Abstract
Objective
Restoration of somatosensory deficits in humans requires a clear understanding of the neural representations of percepts. To characterize the cortical response to naturalistic somatosensation, we examined field potentials in the primary somatosensory cortex of humans.
Methods
Four patients with intractable epilepsy were implanted with subdural electrocorticography (ECoG) electrodes over the hand area of S1. Three types of stimuli were applied, soft-repetitive touch, light touch, and deep touch. Power in the alpha (8–15 Hz), beta (15–30 Hz), low-gamma (30–50 Hz), and high-gamma (50–125 Hz) frequency bands were evaluated for significance.
Results
Seventy-seven percent of electrodes over the hand area of somatosensory cortex exhibited changes in these bands. High-gamma band power increased for all stimuli, with concurrent alpha and beta band power decreases. Earlier activity was seen in these bands in deep touch and light touch compared to soft touch.
Conclusions
These findings are consistent with prior literature and suggest a widespread response to focal touch, and a different encoding of deeper pressure touch than soft touch.
Keywords: Somatosensory, Brain Computer Interface (BCI), Brain Machine Interface (BMI), Electrocorticography, Cortical Stimulation
Introduction
As interest in somatosensory restoration mounts for brain computer interfaces (BCI), neuro-rehabilitation, and related fields, understanding the complex neural mechanisms of somatosensation becomes more imperative. Neural encoding of tactile sensation from primary somatosensory cortex (S1) recordings span multiple spatial and temporal scales and can be studied through both single-unit recordings and field potentials. As with other sensory input paradigms, tuning, feature encoding, and large-scale shifts in frequency bands are likely to represent aspects of cortical processing of sensory information. However, our understanding of these mechanisms in the context of natural sensation is limited.
Single-unit action potentials exhibit several features of encoding, including stimulus orientation tuning (Hsiao et al. 2002), vibrational patterns (Salinas et al. 2000), and flutter patterns (Mountcastle et al. 1969). Similarly, bursts of spike rates (Luna et al. 2005; Salinas et al. 2000), or the gap between these bursts (Birznieks and Vickery 2017), are locked to vibrational tactile stimulus patterns. The intensity of the stimulus appears to be coded by fi ring rate, possibly due to simple summation overactive mechanoreceptors (Bensmaia 2008; Muniak et al. 2007). Individual S1 neurons may encode different features, with some tuned to amplitude, frequency, or both (Alvarez et al. 2015). The field potentials in the high-gamma range are correlated with spikes in somatosensation, suggesting a direct relationship between the two (Ray et al. 2008b).
Oscillatory patterns in the field potentials of the primary somatosensory cortex show changes following somatosensory stimuli in alpha and beta band power, likely reflecting changes in attended stimuli (Bauer et al. 2014; Burton and Sinclair 2000; van Ede et al. 2014), and gamma band power also as an attentional resource with the possible processing of the sensation (Bauer et al. 2006; Hauck et al. 2007; van Ede et al. 2014). Specifically, contralateral depression and rebound of alpha and beta activity reflect attentional shifts to attended stimuli, but not unattended stimuli. Parts of the high-gamma band may also reflect attention. Ray et al., in a study of vibrotactile stimuli with electrocorticography (ECoG) recordings over S1, showed an early high-gamma (HG) power increase, regardless of attention, and a later component, around 400 ms, that was larger when subjects focused on the vibrotactile stimulus (Ray et al. 2008a, b ). Ryun et al. with further S1 ECoG human studies using different vibratory stimuli, found patterns of HG that peaked early (50–100 ms) and attenuated shortly after, but did not show stimulus frequency dependence (Ryun et al. 2017b). In other experiments, electrical stimulation to the human hand showed a relation between HG power and stimulus intensity, suggesting the gamma band power directly encodes stimulus intensity (Rossiter et al. 2013).
Directly comparing cortical changes during different types of tactile stimuli has been achieved in human and NHP studies, but often with unnatural vibrotactile stimuli. Ray et al., in a NHP study, found HG responses to vibrotactile stimuli, but no changes in power associated with changes in stimulus. Timing of the changes in field potentials were not evaluated directly, but more prolonged changes in beta and HG were suggested from their analysis (Ray et al. 2008). In human S1, a greater HG response was noted during noxious stimuli compared to non-noxious stimuli, and the response was higher with more severe pain (Liu et al. 2015). In another human study, vibrotactile stimuli patterns were compared to natural sensations from touching textured patterns in S1, showing similar changes in HG (Ryun et al. 2017). Here, a courser texture showed greater power in S1 than a finer texture, with changes in HG that lasted for the duration of the stimulus. Although similar findings were seen with natural vibratory stimuli compared to unnatural, it remains to be seen how non-vibratory stimuli alter the neural activity in S1.
Direct electrical S1 stimulation, whether through microelectrode or electrocorticography, is a complementary tool to explore how electrical changes in the cortex translate into percepts. With relatively large electrodes and correspondingly large current amplitudes (~ 1–10 mA), stimulation through ECoG electrodes has produced percepts described with artificial qualities such as “buzzing” or “electrical” sensations. Changing the parameters of frequency, amplitude, and pulse-width were noted to increase the intensity of the percept, and sometimes widened the receptive field of the percept, but did not change the type of somatosensation perceived (Lee et al. 2018). Stimulation through microelectrodes, with much smaller amplitudes of electric current (~ 10–100 μA), produced a wider range of sensations including some with naturalistic descriptions (Armenta Salas et al. 2018; Flesher et al. 2016). Here, higher amplitudes were associated with a proprioceptive sensation, but the relationship was not consistent (Armenta Salas et al. 2018).
With some exceptions as described above, studies of somatosensation in the human cortex are often conducted using magnetoencephalography or electroencephalography, which lack the spatial resolution for a more detailed analysis of cortical changes. Furthermore, because it has been important to understand basic neural processing, most of these studies have used easily quantified but unnatural-feeling vibrotactile stimuli; however, natural stimuli may produce different neural dynamics. Here, we conducted a study in epileptic humans with realistic tactile stimuli while recording ECoG signals from the hand area of S1. The goal of the study was to characterize changes in the spectral power of these signals to better understand how the brain processes natural tactile stimulation.
Methods
Subjects and implantation
Six patients with intractable epilepsy required surgical implantation of subdural ECoG grids for seizure localization and gave informed consent to participate in this IRB-approved study (study approval HS-13–00528) as part of a larger study to evaluate ECoG for somatosensory BCI. All subjects were right-hand dominant, self-reported normal somatosensation, and exhibited no clinical deficits in somatosensation on examination. During implantation of the subdural electrodes, an additional grid was placed over S1 hand area for research. The research grid placed over S1 was placed with the assistance of neuronavigation software. For five participants (S01 and S03 through S06), the additional grid was a 64-channel “mini”-ECoG grid consisting of 2 mm contacts (1.2 mm exposed), spaced 3 mm apart center-to-center (FG64C-MP03, Ad-Tech Medical Instrumentation Corporation, Oak Creek, WI, USA). In one of these (S05), the grid was split in half due to surgical concerns of disrupting adhesions with the larger grid. In the remaining participant (S02), a standard 20-channel ECoG grid with 4.75 mm contacts (1.5 mm exposed), spaced 10 mm apart center-to-center (AU4 × 5P2, LifeSciences, Plainsboro, NJ, USA) was implanted. Grids were anchored to the dura to prevent post-operative movement, and the dura, bone, and scalp were closed in typical water-tight fashion. Patients were transported to the epilepsy monitoring unit where testing occurred following the restoration of their antiepileptic medications. Epileptogenic zones were localized to the mesial structures in S01, S03, S04. In S02, epilepsy was the result of a cavernoma in the parietal lobe distant from the S1 hand area and posterior to S1 in general. S05 had an interhemispheric parietal seizure focus, distinct from S1 on mapping and imaging, and S06 had frontal and temporal seizure foci, related to areas of encephalomalacia that were not in primary motor or sensory cortex.
