Abstract
Recently, the microbiome has been gaining traction as a major player regulating various functions that correlate with many pathological conditions, including cancer. The central gut microbiota population has the capability to regulate normal inflammatory, immune, and metabolic functions, and disturbance in the balance of the normal microbiota population can subsequently induce pathological responses that closely relate with the mechanistic development and progression of cancer in various forms and sites. As a disease with major socioeconomic burden partly due to its current therapeutic options, modulating the imbalanced gut microbiota represents a novel option not only as an adjuvant therapy to relieve cancer treatment–related symptoms but also to influence cancer progression itself. In this review, we will discuss how the microbiome, specifically the gut microbiota, could affect cancer pathogenesis and what the effect of gut microbiota–targeting treatment options have on the many aspects of cancer pathologies based on the knowledge of recent years.
Keywords: dysbiosis, short chain fatty acids, estrobolome, probiotics, fecal microbiota transplantation
Current Situation of Cancer Therapy
Cancer is one of the fastest-growing types of disease that can affect the human body. As one of the main health problems worldwide, cancer is not only the second leading cause of death in the United States,1 but is also in the top 3 in the world.2 In 2018, the incidence of all types of cancer rose up to 18.1 million, with 9.6 million cancer deaths.3 Among the various forms of cancer occurring in many organs, it is well accepted that lung cancer has the highest prevalence, where in 2018, the World Health Organization has reported that there were 2.094 million new cases of lung cancer, followed by breast cancer (2089 million), colorectal cancer (1.8 million), prostate cancer (1.3 million), stomach cancer (1 million), liver cancer (841 080), esophageal cancer (572 043), and cervical cancer (569 847).3
One of the currently most problematic cancer-related issues is its treatment aspect, including its clinical efficacy and cost-effectiveness. One study estimated that the average expenditure of current cancer treatment per visit for inpatients in the United States ranged from $1157.7 to $7975.4 Another retrospective observational study by Yin et al4 in China confirmed the high cost of cancer care. As is the case with the overall incidence, lung cancer took up the highest percentage of treatment cost. The study by Yin et al4 identified that lung cancer had the highest cost (15% of overall cancer costs), followed by breast cancer (12%) and colorectal cancer (10%). Moreover, although about 30% to 40% of patients with cancer can be effectively cured using the current cancer chemotherapy,5 or an even higher percentage by utilizing comprehensive treatment options, including radiation and surgery, the overall cost of cancer care can cause a socioeconomic burden, with a less than ideal overall long-term survival rate, especially for several forms of cancer.6 Due to the nature of this disease that will affect all aspects of human life, including low quality of life, psychology, and financial toxicity, the eradication of cancer is important and valuable. To do so, novel treatment options to both treat and prevent cancer are needed to relieve the major burden of cancer.
Current Understanding of Microbiome and Gut Microbiota
The microbiome is the set of genomes from all the microorganisms found in a certain biosphere.7 On the other hand, microbiota refers to specific microorganisms that are located in specific environment.7,8 As such, all microorganisms could be called microbiota, such as bacteria, viruses, fungi, and parasites.7,8 Microbiota or microbes can be found in many parts of the human body, with the primary sites being the external and internal surfaces of the body, including gastrointestinal tract, skin, saliva, oral mucosa, vagina, and conjunctiva.9 In total, the number of human microbiota is estimated at up to 100 trillion symbiotic microbial cells.10 Host-microbe interactions occur primarily along mucosal surfaces, and one of the largest interfaces is the human intestinal mucosa.11 Because of that, it makes sense that the vast majority of commensal bacteria reside in the colon.12 From 1200 different bacterial species that have been identified, it is estimated that an individual has at least 160 different species in the gut.13,14 The gut microbial community is composed by 5 phyla, Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Verrucomicrobia.15 In normal conditions, Bacteroidetes and Firmicutes are the more dominant microbiota in the human gut.16 However, an imbalance in the gut microbial community, termed dysbiosis, could occur in the presence of a disease.17
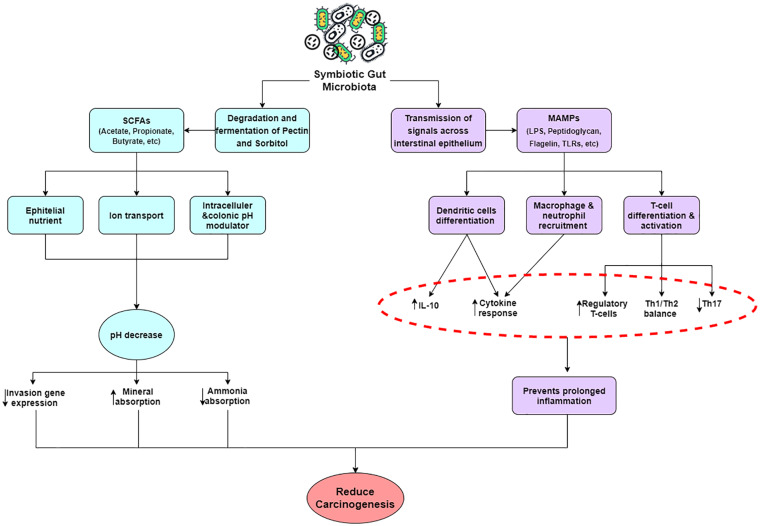
It has been established recently that there is a close relationship between host (human) and microbiota, and this forgotten organ plays novel roles in human health.11 Among the variable microbiomes in specific parts of the body, the gut microbiota has been known to play important roles in modulating immune responses of not only the local gastrointestinal tract but the whole body itself.14 Indeed, several groundbreaking findings have pointed out the critical role that the gut microbiome has in many pathological conditions. It has been implied in many reports that the gut microbiota mechanistically plays its important role in several ways. First, the microbiome harbored in the gut can help in biodegradation of complex sugars and glycans,13 for example, degradation of pectin and sorbitol.18 The long linear chains of α-1,4-glycoside-linked d-galacturonic acid (pectin) are also fermented by microflora.19 The major end product are the short-chain fatty acids (SCFAs); acetate, propionate and butyrate, the gases H2 and CO2, ammonia, amines, and phenols.20 In fact, the SCFAs have several different functions, including as nutrients for the colonic epithelium, modulators of colonic and intracellular pH, cell volume, and other functions associated with ion transport. In addition, the SCFAs are also regulators of proliferation, differentiation, and gene expression.21 The increase of SCFAs in the human body results in decreased pH, which indirectly influences the composition of the colonic microflora (the more acidic the pH, more the potentially pathogenic clostridia are reduced), decreases solubility of bile acids, increases absorption of minerals (indirectly), and reduces ammonia absorption by the protonic dissociation of ammonia and other amines (Figure 1).22,23
Figure 1.
