Abstract
Background:
This randomized single-blinded clinical trial aimed to evaluate the effectiveness of diode laser and fluoride in the treatment of dentin hypersensitivity (DH) due to gingival recession.
Materials and Methods:
Twenty-eight individuals randomly distributed across three groups participated: 10 individuals who were treated with diode laser, nine who were treated with fluoride, and nine who received placebo. Pain was assessed with the Visual analog scale (VAS). Evaporative stimulus and tactile stimulus were evaluated with the verbal rating scale (VRS). VAS was applied shortly after, 6 h after, 12 h after, and 24 h after the single-session treatment for DH, whereas VRS was applied shortly after, 15 min and 7 days after the treatment. Participants' quality of life was assessed with the validated Brazilian version of the Dentine Hypersensitivity Experience Questionnaire assessing functional limitations, coping behaviors, emotional, and social impacts caused by DH. Descriptive statistics and the ANOVA test were used. Values of P < 0.05 were statistically significant.
Results:
Diode laser significantly reduced the DH to the evaporative stimulus (P = 0.002). The application of fluoride did not change the degree of DH to evaporative and tactile stimuli (P > 0.05). The group of individuals who were treated with diode laser presented a higher reduction in DH (25.4%) when compared to the group of individuals treated with fluoride (17.1%), and the group of individuals among whom placebo had been used (2.9%). Descriptive analysis indicated that the items measuring the emotional and social impacts of DH were those with a more negative impact on the individuals.
Conclusion:
Therapy with diode laser was more effective in reducing DH than therapy with fluoride.
Keywords: Dentin sensitivity, fluorine, lasers, quality of life
INTRODUCTION
Dentin hypersensitivity (DH) has been defined as pain arising from the exposed dentin in response to tactile, thermal, evaporative, or osmotic stimuli, which cannot be explained by another oral pathology.[1] It is a common clinical condition, in particular among individuals who have gingival recession.[2] The prevalence of DH varies from 5% to 85% in the adult population,[3] being more prevalent among individuals between the ages of 20 and 50.[4]
Pain results from the activation of the nerve fibers A-δ in the dentin tubules. The A-δ fibers are likely activated by means of the hydrodynamic mechanism, represented by the flow of fluids within the dentin tubules caused by external stimuli. Thus, the activation is directly associated with the presence of open dentin tubules.[5]
Loss of enamel and removal of root cement with consequent dentin exposure is the main contributing factor for DH. This may be a consequence of inadequate tooth brushing, periodontal treatment, psychological factors, and the existence of exogenous and endogenous acids in the diet.[6] The diagnosis is based on the combination of anamnesis with clinical examination, during which other outcomes, such as dental fracture, dental caries, and pulpitis should be ruled out.[7]
The ideal DH treatment should be one of easy application, rapid, and effective for long periods. Pulp irritation, pain, and tooth staining should also be avoided.[4] A plethora of methods to reduce or to eliminate DH, such as guidance on the diet as well as the use of toothpaste with potassium, fluoride, resin, and laser has been acknowledged in the literature.[8,9,10,11]
Diode laser was first used as a method of therapeutic intervention for DH in 1985.[9] The interaction of laser with tissue leads to tissue reactions, which vary according to the laser wavelength and amount of energy applied. The interaction of laser with the dental pulp causes a photobiomodulation effect, increasing the metabolic activity of odontoblasts, and the production of tertiary dentin with consequent obliteration of the dentin tubules.[12,13] Fluoride is also widely used in dentistry. The mechanism of action for DH treatment consists of the chemical capacity of fluoride in reducing the fluid movements in the dentin tubules by means of the deposit of minerals on the open dentin tubules.[14]
DH can also impair oral health-related quality of life (OHRQoL), which is the individual's understanding of how the oral condition impacts his/her daily life.[15] Individuals with sensitive teeth report impairment of their OHRQoL. Conversely, DH treatment may be associated with the improvement of the individual's well-being.[16]
Therefore, the objective of the present study was to evaluate the effectiveness of diode laser and fluoride gel in the treatment of individuals with DH.
MATERIALS AND METHODS
Study design protocol and registration
The reporting of this randomized single-blinded clinical trial conforms to the guidelines of the Consolidated Standards of Reporting Trials.[17] The study was registered in the Brazilian Registry of Clinical Trials under the number RBR-9sg585.