Of the six original participants, S05 and S06 were excluded from further analysis. In S05, five electrodes were clinically mapped to the somatosensory cortex, but four exhibited signal-corrupting noise (see Signal Processing for noise rejection methods), and the fifth showed no statistically significant changes. For S06, clinical stimulation mapping found no electrodes with somatosensory percepts, and an initial dermatomal tactile test found no statistically significant changes in any recording channels. See Table 1 for demographic details of the remaining four patients evaluated.
Table 1.
Subject demographics and testing location
| Subject | Age | Gender | Handedness | Recording side | Seizure focus | Epilepsy duration (years) | Type of grid* | Channels over S1 | Location of testing |
|---|---|---|---|---|---|---|---|---|---|
| S01 | 21 | Female | Right | Left | MTS | 3 | M | 11 | Tip of digit 1 |
| S02 | 25 | Male | Right | Right | Parietal cavernoma | 3 | S | 6 | Hypothenar eminence |
| S03 | 50 | Female | Right | Left | MTS | 45 | M | 25 | Mid palm |
| S04 | 43 | Male | Right | Left | Amygdala | 24 | M | 18 | Tip of digit 3 |
Subjects all had normal somatosensation and seizure foci distant from S1, except S03 who had a cavernoma in his parietal lobe posterior to the inferior portion S1. The area of the hand chosen for testing was determined by mapping of the ECoG grid, where electrical stimulation was used to evaluate which electrodes were over the primary somatosensory cortex. Following that mapping, the areas of the hand where percepts were elicited with electrical stimulation were presumed to be somatotopically aligned to cortex underneath those electrodes, and were chosen for testing with mechanical stimulation
M Mini electrocorticography grid, 2 mm contacts spaced 3 mm apart, 64 electrodes; S “Standard” electrocorticography grid, 4.75 mm contacts spaced 10 mm apart, 20 electrodes. S1 = Somatosensory cortex. MTS Mesial temporal sclerosis
Experimental design
In conjunction with the clinical mapping, the grid over the S1 hand area was mapped using standard clinical stimulation mapping parameters (Agnew and McCreery 1987; Wyllie et al. 1988). In areas with somatosensation-only percepts, stimulation was tested with bipolar electrode pairs using charge-balanced, alternating anodic-or cathodic-first pulse trains. Frequency (50 Hz) and pulse width (500 μs) were held constant, and duration (1–3 s) and amplitude (0.5–12 mA) were varied systematically. Percepts and somatotopic location were described by the subject for each setting. Amplitude increases halted at the maximum value, or when involuntary motor action was noted or upon patient request (however, none of the subjects made such a request). From these data, an electrode pair with a stable percept over five repetitions on the glabrous skin of the hand, was chosen as the “primary” electrode pair (see Table 1).
For tactile testing, subjects laid their hand out palm up in a comfortable manner, within their field of view, and were instructed to relax, keep their eyes open, and not move their hand. The dermatomal hand area chosen for testing was then subject to 15 repetitions each of three tactile stimuli: Soft Touch (ST) with a cotton probe, Light Touch (LT, minimal or no indentation of the skin) with a smoothed balsa-wood probe, and Deep Touch (DT, indentation of the skin) with the same wooden probe. The contact area of the wooden probe and the cotton probe were 3 mm × 0.5 mm. Contact with the skin lasted 1–1.6 s, and intervals between touch varied between 1.5 and 2.5 s. For DT and LT, after initial contact, no further movement occurred until release. ST consisted of a repeat brushing motion at a rate of between 1 and 2 Hz, for three or four brushes in each stimulus trial. The affected dermatomal area was the same for DT and LT, whereas ST brushing covered a patch of 3 mm × 1 cm. The timing for touch lengths, brushing speed, brushing quantity, and timing between touches were variedby the experimenter to prevent anticipation/expectation by the subject.Although the touches were not automated, video of the touches were reviewed to ensure that all were within the predesignated range of parameters (e.g. 1–2 Hz). All stimuli remained within the comfort level of the patient as self-reported. All touches were performed by the same experimenter (DRK). Touch pressure was estimated (e.g. no skin indentation vs skin indentation) due to two logistical limitations; (1) touch locations were determined after implantation, and were thus not always accessible to automation, and (2) touch pressure relies on counter pressure to the hand (i.e. hand restriction), which could not be done safely in ICU patients because of venous catheter lines and other medical equipment-or procedure-related restrictions. Force was also difficult to estimate given the varied locations of touch (e.g. fingertip vs palm), and patient factors like volume status. For these same reasons, the speed of indentation was not possible to know exactly, as that is partly dependent on the counter force. However, indentation was verified to occur between 0.2 and 0.35 s after touch onset. Although a significant limitation, we postulated that the difference between the three types of touch, no skin-indentation, skin-indentation, and a brushing, would sufficiently estimate their natural perceptual differences.
Signal processing
Neural activity was recorded at 2000 samples/s (N = 2) or 256 samples/s (N = 2), through a 128 channel amplifier (Xltek EMU128FS, Natus Neurology Incorporated, Warwick, RI, USA), with a 0.065 Hz analog DC high-pass filter, and a digital anti-aliasing filter. A needle-electrode placed in the subcutaneous and muscular tissue of the neck was used as ground, and a reference electrode of a contact with relative inactivity and distant from the recording electrodes (i.e. on a grid or strip farthest from the S1 grid).
Recordings were evaluated offline and visually inspected for seizures or other corrupting signals by an epileptologist. Using MATLAB software (The Mathworks, Natick, MA, USA), notch filters (8th order Butterworth filter, using the filtfilt function in MATLAB) at 60 and 120 Hz were applied to remove electrical line noise. Power spectra were calculated between 0 and 125 Hz, for − 0.5 to 2.0 s relative to the onset of touch, using the Chronux package (http://chronux.org/, Mitra and Bokil 2008) with 0.2 s-window, step-size 0.005 s, time-bandwidth parameter 2, tapers 3, and pad 1; and a second time, separately, with 0.5 s window and 0.005 s step size (all other parameters the same) to gain better resolution of the power in the lower frequency bands. For each stimulus type, the signal was trial-averaged, z-scored, and normalized to the inter-trial intervals (ITI). The ITIs were visually inspected, and those channels without an expected fall off in power of approximately 1/frequency were reviewed by an epileptologist. If the channel did not show expected neural activity or was corrupted by substantial artifact, it was excluded from analysis. For analysis, to ensure that the ITI was not biased to systematic changes in the bands (e.g. beta rebound occurring after the touch was released), the ITI power spectra were averaged across trials, and then compared along each frequency bin to an ITI without the first 300 ms, using a Wilcoxon rank sum. No channels over S1 had significant differences in any frequency bands (p > 0.05 for all). To further reduce the variance across the ITI, each frequency bin was made uniform, as the average of all time points across the ITI.