Schematic diagram of the various signaling pathways and products maintained by an intact gut microbiota.
The homeostatic relationship between the gut microbiota and intestinal mucosal immune system is important in maintaining normal conditions of the body. The disruption of this interaction might link to various diseases.24,25 This begins with the transmission of gut microbiota signals across the intestinal epithelium.16 Microbe-associated molecular patterns such as lipopolysaccharide, peptidoglycan, flagellin, or other structural components are recognized by pattern-recognition receptors, such as Toll-like receptors (TLRs), NOD-like receptors, or RIG-1–like receptors, on epithelial and immune cells.26 Remarkably, lipopolysaccharides derived from different gut microbial species induce TLR4 signaling differently27 and might also have distinct effects early in life.28 Only a fraction of microbial signaling can be attributed to general recognition of microbial derivatives through pattern-recognition receptors,29 and there are probably more specific microbial signals that regulate host transcription.
Moreover, several studies have suggested that the gut microbiota has the ability to produce important cytokines that regulates intestinal mucosal homeostasis and provides resistance to the fungus Candida albicans. In addition, Lactobacilli have been known as a catabolizing agent of the amino acid tryptophan into the metabolite indole-3-aldehyde, a ligand to the aryl hydrocarbon receptor (AHR). AHR is expressed by innate lymphoid cells group 3 (ILC3s), and its activation induces the expression of the aforementioned cytokine interleukin (IL)-22. In turn, IL-22 mediates a pivotal innate antifungal resistance so the host can survive from “the fungus-shifted-induced-diseases,” and protect the intestinal mucosa from inflammation.30-32 Taken together, all of the aforementioned mechanistic insights provide proofs that maintaining a proper gut microbiota population could go a long way toward maintaining proper homeostatic balance of various functions of the body.
The Link Between the Microbiome and Cancer
During the past few years, numerous researchers have analyzed the correlation between cancer and microbiota, due to the connection between cancer and immune responses, particularly the central gut microbiota population. Several groups have tried to link a change in gut microbiota population with cancer occurrence and progression. Dysbiosis or disturbance of gut microbiota can increase the risk of a person to develop inflammatory, autoimmune, and malignant diseases.33,34 Although one would logically think that gut microbe dysbiosis is associated with gastrointestinal tract malignancies, which has been shown, much evidence also suggest that disturbances in the gut microbiota population could also be related to cancer of other organs, such as breast cancer, lung cancer, and adult T-cell leukemia.35-37
As mentioned previously, due to the fact that there is a specific set of bacteria that normally inhabit the gut mucosal layers, any changes that can cause a shift in the bacterial population toward any “unwanted” bacteria could induce pathogenic reactions and this so-called pathogenic reaction could cause different reactions and induce different forms of cancer in various sites. Mechanistically, there are several proposed pathways in play to explain the link between cancer occurrence and gut microbiota dysbiosis, especially to explain the manner in which some specific bacterium could induce and modulate cancer occurrence and progression. In general, the mechanism of how unwanted microbiota could modulate cancer pathophysiology can be divided into 3 classes of action:
Class A is defined as involving immunologic tissues, in which the bacteria stimulate chronic inflammation. Inflammatory mediators produced in this process cause or facilitate cell proliferation, mutagenesis, oncogene activation, and angiogenesis.38,39
Class B requires direct microbial interactions with parenchymal cells. Bacteria may affect cell proliferation that could activate pro-inflammatory and procarcinogenic NF-κB pathway and inhibit cellular apoptosis.38,39
Class C involves distant effects from local gut microbiota interactions. Bacteria generate various substances, including hormonal intermediates and metabolites, that could act in a carcinogenic manner to distant sites.38,39
With regard to pathogenic bacteria, several strains have been linked to cancer. The most well-known bacterium associated with development of cancer in human is Helicobacter pylori. This class I carcinogen bacterium, which is the main cause of chronic gastritis and peptic ulcer, could also induce further development of gastric adenocarcinoma, gastric mucosa–associated lymphoid tissue, and lymphoma with intestinal metaplasia.40 Additionally, this particular bacterium can also be found in the oral cavity.