Sampling, setting, and period of the study
This study was conducted between August 2018 and June 2019. The sample consisted of 28 individuals with DH due to gingival recession randomly distributed across three groups: ten individuals who were treated with diode laser, nine who were treated with fluoride, and nine who received placebo. The distribution of participants among groups was performed randomly with a sealed envelope, in which numbers corresponding to the treatment modalities were placed. Treatment was carried out in a single session. The GaAlAs infrared semiconductor laser, 808-nm wavelength was applied over the exposed root region at a central point for 60 s [Figure 1]. The application of acidulated fluoride phosphate 1.23% was performed under isolation with cotton rolls and after the procedure the tooth was dried with a piece of cotton. Application of the fluoride was performed with a small sterile cotton ball on the exposed root surface for 60 s. After application, the patient was instructed to spit exhaustively for 1 min [Figure 2]. For the placebo, the placement of a layer of acrylic resin blocked the photons. The application of the placebo gel was similar to the application of fluoride. However, the cotton used for the application of the placebo on the tooth had no medication for DH.
Figure 1.
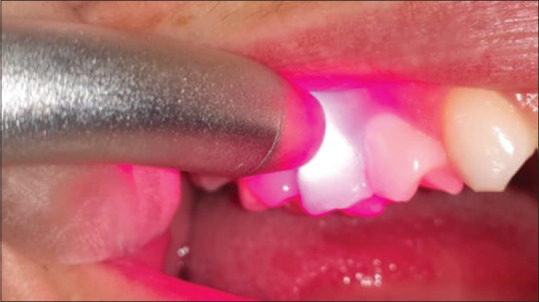
Laser application
Figure 2.
Fluoride application
Participants were unaware to which treatment they would be submitted. The inclusion criteria were individuals diagnosed with a score of DH higher than or equal to two in the verbal rating scale (VRS) after evaporative and tactile stimulation in at least one sound tooth with gingival recession, individuals older than 18 years and those who accepted to participate in the study after signing of the informed consent form. The exclusion criteria were individuals submitted to periodontal treatment or the treatment of DH within the last 30 days, pregnant women and individuals with decayed or filled teeth.
Ethical issues
The present study was conducted according to the ethics principles of the Helsinki Declaration. Approval of the Ethics Research Committee from the University was obtained (CAAE 87761718.1.0000.5149). Participants signed a consent form.
Biological, social and behavioral data
For each participant, biological, social, and behavioral data were collected by means of a structured questionnaire in the form of an interview. This questionnaire retrieved data on participants' age, sex, schooling, family income, use of any medications, and history of dental visits. Gingival recession was measured with a periodontal probe (UNC-15, Hu-Friedy®).[18]
Pain evaluation
The visual analog scale (VAS) and the VRS were used to measure pain. The VAS represents a simplified and validated tool for this purpose.[19] The participant indicated his/her level of pain perception on a horizontal line ranging between 0 and 10 mm. Participants were instructed that zero indicated no pain and no discomfort and ten indicated very intense pain and much discomfort. The patient was advised to record the pain intensity shortly after (TO), 6 h after (T1), 12 h after (T2), and 24 h after (T3) treatment for DH. The following threshold values were adopted: Absence of pain = 0; mild pain = 0.1–3.9; moderate pain = 4.0–6.9; and intense pain = 7.0–10.0.
The VRS allowed us to measure the pain level after the application of evaporative stimulus (air) and tactile stimulus. During the assessment of the evaporative stimulus, the selected tooth was isolated with cotton rolls, dried, and air was applied within a distance of 1 cm.[20] During the assessment of the tactile stimulus, the root surface of the tooth was scaled with a periodontal probe. Pain intensity was recorded shortly after (T0), 15 min after (T1), and 7 days after (T2) the single-session treatment. The VRS ranged in a scale from zero to three, in which zero denoted no discomfort, but the patient felt stimulation; one denoted little discomfort, but not painful; two denoted pain during the application of stimulus; and three denoted pain during and shortly after stimulus application.
Oral health-related quality of life assessment
In addition, the validated Brazilian version of the Dentine Hypersensitivity Experience Questionnaire (DHEQ-15) assessing functional limitations, coping behaviors, emotional, and social impacts caused by DH was applied. The DHEQ-15 consists of 15 items distributed across five subscales: “constraints,” “adaptation,” “social impact,” “emotional impact,” and “identity.” The response options for each item are given on a seven-point Likert scale as follows: 1 = “I strongly disagree;” 2 = “I disagree;” 3 = “I agree a little;” 4 = “I do not agree or disagree;” 5 = “I agree a little;” 6 = “I agree;” and 7 = “I agree a lot.”[15] A higher score indicated a more negative perception of the individual regarding the impact of DH on himself/herself.