Statistical analysis
To identify regions of interest in time and frequency from the power spectra, a cluster-mass based permutation test was performed (Maris and Oostenveld 2007). Data were separated into trials, starting at the onset of touch and ending at the removal of touch, and the ITI was from the end of touch until the onset of touch. For each stimulus type, the normalized power in each time–frequency bin was compared to the time-averaged normalized power in the same frequency bin from the ITI using a two-tailed t-test (MATLAB) with a significance threshold of p < 0.05. Statistically, significant time–frequency bins were then clustered using the bwconncomp function (MATLAB). Clusters were compared to a cluster-mass generated by permutation test as follows. For N = 5000 repetitions, trial and ITI labels were shuffled and the data were subjected to the same clustering procedure described above. For each repetition, the cluster-mass statistic, i.e., the summed t-statistic from the largest cluster (most time–frequency bins) was recorded. A p-value was computed by comparing the observed cluster-mass statistic from the unshuffled data to the population of cluster-mass statistics computed from the shuffled data, with the alpha-level set to 0.05. Channels were evaluated for significant clusters that included the frequency bands alpha (8–15 Hz), beta (15–30 Hz), low-gamma (30–50 Hz), and high-gamma (50–125 Hz).
Next, to evaluate the significance of changes in power over time and frequency, channels with statistically significant clusters were analyzed to identify the extrema in power following touch onset within the same frequency bands alpha (8–15 Hz), beta (15–30 Hz), low-gamma (30–50 Hz), and high-gamma (50–125 Hz). To capture the timing of the changes, the raw signal was squared and bandpass filtered using a Butterworth filter (filtfilt function; MATLAB), to reflect the power in those bands, then z -scored. Power in these bands was smoothed with a trapezoidal integral (the function trapz in Matlab) with a moving window of 100 ms, sliding 5 ms for − 0.5 s to 2 s relative to touch onset. Next, 0.4 s of data was removed from both the beginning and end of these windows to account for edge effects. The data were then analyzed to identify the time and magnitude of the largest and smallest integral values, and the differences in time and magnitude among the different stimuli conditions were assessed using analysis of variance (anova1 function; MATLAB).
Results
Somatosensory cortex/grid results
G rid locations were verified post-operatively by fusing a pre-operative MRI and a post-operative CT. The central sulcus was identified with verification from a senior neuroradiologist, and contacts presumed to rest over S1 were identified based on anatomic location and results from mapping (see Online Resource Fig. 1 for details of the mapping). Electrodes chosen for analysis were those that showed clear somatosensory-only responses from the patient during mapping, and verified on imaging to be over S1. All electrodes with somatosensory responses, even if not in the hand area, were included for completeness (see Fig. 1). Some electrodes had clear somatosensory percepts on stimulation, without motor involvement on repeat stimulation (N> 5 stimulations), however, on imaging, appeared to be in the primary motor cortex. Given the inherent error in localizing electrodes of small size and pitch, if these electrodes were close to S1 they were included (e.g., S04 in Fig. 3). Stimulation mapping was done just prior to testing, and was therefore expected to be accurate.
Fig. 1.
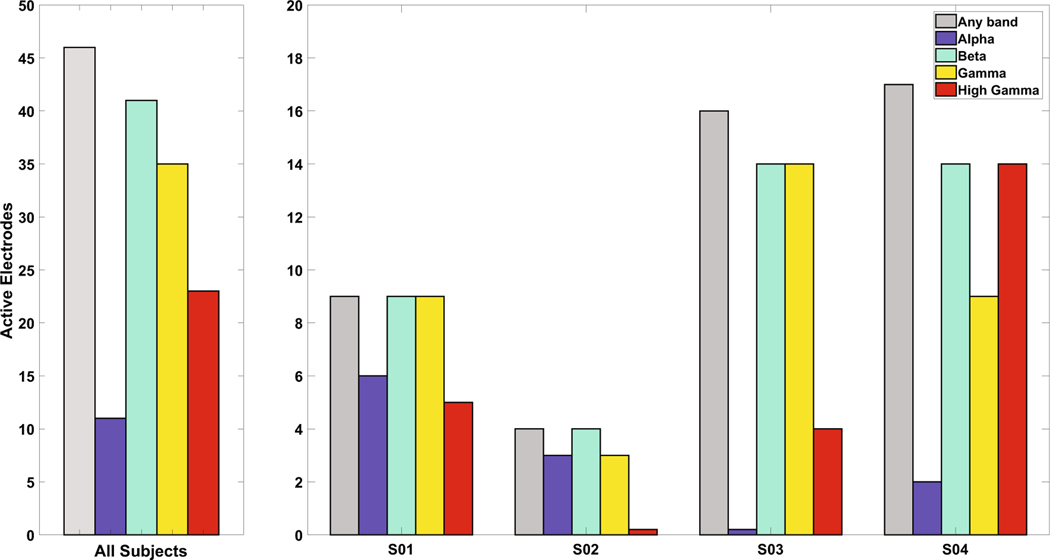
Number of electrodes with statistically significant changes in the frequency bands. Number of electrodes (out of 64 electrodes for S01, S03, S04 and 20 electrodes for S02) with S1 activity in any frequency band, as determined by a cluster-mass based permutation test. Forty-eight of the 60 electrodes (80.0%) over S1 had activity in one of the bands. Beta activity was the most frequent (90% of those electrodes with activity), followed by gamma. S02 had no statistically significant activity in high gamma, and S03 had no activity in the alpha band
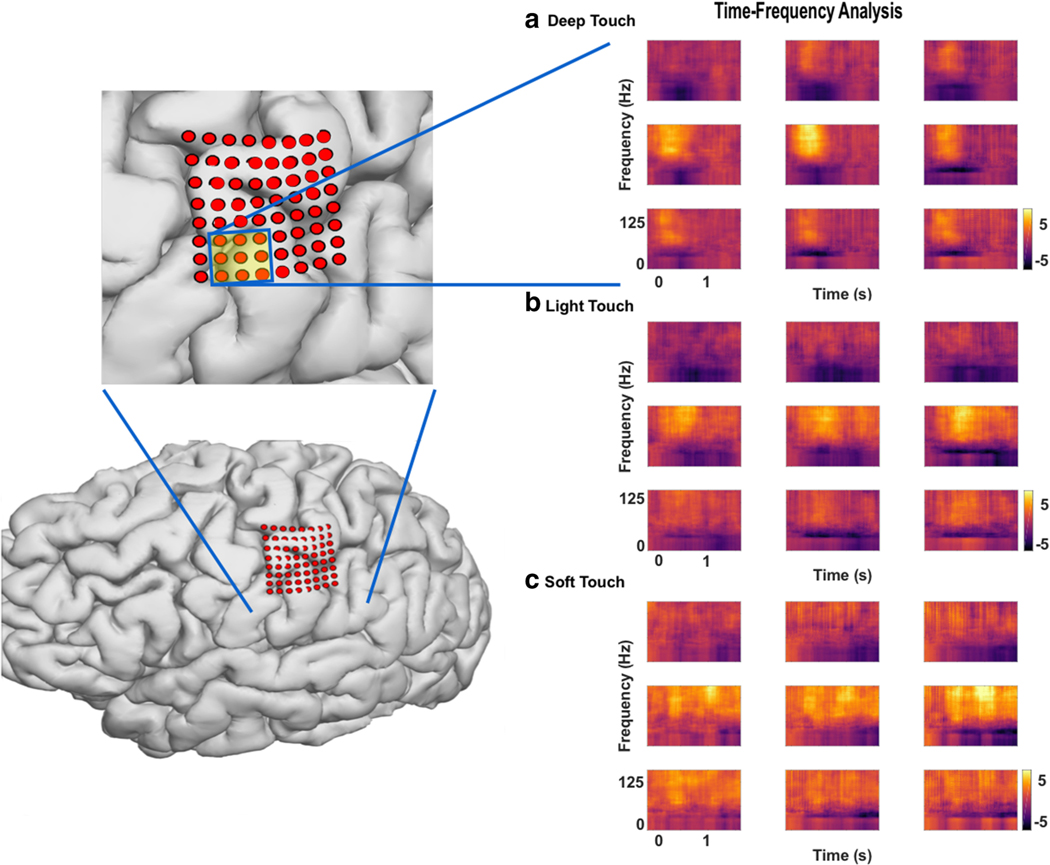
Fig. 3.