In other examples, various studies have also identified some specific species that remarkably correlate with oral squamous cell carcinoma (OSCC), such as Streptococcus sp, Peptostreptococcus sp, Prevotella sp, Fusobacterium sp, Porphyromonas gingivalis, and Capnocytophaga gingivalis.40-43 Remarkably, the discovery of specific bacterial species in OSCC samples from humans have been reported. One study performed immunohistochemical staining to investigate the presence of P gingivalis. The result showed that P gingivalis was significantly positive only in the OSCC sample in comparison to controls.44 Furthermore, other studies also have found that 3 specific species were increased in the saliva from 80% of individuals with OSCC; which are Capnocytophaga gingivalis, Prevotella melaninogenica, and Streptococcus mitis. With 80% sensitivity and 82% specificity, it might become a diagnostic indicator of OSCC and a true proof that a specific set of bacteria is needed to induce OSCC.42 In addition to microbe-associated OSCC, microbiota also have been linked with esophageal diseases such as Barret’s esophagus (BE), esophageal squamous cell cancers, and esophageal adenocarcinoma. Plenty of research has reported the correlation of microbe and cancerous esophagus diseases. For instance, researchers from the Esophageal and Lung Institute, Canonsburg, PA, found that Escherichia coli was detected in BE and esophageal adenocarcinoma patient groups but was absent in the tumor-adjacent normal epithelium, dysplasia, and the gastroesophageal reflux disease groups, implicating the need for E coli presence for BE development to occur.45
Moving to another organ, it has already been established that breast cancer pathology is associated with estrogen, and interestingly, the systemic estrogens are also modulated by gut microbiota.35 The connection of breast cancer with gut microbiota is bridged by a set of enteric genes whose products are of capable metabolizing estrogen, termed the estrobolome. The estrobolome enteric bacteria possess β-glucuronidases and β-glucosidases, hydrolytic enzymes involved in the deconjugation of estrogens. An estrobolome enriched in enzymes favoring deconjugation would promote reabsorption of free estrogens, and thus increase relative total estrogen burden.35 Because estrogen is widely recognized as a causal factor in the etiology of hormone receptor–positive breast cancer and plays an important role in the initiation and promotion of neoplastic growth, the increase in total estrogen burden would be disadvantageous.46 Based on an integrated microbial genomes database, there are more than 50 bacteria colonizing the human intestinal tract that encode β-glucuronidases and/or β-glucosidases including Alistipes, Bacteroides, Bifidobacterium, Citrobacter, Clostridium, Dermabacter, Escherichia, Faecalibacterium, Lactobacillus, Marvinbryantia, Propionibacterium, Roseburia, Tannerella, and many more.35 Any overabundance found in this set of bacteria could induce further imbalance in the estrogen burden and subsequently promote breast cancer.
Moreover, not only the rise of the pathogenic bacteria but also the decrease in different normal inhabitants of the gut or probiotics could also induce an imbalance in the aforementioned normal inflammatory and immune responses of the body, both of which are strongly related to carcinogenesis. As an example, the correlation between microbiota and lung cancer has been recently reported as being related to such an imbalance. A study held by Zhuang et al36 found that although there was no difference in gut microbial alpha diversity, microbial composition, nevertheless, showed significant differences compared with healthy controls. These differences were mainly caused by Actinobacteria (phylum level), Bifidobacterium, and Enterococcus (genus level), which might have a significant potential as biomarkers for lung carcinogenesis.36 Actinobacteria was found as the strongest marker in healthy controls, and it was elevated in healthy individuals. Bifidobacteriales disclosed a major abundance in healthy controls, whereas the elevated bacteria in the lung cancer groups were Enterococcaceae.36 The decrease of the phylum Actinobacteria in the human gut may also be involved in the pathogenesis of lung cancer. This notion is supported by a finding by Zhou et al,47 where they found that the Actinobacteria produce cancer-killing substance in the human intestine, while its bioactive secondary metabolites have potent cancer-suppressing activity. As such, not only it is important to minimize the growth of pathogenic bacteria in the gut microbiota, but it is also essential that normal bacteria population to be maintained to achieve optimal microbiota function.
As mentioned, the difference in gut microbiota composition could also affect the immune response to various pathogens, including those related to cancer pathogenesis. One aspect that has been recently studied is the immune checkpoints, key regulators of the immune responses in part responsible for carcinogenesis. In particular, 2 molecules have been well studied up to this point, CTLA-4 and PD-1.48 CTLA-4, a receptor constitutively expressed in regulatory T-cells, is known to play a role in dampening T-cell activation and subsequent responses via its capability to act as a CD28 antagonist.49 One of the main consequences of this is the decrease of the key cytokine IL-2 that is already known to be pivotal in modulating the differentiation of CD4+ regulatory T-cells into T-helper 1 or T-helper 2 cells while subsequently inhibiting T-helper 17 differentiation, thereby serving as a so-called “regulator” for Th1- and Th2-regulated immune responses.50,51 On the other hand, PD-1 is a transmembrane receptor with known ligands PD-L1 and PD-L2 that acts as a regulator in the event of infection.48,49 The PD-1/PD-L1 interactions will inhibit the activation and differentiation of effector T-cells and their subsequent functions, rendering them exhausted. On this aspect, the impact of gut microbiota populations has been recently studied by several groups, especially in the condition of the blockade of CTLA4 or PD-1 using therapeutic agents (also known as immune checkpoint inhibitors [ICIs]).48,49 As mentioned later, several microbiotas are known to be able to modulate the efficacy of ICI therapy in cancer conditions due to their various functions, which will be elaborated further on.
Modulating Gut Microbiota as Treatment Strategy of Cancer
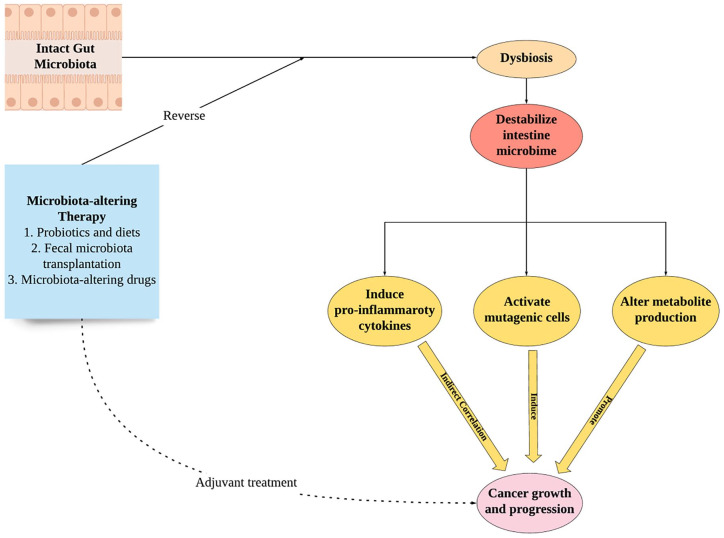
It has been shown how disturbances in the gut microbiota population balance could cause unwanted bacteria to prosper and exert their pathological and carcinogenic effects; thus, maintaining an intact and normal gut microbiota is essential to prevent such phenomena.52 As such, the capability to modulate or reverse the unbalanced gut microbiota population becomes important to achieve, or reacquire, said normalcy. There are several ways to modulate the gut microbiota population clinically.53 The most well-known and established method to alter gut microbiota, which is the consumption of probiotics and other specific dietary products, such as yogurt or fiber-rich food, has previously been explored in several conditions, such as cardiovascular diseases, chronic kidney disease, brain injury, and obesity, among others, with varying degrees of success.54-57 Another option to modulate microbiota is via the fecal microbiota transplantation (FMT), in which liquefied and filtered stool from a healthy donor would be transplanted to recipients during various procedures, such as colonoscopy or enema administration.58 FMT is currently considered as a treatment option in recurrent Clostridium difficile–infected patients.58 Together with probiotics administration, FMT is also considered an effective option to alter gut microbiota and, subsequently, other local microbiota populations (Figure 2).
