ABSTRACT
Background
Observational evidence suggests higher nut consumption is associated with better glycemic control; however, it is unclear if this association is causal.
Objectives
We aimed to conduct a systematic review and meta-analysis of randomized controlled trials to examine the effect of tree nuts and peanuts on markers of glycemic control in adults.
Methods
A systematic review and meta-analysis of randomized controlled trials was conducted. A total of 1063 potentially eligible articles were screened in duplicate. From these articles, 40 were eligible for inclusion and data from these articles were extracted in duplicate. The weighted mean difference (WMD) between the nut intervention and control arms was determined for fasting glucose, fasting insulin, glycated hemoglobin (HbA1c), and homeostasis model assessment of insulin resistance (HOMA-IR) using the DerSimonian and Laird random-effects method. For outcomes where a limited number of studies were published, a qualitative synthesis was presented.
Results
A total of 40 randomized controlled trials including 2832 unique participants, with a median duration of 3 mo (range: 1–12 mo), were included. Overall consumption of tree nuts or peanuts had a favorable effect on HOMA-IR (WMD: −0.23; 95% CI: −0.40, −0.06; I2 = 51.7%) and fasting insulin (WMD: −0.40 μIU/mL; 95% CI: −0.73, −0.07 μIU/mL; I2 = 49.4%). There was no significant effect of nut consumption on fasting blood glucose (WMD: −0.52 mg/dL; 95% CI: −1.43, 0.38 mg/dL; I2 = 53.4%) or HbA1c (WMD: 0.02%; 95% CI: −0.01%, 0.04%; I2 = 51.0%).
Conclusions
Consumption of peanuts or tree nuts significantly decreased HOMA-IR and fasting insulin; there was no effect of nut consumption on HbA1c or fasting glucose. The results suggest that nut consumption may improve insulin sensitivity. In the future, well-designed clinical trials are required to elucidate the mechanisms that account for these observed effects.
Keywords: tree nuts, peanuts, glucose, insulin, HbA1c, insulin sensitivity, HOMA-IR, diabetes, meta-analysis, systematic review
Introduction
Over 1 million deaths were attributable to diabetes worldwide in 2016, an increase of 31% since 2006 (1). Premature mortality due to diabetes has increased by 25% in the past decade and in 2016 accounted for 28.8 million y of life lost (1). This parallels the ∼4-fold increase in diabetes prevalence since 1980. The most recent reports estimate that 422 million people worldwide are living with diabetes (2). In addition, the WHO reported ∼1.9 billion adults are overweight, including 600 million that are obese, and thus, at heightened risk of diabetes. In the United States, it is estimated that 30.2 million adults have diabetes, 90% of whom have type 2 diabetes, and 7.2 million are living with undiagnosed diabetes (3).
Some epidemiologic studies suggest that nut consumption reduces diabetes risk and mortality from diabetes, although this evidence remains inconclusive (4–6). A systematic review and meta-analysis of 5 prospective cohort studies and 1 randomized controlled trial showed a 13% reduction in type 2 diabetes risk with consumption of 4 servings (28.4 g/serving) of nuts per week (RR: 0.87; 95% CI: 0.81, 0.94) (4). A similar meta-analysis of prospective cohort studies reported a 12% reduction in type 2 diabetes risk per 1 serving/d (28 g) of nuts (RR: 0.88; 95% CI: 0.84, 0.92), although upon further analyses adjusted for BMI, the association was attenuated to nonsignificance (RR: 1.03; 95% CI: 0.91, 1.16) (6). More recently, Aune et al. (5) showed in a systematic review and meta-analysis of 4 studies (n = 202,751) that per 28 g/d of peanuts and tree nuts, risk of diabetes mortality was reduced by 39% (RR: 0.61; 95% CI: 0.43, 0.88).
Despite observational evidence to suggest higher nut consumption may be protective against type 2 diabetes, it is unclear if this association is causal. Viguiliouk et al. (7) conducted a systematic review and meta-analysis of studies that compared a diet including tree nuts to an isocaloric diet without tree nuts in individuals with type 2 diabetes. In this pooled analysis of 12 randomized controlled trials, tree nut consumption reduced fasting glucose by 2.7 mg/dL (95% CI: −4.9, −0.4 mg/dL) and glycated hemoglobin (HbA1c) by 0.07% (95% CI: −0.10%, −0.03%). The current systematic review will update this analysis and broaden the scope to include studies of individuals with and without diabetes to increase the generalizability of the results and inform population dietary recommendations. The aim was to conduct a systematic review and meta-analysis of randomized controlled trials to examine the effect of tree nuts and peanuts on markers of glycemic control in adults.
Methods
A systematic review and meta-analysis was performed to examine the effect of tree nut and peanut consumption on markers of glycemic control. Peanuts were included because, despite being a legume botanically, their consumption in the diet is more similar to tree nuts, and as stated in the 2015–2020 Dietary Guidelines for Americans peanuts and peanut butter along with almonds and mixed nuts are the most commonly consumed nuts in the United States (8). This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (9).
Eligibility criteria
Randomized controlled trials that compared intake of peanuts and/or tree nuts (almonds, brazil nuts, cashews, hazelnuts, macadamia nuts, pecans, pine nuts, pistachios, walnuts, or a combination of these nuts), including whole nuts, nut butters, and nut oils, to a control group not consuming nuts were included. Eligible trials studied adults >18 y of age and had a minimum follow-up of 3 wk. In addition, included trials reported ≥1 of the following outcome measurements: fasting glucose; fasting insulin; β cell function from the homeostasis model of assessment (HOMA-β); HOMA-IR; HbA1c; insulin resistance status; 2-h glucose; 2-h insulin; measures of insulin sensitivity (e.g., Matsuda index); fructosamine; or C-peptide. Studies were not excluded because of subject characteristics, and no date restrictions were applied. Only studies published in English were eligible for inclusion.
Studies were excluded if they used a nonrandomized treatment allocation, had an intervention period <3 wk, or lacked a control group whereby subjects did not consume nuts. Studies where 2 different nuts were compared without a control group that did not consume nuts were excluded. In addition, studies that tested other dietary components or dietary patterns in addition to nuts were excluded because the specific effect of nuts could not be determined in these trials. Retracted articles were also excluded.
Search strategy and study selection
A systematic search was conducted using PubMed, the Cochrane Collaboration Library, and CINAHL from the index date of each database through 20 May, 2018. See the Supplemental Material for the search terms used. Three authors (KSP, AMT, and EAJ) screened the titles and abstracts of articles identified in the search in duplicate. The full texts of articles identified as potentially eligible were also reviewed in duplicate. Disagreements were resolved by the third author.
Data extraction
Data were extracted from eligible studies in duplicate by 3 authors (KSP, AMT, and EAJ). Extracted data were entered into a standardized spreadsheet. The following items were extracted: nut studied and dose; study design (parallel; crossover); intervention type (supplementation ± nuts provided; controlled feeding); control group conditions; study population (healthy, overweight, or obese, metabolic syndrome, type 2 diabetes, prediabetes); number of subjects included in analyses; number and percentage of male subjects; mean age of subjects; mean BMI or body weight; length of follow-up; and funding source. For each outcome measure, the mean (or median), variance, and number of subjects in the treatment and control groups at follow-up were extracted. If this was not reported, change from baseline, variance, and number of subjects in each group was extracted. For crossover studies, the P value from the paired analyses was extracted. In studies where >1 dose of nuts was tested, data for the treatment providing the greatest quantity of nuts were extracted. Further, data for non-nut active treatment arms were not extracted. In studies where participants were followed-up more than once, data from the greatest time since baseline were included. However, in studies where participants were followed up after a period of time since the completion of an active phase, data from the end of the active treatment phase were used.
Risk of bias
Risk of bias in the included studies was assessed using the Cochrane Risk of Bias Tool (10). In duplicate, 3 authors (KSP, AMT, and EAJ) assessed the included studies to determine whether there was low, high, or unclear risk of bias based on the methods used to generate the random sequence, conceal allocation, and blind participants and outcome assessors, as well as incomplete outcome data. Disagreements were resolved by the third author.
Statistical analyses
The primary effect measure was mean difference between the intervention group and the control group at follow-up. Where this was not reported, the difference in change from baseline between the groups was used. The mean and SD were extracted from articles; where data were reported in a different format, standard calculations were done to derive the mean and SD (10, 11). For trials with >1 control group, the groups were combined by applying a weighted average to enable a single pairwise comparison. Similarly, for trials that included >1 intervention arm that had the same type and dose of nut but provided the nuts at different times of the day (e.g., breakfast, lunch, dinner, snacks), the intervention groups were combined. Only 1 of the included studies (12) examined the effect of consumption timing, and there was no difference in the glycemic control outcomes by time of nut consumption and thus the nut treatment arms were combined. For trials that had multiple arms comparing different nuts, the data were pooled for the primary analyses; however, for the subgroup analysis by nut type, data from the different nut treatments were included separately.