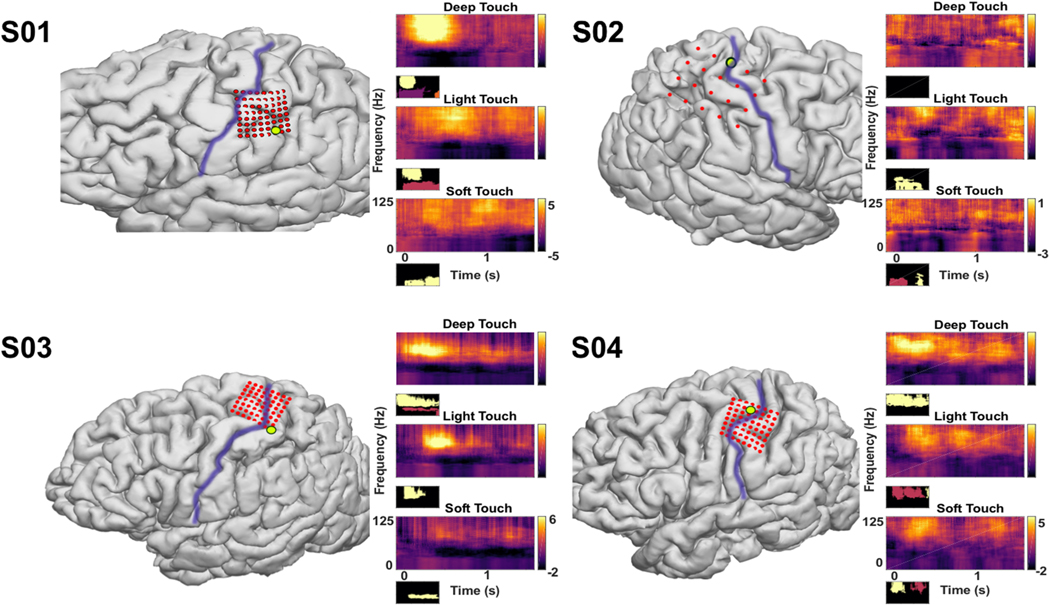
Time–frequency plots for selected channels over somatosensory cortex Deep touch, with indentation of the skin, light touch, without indentation of the skin, and soft touch, with a continuous brushing motion, are shown for one channel in each subject. The purple line represents the central sulcus. Some electrodes appear over motor cortex, but with variability from three-dimensional imaging fusion techniques for electrode localization, bias was given toward cortical mapping results when identifying electrodes as S1 contacts. High-gamma elevates shortly after touch onset for DT and LT, and later for ST, with streaks of HG prior to the statistically significant elevation, suggesting a building of power. The return to baseline of HG power is noted while the touch itself is still occurring. Power in the alpha and beta bands are both decreased, starting after the increase in HG power. Gamma band power is also decreased, although part of it appears in continuity with the beta band. Below each plot is a mask of the statistically significant signal as analyzed through a cluster-mass permutation test (N = 5000); different colors indicate different contiguous clusters. **Smoothed normalized power, arbitrary units. * p < 0.01
Of 212 electrodes (all electrodes on the grids being evaluated), 60 had somatosensory-only percepts during mapping (28.3%, see Fig. 1). Of these, 46(76.7%) had a statistically significant change, based on the cluster-mass based permutation test, in one or more of the analyzed frequency bands (alpha, beta, gamma, or high-gamma). Nearly all electrodes exhibited statistically significant changes in beta band power (15–30 Hz) with respect to ITI (43 electrodes, 93.5%; Fig. 1). The same was also true per patient: the largest proportion of significant changes in each patient’s data was from beta band power. Half of all electrodes (23 electrodes, 50.0%) showed significant changes in the HG (50–120 Hz) band. Alpha band (8–15 Hz) power changes were seen in 11 electrodes (22.9%), and gamma (30–50 Hz) in 36 electrodes (78.3%). S02 had no changes in the HG frequency band, and S03 had no changes in the alpha band. All statistically significant changes in HG corresponded to elevations in power whereas changes in alpha, beta, and gamma all corresponded to decreases inpower.
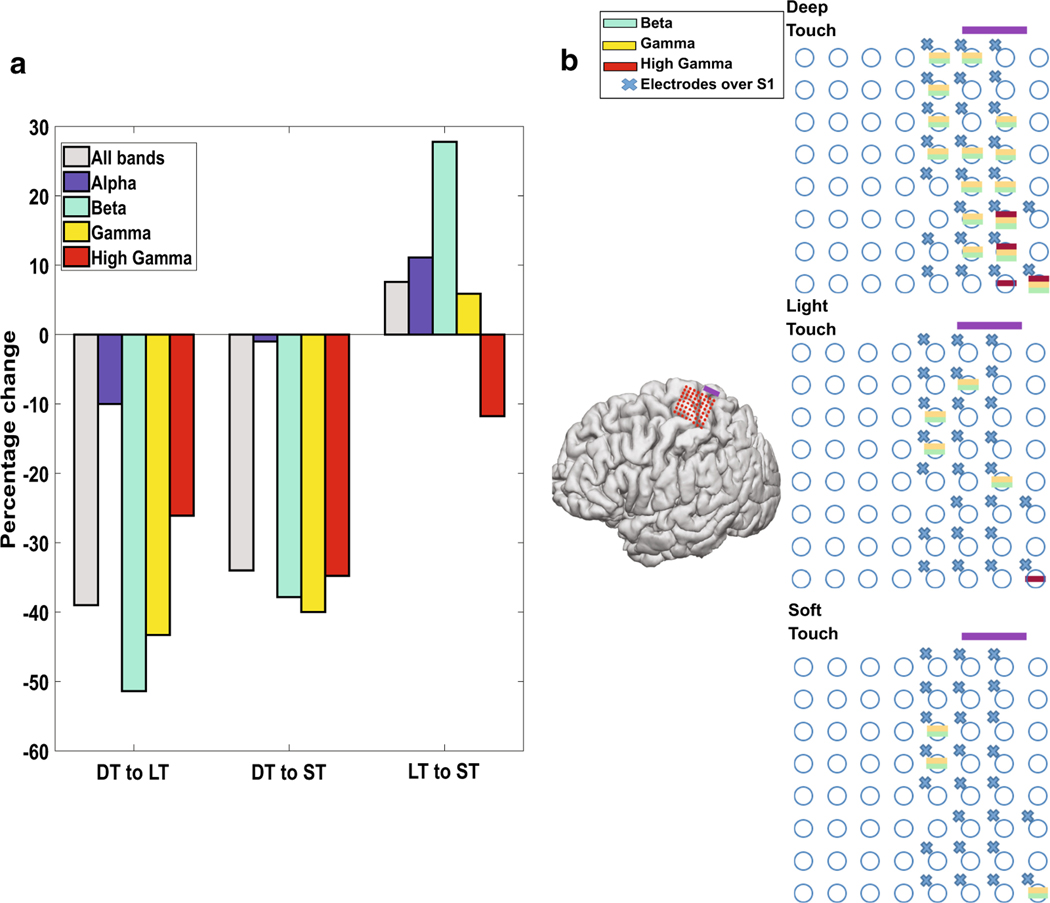
Differences between the types of touch were noted, with respect to number of electrodes with significant changes in each frequency band. DT had the highest number of electrodes with significant changes in all four bands examined, with LT and ST showing a 39% and 34% drop-off respectively, in total electrodes with significant changes in power (see Fig. 2a). Compared to LT, ST had a relative increase in electrodes with alpha, beta, and gamma band changes. The most significant reduction was in electrodes with beta band power, where LT had about half as many as DT (51.4%), and a third as many as ST (27.8%). The pattern of widespread changes in DT and a reduction in LT and ST could be steep, as seen in S03 (see Fig. 2b). Several of the electrodes with beta and gamma changes were shown to have activity in areas far from the hand (e.g. face, see Online Resource Figs.1, 2b). The locations of the changes were relatively stable, in that the electrode quantity was reduced, but otherwise involved the same electrodes (see Fig. 2b). Only three electrodes had changes in ST that did not overlap with those in DT, and none in LT.