Figure 2.
Schematic diagram of the correlations between gut microbiota, cancer, and its modulation as a novel therapeutic approach.
It is interesting to note that most, if not all, of the treatment options that have been explored in the field of microbiota mainly modulate the gut microbiota, rather than the local microbiota population of various target organs/cells. This is mostly due to the function of the gut microbiota as a central regulator for local populations through its ability to centrally modulate the immune response and subsequent cellular gene expression patterns, as previously explained.34 In the intact gut microbiota condition, it has been shown in many studies that proper microbiota-driven innate immunity activation, through the regulation of CD4+ and CD8+ T-cells functions, could act as both a sensor and an inducer of needed reactions to defend host organisms, both locally and systemically, while during dysbiosis or imbalanced gut microbiota condition, the balance of this function would also be disturbed and give rise to self-reactive T-cells, which could potentially induce prolonged local and systemic pro-inflammatory and carcinogenic effects.33,52
Another example of how gut microbiota population affects cancer treatment is in the aforementioned immune checkpoints and the ICIs, as previously mentioned. Several studies have proven how specific microbiota activities could positively affect ICI efficacy in immunocompromised patients, including those suffering from cancers, and this affects both CTLA-4 inhibitor and PD-1 inhibitor groups. First, a 2015 study from Vétizou et al59 revealed that in the presence of Bacteroides thetaiotaomicron and Bacteroides fragilis, CTLA-4–specific 9D9 antibody has an improved capability of binding and blocking CTLA-4 activity in antibiotic-treated mice with tumors. This effect was attributed to decreased subclinical colitis signs, increased Th1 immune response activities, and promotion of the maturation of intratumor dendritic cells. The authors similarly applied FMT from donor patients to mice and found that mice transplanted with feces from patients with Bacteroides-rich microbiota population responded better to CTLA-4 inhibitor treatment. In a more clinical setting, it has been shown by Gopalakrishnan et al that patients with favorable PD-1 inhibitor response have a distinct microbiota population in comparison to those with unfavorable responses.60 Specifically, they found that responders to PD-1 inhibitor therapy have enrichment in Faecalibacterium genus, Ruminococcaceae family and Clostridiales order. Enrichment of the aforementioned kinds of microbiota were revealed to increase CD4+ and CD8+ effector cells with preserved cytokine responses to anti–PD-1 therapy. Conversely, those with unfavorable anti-PD-1 responses have an abundance of Bacteroidales population with subsequent increase in regulatory T-cells and blunted cytokine responses. In short, the capability of gut microbiota in modulating not only local but also systemic immune response as some sort of central regulator is what drives the current treatment options to also be focused on this particular population of bacteria. As will be discussed later, the ways to alter gut microbiota would include probiotics, FMT, and other microbiota-altering agents.
Probiotics
Utilizing the aforementioned gut microbiota–altering agents or therapies in cancer conditions has been explored or is being explored as an adjuvant therapy to directly affect the progression and growth of cancer cells. Among the modalities available to alter gut microbiota, probiotics have been the most extensively studied, due to their availability, low cost, and overall safe nature, although other microbiota-altering dietary products such as yogurt or fibers are also available.34,53,61 One trial held in Monza, Italy, analyzed the administration of a probiotic mixture of Bifidobacterium longum and Lactobacillus johnsonii perioperatively in colorectal cancer patients undergoing surgery and found, in conjunction with a shift of the colonic mucosal microbiota population in the probiotic-treated group, a higher expression of CD3, CD4, CD8, and naïve and memory lymphocyte subsets compared with the placebo-treated group.62 Moreover, the proliferative capabilities of the ex vivo colonic mucosal cells were also dramatically reduced in the probiotics-treated group. Similarly, other groups have also shown that by treating colorectal cancer patients with a postoperative probiotic mix, a marked reduction of circulating pro-inflammatory cytokines such as IL-6, tumor necrosis factor–α (TNF-α), IL-17A, IL-17C, and IL-22 could be observed.63 All of these results collectively suggest that altering the microbiota could affect the progression of cancer through shifting of inflammatory and immune responses toward the anticarcinogenic phenotype clinically.
In a more basic and translational setting, a study by Li et al64 showed that administering a probiotic mix in vivo, this time a novel mix called Prohep consisting of Lactobacillus rhamnosus GG, viable E coli Nissle 1917, and heat-inactivated VSL#3, could affect the progression of hepatocellular carcinoma cell growth after subcutaneous tumor inoculation in mice in a manner almost similar to cisplatin treatment. This effect is caused by the ability of Prohep to alter T-helper 17 cell distribution and polarization toward the anti-migratory and subsequent anti-inflammatory state, which is important because Th17 is the T-cell with the ability to secrete the pro-inflammatory and pro-angiogenesis IL-17 cytokine that is important in hepatocellular carcinoma and various other cancer development.65 Prohep could induce this positive effect due to its ability to alter gut microbiota composition, in which it showed the increase of the Bacteroidetes phylum, the phylum important in producing acetate and propionate from fiber.64 Moreover, several major anti-inflammatory bacterial genera were significantly increased in the gut population after Prohep treatment, including Butyricimonas and Prevotella. This study underscores greatly how probiotic treatment could affect the immune, metabolic, and inflammatory responses of the whole body with concurrent anticarcinogenic effect in vivo.
