MPN patients with mental health difficulties manage their depression and anxiety. These results are encouraging, as app-based meditation may serve as a feasible, low-intensity, nonpharmacological intervention that could be easily available to cancer patients who are struggling with mental health. Meditation apps, such as Calm, could also be incorporated into standard cancer care to serve as a supplementary tool for individuals having ongoing difficulties with mental health and requiring consistent treatment to manage their symptoms. Future research should include large-scale efficacy trials to provide a better understanding of whether Calm and other meditation apps can improve mental health in cancer patients, the mechanisms that drive those effects, and the populations that most benefit from app-based meditation interventions.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Megan E. Puzia states that she is independently contracted by Calm. Jennifer Huberty states that she is the Director of Science with Calm. The other authors have no conflicts of interest to disclose.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Megan E. Puzia  https://orcid.org/0000-0002-5188-0445
https://orcid.org/0000-0002-5188-0445
Ryan Eckert  https://orcid.org/0000-0001-9105-5354
https://orcid.org/0000-0001-9105-5354
Linda Larkey  https://orcid.org/0000-0001-7681-6813
https://orcid.org/0000-0001-7681-6813
References
- 1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55:595-600. [DOI] [PubMed] [Google Scholar]
- 2. Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted county study, 1976-1995. Am J Hematol. 1999;61:10-15. [DOI] [PubMed] [Google Scholar]
- 3. Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118:401-408. [DOI] [PubMed] [Google Scholar]
- 4. Brochmann N, Flachs EM, Christensen AI, et al. Anxiety and depression in patients with Philadelphia-negative myeloproliferative neoplasms: a nationwide population-based survey in Denmark. Clin Epidemiol. 2019;11:23-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McFarland DC, Polizzi H, Mascarenhas J, Kremyanskaya M, Holland J, Hoffman R. Psychological symptoms among patients with BCR-ABL–negative myeloproliferative neoplasms. J Natl Compr Canc Netw. 2016;14:1563-1570. [DOI] [PubMed] [Google Scholar]
- 6. Scherber RM, Kosiorek HE, Senyak Z, et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer. 2016;122:477-485. [DOI] [PubMed] [Google Scholar]
- 7. Brintzenhofe-Szoc KM, Levin TT, Li Y, Kissane DW, Zabora JR. Mixed anxiety/depression symptoms in a large cancer cohort: prevalence by cancer type. Psychosomatics. 2009;50:383-391. [DOI] [PubMed] [Google Scholar]
- 8. Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs) an international internet-based survey of 1179 MPD patients. Cancer. 2007;109:68-76. [DOI] [PubMed] [Google Scholar]
- 9. Mesa R, Miller CB, Thyne M, et al. Impact of myeloproliferative neoplasms (MPNs) on patients’ overall health and productivity: results from the MPN Landmark Survey in the United States. Blood. 2014;124:3183.25202141 [Google Scholar]
- 10. Scherber RM, Geyer HL, Mesa RA. Quality of life in MPN comes of age as a therapeutic target. Curr Hematol Malig Rep. 2014;9:324-330. [DOI] [PubMed] [Google Scholar]
- 11. Gowin KL, Langlais BT, Kosiorek HE, et al. Sleep and psychiatric disturbance in chronic Philadelphia negative myeloproliferative neoplasms. Blood. 2017;130 (suppl 1):4189. [Google Scholar]
- 12. Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: a review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. 2019;23:2324-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36:1059-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McFarland DC, Shen MJ, Polizzi H, et al. Preferences of patients with myeloproliferative neoplasms for accepting anxiety or depression treatment. Psychosomatics. 2017;58:56-63. [DOI] [PubMed] [Google Scholar]
- 15. De Las Cuevas C, Motuca M, Baptista T, de Leon J. Skepticism and pharmacophobia toward medication may negatively impact adherence to psychiatric medications: a comparison among outpatient samples recruited in Spain, Argentina, and Venezuela. Patient Prefer Adherence. 2018;12:301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moncrieff J, Cohen D, Porter S. The psychoactive effects of psychiatric medication: the elephant in the room. J Psychoactive Drugs. 2013;45:409-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kabat-Zinn J. Wherever You Go, There You Are: Mindfulness Meditation in Everyday Life. Hachette Books; 2009. [Google Scholar]
- 18. Blake MJ, Trinder JA, Allen NB. Mechanisms underlying the association between insomnia, anxiety, and depression in adolescence: implications for behavioral sleep interventions. Clin Psychol Rev. 2018;63:25-40. [DOI] [PubMed] [Google Scholar]
- 19. Blanck P, Perleth S, Heidenreich T, et al. Effects of mindfulness exercises as stand-alone intervention on symptoms of anxiety and depression: systematic review and meta-analysis. Behav Res Ther. 2018;102:25-35. [DOI] [PubMed] [Google Scholar]