Fig. 2.
Changes in the number of involved electrodes based on type of touch. a The percentage change in the number of electrodes with statistically significant activity as a function of the type of touch. Deep touch (DT) had the most of each type, with fewer involved electrodes in light touch (LT) and soft touch (ST). LT had the least number of active electrodes during touches. Overall, beta band had the greatest amount of change between the touch types. b Subject S03 shows a representative change in the location of active electrodes. The purple bar denotes the orientation on the 3-D mapped grid. An “x” indicates those electrodes with S1 activity. Decreases are seen in the number of electrodes with beta, gamma, and high-gamma activity in ST and LT compared to DT (no significant alpha activity was observed in this subject). A broad spread of activity in beta and gamma activity is noted with DT
High-gamma band
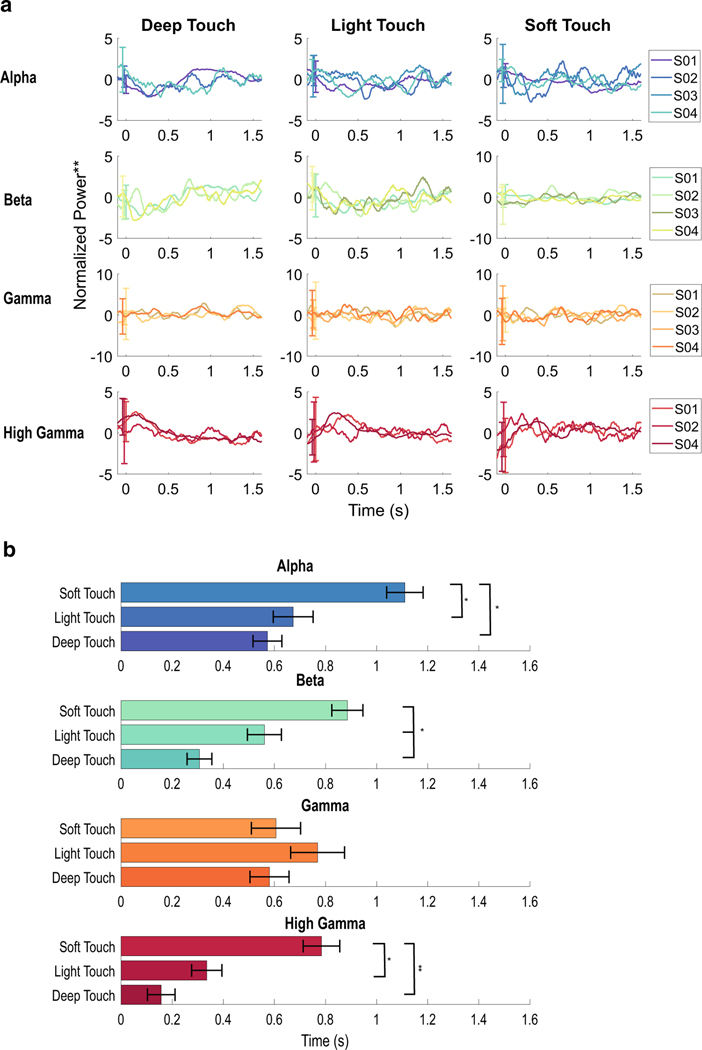
High-gamma activity is noted in three of four subjects (Fig. 1). Elevations in HG power are seen within select electrodes over S1, lasting between around 300–500 ms, but extinguishing prior to the end of tactile stimulus (Fig. 3). The latency from stimulus onset to the earliest changes was minimal, often 50 ms or less, and particularly in DT. The peak and nadir of the signal were assessed as a moving integral of the mean band-passed signal over 100 ms windows (5 ms step) and taken as the maximum and of the integral in each window. Peaks for HG occurred between 160 ms and 780 ms, on average, depending on the type of touch. During DT, the latency from stimulus onset to the peak was 160 ± 50 ms, and for LT 340 ± 60 ms, and ST 780 ± 70 ms. DT and LT differed significantly from ST (p < 0.0001, see Fig. 4), but not from each other (p= 0.09). Although these peaks were found through calculating the extrema, on visual inspection, ST had a pattern of multiple peaks. Soft touch had repetitive streaks of HG power elevations, often with either two statistically significant clusters (see Fig. 3, S04), or with the first peak failing to reach significance (see Fig. 5, statistical masking not shown). The calculated peak of 780 ± 70 ms indicates that the strongest peak, on average, occurred later in the trial.
Fig. 4.
Differences between the timing of greatest change for deep touch (DT), light touch (LT), and soft touch (ST) a Average power across the frequency bands during each type of touch for each patient. Normalized power, smoothed, for each patient during the different touches, separated into frequency bands through band filtering. The trends for high gamma increases and alpha and beta decreases are evident. Not all subjects had statistically significant changes in all frequency bands during certain types of touch (e.g. S03 did not have changes in the beta band during DT). b Timing of the peaks or nadirs of each frequency band across all patients and all channels. The means (bars = SD) of the peak or nadir for a moving integral taken of the power. Decreases in the power for frequency bands: alpha, beta, and gamma, and increases in the power for the frequency band high-gamma are shown. In alpha, beta, and high gamma, DT and LT occurred earlier than ST; in beta band changes, DT occurred earlier that LT
Fig. 5.
Time–frequency plots in neighboring channels in S01 during the three types of touch The grid location on the cortex, with an area zoomed in on the grid, highlighting the six electrodes displayed on the right. Elevations in HG activity, and decreases in alpha, beta, and gamma power, can be seen in several channels. During cortical mapping, the channels shown caused percepts in the digit used for testing. Although the area of the digit being tested was small, changes spread through the electrodes
Beta band
Decreases in beta band activity were most common among all stimulus conditions and patients. In channels with elevations in HG, decreases in beta power were seen concurrently, even if these decreases did not reach significance (see Fig. 3, S01, S03, S04). Although beta power is seen to decrease concurrently with HG, it generally reaches its extreme value later than the HG power extremum and is sustained longer (Fig. 4). The nadirs of beta power occurred earliest in DT (310 ± 50 ms), later for LT (560 ± 70 ms), and later still for ST (890 ± 61 ms, p < 0.0001 for DT vs ST and p < 0.01 for DT vs LT and for LT vs ST). Unlike HG changes, the changes in beta power are more well-defined “streaks”, with less variability across time. This is most evident in the changes in ST, with a more sustained response to continued motion during touch (in contrast to the static stimulus of DT and LT; see Figs. 3, 4).
Alpha band
Changes in the alpha power band were less common than changes in beta, but were concurrent with beta. No electrodes showed changes in alpha without also exhibiting changes in beta band power. However the nadirs of alpha power occurred later than beta nadirs by around 200 ms for each stimulus condition. Whereas beta changes appear to remain stable over time, alpha changes are more punctuated and occur after the start of the HG peaks when co-occurring. This can be seen well in Fig. 5, during ST, where the alpha changes are punctuated following the HG changes. The nadir of DT changes (570 ± 60 ms) and LT changes (670 ± 80 ms) were found earlier than ST changes (1110 ± 70 ms, p< 0.001 for DT vs ST and LT vs ST), but not significantly different from each other (p = 0.9 DT vs LT).