Statistical analysis
Data analysis was performed using the Statistical Package for the Social Sciences (version 23.0, IBM Corp., Armonk, NY, USA). Descriptive analysis was performed. The ANOVA test for independent samples was used to compare groups with respect to the variables age and recession. In order to compare groups with respect to the variables sex, schooling, and family income, the Chi-square test was used.
For intergroup comparisons regarding evaporative stimulus, tactile stimulus, and pain evaluation, the ANOVA test for independent samples was used. The ANOVA test for repeated measures was used for intragroup comparisons of evaporative stimulus, tactile stimulus, and pain assessment. Values of P < 0.05 were considered statistically significant.
RESULTS
The characteristics of the sample according to the variables of interest are presented in Table 1. The mean age of the 28 participants was 48.4 years. There was no difference among groups with respect to participants' age. In addition, the groups were similar with respect to sex, schooling, family income, and gingival recession.
Table 1.
Characteristics of the sample with respect to the variables of interest
| Variables | Total sample | Fluorine group | Laser group | Placebo group | P |
|---|---|---|---|---|---|
| n (%) | 28 (100) | 9 (32.1) | 10 (35.7) | 9 (32.1) | |
| Age (years) | 48.4 | 47.7 | 48.9 | 48.7 | 0.971* |
| Sex | |||||
| Female | 22 | 8 | 6 | 8 | 0.093† |
| Male | 6 | 1 | 4 | 1 | |
| Schooling (years) | |||||
| <8 | 7 | 4 | 2 | 1 | 0.311† |
| ≥8 | 21 | 5 | 8 | 8 | |
| Family income (SM) | |||||
| <3 | 22 | 5 | 9 | 8 | 0.124† |
| ≥3 | 6 | 4 | 1 | 1 | |
| Gingival recession (mm) | 3.5 | 2.8 | 3.8 | 3.9 | 0.123* |
*ANOVA test for independent samples; †Chi-square test, ‡P≤0.05. n – Number; SM – Minimum wages; mm – millimeter
Table 2 shows the distribution of the participants according to their answers regarding the 15 items of the DHEQ-15. The “I strongly agree” and “I agree” response options were the most prevalent answers among most fifteen items of the questionnaire. The descriptive analysis demonstrated that the items measuring the emotional and social impacts of DH were those, in which the participants indicated a more negative impact.
Table 2.
Distribution of the responses of the overall sample to the items of the Dentine Hypersensitivity Experience Questionnaire-15 questionnaire
| Questions | Total sample | ||||||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | |
| Question 1 | 3 | 0 | 2 | 1 | 0 | 7 | 15 |
| Question 2 | 3 | 3 | 2 | 2 | 1 | 7 | 10 |
| Question 3 | 1 | 1 | 0 | 2 | 4 | 4 | 16 |
| Question 4 | 1 | 8 | 1 | 3 | 3 | 6 | 8 |
| Question 5 | 1 | 9 | 3 | 2 | 3 | 4 | 6 |
| Question 6 | 0 | 3 | 2 | 2 | 2 | 7 | 12 |
| Question 7 | 3 | 5 | 3 | 1 | 3 | 9 | 4 |
| Question 8 | 2 | 6 | 2 | 5 | 1 | 7 | 5 |
| Question 9 | 5 | 5 | 0 | 3 | 2 | 6 | 7 |
| Question 10 | 3 | 5 | 0 | 1 | 4 | 6 | 9 |
| Question 11 | 1 | 1 | 1 | 1 | 2 | 7 | 15 |
| Question 12 | 0 | 4 | 2 | 5 | 0 | 7 | 10 |
| Question 13 | 6 | 7 | 3 | 3 | 2 | 2 | 5 |
| Question 14 | 1 | 4 | 0 | 6 | 4 | 7 | 6 |
| Question 15 | 7 | 11 | 1 | 2 | 0 | 7 | 0 |
R1 – Strongly disagree; R2 – Disagree; R3 – Agree a little; R4 – Do not agree or disagree; R5 – I agree a little; R6 – I agree; R7 – I agree a lot
Table 3 portrays the DH of the participants in the three groups according to the evaporative and tactile stimuli before and after the treatment. Diode laser significantly reduced the DH to the evaporative stimulus (P = 0.002). The topical application of fluoride did not change the degree of DH to evaporative and tactile stimuli (P > 0.05).