Paired analyses were conducted for all crossover studies (13). No study reported the correlation between outcome measures in the intervention and control treatment arms so the correlation was derived from the variance of the within-subject difference, where possible, or a conservative estimate of 0.5 was used (13).
For each outcome, the values from each trial were pooled and analyzed using the DerSimonian and Laird random-effects method because of the heterogeneity present. The I2 statistic was used to explore the percentage of variability due to heterogeneity between studies rather than sampling error. To explore sources of heterogeneity, subgroup analyses were performed according to type of nut studied, diabetes status of the subjects, whether an isocaloric control was provided, and the weight status of subjects during the intervention period (neutral: weight stable; positive: weight gain; negative: weight loss). To determine whether inclusion of any one trial changed the main result, an analysis was conducted whereby each study was individually removed for recalculation of the weighted mean difference (WMD). To determine whether a dose–response relation existed, the dose of nuts provided was plotted against the mean difference for each outcome using meta-regression. The risk of publication bias was assessed by examination of funnel plots of the WMD against the SE of the WMD. Results are presented as WMD and 95% CI unless otherwise stated. Data analyses were performed using STATA version 13.1 (StataCorp). P values of <0.05 were considered statistically significant.
Systematic search
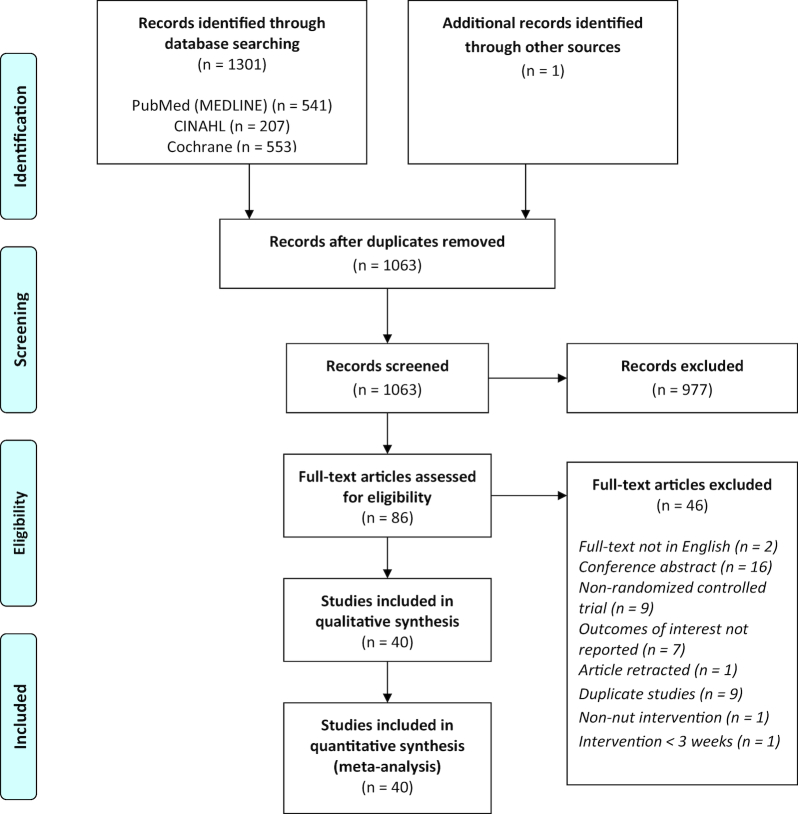
The initial database search returned 1301 articles and 1 article was identified by a hand search; after duplicates were removed 1063 articles remained. Screening based on the title and abstract yielded 86 articles for full-text review. After full-text review, 40 articles remained and were included in the qualitative and quantitative synthesis (Figure 1). The characteristics of the included studies are summarized in Table 1.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of included studies.
TABLE 1.
Characteristics of the studies included in the meta-analysis1
| Authors | Treatment | Dose of nuts (g) | Type of intervention | Study design | Control treatment | Subjects | n | n male (%) | Mean age (y) | Mean BMI | Length of follow-up (mo) | Funding source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Almonds | ||||||||||||
| Abazarfard et al. (14) | Almonds | 50 g/d | Supplementation, nuts provided + 1000 kcal/d restriction | 2-arm parallel | No nuts + 1000 kcal/d restriction | Overweight and obese premenopausal women | 100 | 0 (0) | ∼42 | 29.6 | 3 | Shiraz University of Medical Sciences |
| Chen et al. (15) | Almonds | 60 g/d | Controlled feeding | 2-arm crossover | National Cholesterol Education Program step II diet; isocaloric | T2DM prescribed oral hypoglycemic agents | 33 | 13 (39) | 54.9 | 25.5 | 3 | Almond Board of California and USDA/Agricultural Research Service |
| Cohen and Johnston (16) | Almonds | 28 g/d, 5 d/wk | Supplementation, nuts provided | 2-arm parallel | 2 cheese sticks/d 5 d/wk | T2DM | 13 | 7 (54) | 66 | 34.8 | 3 | Lloyd S. Hubbard Nutrition Fund of the Arizona State University Foundation. Nuts provided by California Almond Board |
| Damasceno et al. (17) | Almonds | 50–75 g/d | Supplementation, nuts provided | 3-arm crossover | 35–50 g virgin olive oil/d | Men and postmenopausal women with hypercholesterolemia | 18 | 9 (50) | 56 | 25.7 | 1 | International Nut and Dried Fruit Council, Spanish Ministry of Health, and Fundació Privada Catalana de Nutrició i Lípids |
| Jenkins et al. (18) | Almonds | 73 g/d | Supplementation, nuts provided | 3-arm crossover2 | Self-selected low-fat diet + isocaloric muffin | Hyperlipidemic mean and postmenopausal women | 27 | 15 (56) | 64 | 25.7 | 1 | Canadian Research Chair Endowment of the Federal Government of Canada and the Almond Board of California |
| Li et al. (19) | Almonds | 20% of energy (∼60 g/d) | Controlled feeding | 2-arm crossover | National Cholesterol Education Program step II diet; isocaloric | T2DM + mild hyperlipidemia | 20 | 9 (45) | 58 | 26 | 1 | Almond Board of California and LEARN weight management foundation |
| Lovejoy et al. (20) | Almonds + high-fat diet (37% fat) | 53–113 g/d (10% of total fat from almonds) | Controlled feeding | 4-arm crossover | High-fat diet (37% fat, 10% from olive or canola oil) | T2DM | 30 | 13 (43) | 53.8 | 33 | 1 | Almond Board of California |
| Almonds + low-fat diet (25% fat) | Low-fat diet (25% fat, 10% from olive or canola) | |||||||||||
| Sabate et al. (21) | Almonds + Step 1 diet | 20% of energy | Controlled feeding | 3-arm crossover3 | Isocaloric Step 1 diet | Healthy | 25 | 14 (56) | 41 | <30 | 1 | Almond Board of California |
| Tan and Mattes (12) | Almonds at breakfast | 43 g/d | Supplementation, nuts provided | 5-arm parallel | No nuts or seeds consumed | At risk of T2DM | 137 | 48 (35) | ∼30 | 28.2 | 1 | Almond Board of California |
| Almonds at lunch | ||||||||||||
| Almonds as morning snack | ||||||||||||
| Almonds as afternoon snack | ||||||||||||
| Wien et al. (22) | Almonds + ADA diet | 20% of energy | Supplementation, nuts provided | 2-arm parallel | ADA diet without nuts | Prediabetes | 65 | 17 (26) | 54 | 29.5 | 4 | Almond Board of California |
| Wien et al. (23) | Almonds + low-calorie formula-based diet | 84 g/d | Supplementation, nuts provided | 2-arm parallel | Self-selected complex CHO and safflower oil + low-calorie formula-based diet; isocaloric | Overweight or obese | 65 | 28 (43) | 55 | 38 | 6 | General Clinical Research Center Grant (NIH) and the Almond Board of California |
| Cashews | ||||||||||||
| Mohan et al. (24) | Cashews | 30 g/d | Supplementation, nuts provided | 2-arm parallel | Nut-free, standard diabetic diet | T2DM <10 y prescribed oral antihypoglycemic agents, HbA1c <10% | 269 | 145 (54) | 58.8 | 25.9 | 3 | Cashew Export Promotion Council of India, the Department of Commerce, Ministry of Commerce & Industry, Government of India; Samsons Trading Company Pvt. Ltd.; Kerala Nut Food Company; Alphonsa Cashew Industries; Cashew Manufacturers Association; Western India Cashew Company Pvt. Ltd.; Intersnack Procurement BV |
| Mukuddem-Petersen et al. (25) | Cashews | 20% of energy | Controlled feeding | 3-arm parallel | Isocaloric diet | MetS | 64 | 29 (45) | 45 | 35 | 2 | The National Research Foundation (NRF) and the South African government's Technology and Human Resources for Industry Program (THRIP). Various food donations: Tiger Brands, Pick ’n Pay, Clover, and Unilever |
| Hazelnuts | ||||||||||||
| Damavandi et al. (26) | Hazelnuts | 10% of energy (∼29 g/d) | Controlled feeding | 2-arm parallel | Self-selected | T2DM | 48 | 15 (31) | 56 | 28.3 | 2 | None reported |
| Mixed nuts | ||||||||||||
| Agebratt et al. (27) | Mixed | 7 kcal · kg weight–1 · d–1 (1.24 g nuts · d–1 · kg body weight–1) | Supplementation, reimbursement provided | 2-arm parallel | Fresh fruit 7 kcal · kg–1 · d–1 | Healthy | 30 | 18 (60) | 24 | 22.3 | 2 | The County Council of Östergötland and Linköping University, Department of Medical and Health Sciences |
| Casas-Agustench et al. (28) | Mixed | 30 g/d (15 g walnuts, 7.5 g almonds, 7.5 g hazelnuts) | Supplementation, nuts provided | 2-arm parallel | Prudent diet, no nuts | ≥3 ATP III criteria for MetS | 50 | 28 (56) | ∼52 | 30.8 | 3 | Spanish Ministry of Education and Science, Spanish Ministry of Health, International Nut Council |