Gamma band
Decreases in the gamma band power also co-occurred with beta band changes. In the time–frequency plots in Figs. 3 and 4, changes within this band are relatively coincident with the changes in the beta band. Although that suggests a continuity between beta and gamma (i.e. gamma is a reflection of changes in beta), the timing of the changes do not mirror that of beta; the nadir occurred at 580 ± 80 ms in DT, 780 ± 80 ms in LT, and 600 ± 100 ms in ST. None were statistically significant from each other. This trend is reflected in the relatively flat response seen in Fig. 4a. The time–frequency plots generally show an area of transition from the decreases seen in beta and the increases in HG power, much of which makes up the gamma frequency band.
Discussion
Here we sought to understand the neural dynamics in S1 during realistic, mechanical stimuli. We observed focal increases in HG power with subsequent broader decreases in alpha, beta, and gamma power in ECoG signals recorded in human cortex over S1. Three tactile stimuli were tested: deep pressure with indentation of the skin (DT); lighter touch without indentation of the skin (LT), and repetitive soft grazing with a cotton probe (ST). Among electrodes localized to S1, a large portion showed activation in one or more frequency bands. Consistent with the concept of a common pathway for conveying sensory information described in prior work, similar patterns of neural activity—elevations in HG and decrease in alpha and beta band power—were observed in all three task conditions. However, there were notable exceptions in the timing of the changes between conditions. Deep touch and light touch showed an earlier response compared to a soft, repetitive touch in alpha, beta (both decreases in power) and HG (elevation in power). Changes in the neural activity in DT and LT often attenuated earlier than the release of the stimulus, similar to previous work (Ryun et al. 2017), whereas the ST pattern, a repetitive stimulus, appeared to have sustained changes.
High-gamma and gamma frequency bands
Oscillations in HG in cortical field potentials are believed to coordinate information across cortical areas and are associated with selective attention(Crone et al. 1998; Engel et al. 2001; Fries 2015; Fries et al. 2001; Krebber et al. 2015; Tallon-Baudry and Bertrand 1999). Our results here are concordant with other somatosensory studies in both nonhuman primates and humans (Bauer et al. 2006; Hauck et al. 2007; Ray et al. 2008a, b; Ryun et al. 2017; van Ede et al. 2014), and support that HG plays a role in somatosensory processing. For example, early modulation of HG power during the DT and LT conditions could be explained by a summary of the response of peripheral afferent receptors. In modeling the encoding of the depth of pressure from the activity of peripheral mechanoreceptors, it has been shown that a summary of the activity of all local receptors is the most accurate model (Muniak et al. 2007). The activity we have seen here, with an earlier increase in HG, may be a cortical reflection of the higher peripheral activity: as pressure depth increases, more peripheral mechanoreceptor activity equates to an increase in total input to S1. The increased input rate may then reach a triggering threshold faster, leading to earlier activation of HG. By similar logic, lower input rate from soft touch would correspond to later activation.
Support for this model is reflected in studies of electrical stimulation of the hand. Activation of slowly adapting afferents can produce the perception of increased pressure as the electrical stimulation pulse frequency increases on the peripheral receptors (Macefield et al. 1990. Here, an amplitude-dependent response in HG power, even at sub-threshold pain levels, indicated that the HG response stemmed from the intensity rather than a secondary feature like pain (Rossiter et al. 2013). During electrical stimulation of S1 directly, increasing the frequency, amplitude, and pulse-width have all shown an increase in the intensity felt by subjects (Lee et al. 2018) and may reflect a similar, but reverse, process of delivering more “charge” from the peripheral receptors to the cortex.
The separation between gamma and HG is poorly understood. In motor, visual, and auditory tasks, elevation in gamma activity in the 30–60 Hz range is associated with top-down, attentional processing (Debener et al. 2003; Gruber et al. 1999; Pfurtscheller et al. 2003). However, many of these studies involve scalp recordings which filter out high-frequency oscillations making it difficult to clearly separate activity in these higher frequency bands. A clear separation between low-and high-gamma has been shown in visual processing, where HG is highly correlated with spiking activity (Ray and Maunsell 2011). Similarly, in a walking task, electroencephalography recordings showed alternating activity in low-and high-gamma (24–40 Hz and 70–90 Hz respectively) with low-gamma suppression during high-gamma activity (Seeber et al. 2015). However, prior ECoG recordings in a somatosensory task exhibited no changes in gamma (Menon et al. 1996), or changes in HG only (Ray et al. 2008a, b; Ryun et al. 2017a, b). Here we observed separation of gamma and HG. Power increases during a stimulus occurred over a relatively broad frequency band, leading to the definition of HG used in this report (50–125 Hz). We chose a relatively conservative band for gamma (30–50 Hz). Decreases in gamma were often concurrent with beta changes and may extend the activity in this band rather than a separate process. As seen in Figs. 3 and 5, there is a clear, relatively inactive separation between the decrease in the lower frequency bands (alpha and beta) and the increase in HG, similar to prior work (Ray et al. 2008a, b; Ryun et al. 2017). In somatosensory perception, part of the gamma band is relatively inert and suggests the changes in gamma are more reflective of changes in the lower frequency bands, than their own separate process in this study.
Alpha and beta frequency bands
Decreases in alpha and beta activity have been associated with changes in focused attention. Alpha rhythms are thought to have an inhibitory effect (Haegens et al. 2011, 2012; Handel et al. 2011), preventing gamma band excitation from escaping a local network and activating distal networks (Fries 2015). Both alpha and beta-band decreases are also seen in motor activation while HG band activity increases (Crone et al. 1998) and may reflect a release of inhibition (Brinkman et al. 2014; Khanna and Carmena 2017; Pfurtscheller et al. 1997, 2003). In somatosensation, both alpha and beta oscillation decreases have been seen with tactile stimulation in humans (Bauer et al. 2006; Haegens et al. 2011; Hauck et al. 2007). Alpha changes are thought to be under top-down control, correlated to anticipation and performance on a vibrotactile discrimination task (Haegens et al. 2011) and with attended stimuli (Peng et al. 2014). Beta has a similar profile, with a decrease during anticipation, attention shifting, and stimulus onset, but with an additional modulation during the processing of an attended stimulus, as opposed to alpha which has been seen to primarily change during anticipation (van Ede et al. 2014). Similar to these studies, we observed decreases in both the traditional alpha and beta bands which could reflect attention processes related to the touch stimulus. Here, our unattended stimuli (that is, passive stimuli, subjects had no task related to the stimuli) corresponded to alpha and beta decreases after stimulus onset. If alpha and beta are modulated prior to attended stimuli, or after stimulus onset for unattended stimuli (van Ede et al. 2014), it is possible that an unattended tactile stimulus draws attention, reflected by the decrease in alpha and beta power.
Timing and location
Soft touch exhibited a pattern of increasing power in HG, taking longer to peak than DT or LT. Alpha and beta also showed delayed nadirs in ST.Visual inspection shows a patternof multiple peaks in HG, seen most clearly in S01 and S04 (see Figs. 3, 5), or a steady elevation in HG. Since the calculated peak occurred around 800 ms after onset, it suggests that the first, or earlier part of the peak, was not as strong as the later part. This indicates that with a repetitive motion, the HG (and beta and alpha) activity builds. The attenuation of the signal in DT and LT is not present in a continuously brushing stimulus, but is present in these more sustained types of touch. Whether these bands are part of an attentional process, stimulus encoding, or some other, they are present in S1 during a sustained brushing stimulus, but not in an unmoving deep or light stimulus. It should be noted that the repetitive brushing of ST and the stationary DT/LT are quite different in terms of dermatomes enlisted, and nature of the touch (sustained vs active), and are thus impossible to directly compare. The extended activity, and later/multiple peaks of ST may simply be due to the spatial and temporal changes associated with the inherent variability in the stimulus.