Probiotics have also been shown to positively affect various pathological conditions related to cancer and/or conventional cancer treatment modalities. One such condition is gastrointestinal disturbance, including nausea, vomiting, diarrhea, and/or constipation, in which changing the microbiota population has demonstrated success. One study from Canada observed the effect of probiotics in pelvic cancer patients undergoing radiation therapy, in which radiation-induced diarrhea is a common occurrence.66,67 Remarkably, probiotic administration could reduce the incidence of diarrhea with no apparent side effects. Similar studies analyzed the effects of probiotics on other possible side effects in lung cancer patients undergoing chemotherapy, in addition to reducing the systemic inflammatory responses observed from the neutrophil and lymphocyte counts.68 Taken together, probiotics are a promising pathway toward maintaining healthy gut microbiota and concurrent anticarcinogenic effects. As there are currently various trials analyzing the effect of probiotics in cancer, it will be interesting to see future developments of probiotic use in this condition.
Fecal Microbiota Transplantation
In contrast to probiotics, FMT is not as commonly explored in the field of cancer therapy in comparison to the aforementioned probiotics or other dietary products.69 This is partly due to the perceived possible infection risk of translocating bacteria from a different individual, especially in immunocompromised individuals. Due to the need to perform colonoscopy or endoscopy to infuse the donor feces, studies have also highlighted the possible risk of FMT procedure-related adverse effects.70,71 In addition to that, the possible adverse effect of incurring other noninfectious diseases by modulating the microbiota has also been mentioned, although studies reporting this phenomenon have been rare.70 Indeed, due to these issues, several studies have excluded immunocompromised patients from their FMT trials, and recommendations from several health organization followed suit with the cautionary approach to FMT treatment in cancer patients.71
Even so, FMT has been recently utilized in a basic translational setting to positive effect by Riquelme et al72 in their study on pancreatic cancer. In their study utilizing FMT in pancreatic cancer patients with donor controls, they confirmed the ability of gut microbiota to modulate the local tumor microbiota environment and subsequently alter the responses needed for tumor growth, evidenced by the changes in gene expression patterns of various inflammatory pathways The group receiving FMT from long-term survivor pancreatic cancer patients had considerably lower procarcinogenic features in comparison to those receiving the short-term survivor pancreatic cancer patients’ FMT.72 Similarly, another study from Li et al64 found that FMT of fecal samples from colorectal patients could cause enhanced progression of intestinal adenoma in vivo. Additionally, one other study also found that the use of FMT as an adjuvant therapy to chemotherapy treatment with 5-fluorouracil (5-FU) could prevent 5-FU–induced gut dysbiosis.73 This is another indication that having an intact, healthy microbiota population could help in terms of halting cancer progression.
Mechanistic insights with regard to the FMT-cancer link showed that, among the various inflammatory and immune pathways that are modulated after FMT, restoring the balance of TLR signaling pathways represent one major advantage of FMT application in cancer.71 It is well known that TLR4 signaling can cause aberrant immune responses skewing toward pro-inflammatory pathways, but other forms of TLRs, such as TLR2, have been linked with anti-inflammatory pathway activation.27,74 As such, FMT represents one alternative to restoring the so-called anti-inflammatory pathway and prevent further progression of cancer.
Unfortunately, limited clinical evidence currently exists of FMT application due to the aforementioned perceived risk of infection beyond its usage for recurrent Clostridium difficile infection, highlighted by the fact that most of the studies conducted have been limited to human-to-mice transplantation. As controversial as it is, several groups are trying to show that the benefits of human-to-human FMT in cancer patients outweigh its risk. Currently, several trials are ongoing in the clinical application for FMT in cancer patients. For example, one group from Israel has reported their preliminary findings from 3 anti-PD-1 refractory patients undergoing FMT from anti–PD-1 responsive donors.75 Preliminary reports from the investigators suggested the overall safety of this combined approach with increased tumor CD68+ and CD8+ T-cell infiltrations.75 Nevertheless, while promising, it is understandable that a cautious approach is being taken to this gut microbiota-altering therapeutic alternative.
Other Treatments Targeting Microbiota
In addition to probiotics, diet changes, and FMT, many drugs are known to change the population of the microbiota. One logical example would be the antibiotics. Several classes of antibiotics have been known to have the effects, or side effects, of shifting gut microbiota population. One such class is the macrolides, in which one study has shown that there is a shift toward certain phyla (ones that includes E coli and Campylobacter) in microbiota population among infants prescribed azithromycin.76 This microbiota-altering phenomenon is not exclusive to antibiotics. For example, it is known that statins could also alter microbiota, which is hypothesized to be related to the changes in lipid and glucose metabolism induced by statins.77
Unfortunately, although most of the aforementioned drugs have the potential to alter the microbiota population, most of the alterations reported have a negative effect toward microbiota population balance, meaning that rather than shifting the population toward the needed population for a positive health outcome, those therapies could rather induce dysbiosis and subsequent pathological consequences. The aforementioned statin treatments, in this case atorvastatin and rosuvastatin, could induce a shift in microbiota population toward the Bacteroides and Mucispirillum, both of which induce pro-inflammatory cytokine expression and release, such as TGF-β and IL-1β.77 The subsequent changes in metabolite availability, namely, the SCFAs, due to statin-induced microbiota composition shift is thought to induce the pro-inflammatory responses of the host immune system.77 In addition, reported effects of antibiotics have also highlighted the possibility of dysbiosis, or rather a shift toward the so-called “unwanted” bacterial populations, which would not be beneficial.76 Even so, the promise of microbiota-altering drugs remains high, especially considering the increased efficacy these drugs can potentially have in comparison to probiotics or dietary changes alone. As such, many researchers are taking interest in future utilization of antibiotics’ effect on gut microbiota (Table 1).