| Lee et al. (29) | Mixed | 30 g/d (15 g walnuts, 7.5 g almonds, 7.5 g hazelnuts) + prudent diet | Supplementation, nuts provided | 2-arm parallel | Prudent diet | MetS and BMI > 23 | 60 | 0 (0) | Included 35–65 y | 27.1 | 1.5 | Ministry of Food and Drug Safety |
| Peanuts | ||||||||||||
| Barbour et al. (30) | Peanuts (high oleic) | 56–84 g/d (∼15–20% of energy), 6 d/wk | Supplementation, nuts provided | 2-arm crossover | Habitual diet devoid of nuts | Healthy overweight males and postmenopausal women | 61 | 29 (48) | 65 | 31 | 3 | Australian Research Council and Peanut Company of Australia |
| Johnston et al. (31) | Peanuts | 1 oz (28 g/d)/d | Supplementation, nuts provided | 2-arm parallel | Grain bar (1.4 oz); not isocaloric; high CHO; low-fat diet counseling based on BMR | Healthy, overweight, and obese | 44 | 16 (36) | 41 | 31.8 | 2 | National Peanut Board, Atlanta, GA |
| Moreira Alves et al. (32) | Conventional peanuts; high-oleic peanuts | 56 g/d | Supplementation, nuts provided + 250 kcal/d restriction | 3-arm parallel | Isocaloric + 250 kcal/d restriction | Healthy males, some with insulin resistance | 65 | 65 (100) | 27 | 29.5 | 1 | Peanut Collaborative Research Support Program; CAP agroindustrial provided nuts |
| Wien et al. (33) | Peanuts | 20% energy (∼46 g/d) | Supplementation, nuts provided | 2-arm parallel | ADA diet without peanuts + dietary counseling | T2DM > 6 mo with HbA1c < 9% | 60 | 30 (50) | 61.5 | 32 | 6 | National Peanut Board, Atlanta, GA |
| Pecans | ||||||||||||
| McKay et al. (34) | Pecans | 15% of energy (∼42.5 g/d) | Controlled feeding | 2-arm crossover | Average American Diet; isocaloric | Metabolically at-risk men and postmenopausal women | 26 | 21 (81) | 59.7 | 29.2 | 1 | USDA/Agricultural Research Service and the National Pecan Shellers Association |
| Pistachios | ||||||||||||
| Gulati et al. (35) | Pistachios | 20% of energy | Supplementation, nuts provided + dietary counseling | 2-arm parallel | Dietary counseling | MetS | 68 | 37 (54) | 42.5 | 30.9 | 6 | Full funding from Paramount Farms Inc., California |
| Hernández-Alonso et al. (36) | Pistachios | 57 g/d | Supplementation, nuts provided | 2-arm crossover | Isocaloric diet | Prediabetes | 54 | 29 (54) | 55 | 28.9 | 4 | American Pistachio Growers (formerly Western Pistachio Association) and Paramount Farms |
| Kasliwal et al. (37) | Pistachios | 40 g/d | Supplementation, nuts provided + lifestyle counseling | 2-arm parallel | Lifestyle counselling, avoidance of nuts | Mild dyslipidemia | 42 | 35 (82) | ∼39 | 27 | 3 | Paramount Farms Inc., California |
| Li et al. (38) | Pistachios | 53 g/d | Supplementation, nuts provided + 500 kcal/d restriction | 2-arm parallel | 56 g/d pretzels + 500 kcal/d restriction | Healthy, overweight | 52 | 13 (19) | ∼46 | 30.5 | 3 | Not reported |
| Parham et al. (39) | Pistachios | 50 g/d | Supplementation, nuts provided | 2-arm crossover | Normal diet, no nuts | T2DM ≥ 1 y | 44 | 11 (25) | 51.5 | 31 | 3 | Not reported |
| Sauder et al. (40) | Pistachios | 20% energy (59–128 g/d) | Controlled feeding | 2-arm crossover | Based on American Heart Association's Therapeutic Lifestyle Change diet (26.9% total fat, 6.7% saturated fat, 186 mg/d cholesterol), isocaloric | Men and postmenopausal women with well-controlled T2DM | 30 | 15 (50) | 56 | 31.2 | 1 | American Pistachio Growers and NIH |
| Wang et al. (41) | Pistachios | 70 g/d | Supplementation, nuts provided | 3-arm parallel4 | AHA Step 1 diet, no nuts | MetS ± T2DM | 59 | 25 (42) (46) | 51 | 28 | 3 | Paramount Farms, Los Angeles, CA |
| Walnuts | ||||||||||||
| Bamberger et al. (42) | Walnuts | 43 g/d | Supplementation, nuts provided | 2-arm crossover | Nut free, Western-type diet | Men and postmenopausal women with LDL < 190 mg/dL, triglycerides < 350 mg/dL, and BMI < 35 | 204 | 60 (31) | 63 | 25.4 | 2 | California Walnut Commission |
| Damasceno et al. (17) | Walnuts | 40–65 g/d | Supplementation, nuts provided | 3-arm crossover | 35–50 g virgin olive oil/d | Men and postmenopausal women with hypercholesterolemia | 18 | 9 (50) | 56 | 25.7 | 1 | International Nut and Dried Fruit Council, Spanish Ministry of Health, and Fundació Privada Catalana de Nutrició i Lípids |
| Katz et al. (43) | Walnuts | 56 g/d | Supplementation, nuts provided | 2-arm crossover | Ad libitum diet | Enlarged waist circumference + 1 risk factor for MetS | 46 | 18 (39) | 57 | 33.2 | 2 | California Walnut Commission |
| Ma et al. (44) | Walnuts | 56 g/d | Supplementation, nuts provided | 2-arm crossover | Ad libitum diet | T2DM for 1–5 y | 24 | 10 (42) | 58 | 32.5 | 2 | Not reported |
| Mukuddem-Petersen et al. (25) | Walnuts | 20% of energy | Controlled feeding | 3-arm parallel | Isocaloric diet | MetS | 64 | 29 (45) | 45 | 35 | 2 | The National Research Foundation (NRF) and the South African government's Technology and Human Resources for Industry Program (THRIP). Various food donations: Tiger Brands, Pick ’n Pay, Clover, and Unilever |
| Njike et al. (45) | Walnuts | 56 g/d | Supplementation, nuts provided | Crossover Latin square | Ad libitum diet | High risk of T2DM | 112 | 31 (28) | 55 | 30 | 6 | California Walnut Commission |
| Rock et al. (46) | Walnuts | 18% of energy | Supplementation, nuts provided + dietary counseling | 3-arm parallel | Dietary counseling + lower-fat, higher-CHO dietDietary counseling + lower-CHO, higher-fat diet | Overweight and obese men and women without T2DM | 245 | 0 (0) | 50 | 33.5 | 12 | National Cancer Institute, California Walnut Commission |
| Tapsell et al. (47) | Walnuts | 30 g/d | Supplementation, nuts provided + interdisciplinary advice | 3-arm parallel5 | Interdisciplinary advice | Overweight or obese | 101 | (∼26) | 45 | 32 | 12 | Illawarra Health and Medical Research Institute and the California Walnut Commission |
| Tapsell et al. (48) | Walnuts | 30 g/d | Supplementation, nuts provided + low-fat dietary advice | 2-arm parallel | Low-fat dietary advice | T2DM | 35 | Not reported | 54 | 33.1 | 12 | California Walnut Commission |
| Tapsell et al. (49) | Walnuts | 30 g/d | Supplementation, nuts provided | 3-arm parallel | Low-fat dietModified fat diet using exchange lists with fatty acid considerations | T2DM > 1 y with HbA1c < 9% | 58 | 34 (59) | 59 | 30 | 6 | California Walnut Commission and Australian Research Council |
| Wu et al. (50) | Walnuts | 30 g/d | Supplementation, nuts provided + lifestyle counseling | 3-arm parallel6 | Lifestyle counseling | MetS | 189 | 105 (56) | 48 | 25.5 | 12 | California Walnut Commission |
| Wu et al. (51) | Walnuts | 43 g/d | Supplementation, nuts provided | 2-arm crossover | Western-type diet | Healthy | 40 | 10 (25) | 60 | 24.9 | 2 | California Walnut Commission |
| Nut oils | ||||||||||||
| Mullner et al. (52) | Walnut oil | 9 g/d (n–3:n–6 ratio: 1.3:6.1) | Supplementation, oil provided | 2-arm parallel | Mixed oil (corn, sunflower, and linseed oils); n–3:n–6 ratio: 0.6:5.7 | T2DM, HbA1c < 9.5%, treated with oral medication or insulin | 92 | 38 (41) | 63 | Body weight: ∼89 kg | 2.5 | EU, through the cross-border cooperation program Slovakia–Austria |
1BMI measured in kg/m2. ADA, American Diabetes Association; ATP III, Adult Treatment Panel III; BMR, basal metabolic rate; CHO, carbohydrate; MetS, metabolic syndrome; T2DM, type 2 diabetes.