A large percentage of electrodes showed changes in at least one frequency band (93.5%). This is surprising given the dermatomal area of the hand used for stimulation was considerably smaller than the S1 areas that exhibited a response from cortical stimulation during mapping. Elevations in HG were seen in areas that, on stimulation, were mapped to other digits or the palm. Using this high-density grid, we showed changes that spread over large areas of the S1 hand area, suggesting either a broad response or a response strong enough to be picked up on multiple channels. As seen in Figs. 2 and 5, although the changes in adjacent channels were similar, they were not identical, with more distinct beta changes appearing where HG was most responsive. Overall, changes in the beta band were the most common (see Fig. 1), and changes in the alpha band were the least. This suggests a broader role for the effects of beta band oscillations. Beta oscillations play a role in anticipation and attention (van Ede et al. 2014), and the widespread changes may reflect a global shift of attentional resources to sensation in the hand (for example, to anticipate and attend another stimulus). These results are indirectly supported by other human ECoG studies, since standard grid spacing (10 mm), and the special discrimination of electroencephalography or magnetoencephalography are not fi ne enough to show distinct regions within a finger or palm, and yet exhibit the same characteristics shown here. An alternative explanation is that the lower frequencies have lower spatial resolution and more propagation through neural tissue, and are thus less prone to change. Our finding that beta band exhibited more change than gamma band would seem to conflict with such a hypothesis, unless this behavior is prominent in the low (alpha) frequencies but attenuated above this range. On the other hand, if beta acts in an inhibitory role and alpha in an attentional resource role (Brinkman et al. 2014; van Ede et al. 2014; Zhang et al. 2008), we would expect beta to be more spatially distinct, along with high-gamma, and alpha to have more broad changes.
Following DT, more electrodes were activated compared to LT or ST. Generally, the largest difference between conditions occurred in the beta band, but also frequently in HG (see Fig. 2). The number of subjects was limited, however these results suggest an interesting trend. Since the electrodes that showed these changes were often in cortical areas distant from the electrodes associated with the area of touch (including some from cortical areas that activated face, see Fig. 2 and Online Resource Fig. 1), it suggests a broad role for these frequency bands, that is at least partially dependent on the depth of touch. Prior studies have shown HG to be more spatially specific than alpha or beta (Crone et al. 2006), but here we see modality changes in beta and HG. One interpretation of this is that beta and HG act to inhibit (Brinkman et al. 2014; Zhang et al. 2008) and share downstream information (Fries 2015; Schoffelen et al. 2011) respectively. Desynchronization of beta, and synchronization in HG, reflect less inhibition and more information sharing, and a deeper or repetitive touch both contain more sensory “information” to be dispersed through the cortex. Alternatively, more cutaneous receptors may be activated from DT and ST, and the cortical activation may therefore be seen in a larger region.
One of the subjects, S02, did not show statistically significant changes in HG power, with changes seen only in alpha, beta, and gamma bands. This may reflect a variation in the strength of the effect, or inconsistency across subjects. It may be due to differences in electrodes; S02 had a standard ECoG grid with larger contacts (4.75 mm contacts) and larger spacing (10 mm) between contacts than the other subjects (2 mm contacts and 3 mm spacing). With these physical changes, relevant activity in the higher frequency bands may have occurred between the electrodes or averaged out in the larger volume of sensitivity. Alternatively, inflammation from the grid, or poor contact with the cortical surface, may have inhibited recording of higher-frequency responses, however this same subject engaged in a motor task as part of a separate study and showed HG changes in one electrode (data not shown). Additionally, recordings from S02 exhibited increases in HG that did not reach significance (see Fig. 3).
Limitations
This study has several constraints. First, automation was a necessary sacrifice for natural, realistic sensations, and vibration and force (e.g. indentation of the skin) had to be estimated, which introduces variation in the stimulus. Second, without direct visual access to S1 during grid placement, electrode location and selection relied on imaging, with some error likely in estimated vs actual electrode positions. For example, two subjects showed no modulation in the ECoG signal during the mechanical stimuli, and this was likely due to suboptimal placement of the grids. Third, attention was not well controlled. Trial lengths and inter-trial intervals were varied to reduce anticipation, but no efforts were made to divert or focus attention to simulate natural somatosensation as much as possible. Ideally, more detailed experiments would alter attention and provide consistent indentation pressure during the tactile stimuli. However, with limited time per patient, under the constraints of an ICU setting with patients in recovery from implant surgery, we chose realistic sensations and exercised as much control as possible. Future endeavors will focus on these aspects to differentiate these sources of attention.
Finally, the nature of creating a brushing motion alters the spatial area on the skin involved from that involved in DT/LT, limiting the comparison between the modalities. The intention was to describe the difference between natural sensations, which inherently reduces the ability to control such features.
Conclusion
We used realistic, mechanical stimuli and observed changes in ECoG activity recorded over S1. All three types of touch (deep, light and soft) showed characteristic HG band activation, with depression and recovery of alpha and beta band activity, similar to prior studies. Deep and light touch occurred earlier than soft touch. Early peaks in DT and LT attenuated prior to the release of touch. Repetitive ST exhibited a building pattern in the HG range. Together these findings support a role for HG, beta, and alpha in encoding features of different natural tactile stimuli.
Supplementary Material
Acknowledgments
Funding We wish to acknowledge the generous support of Cal-BRAIN: A Neurotechnology Program for California, National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health (KL2TR001854), National Institutes of Health (R25 NS099008–01), The Neurosurgery Research and Education Foundation (NREF), the Tianqiao and Chrissy Chen Brain-machine Interface Center at Caltech, the Boswell Foundation and the Della Martin Foundation, and the University of Southern California Neurorestoration Center. None of the listed sources of funding had a role in study collection, analysis, interpretation of data, or writing of the manuscript.
Footnotes
Compliance with ethical standards
Ethical approval All research herein complies with institutional and international guidelines on research involving human participants, and was conducted after approval by the institutional review board (study approval HS-13–00528). Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00221-019-05495-1) contains supplementary material, which is available to authorized users.
Publisher’s Note S pringer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Conflict of interest The authors declare that they have no conflict of interest involved with this or related work.