Table 1.
Examples of Gut Microbiota Alteration-Based Therapy Application in Various Cancers.
| Cancer Type | Therapy | Response to Therapy | Subject | Reference |
|---|---|---|---|---|
| Colorectal cancer | Probiotics (Bifidobacterium longum + Lactobacillus johnsonii) | Greater expression of CD3, CD4, CD8, naïve and memory lymphocytes. Decrease of CD83-123, CD83-HLADR, and CD83-11c | Human | Gianotti et al62 |
| Colorectal cancer | Probiotics (Lactobacillus and Bifidobacteria mix) | Postsurgical reduction in circulating inflammatory markers (eg, TNF-α, IL-6, IL-17a, IL-17c) | Human | Zaharuddin et al63 |
| Lung cancer | Probiotics (Clostridium butyricum) | Reduced lymphocyte count, platelet/lymphocyte ratio, and neutrophil/lymphocyte ratio. Decrease in pathogenic genera and increase in SCFA-producing genera | Human | Tian et al68 |
| Gastric cancer | Fiber-rich diet with/without probiotics | Reduced chemotherapy-induced gastrointestinal disorders | Human | Zhao et al78 |
| Breast cancer | Probiotics-rich diet | Reduced incidence of sarcopenic obesity due to antiestrogenic medication | Human | Artene et al79 |
| Pelvic cancer | Probiotics (Lactobacillus acidophilus + Bifidobacterium longum) | Reduced radiation-induced grade 2-4 diarrhea | Human | Demers et al66 |
| Colorectal cancer | Probiotics (Lactobacillus paracasei K5) | Antiproliferative and apoptotic in vitro effects | Caco-2 cells | Chondrou et al80 |
| Colorectal cancer | Probiotics (Lactobacillus rhamnosus+ Lactobacillus acidophilus) with Celecoxib | Reduction of tumor burden and multiplicity in addition to increased apoptosis activity | Rats | Sharaf et al81 |
| Colorectal cancer | FMT from colorectal cancer patients or donor | Increased intestinal tumor proliferation with decreased apoptosis and increased pro-inflammatory cytokines expression through the Wnt signaling activation | Mouse | Li et al64 |
| Pancreatic cancer | FMT from long-term survivor of pancreatic cancer | Gut microbiota composition shift toward a more favorable population for inhibiting tumor growth through CD8 T-cells recruitment and activation | Mouse | Riquelme et al72 |
| Epithelial cancers | FMT from PD-1 blockade-responding patients and Probiotics (Akkermansia muciniphila) | Reduced tumor growth activity and increased apoptosis, with additional Akkermansia Muciniphila–driven Th1 immunosurveilence responses | Mouse | Routy et al82 |
Abbreviations: SCFA, short-chain fatty acid; FMT, fecal microbiota transplantation.
Altering Gut Microbiota as Cancer Prevention
Gut microbiota composition and altering the gut microbiota population is not only a treatment option but has also been recently shown to be beneficial in preventing several kinds of cancer. A study by Yang et al83 pooling cohorts from 10 countries examined how dietary patterns of yogurt and fiber consumption, 2 gut microbiota–altering agents, could have a long-term effect in lung cancer occurrence. In relation to that study, various studies have found that with increased population-altering dietary consumption, such as yogurt and fiber consumption amount, there is an intact, healthy gut microbiota population composed of mainly of the normal bacterial population.61 As mentioned, these bacteria are responsible for the maintenance of healthy immune response and production of various metabolic products, in addition to suppression of aberrant inflammatory responses.
The positive effect of gut microbiota–altering treatments has not only been studied in lung cancer but also in other forms of cancer, such as colorectal and oral cancers, among others.84,85 Moreover, in an interesting development, a group from Japan proposed utilizing recombinant Bifidobacterium displaying Wilms’ Tumor 1 (WT1) protein, a protein associated with pediatric renal cancer cells, as a vaccine via its gut microbiota function and population-altering capability.86 These examples, combined with other emerging evidence, highlight the potential of normal gut microbiota composition maintenance in preventing carcinogenesis in various sites.
Conclusion
Modulating gut microbiota to relieve the burden of cancer is a novel yet important option as a future therapeutic possibility, especially as an additional therapeutic option to increase the efficacy and safety of other cancer treatment modalities through its central immune modulation mechanism. Additionally, treating dysbiosis of the gut microbiota could also be a novel option for cancer prevention.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Tatsuya Nagano  https://orcid.org/0000-0003-0790-5139
https://orcid.org/0000-0003-0790-5139
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. World health statistics overview 2019: monitoring health for the SDGs. https://apps.who.int/iris/bitstream/handle/10665/311696/WHO-DAD-2019.1-eng.pdf?ua=1. Updated 2019. Accessed November 13, 2019.
- 3. International Agency for Research on Cancer, World Health Organization. Latest global cancer data: Cancer burden rises to 18.1 million new and 9.6 million cancer deaths in 2018. https://www.who.int/cancer/PRGlobocanFinal.pdf. Accessed April 14, 2020.
- 4. Yin X, Xu Y, Man X, et al. Direct costs of both inpatient and outpatient care for all type cancers: the evidence from Beijing, China. Cancer Med. 2019;8:3250-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dahl BJ. The cost of oncology drugs: a pharmacy perspective, part I. Fed Pract. 2016;33(suppl 1):22S-25S. [PMC free article] [PubMed] [Google Scholar]
- 6. Paolillo M, Boselli C, Schinelli S. Glioblastoma under siege: an overview of current therapeutic strategies. Brain Sci. 2018;8:E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fios Genomics. Microbiome vs microbiota. https://www.fiosgenomics.com/microbiome-vs-microbiota/. Accessed November 13, 2019.