2Data from the half-dose-almond group not included in the meta-analysis.
3Data from the low-almond-diet group not included in the meta-analysis.
4Data from the low-dose-pistachio group not included in the meta-analysis.
5Data from the usual-care control group not included in the meta-analysis.
6Data from the flaxseed-supplemented group not included in the meta-analysis.
The trials included 2832 unique participants, had a median duration of 3 mo (range: 1–12 mo), and the dose of nuts tested varied from 20 to 113 g/d (median: 52 g/d). The included randomized controlled trials studied almonds (n = 11), cashews (n = 2), hazelnuts (n = 1), mixed nuts (n = 3), peanuts (n = 4), pecans (n = 1), pistachios (n = 7), walnuts (n = 12), and nut oil (n = 1). Nine studies used a controlled feeding intervention, and 31 studies, contributing 33 comparisons, had participants supplement their diets with nuts. Thirty-one studies received total or partial funding from industry, 4 studies did not disclose a funding source, and 5 studies were funded by a university or government department.
The risk of bias assessment for the included randomized controlled trials is summarized in Supplemental Table 1. The included trials had low (n = 18) or unclear (n = 22) risk of bias based on the methods used to generate the randomization sequence. Thirty-five studies had unclear risk of bias, and 5 studies had low risk of bias due to allocation concealment. With the exception of 1 study, all studies had a high risk of performance bias. There was unclear risk of detection bias in 34 studies. Risk of attrition bias was low in 33 studies, unclear in 5 studies, and high in 2 studies. Examination of funnel plots revealed no evidence of publication bias for fasting glucose, although for HbA1c, insulin, and HOMA-IR there was some indication of publication bias (Supplemental Figure 1).
Results
Fasting glucose
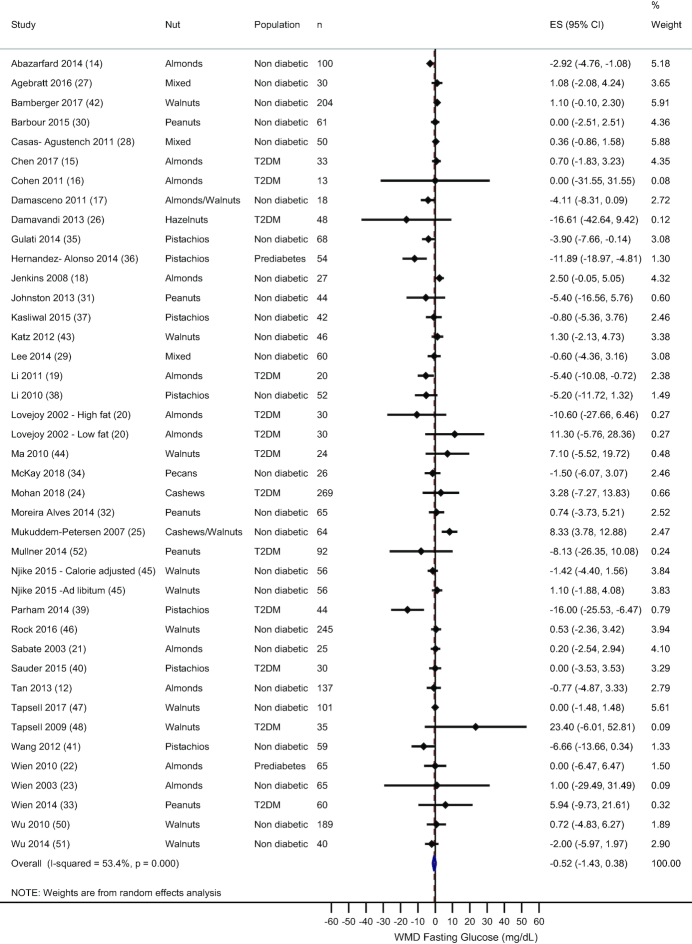
A total of 39 randomized controlled trials, including 41 comparisons, measured fasting glucose. Overall, there was no effect of nut consumption on fasting glucose (WMD: −0.52 mg/dL; 95% CI: −1.43, 0.38 mg/dL; I2 = 53.4%)—see Figure 2. Sensitivity analysis showed that this result remained after individual removal of each study. Subgroup analysis by type of nut studied revealed similar results for almonds, cashews, peanuts, and walnuts, although a significant reduction in fasting glucose was observed with pistachio consumption compared with the control treatment (WMD: −5.18 mg/dL; 95% CI: −8.76, −1.60 mg/dL; I2 = 67%)—see Supplemental Table 2. Subgroup analyses by diabetes status of the sample, provision of an isocaloric control treatment, and the weight status of the subjects during the study showed no deviation from the main result. No association was observed between the mean difference in fasting glucose and nut dose (data not shown).
FIGURE 2.
Random-effects meta-analysis of mean difference in fasting glucose with nut consumption compared to control treatment. DerSimonian and Laird random-effects method used for data analysis. Data expressed as WMD (95% CI). Weights are from random-effects analysis. ES, effect size; T2DM, type 2 diabetes; WMD, weighted mean difference.
HbA1c
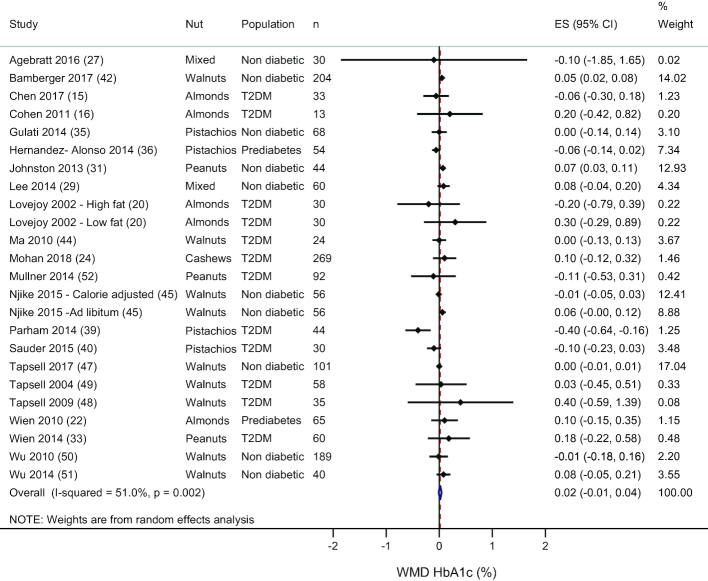
There were 22 studies, including 24 comparisons, that measured HbA1c. There was no effect of nut consumption on HbA1c (WMD: 0.02%; 95% CI: −0.01%, 0.04%; I2 = 51.0%), see Figure 3. Systematic removal of each study showed that no one study changed the main effect. Subgroup analyses by nut type, provision of an isocaloric control, and the weight status of the participants during the study showed similar results—see Supplemental Table 3. However, in studies of healthy individuals, an increase in HbA1c was observed with nut consumption compared to the control treatment (WMD: 0.03%; 95% CI: 0.01%, 0.06%); in subjects with prediabetes or type 2 diabetes there was no significant effect of nuts on HbA1c. Further subgroup analyses for the duration of the treatment period showed that in studies that had a duration of <3 mo (n = 14), HbA1c increased with nut consumption compared with the control treatment (WMD: 0.05%; 95% CI: 0.03%, 0.08%); however, for studies with a duration ≥3 mo (n = 10) there was no difference between the treatments (WMD: −0.01%; 95% CI: −0.04%, 0.03%)—see Supplemental Table 3. In healthy individuals, HbA1c was only increased after nut consumption in studies with a duration <3 mo (WMD: 0.06%; 95% CI: 0.04%, 0.08%); in studies that had a treatment period ≥3 mo there was no difference between the treatments (WMD: 0.001%; 95% CI: −0.01%, 0.01%). In studies that included subjects with type 2 diabetes, subgroup analyses by the duration of the study (≥3 or <3 mo) showed that, regardless of the length of the study, there was no effect of nut consumption on HbA1c (data not shown). Meta-regression showed no significant association between nut dose and mean difference in HbA1c (data not shown).