- 20. Hoge EA, Bui E, Palitz SA, et al. The effect of mindfulness meditation training on biological acute stress responses in generalized anxiety disorder. Psychiatry Res. 2018;262:328-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445:5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Black DS, O’Reilly GA, Olmstead R, Breen EC, Irwin MR. Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: a randomized clinical trial. JAMA Intern Med. 2015;175:494-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zimmermann FF, Burrell B, Jordan J. The acceptability and potential benefits of mindfulness-based interventions in improving psychological well-being for adults with advanced cancer: a systematic review. Complement Ther Clin Pract. 2018;30:68-78. [DOI] [PubMed] [Google Scholar]
- 24. Huberty J, Eckert R, Larkey L, et al. Smartphone-based meditation for myeloproliferative neoplasm patients: feasibility study to inform future trials. JMIR Form Res. 2019;3:e12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the Patient-Reported Outcomes Measurement Information System (PROMIS) global items. Qual Life Res. 2009;18:873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cook KF, Jensen SE, Schalet BD, et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol. 2016;73:89-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schalet BD, Pilkonis PA, Yu L, et al. Clinical validity of PROMIS depression, anxiety, and anger across diverse clinical samples. J Clin Epidemiol. 2016;73:119-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2012;10:6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reeve BB, Hays RD, Bjorner JB, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care. 2007;45(5 suppl):S22-S31. [DOI] [PubMed] [Google Scholar]
- 31. Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437-448. [Google Scholar]
- 32. Baguley T. Standardized or simple effect size: what should be reported? Br J Psychol. 2009;100:603-617. [DOI] [PubMed] [Google Scholar]
- 33. Rush SE, Sharma M. Mindfulness-based stress reduction as a stress management intervention for cancer care: a systematic review. J Evid Based Complementary Altern Med. 2017;22:348-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sanaei H, Hossini SA, Jamshidifar Z. Effectiveness of mindfulness training on self-efficacy of patients infected by breast cancer. Proc Soc Behav Sci. 2014;159:426-429. [Google Scholar]
- 35. Labelle LE, Campbell TS, Faris P, Carlson LE. Mediators of mindfulness-based stress reduction (MBSR): assessing the timing and sequence of change in cancer patients. J Clin Psychol. 2015;71:21-40. [DOI] [PubMed] [Google Scholar]
- 36. White LL, Cohen MZ, Berger AM, Kupzyk KA, Bierman PJ. Self-efficacy for management of symptoms and symptom distress in adults with cancer: an integrative review. Oncol Nurs Forum. 2019;46:113-128. [DOI] [PubMed] [Google Scholar]
- 37. Conley CC, Bishop BT, Andersen BL. Emotions and emotion regulation in breast cancer survivorship. Healthcare (Basel). 2016;4:E56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Y, Yang Y, Zhang R, Yao K, Liu Z. The mediating role of mental adjustment in the relationship between perceived stress and depressive symptoms in hematological cancer patients: a cross-sectional study. PLoS One. 2015;10:e0142913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khoury B, Lecomte T, Fortin G, et al. Mindfulness-based therapy: a comprehensive meta-analysis. Clin Psychol Rev. 2013;33:763-771. [DOI] [PubMed] [Google Scholar]
- 40. Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78:169-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bennett-Levy J, Richards DA, Farrand P, et al. , eds. Low intensity CBT interventions: a revolution in mental health care. In: Oxford Guide to Low Intensity CBT Interventions. Oxford University Press; 2010:3-18. [Google Scholar]
- 43. Bower P, Kontopantelis E, Sutton A, et al. Influence of initial severity of depression on effectiveness of low intensity interventions: meta-analysis of individual patient data. BMJ. 2013;346:f540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weisel KK, Lehr D, Heber E, et al. Severely burdened individuals do not need to be excluded from internet-based and mobile-based stress management: effect modifiers of treatment outcomes from three randomized controlled trials. J Med Intern Res. 2018;20:e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khalil A, Faheem M, Fahim A, et al. Prevalence of depression and anxiety amongst cancer patients in a hospital setting: a cross-sectional study. Psychiatry J. 2016;2016:3964806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shankar A, Dracham C, Ghoshal S, Grover S. Prevalence of depression and anxiety disorder in cancer patients: an institutional experience. Indian J Cancer. 2016;53:432-434. [DOI] [PubMed] [Google Scholar]
- 47. Brandenbarg D, Maass SWMC, Geerse OP, et al. A systematic review on the prevalence of symptoms of depression, anxiety and distress in long-term cancer survivors: implications for primary care. Eur J Cancer Care (Engl). 2019;28:e13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124:2507-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Christodoulou G, Black DS. Mindfulness-based interventions and sleep among cancer survivors: a critical analysis of randomized controlled trials. Curr Oncol Rep. 2017;19:60. [DOI] [PMC free article] [PubMed] [Google Scholar]