References
- Agnew WF, McCreery DB (1987) Considerations for safety in the use of extracranial stimulation for motor evoked potentials. Neurosurgery 20:143–147 [DOI] [PubMed] [Google Scholar]
- Alvarez M, Zainos A, Romo R (2015) Decoding stimulus features in primate somatosensory cortex during perceptual categorization. Proc Natl Acad Sci USA 112:4773–4778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenta Salas M, Bashford L, Kellis S, Jafari M, Jo H, Kramer D, Shanfield K, Pejsa K, Lee B, Liu CY et al. (2018) Proprioceptive and cutaneous sensations in humans elicited by intracortical microstimulation. Elife 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P (2006) Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci 26:490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Stenner MP, Friston KJ, Dolan RJ (2014) Attentional modulation of alpha/beta and gamma oscillations reflect functionally distinct processes. J Neurosci 34:16117–16125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensmaia SJ (2008) Tactile intensity and population codes. Behav Brain Res 190:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birznieks I, Vickery RM (2017) Spike timing matters in novel neuronal code involved in vibrotactile frequency perception. Curr Biol 27:1485–1490.e1482 [DOI] [PubMed] [Google Scholar]
- Brinkman L, Stolk A, Dijkerman HC, de Lange FP, Toni I (2014) Distinct roles for alpha-and beta-band oscillations during mental simulation of goal-directed actions. J Neurosci 34:14783–14792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ (2000) Attending to and remembering tactile stimuli: a review of brain imaging data and single-neuron responses. J Clin Neurophysiol 17:575–591 [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP (1998) Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain 121(Pt 12):2301–2315 [DOI] [PubMed] [Google Scholar]
- Crone NE, Sinai A, Korzeniewska A (2006) High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res 159:275–295 [DOI] [PubMed] [Google Scholar]
- Debener S, Herrmann CS, Kranczioch C, Gembris D, Engel AK (2003) Top-down attentional processing enhances auditory evoked gamma band activity. Neuroreport 14:683–686 [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W (2001) Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2:704–716 [DOI] [PubMed] [Google Scholar]
- Flesher SN, Collinger JL, Foldes ST, Weiss JM, Downey JE, Tyler-Kabara EC, Bensmaia SJ, Schwartz AB, Boninger ML, Gaunt RA (2016) Intracortical microstimulation of human somatosensory cortex. Sci Transl Med 19(8):361ra141 [DOI] [PubMed] [Google Scholar]
- Fries P (2015) Rhythms for cognition: communication through coherence. Neuron 88:220–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R (2001) Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291:1560–1563 [DOI] [PubMed] [Google Scholar]
- Gruber T, Muller MM, Keil A, Elbert T (1999) Selective visual-spatial attention alters induced gamma band responses in the human EEG. Clin Neurophysiol 110:2074–2085 [DOI] [PubMed] [Google Scholar]
- Haegens S, Handel BF, Jensen O (2011) Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J Neurosci 31:5197–5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Luther L, Jensen O (2012) Somatosensory anticipatory alpha activity increases to suppress distracting input. J Cogn Neurosci 24:677–685 [DOI] [PubMed] [Google Scholar]
- Handel BF, Haarmeier T, Jensen O (2011) Alpha oscillations correlate with the successful inhibition of unattended stimuli. J Cogn Neurosci 23:2494–2502 [DOI] [PubMed] [Google Scholar]
- Hauck M, Lorenz J, Engel AK (2007) Attention to painful stimulation enhances gamma-band activity and synchronization in human sensorimotor cortex. J Neurosci 27:9270–9277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao SS, Lane J, Fitzgerald P (2002) Representation of orientation in the somatosensory system. Behav Brain Res 135:93–103. http://chronux.org/ [DOI] [PubMed] [Google Scholar]
- Khanna P, Carmena JM (2017) Beta band oscillations in motor cortex reflect neural population signals that delay movement onset. Elife [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebber M, Harwood J, Spitzer B, Keil J, Senkowski D (2015) Visuotactile motion congruence enhances gamma-band activity in visual and somatosensory cortices. Neuroimage 117:160–169 [DOI] [PubMed] [Google Scholar]
- Lee B, Kramer D, Armenta Salas M, Kellis S, Brown D, Dobreva T, Klaes C, Heck C, Liu C, Andersen RA (2018) Engineering artificial somatosensation through cortical stimulation in humans. Front Syst Neurosci 12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Chien JH, Chang YW, Kim JH, Anderson WS, Lenz FA (2015) Functional role of induced gamma oscillatory responses in processing noxious and innocuous sensory events in humans. Neuroscience 310:389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna R, Hernandez A, Brody CD, Romo R (2005) Neural codes for perceptual discrimination in primary somatosensory cortex. Nat Neurosci 8:1210–1219 [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC, Burke D (1990) Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J Physiol 429:113–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R (2007) Nonparametric statistical testing of EEG-and MEG-data. J Neurosci Methods 164:177–190. [DOI] [PubMed] [Google Scholar]
- Menon V, Freeman WJ, Cutillo BA, Desmond JE, Ward MF, Bressler SL, Laxer KD, Barbaro N, Gevins AS (1996) Spatio-temporal correlations in human gamma band electrocorticograms. Electroencephalogr Clin Neurophysiol 98:89–102 [DOI] [PubMed] [Google Scholar]
- Mitra P, Bokil H (2008) Observed brain dynamics. Oxford University Press, Oxford [Google Scholar]
- Mountcastle VB, Talbot WH, Sakata H, Hyvarinen J (1969) Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol 32:452–484 [DOI] [PubMed] [Google Scholar]
- Muniak MA, Ray S, Hsiao SS, Dammann JF, Bensmaia SJ (2007) The neural coding of stimulus intensity: linking the population response of mechanoreceptive afferents with psychophysical behavior. J Neurosci 27:11687–11699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Hu L, Zhang Z, Hu Y (2014) Changes of spontaneous oscillatory activity to tonic heat pain. PLoS One 9:e91052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G (1997) Foot and hand area mu rhythms. Int J Psychophysiol Jun 26:121–135 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Graimann B, Huggins JE, Levine SP, Schuh LA (2003) Spatiotemporal patterns of beta desynchronization and gamma synchronization in corticographic data during self-paced movement. Clin Neurophysiol 114:1226–1236 [DOI] [PubMed] [Google Scholar]
- Ray S, Maunsell JH (2011) Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol 9:e1000610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Niebur E, Hsiao SS, Sinai A, Crone NE (2008a) High-frequency gamma activity (80–150Hz) is increased in human cortex during selective attention. Clin Neurophysiol 119:116–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Hsiao SS, Crone NE, Franaszczuk PJ, Niebur E (2008b) Effect of stimulus intensity on the spike-local field potential relationship in the secondary somatosensory cortex. J Neurosci 28:7334–7343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HE, Worthen SF, Witton C, Hall SD, Furlong PL (2013) Gamma oscillatory amplitude encodes stimulus intensity in primary somatosensory cortex. Front Hum Neurosci 7:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryun S, Kim JS, Jeon E, Chung CK (2017a) Movement-related sensorimotor high-gamma activity mainly represents somatosensory feedback. Front Neurosci 11:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryun S, Kim JS, Lee H, Chung CK (2017b) Tactile Frequency-Specific High-Gamma Activities in Human Primary and Secondary Somatosensory Cortices. Sci Rep 7:15442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Hernandez A, Zainos A, Romo R (2000) Periodicity and firing rate as candidate neural codes for the frequency of vibrotactile stimuli. J Neurosci 20:5503–5515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelen JM, Poort J, Oostenveld R, Fries P (2011) Selective movement preparation is subserved by selective increases in corti-comuscular gamma-band coherence. J Neurosci 31:6750–6758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeber M, Scherer R, Wagner J, Solis-Escalante T, Muller-Putz GR (2015) High and low gamma EEG oscillations in central sensorimotor areas are conversely modulated during the human gait cycle. Neuroimage 112:318–326 [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O (1999) Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 3:151–162 [DOI] [PubMed] [Google Scholar]
- van Ede F, Szebenyi S, Maris E (2014) Attentional modulations of somatosensory alpha, beta and gamma oscillations dissociate between anticipation and stimulus processing. Neuroimage 97:134–141 [DOI] [PubMed] [Google Scholar]
- Wyllie E, Luders H, Morris HH, Lesser RP, Dinner DS, Rothner AD, Erenberg G, Cruse R, Friedman D, Hahn J et al. (1988) Subdural electrodes in the evaluation for epilepsy surgery in children and adults. Neuropediatrics 19:80–86 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Bressler SL, Ding M (2008) Response preparation and inhibition: the role of the cortical sensorimotor beta rhythm. Neuroscience 156:238–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.