FIGURE 3.
Random effects meta-analysis of mean difference in HbA1c with nut consumption compared to control treatment. DerSimonian and Laird random-effects method used for data analysis. Data expressed as WMD (95% CI). Weights are from random-effects analysis. ES, effect size; HbA1c, glycated hemoglobin; T2DM, type 2 diabetes; WMD, weighted mean difference.
HOMA-IR
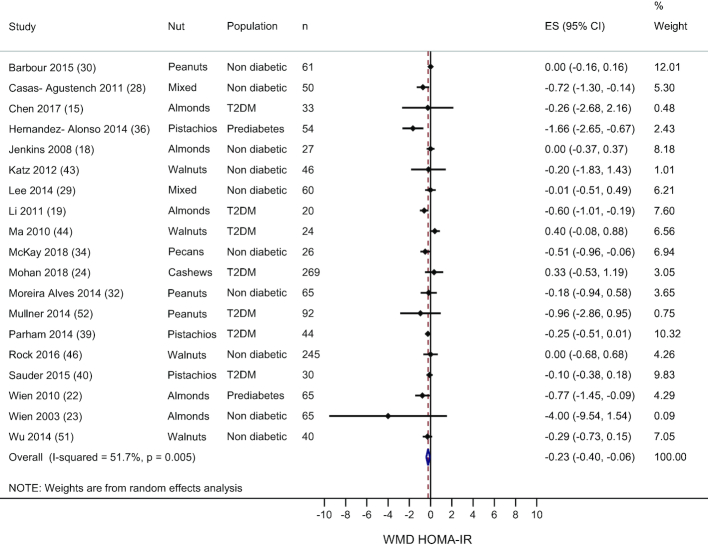
Nineteen studies measured HOMA-IR and overall the treatment effect favored nut consumption (WMD: −0.23; 95% CI: −0.40, −0.06; I2 = 51.7%)—see Figure 4. Exclusion of each study from the analysis showed that no individual study changed the main result. Subgroup analyses by nut type showed no effect of any individual nut on HOMA-IR—see Supplemental Table 4. In the 14 studies that used an isocaloric control treatment, there was a significant reduction in HOMA-IR with nut consumption compared to the control treatment (−0.36; 95% CI: −0.57, −0.15; I2 = 42.8%). Subgroup analysis by diabetes status of the sample showed that in the 2 studies that included individuals with prediabetes, there was a significant reduction in HOMA-IR with nut consumption (−1.14; 95% CI: −2.0, −0.28; I2 = 52.7%). There was no association between nut dose and mean difference in HOMA-IR (data not shown).
FIGURE 4.
Random effects meta-analysis of mean difference in HOMA-IR with nut consumption compared to control treatment. DerSimonian and Laird random-effects method used for data analysis. Data expressed as WMD (95% CI). Weights are from random-effects analysis. ES, effect size; T2DM, type 2 diabetes; WMD, weighted mean difference.
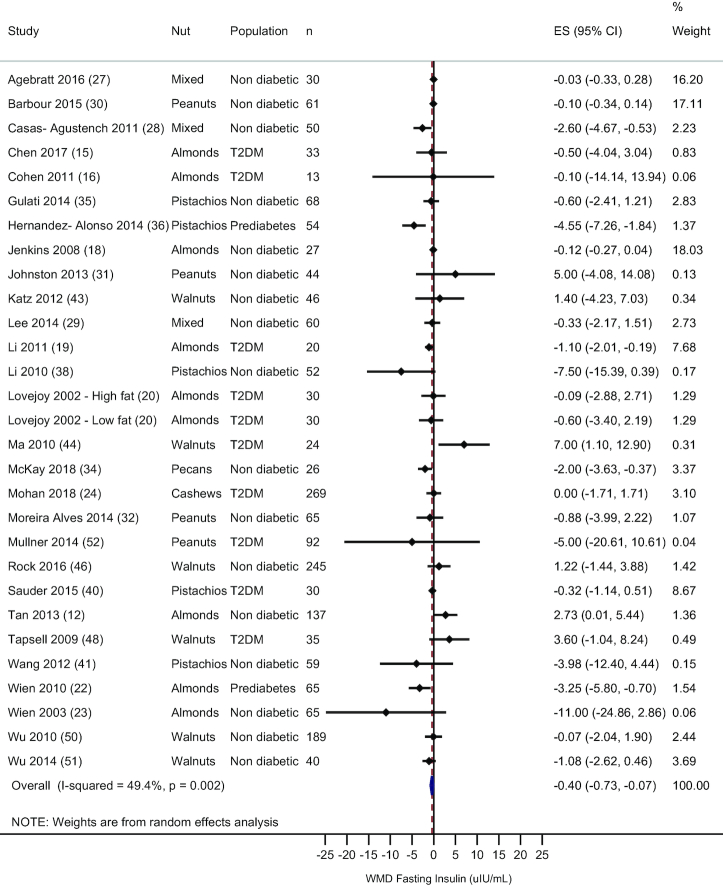
Fasting insulin
A total of 28 studies, including 29 comparisons, measured fasting insulin. Overall, there was a significant reduction in fasting insulin with nut consumption (WMD: −0.40 μIU/mL; 95% CI: −0.73, −0.07 μIU/mL; I2 = 49.4%)—see Figure 5. Removal of each study individually showed no deviation from the main result. Subgroup analysis by nut type showed that no one nut could account for this effect—see Supplemental Table 5. In the 23 trials that used an isocaloric control treatment, the treatment effect favored nut consumption (WMD: −0.65 μIU/mL; 95% CI: −1.07, −0.24 μIU/mL; I2 = 49.4%). In the 2 studies that included subjects with prediabetes, there was a reduction in fasting insulin (WMD: −3.86 μIU/mL; 95% CI: −5.72, −2.0 μIU/mL), but no effect was observed in healthy subjects or those with type 2 diabetes. Meta-regression showed no association between nut dose and mean difference in fasting insulin (data not shown).
FIGURE 5.
Random effects meta-analysis of mean difference in fasting insulin with nut consumption compared to control treatment. DerSimonian and Laird random-effects method used for data analysis. Data expressed as WMD (95% CI). Weights are from random-effects analysis. ES, effect size; T2DM, type 2 diabetes; WMD, weighted mean difference.
Endpoints included in the qualitative synthesis only
Only a small number of studies measured outcomes related to insulin production and HOMA-β cell function (n = 7), glucose response after an oral-glucose-tolerance test (OGTT) (n = 5), insulin response after an OGTT (n = 2), insulin sensitivity (n = 3), and short-term glucose control (n = 2). Because a limited number of trials measured these endpoints and the measurements used were heterogeneous, a meta-analysis was not performed. A qualitative synthesis of these studies is presented in Table 2.
TABLE 2.