- 8. Harvard T.H. Chan School of Public Health. The nutrition source. The microbiome. https://www.hsph.harvard.edu/nutritionsource/microbiome/. Accessed November 13, 2019.
- 9. Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337-340. [DOI] [PubMed] [Google Scholar]
- 10. Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. 2012;70(suppl 1):S38-S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107-133. [DOI] [PubMed] [Google Scholar]
- 13. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eckburg PB, Bik EM, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079-1089. [DOI] [PubMed] [Google Scholar]
- 16. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Messer JS, Chang EB. Microbial physiology of the digestive tract and its role in inflammatory bowel diseases. In: Said HM, ed. Physiology of the Gastrointestinal Tract. 6th ed. London, England: Academic Press; 2018:795-810. [Google Scholar]
- 18. Dongowski G, Lorenz A, Anger H. Degradation of pectins with different degrees of esterification by Bacteroides thetaiotaomicron isolated from human gut flora. Appl Environ Microbiol. 2000;66:1321-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991;70:443-459. [DOI] [PubMed] [Google Scholar]
- 20. Wong JMW, de Souza R, Kendall CWC, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235-243. [DOI] [PubMed] [Google Scholar]
- 21. Cook SI, Sellin JH. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther. 1998;12:499-507. [DOI] [PubMed] [Google Scholar]
- 22. Roberfroid MB. Inulin-type fructans: functional food ingredients. J Nutr. 2007;137(11 suppl):2493S-2502S. [DOI] [PubMed] [Google Scholar]
- 23. Jackson AA. Aminoacids: essential and non-essential? Lancet. 1983;321:1034-1037. [DOI] [PubMed] [Google Scholar]
- 24. Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65-74. [DOI] [PubMed] [Google Scholar]
- 25. Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75-84. [DOI] [PubMed] [Google Scholar]
- 26. Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819-826. [DOI] [PubMed] [Google Scholar]
- 27. Bäckhed F, Normark S, Schweda EKH, Oscarson S, Richter-Dahlfors A. Structural requirements for TLR4-mediated LPS signalling: a biological role for LPS modifications. Microbes Infect. 2003;5:1057-1063. [DOI] [PubMed] [Google Scholar]
- 28. Vatanen T, Kostic AD, d’Hennezel E, et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165:842-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larsson E, Tremaroli V, Lee YS, et al. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut. 2012;61:1124-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372-385. [DOI] [PubMed] [Google Scholar]
- 31. Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116-132. [DOI] [PubMed] [Google Scholar]
- 32. Lee JS, Cella M, McDonald KG, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Francescone R, Hou V, Grivennikov SI. Microbiome, inflammation, and cancer. Cancer J. 2014;20:181-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bultman SJ. Emerging roles of the microbiome in cancer. Carcinogenesis. 2013;35:249-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwa M, Plottel CS, Blaser MJ, Adams S. The intestinal microbiome and estrogen receptor–positive female breast cancer. J Natl Cancer Inst. 2016;108(8). doi: 10.1093/jnci/djw029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhuang H, Cheng L, Wang Y, et al. Dysbiosis of the gut microbiome in lung cancer. Front Cell Infect Microbiol. 2019;9:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manzo VE, Bhatt AS. The human microbiome in hematopoiesis and hematologic disorders. Blood. 2015;126:311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10:324-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y, Wang X, Li H, Ni C, Du Z, Yan F. Human oral microbiota and its modulation for oral health. Biomed Pharmacother. 2018;99:883-893. [DOI] [PubMed] [Google Scholar]
- 40. Karpiński TM. Role of oral microbiota in cancer development. Microorganisms. 2019;7:E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sasaki M, Yamaura C, Ohara-Nemoto Y, et al. Streptococcus anginosus infection in oral cancer and its infection route. Oral Dis. 2005;11:151-156. [DOI] [PubMed] [Google Scholar]
- 42. Mager DL, Haffajee AD, Devlin PM, Norris CM, Posner MR, Goodson JM. The salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 2005;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pushalkar S, Ji X, Li Y, et al. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012;12:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Katz J, Onate MD, Pauley KM, Bhattacharyya I, Cha S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci. 2011;3:209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zaidi AH, Kelly LA, Kreft RE, et al. Associations of microbiota and toll-like receptor signaling pathway in esophageal adenocarcinoma. BMC Cancer. 2016;16:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Furth PA, Cabrera MC, Díaz-Cruz ES, Millman S, Nakles RE. Assessing estrogen signaling aberrations in breast cancer risk using genetically engineered mouse models. Ann N Y Acad Sci. 2011;1229:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou YJ, Zhao DD, Liu H, et al. Cancer killers in the human gut microbiota: diverse phylogeny and broad spectra. Oncotarget. 2017;8:49574-49591. doi: 10.18632/oncotarget.17319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yi M, Yu S, Qin S, et al. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J Hematol Oncol. 2018;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology. 2016;5:e1163462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cote-Sierra J, Foucras G, Guo L, et al. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wong SH, Kwong TNY, Wu CY, Yu J. Clinical applications of gut microbiota in cancer biology. Semin Cancer Biol. 2019;55:28-36. [DOI] [PubMed] [Google Scholar]
- 53. Ma W, Mao Q, Xia W, Dong G, Yu C, Jiang F. Gut microbiota shapes the efficiency of cancer therapy. Front Microbiol. 2019;10:1050. doi: 10.3389/fmicb.2019.01050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120:1183-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li FX, Wang MH, Wang JP, Li R, Zhang Y. Alterations to the gut microbiota and their correlation with inflammatory factors in chronic kidney disease. Front Cell Infect Microbiol. 2019;9:206. doi: 10.3389/fcimb.2019.002069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Angelucci F, Cechova K, Amlerova J, Hort J. Antibiotics, gut microbiota, and Alzheimer’s disease. J Neuroinflammation. 2019;16:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brusaferro A, Cozzali R, Orabona C, et al. Is it time to use probiotics to prevent or treat obesity? Nutrients. 2018;10:E1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gupta A, Khanna S. Fecal microbiota transplantation. JAMA. 2017;318:102. [DOI] [PubMed] [Google Scholar]