Summary of endpoints included in the qualitative synthesis only1
| Study | Outcome measured | Nut | Population | Result | Effect |
|---|---|---|---|---|---|
| Insulin production and β cell function | |||||
| Chen et al. (15) | C-peptide | Almonds | T2DM prescribed oral hypoglycemic agents | No difference by treatment | ↔ |
| Jenkins et al. (18) | C-peptide | Almonds | Men and postmenopausal women with hypercholesterolemia | No difference by treatment | ↔ |
| Jenkins et al. (18) | 24-h C-peptide output | Almonds | Men and postmenopausal women with hypercholesterolemia | No difference by treatment | ↔ |
| Jenkins et al. (18) | Creatinine-corrected 24-h C-peptide output | Almonds | Men and postmenopausal women with hypercholesterolemia | Lower after almond treatments vs. control | ↓ |
| Barbour et al. (30) | HOMA-β cell function | Peanuts (high oleic) | Healthy overweight males and postmenopausal women | No between-treatment effect | ↔ |
| Hernández- Alonso et al. (36) | HOMA-β cell function | Pistachios | Prediabetes | No between-treatment effect | ↔ |
| McKay et al. (34) | HOMA-β cell function | Pecans | Metabolically at-risk men and postmenopausal women | Between-group difference in mean change of −21.34 favoring pecan group | ↓ |
| Rock et al. (46) | HOMA-β cell function | Walnuts | Overweight and obese men and women without T2DM | No between-group effect | ↔ |
| Wien et al. (22) | HOMA-β cell function | Almonds | Prediabetes | Almond group: −13.2; control group: +22.3; P < 0.05 | ↓ |
| Glucose concentrations after 75-g OGTT | |||||
| Casas-Agustench et al. (28) | 2-h glucose | 30 g/d (15 g walnuts, 7.5 g almonds, 7.5 g hazelnuts) | ≥3 ATP III criteria for MetS | No between-group effect | ↔ |
| Lovejoy et al. (20)—high-fat diet | 2-h glucose | Almonds | T2DM | No main effect for fat source (almond vs. oil) or fat level (high or low). Significant interaction (fat source × fat level), 2-h glucose lower after the low-fat control treatment vs. low-fat almond and high-fat control diets | ↔ |
| Lovejoy et al. (20)—low-fat diet | 2-h glucose | Almonds | T2DM | As above | ↑ |
| Mukuddem-Petersen et al. (25) | 2-h glucose | Cashews Walnuts | MetS | No between-group effect | ↔ |
| Wang et al. (41) | 2-h glucose | Pistachios | MetS ± T2DM | No between-group effect | ↔ |
| Sauder et al. (40) | Glucose AUC | Pistachios | Men and postmenopausal women with well-controlled T2DM | No between-treatment effect | ↔ |
| Insulin concentrations after 75-g OGTT | |||||
| Lovejoy et al. (20)—high-fat diet | 2-h insulin | Almonds | T2DM | No main effect for fat source (almond vs. oil), fat level (high or low), or interaction (fat source × fat level) | ↔ |
| Lovejoy et al. (20)—low-fat diet | 2-h insulin | Almonds | T2DM | As above | |
| Sauder et al. (40) | Insulin AUC | Pistachios | Men and postmenopausal women with well-controlled T2DM | No between-treatment effect | ↔ |
| Insulin sensitivity | |||||
| Barbour et al. (30) | HOMA-IS | Peanuts (high oleic) | Healthy overweight males and postmenopausal women | No between-treatment effect | ↔ |
| Sauder et al. (40) | Matsuda Index | Pistachios | Men and postmenopausal women with well-controlled T2DM | No between-treatment effect | ↔ |
| Rock et al. (46) | Insulin resistance status | Walnuts | Overweight and obese men and women without T2DM | No between-group effect | ↔ |
| Short-term glucose control | |||||
| Mukuddem-Petersen et al. (25) | Fructosamine | Cashews Walnuts | MetS | No between-group effect | ↔ |
| Sauder et al. (40) | Fructosamine | Pistachios | Men and postmenopausal women with well-controlled T2DM | Pistachio: 228.5 μmol/L vs. control: 233.5 μmol/L; P = 0.034 | ↓ |
1↔, no change; ↓, reduction or lower; ↑, increase or higher; ATP III, Adult Treatment Panel III; HOMA-IS, homeostasis model of assessment insulin sensitivity; HOMA-β, β cell function from the homeostasis model of assessment; MetS, metabolic syndrome; OGTT, oral-glucose-tolerance test; T2DM, type 2 diabetes.
Jenkins et al. (18) measured C-peptide, 24-h C-peptide output, and creatinine-corrected 24-h C-peptide output, and observed lower C-peptide output after creatinine correction with almond consumption compared with the control treatment. Chen et al. (15) also measured C-peptide and found no effect of almond consumption on C-peptide compared with the control treatment. Five studies measured HOMA-β cell function (22,30, 34, 36,46) and 3 of these found no difference between the nut and control treatments (30, 36, 46). Wien et al. (22) and McKay et al. (34) observed a reduction in HOMA-β cell function with almond and pecan consumption, respectively, compared with the control treatments. Five studies measured 2-h glucose concentrations after a 75-g OGTT and no difference was observed between the nut and control treatments in 4 of these studies (25,28,40, 41). Lovejoy et al. (20) showed that 2-h glucose was lower after the low-fat control treatment compared with the low-fat almond treatment and the high-fat control treatment. Two studies showed no difference in 2-h insulin concentrations with nut consumption (20, 40). Similarly, 3 studies reported there was no effect of nuts on measures of insulin sensitivity (30,40,46). Two studies measured fructosamine, a short-term measure of glucose control, and 1 study showed a reduction with pistachio consumption in individuals with type 2 diabetes (40). Mukuddem-Petersen et al. (25) showed no change in fructosamine with walnut or cashew consumption.
Discussion
In this systematic review and meta-analysis of 40 randomized controlled trials, including 2832 participants and a median dose of 52 g/d (range: 20–113 g/d) of peanuts or tree nuts, a significant decrease in HOMA-IR and fasting insulin was observed, whereas we found no effect on HbA1c and fasting glucose. Meta-regression showed that the dose of nuts provided was not associated with the mean difference for any outcome measure. Subgroup analyses by nut type revealed no deviation from the main result for HbA1c, HOMA-IR, or fasting insulin. For fasting glucose, the type of nut provided modified the main effect such that pistachios reduced fasting glucose, whereas almonds, cashews, peanuts, walnuts, and mixed nuts had no effect. The main result for all outcomes was not modified by the weight status of participants during the study; however, in studies that provided an isocaloric control treatment, the effect favored the nut group for HOMA-IR and fasting insulin, whereas for fasting glucose and HbA1c the effect did not differ based on the caloric status of the control treatment. In studies that included individuals with prediabetes, the treatment effect favored the nut group for HOMA-IR and fasting insulin, whereas no differential effect by diabetes status was observed for fasting glucose.
A recent review suggested the substitution of unsaturated fatty acids for SFAs and carbohydrates may be responsible for the association between nut consumption and improvements in insulin sensitivity (53). The authors suggested increased α-linolenic acid, found in some tree nuts including walnuts (∼9 g/100 g) and pistachios (∼0.3 g/100 g), modifies microRNAs that may play a role in modulating insulin sensitivity. α-Linolenic acid can improve insulin action through induction of insulin-like growth factor 1. In a recent study, supplementation with 57 g pistachios/d for 4 mo modified circulating microRNAs that were correlated with favorable changes in HOMA-IR, insulin, and fasting glucose in participants with prediabetes (54). Ribeiro et al. (55) conducted a systematic review of pistachio consumption and glucose metabolism in individuals with prediabetes and type 2 diabetes, and the authors suggested that the unique distribution of MUFAs, PUFAs, polyphenols, and carotenoids present in pistachios may modulate specific microRNA, and increase insulin sensitivity through the phosphoinositide 3-kinase–protein kinase B/AKT signaling pathway. Phosphoinositide 3-kinase is activated after insulin binds to its receptor and, in turn, phosphorylates AKT that stimulates the translocation of glucose transporter 4 and glucose uptake.
The fatty acid profile and bioactive components in nuts may also influence fasting blood glucose through improvement in insulin sensitivity. However, the direction of the relation is unclear. Nuts may primarily act by improving insulin sensitivity, thus promoting increased glucose uptake and lowering fasting glucose concentrations. Conversely, nut consumption also affects fasting glucose through non–insulin-mediated mechanisms. Evidence suggests that nuts lower postprandial glucose excursions owing to delayed gastric emptying, which may also explain improvements in fasting glucose particularly when nuts are consumed as part of a meal (56, 57). One previous systematic review (7) and several narrative reviews (58–60) have suggested that nut consumption has a favorable effect on fasting glucose, yet we did not see improvements in fasting glucose with overall nut consumption. The previous meta-analysis by Viguiliouk et al. (7) only included individuals with type 2 diabetes. We did not exclude studies based on the population recruited to increase the generalizability of our meta-analysis; however, this may have lessened the overall effect, although subgroup analysis showed this result remained in healthy individuals and those with prediabetes or type 2 diabetes. It is likely that the discordant results observed between this updated meta-analysis and Viguiliouk et al.’s meta-analysis are due to the inclusion of studies published since completion of their article search in April 2014.
Moderate heterogeneity was observed between studies for all outcomes; the I2 value of the main effects ranged from 49.4% to 53.4%. Subgroup analyses were conducted to explore this between-study variability. Comparisons by nut type revealed that pistachios had the greatest effect on lowering fasting glucose, but all nut varieties appeared to affect other outcomes similarly. Although this review included peanut and tree nut studies with diabetes-related outcomes, almonds, pistachios, and walnuts were the most-studied nuts whereas other nuts were not well-represented. The diabetes status of the subjects did not modify the effect of nuts on fasting glucose, but 2 studies that included participants with prediabetes observed improved HOMA-IR and fasting insulin with nut consumption. Overall, there was not a consistent subpopulation that benefited more from nut consumption. An earlier review by Viguiliouk et al. (7) included only studies that used an isocaloric control, whereas the present review did not exclude studies based on the control used. Therefore, we examined whether the presence or absence of an isocaloric control affected the glycemic response to nut consumption. The caloric status of the control group did not result in a different response to nut consumption for fasting glucose and HbA1c; however, for HOMA-IR and fasting insulin a significant improvement was only observed in the studies that provided an isocaloric control. This may be due to the diverse controls used. For example, Agebratt et al. (27) used an isocaloric control of fresh fruit whereas Hernández-Alonso et al. (36) used a control that was mainly olive oil.
There has been concern about weight gain with regular nut consumption due to the energy density of nuts, although some studies have shown nut consumption to be beneficial for weight loss (14, 61). Weight gain may attenuate any beneficial effects of nuts on glycemic outcomes. To understand if weight status during the intervention played a role in the efficacy of nut consumption on glycemic control, we completed subgroup analyses by the weight status of the participants during the intervention in the included trials and observed no difference in the effect size for any of the main outcomes. Trials that reported weight gain (positive change) and weight loss (negative change) did not differ with respect to the effect of nuts on fasting glucose, HbA1c, fasting insulin, and HOMA-IR. This suggests that inclusion of nuts in the absence of weight loss has the same effect on glycemic control as nut consumption with weight loss, but further studies are needed to confirm this finding.