- 59. Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Klement RJ, Pazienza V. Impact of different types of diet on gut microbiota profiles and cancer prevention and treatment. Medicina (Kaunas). 2019; 55:E84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gianotti L, Morelli L, Galbiati F, et al. A randomized double-blind trial on perioperative administration of probiotics in colorectal cancer patients. World J Gastroenterol. 2010;16:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zaharuddin L, Mokhtar NM, Nawawi KNM, Ali RAR. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019;19:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li L, Li X, Zhong W, et al. Gut microbiota from colorectal cancer patients enhances the progression of intestinal adenoma in Apcmin/+ mice. EBioMedicine. 2019;48:301-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Guéry L, Hugues S. Th17 cell plasticity and functions in cancer immunity. Biomed Res Int. 2015;2015:314620. doi: 10.1155/2015/314620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Demers M, Dagnault A, Desjardins J. A randomized double-blind controlled trial : Impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr. 2014;33:761-767. doi: 10.1016/j.clnu.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 67. Ciorba MA, Hallemeier CL, Stenson WF, Parikh PJ. Probiotics to prevent gastrointestinal toxicity from cancer therapy: an interpretive review and call to action. Curr Opin Support Palliat Care. 2015;9:157-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tian Y, Li M, Song W, Jiang R, Li YQ. Effects of probiotics on chemotherapy in patients with lung cancer. Oncol Lett. 2019;17:2836-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen D, Wu J, Jin D, Wang B, Cao H. Fecal microbiota transplantation in cancer management: current status and perspectives. Int J Cancer. 2019;145:2021-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. van Nood E, Speelman P, Nieuwdorp M, Keller J. Fecal microbiota transplantation: facts and controversies. Curr Opin Gastroenterol. 2014;30:34-39. [DOI] [PubMed] [Google Scholar]
- 71. Wardill HR, Secombe KR, Bryant RV, Hazenberg MD, Costello SP. Adjunctive fecal microbiota transplantation in supportive oncology: emerging indications and considerations in immunocompromised patients. EBioMedicine. 2019;44:730-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Riquelme E, Zhang Y, Zhang L, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178:795-806.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Le Bastard Q, Ward T, Sidiropoulos D, et al. Fecal microbiota transplantation reverses antibiotic and chemotherapy-induced gut dysbiosis in mice. Sci Rep. 2018;8:6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hoppstädter J, Dembek A, Linnenberger R, et al. Toll-like receptor 2 release by macrophages: an anti-inflammatory program induced by glucocorticoids and lipopolysaccharide. Front Immunol. 2019;10:1634. doi: 10.3389/fimmu.2019.01634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Baruch EN, Youngster I, Ortenberg R, et al. Abstract CT042: Fecal microbiota transplantation (FMT) and re-induction of anti-PD-1 therapy in refractory metastatic melanoma patients—preliminary results from a phase I clinical trial (NCT03353402). In: Proceedings of the American Association for Cancer Research Annual Meeting; March 29 to April 3, 2019; Atlanta, GA. [Google Scholar]
- 76. Parker EPK, Praharaj I, John J, et al. Changes in the intestinal microbiota following the administration of azithromycin in a randomised placebo-controlled trial among infants in south India. Sci Rep. 2017;7:9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim J, Lee H, An J, et al. Alterations in gut microbiota by statin therapy and possible intermediate effects on hyperglycemia and hyperlipidemia. Front Microbiol. 2019;10:1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhao R, Wang Y, Huang Y, et al. Effects of fiber and probiotics on diarrhea associated with enteral nutrition in gastric cancer patients: a prospective randomized and controlled trial. Medicine (Baltimore). 2017;96:e8418. doi: 10.1097/MD.0000000000008418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Artene DV, Bordea CI, Blidaru A. Results of 1-year diet and exercise interventions for ER+/PR±/HER2-breast cancer patients correlated with treatment type. Chirurgia (Bucur). 2017;112:457-468. [DOI] [PubMed] [Google Scholar]
- 80. Chondrou P, Karapetsas A, Kiousi DE, et al. Lactobacillus paracasei K5 displays adhesion, anti-proliferative activity and apoptotic effects in human colon cancer cells. Benef Microbes. 2018;9:975-983. [DOI] [PubMed] [Google Scholar]
- 81. Sharaf LK, Sharma M, Chandel D, Shukla G. Prophylactic intervention of probiotics (L acidophilus, L rhamnosus GG) and celecoxib modulate Bax-mediated apoptosis in 1,2-dimethylhydrazine-induced experimental colon carcinogenesis. BMC Cancer. 2018;18:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91-97. [DOI] [PubMed] [Google Scholar]
- 83. Yang JJ, Yu D, Xiang YB, et al. Association of dietary fiber and yogurt consumption with lung cancer risk: a pooled analysis. JAMA Oncol. 2020;6:e194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li J, Sung CYJ, Lee N, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A. 2016;113:E1306-E1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wei D, Heus P, van de Wetering FT, van Tienhoven G, Verleye L, Scholten RJ. Probiotics for the prevention or treatment of chemotherapy- or radiotherapy-related diarrhoea in people with cancer. Cochrane Database Syst Rev. 2018;8:CD008831. doi: 10.1002/14651858.CD008831.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kitagawa K, Oda T, Saito H, et al. Development of oral cancer vaccine using recombinant Bifidobacterium displaying Wilms’ tumor 1 protein. Cancer Immunol Immunother. 2017;66:787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]