A limited number of trials included other endpoints related to glycemic control; therefore, these outcomes were not included in the meta-analysis. However, almond consumption reportedly improved C-peptide output and 2-h glucose concentrations in 2 separate studies and pistachio treatment reduced fructosamine in 1 other study. C-peptide is a component of the protein, proinsulin, and is formed after cleavage of insulin from proinsulin, therefore, it serves as a marker of insulin production. Increased insulin sensitivity and fasting insulin reported in this meta-analysis suggest that glucose clearance may also be improved with nut consumption. C-peptide and 2-h glucose amelioration reflects increased insulin sensitivity and quicker clearance of glucose, respectively. The majority of other endpoints were not affected by nut consumption.
There are several strengths and limitations to this systematic review and meta-analysis. This review includes the most current data from randomized controlled trials examining the effect of peanut and tree nut consumption on glycemic outcomes and because only randomized controlled trials were included, this meta-analysis provides the highest-quality evidence. Another strength of this review is that the systematic review and data extraction were completed in duplicate. In addition, trials were not excluded based on the population studied and therefore the results are generalizable. Examination of funnel plots revealed minimal evidence of publication bias. This meta-analysis is limited by the moderate to large heterogeneity observed among studies. The included studies had a median duration of 3 mo and therefore the long-term effect of nut consumption on glycemic outcomes remains unclear. In addition, owing to the small number of trials included, subgroup analyses were likely underpowered and the findings should be investigated further. An aggregate meta-analysis was conducted; thus, the subgroup analyses are prone to ecological bias. In addition, in many of the studies, compliance to the intervention was not reported or was self-reported and thus, a subgroup analysis by treatment compliance could not be reliably conducted. The risk of bias was rated as high or unclear in many domains of assessment for several of the included studies. Finally, none of the included studies investigated the effect of nut butters on markers of glycemic control, and only 1 study examined nut oil. Inclusion of the study that tested the effect of nut oil on fasting glucose, HbA1c, HOMA-IR, and fasting insulin did not change the main effect for any of these comparisons.
In conclusion, this systematic review and meta-analysis of 40 randomized controlled trials showed peanuts or tree nuts significantly decreased HOMA-IR and fasting insulin; there was no effect of nut consumption on HbA1c or fasting glucose. The results of this meta-analysis suggest that nut consumption may play a role in improving insulin sensitivity and thus delay the development and progression of type 2 diabetes. In the future, well-designed clinical trials investigating the effect of nut consumption in individuals with and without diabetes are required to elucidate the mechanisms behind the observed effect of nuts on markers of glycemic control.
Supplementary Material
Acknowledgements
The authors’ contributions were as follows—all authors: designed the research (project conception, development of the overall research plan); AMT, EAJ, and KSP: conducted the research (conducted the systematic search, screened the articles, and extracted the data) and drafted the paper; KSP: performed the statistical analyses and had primary responsibility for final content; and PMK-E: critically reviewed the manuscript. PMK-E currently has research funding from the California Walnut Commission. None of the other authors reported a conflict of interest related to the study.
Notes
The authors reported no funding received for this study.
Supplemental Tables 1–5 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
AMT and EAJ contributed equally to this work.
Abbreviations used: HbA1c, glycated hemoglobin; HOMA-β, β cell function from the homeostasis model of assessment; OGTT, oral-glucose-tolerance test; WMD, weighted mean difference.
References
- 1. GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Global report on diabetes. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 3. National Center for Chronic Disease Prevention and Health Promotion. National Diabetes Statistics Report, 2017. Centers for Disease Control and Prevention; 2017. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. [Google Scholar]
- 4. Afshin A, Micha R, Khatibzadeh S, Mozaffarian D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100(1):278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aune D, Keum N, Giovannucci E, Fadnes L, Boffetta P, Greenwood D, Tonstad S, Vatten L, Riboli E, Norat T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Medicine. 2016;14(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luo C, Zhang Y, Ding Y, Shan Z, Chen S, Yu M, Hu FB, Liu L. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100(1):256–69. [DOI] [PubMed] [Google Scholar]
- 7. Viguiliouk E, Kendall CW, Blanco Mejia S, Cozma AI, Ha V, Mirrahimi A, Jayalath VH, Augustin LS, Chiavaroli L, Leiter LA et al.. Effect of tree nuts on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled dietary trials. PLoS One. 2014;9(7):e103376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Department of Health and Human Services. 2015–2020 Dietary Guidelines for Americans. Washington (DC): US Department of Health and Human Services and US Department of Agriculture, Office of Disease Prevention and Health Promotion; 2016. [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 10. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Chichester, UK: John Wiley & Sons; 2011. [Google Scholar]
- 11. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan SY, Mattes RD. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: a randomized, controlled trial. Eur J Clin Nutr. 2013;67(11):1205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31(1):140–9. [DOI] [PubMed] [Google Scholar]
- 14. Abazarfard Z, Salehi M, Keshavarzi S. The effect of almonds on anthropometric measurements and lipid profile in overweight and obese females in a weight reduction program: a randomized controlled clinical trial. J Res Med Sci. 2014;19(5):457–64. [PMC free article] [PubMed] [Google Scholar]
- 15. Chen C-M, Liu J-F, Li S-C, Huang C-L, Hsirh A-T, Weng S-F, Chang M-L, Li H-T, Mohn E, Chen CYO. Almonds ameliorate glycemic control in Chinese patients with better controlled type 2 diabetes: a randomized, crossover, controlled feeding trial. Nutr Metab (Lond). 2017;14:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen AE, Johnston CS. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A1c in individuals with well-controlled type 2 diabetes mellitus. Metabolism. 2011;60(9):1312–17. [DOI] [PubMed] [Google Scholar]
- 17. Damasceno NR, Pérez-Heras A, Serra M, Cofán M, Sala-Vila A, Salas-Salvadó J, Ros E. Crossover study of diets enriched with virgin olive oil, walnuts or almonds. Effects on lipids and other cardiovascular risk markers. Nutr Metab Cardiovasc Dis. 2011;21(Suppl 1):S14–20. [DOI] [PubMed] [Google Scholar]
- 18. Jenkins DJ, Kendall CW, Marchie A, Josse AR, Nguyen TH, Faulkner DA, Lapsley KG, Singer W. Effect of almonds on insulin secretion and insulin resistance in nondiabetic hyperlipidemic subjects: a randomized controlled crossover trial. Metabolism. 2008;57(7):882–7. [DOI] [PubMed] [Google Scholar]
- 19. Li SC, Liu YH, Liu JF, Chang WH, Chen CM, Chen CY. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism. 2011;60(4):474–9. [DOI] [PubMed] [Google Scholar]
- 20. Lovejoy JC, Most MM, Lefevre M, Greenway FL, Rood JC. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am J Clin Nutr. 2002;76(5):1000–6. [DOI] [PubMed] [Google Scholar]
- 21. Sabate J, Haddad E, Tanzman JS, Jambazian P, Rajaram S. Serum lipid response to the graduated enrichment of a Step I diet with almonds: a randomized feeding trial. Am J Clin Nutr. 2003;77(6):1379–84. [DOI] [PubMed] [Google Scholar]
- 22. Wien M, Bleich D, Raghuwanshi M, Gould-Forgerite S, Gomes J, Monahan-Couch L, Oda K. Almond consumption and cardiovascular risk factors in adults with prediabetes. J Am Coll Nutr. 2010;29(3):189–97. [DOI] [PubMed] [Google Scholar]
- 23. Wien MA, Sabate JM, Ikle DN, Cole SE, Kandeel FR. Almonds vs complex carbohydrates in a weight reduction program. Int J Obes Relat Metab Disord. 2003;27(11):1365–72. [DOI] [PubMed] [Google Scholar]
- 24. Mohan V, Gayathri R, Jaacks LM, Lakshmipriya N, Anjana RM, Spiegelman D, Jeevan RG, Balasubramaniam KK, Shobana S, Jayanthan M et al.. Cashew nut consumption increases HDL cholesterol and reduces systolic blood pressure in Asian Indians with type 2 diabetes: a 12-week randomized controlled trial. J Nutr. 2018;148(1):63–9. [DOI] [PubMed] [Google Scholar]
- 25. Mukuddem-Petersen J, Stonehouse Oosthuizen W, Jerling JC, Hanekom SM, White Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: a controlled feeding trial. Br J Nutr. 2007;97(6):1144–53. [DOI] [PubMed] [Google Scholar]
- 26. Damavandi RD, Eghtesadi S, Shidfar F, Heydari I, Foroushani AR. Effects of hazelnuts consumption on fasting blood sugar and lipoproteins in patients with type 2 diabetes. J Res Med Sci. 2013;18(4):314–21. [PMC free article] [PubMed] [Google Scholar]
- 27. Agebratt C, Strom E, Romu T, Dahlqvist-Leinhard O, Borga M, Leandersson P, Nystrom FH. A randomized study of the effects of additional fruit and nuts consumption on hepatic fat content, cardiovascular risk factors and basal metabolic rate. PloS One. 2016;11(1):e0147149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Casas-Agustench P, López-Uriarte P, Bulló M, Ros E, Cabré-Vila JJ, Salas-Salvadó J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2011;21(2):126–35. [DOI] [PubMed] [Google Scholar]
- 29. Lee YJ, Nam GE, Seo JA, Yoon T, Seo I, Lee JH, Im D, Bahn KN, Jeong SA, Kang TS et al.. Nut consumption has favorable effects on lipid profiles of Korean women with metabolic syndrome. Nutr Res. 2014;34(9):814–20. [DOI] [PubMed] [Google Scholar]
- 30. Barbour JA, Howe PR, Buckley JD, Bryan J, Coates AM. Effect of 12 weeks high oleic peanut consumption on cardio-metabolic risk factors and body composition. Nutrients. 2015;7(9):7381–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnston CS, Trier CM, Fleming KR. The effect of peanut and grain bar preloads on postmeal satiety, glycemia, and weight loss in healthy individuals: an acute and a chronic randomized intervention trial. Nutr J. 2013;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moreira Alves RD, Boroni Moreira AP, Macedo VS, Bressan J, Cássia Gonçalves Alfenas R, Mattes R, Brunoro Costa NM. High-oleic peanuts: new perspective to attenuate glucose homeostasis disruption and inflammation related obesity. Obesity (Silver Spring). 2014;22(9):1981–8. [DOI] [PubMed] [Google Scholar]
- 33. Wien M, Oda K, Sabate J. A randomized controlled trial to evaluate the effect of incorporating peanuts into an American Diabetes Association meal plan on the nutrient profile of the total diet and cardiometabolic parameters of adults with type 2 diabetes. Nutr J. 2014;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McKay DL, Eliasziw M, Chen CYO, Blumberg JB. A pecan-rich diet improves cardiometabolic risk factors in overweight and obese adults: a randomized controlled trial. Nutrients. 2018;10(3):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gulati S, Misra A, Pandey RM, Bhatt SP, Saluja S. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: a 24-wk, randomized control trial. Nutrition. 2014;30(2):192–7. [DOI] [PubMed] [Google Scholar]
- 36. Hernández-Alonso P, Salas-Salvadó J, Baldrich-Mora M, Juanola-Falgarona M, Bulló M. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: a randomized clinical trial. Diabetes Care. 2014;37(11):3098–105. [DOI] [PubMed] [Google Scholar]
- 37. Kasliwal RR, Bansal M, Mehrotra R, Yeptho KP, Trehan N. Effect of pistachio nut consumption on endothelial function and arterial stiffness. Nutrition. 2015;31(5):678–85. [DOI] [PubMed] [Google Scholar]
- 38. Li Z, Song R, Nguyen C, Zerlin A, Karp H, Naowamondhol K, Thames G, Gao K, Li L, Tseng CH et al.. Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J Am Coll Nutr. 2010;29(3):198–203. [DOI] [PubMed] [Google Scholar]
- 39. Parham M, Heidari S, Khorramirad A, Hozoori M, Hosseinzadeh F, Bakhtyari L, Vafaeimanesh J. Effects of pistachio nut supplementation on blood glucose in patients with type 2 diabetes: a randomized crossover trial. Review Diabet Stud. 2014;11(2):190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sauder KA, McCrea CE, Ulbrecht JS, Kris-Etherton PM, West SG. Effects of pistachios on the lipid/lipoprotein profile, glycemic control, inflammation, and endothelial function in type 2 diabetes: a randomized trial. Metabolism. 2015;64(11):1521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang X, Li Z, Liu Y, Lv X, Yang W. Effects of pistachios on body weight in Chinese subjects with metabolic syndrome. Nutr J. 2012;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bamberger C, Rossmeier A, Lechner K, Wu L, Waldmann E, Stark RG, Altenhofer J, Henze K, Parhofer KG. A walnut-enriched diet reduces lipids in healthy Caucasian subjects, independent of recommended macronutrient replacement and time point of consumption: a prospective, randomized, controlled trial. Nutrients. 2017;9(10):1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Katz DL, Davidhi A, Ma Y, Kavak Y, Bifulco L, Njike VY. Effects of walnuts on endothelial function in overweight adults with visceral obesity: a randomized, controlled, crossover trial. J Am Coll Nutr. 2012;31(6):415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ma Y, Njike VY, Millet J, Dutta S, Doughty K, Treu JA, Katz DL. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care. 2010;33(2):227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Njike VY, Ayettey R, Petraro P, Treu JA, Katz DL. Walnut ingestion in adults at risk for diabetes: effects on body composition, diet quality, and cardiac risk measures. BMJ Open Diabetes Res Care. 2015;3(1):e000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rock CL, Flatt SW, Pakiz B, Quintana EL, Heath DD, Rana BK, Natarajan L. Effects of diet composition on weight loss, metabolic factors and biomarkers in a 1-year weight loss intervention in obese women examined by baseline insulin resistance status. Metabolism. 2016;65(11):1605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tapsell LC, Lonergan M, Batterham MJ, Neale EP, Martin A, Thorne R, Deane F, Peoples G. Effect of interdisciplinary care on weight loss: a randomised controlled trial. BMJ Open. 2017;7(7):e014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tapsell LC, Batterham MJ, Teuss G, Tan SY, Dalton S, Quick CJ, Gillen LJ, Charlton KE. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr. 2009;63(8):1008–15. [DOI] [PubMed] [Google Scholar]
- 49. Tapsell LC, Gillen LJ, Patch CS, Batterham M, Owen A, Bare M, Kennedy M. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care. 2004;27(12):2777–83. [DOI] [PubMed] [Google Scholar]
- 50. Wu H, Pan A, Yu Z, Qi Q, Lu L, Zhang G, Yu D, Zong G, Zhou Y, Chen X et al.. Lifestyle counseling and supplementation with flaxseed or walnuts influence the management of metabolic syndrome. J Nutr. 2010;140(11):1937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu L, Piotrowski K, Rau T, Waldmann E, Broedl UC, Demmelmair H, Koletzko B, Stark RG, Nagel JM, Mantzoros CS et al.. Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: a randomized controlled cross-over clinical trial. Metabolism. 2014;63(3):382–91. [DOI] [PubMed] [Google Scholar]
- 52. Mullner E, Plasser E, Brath H, Waldschutz W, Forster E, Kundi M, Wagner KH. Impact of polyunsaturated vegetable oils on adiponectin levels, glycaemia and blood lipids in individuals with type 2 diabetes: a randomised, double-blind intervention study. J Hum Nutr Diet. 2014;27(5):468–78. [DOI] [PubMed] [Google Scholar]
- 53. Kim Y, Keogh JB, Clifton PM. Benefits of nut consumption on insulin resistance and cardiovascular risk factors: multiple potential mechanisms of actions. Nutrients. 2017;9(11):1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hernández-Alonso P, Giardina S, Salas-Salvadó J, Arcelin P, Bulló M. Chronic pistachio intake modulates circulating microRNAs related to glucose metabolism and insulin resistance in prediabetic subjects. Eur J Nutr. 2017;56(6):2181–91. [DOI] [PubMed] [Google Scholar]
- 55. Ribeiro PVM, Silva A, Almeida AP, Hermsdorff HH, Alfenas RC. Effect of chronic consumption of pistachios (Pistacia vera L.) on glucose metabolism in pre-diabetics and type 2 diabetics: a systematic review. Crit Rev Food Sci Nutr. 2017:1–9. [DOI] [PubMed] [Google Scholar]
- 56. Kendall CW, Esfahani A, Josse AR, Augustin LS, Vidgen E, Jenkins DJ. The glycemic effect of nut-enriched meals in healthy and diabetic subjects. Nutr Metab Cardiovasc Dis. 2011;21(Suppl 1):S34–9. [DOI] [PubMed] [Google Scholar]
- 57. Kendall CW, Josse AR, Esfahani A, Jenkins DJ. The impact of pistachio intake alone or in combination with high-carbohydrate foods on post-prandial glycemia. Eur J Clin Nutr. 2011;65(6):696–702. [DOI] [PubMed] [Google Scholar]
- 58. Alkhatib A, Tsang C, Tiss A, Bahorun T, Arefanian H, Barake R, Khadir A, Tuomilehto J. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients. 2017;9(12):1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kamil A, Chen CY. Health benefits of almonds beyond cholesterol reduction. J Agric Food Chem. 2012;60(27):6694–702. [DOI] [PubMed] [Google Scholar]
- 60. Riserus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dhillon J, Tan SY, Mattes RD. Almond consumption during energy restriction lowers truncal fat and blood pressure in compliant overweight or obese adults. J Nutr. 2016;146(12):2513–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.