Table 3.
Assessment of dentin hypersensitivity in the three groups according to evaporative and tactile stimuli
| Fluoride group | Diode laser group | Placebo group | P | |
|---|---|---|---|---|
| Evaporative | ||||
| T0 | 2.1 | 2.5 | 2.1 | 0.044*,‡ |
| T1 | 1.9 | 1.6 | 1.4 | 0.316* |
| T2 | 1.3 | 1.0 | 1.2 | 0.201* |
| P | 0.423† | 0.002†,‡ | 0.148† | |
| Tactile | ||||
| T0 | 1.4 | 1.3 | 1.4 | 0.880* |
| T1 | 1.3 | 1.2 | 1.3 | 0.849* |
| T2 | 0.8 | 0.8 | 0.8 | 0.804* |
| P | 0.391† | 0.079† | 0.444† |
*ANOVA test for independent samples; †ANOVA test for repeated measures; ‡P≤0.05. T0 – Shortly after, T1 – 15 min after, T2 – 7 days after
The results of the assessment with VAS are shown in Table 4. In the three groups, no statistically significant difference in the level of pain at the times analyzed was observed. However, the group of individuals who were treated with diode laser presented a higher percentage of reduction in DH (25.4%) when compared to the group of individuals treated with fluoride (17.1%) and the group of individuals among whom placebo had been used (2.9%).
Table 4.
Assessment of pain with the visual analog scale in the three groups
| Time | Fluoride group | Diode laser group | Placebo group | P |
|---|---|---|---|---|
| T0 | 4.1 | 5.1 | 3.5 | 0.134* |
| T1 | 3.4 | 4.1 | 3.6 | 0.916* |
| T2 | 3.6 | 3.8 | 3.3 | 0.797* |
| T3 | 3.4 | 3.8 | 3.4 | 0.871* |
| P | 0.429† | 0.248† | 0.402† |
*ANOVA test for independent samples; †ANOVA test for repeated measures; ‡P≤0.05. T0 – Shortly after; T1 – 6 h after; T2 – 12 h after; T3 – 24 h after
DISCUSSION
DH is a common clinical condition, characterized by acute pain due to the exposure of dentin to evaporative, tactile, thermal, chemical, or osmotic stimuli. The etiology is multifactorial and the interaction among predisposing factors and stimuli is associated with its severity. To eliminate the painful symptoms of DH, treatment aims to obliterate the open tubules, reducing the flow of fluids within the dentin.[21,22]
There are several treatment options for DH, such as the use of specific toothpastes, therapy with laser, therapy with fluoride, use of desensitizers, gingival surgery, or restorations.[1,23] To be successful, treatment must be effective on the short term, since DH is characterized by acute pain associated with great discomfort and with a significant negative impact on the affected individual's quality of life. The present study evaluated and compared the effectiveness of diode laser and therapy with fluoride on DH.
In the baseline, the pain level measured with the VRS scale was very much similar among groups. The improvement in pain intensity occurred in individuals of the three groups evaluated. When the groups were compared with respect to the tactile stimulus, there was no significant difference among them shortly after, 15 min after, and 7 days after treatment. Based on the assessment of evaporative stimulus, treatment with diode laser showed a greater reduction in DH 15 min after treatment (36%) when compared with treatment with fluoride (9.5%) and treatment with placebo (33.3%). After 7 days, treatment with diode laser was also more effective, with a reduction of 37.5% in the DH, while treatment with fluoride led to a reduction of 31.6% and treatment with placebo led to a reduction of 14.3%. Similar results were demonstrated by Pesevska et al., who observed the reduction of DH in 86.6% of the individuals treated with diode laser and 26.6% in the group of individuals treated with fluoride.[24] Sicilia et al. also demonstrated a greater reduction in DH 14 days after treatment with laser (71.7%).[25] In our study, when VAS was analyzed, a reduction in DH over time in the three groups was observed. However, no treatment fully reduced the DH within 24 h. Despite the small sample size, one can state that the obliteration induced by treatment with diode laser lasts longer, which was evidenced by the reduction of pain related to the DH.[5] The deposits of calcium fluoride may also have contributed to the obliteration of the dentin tubules. However, the deposits are small in size (nearly 0.05 micrometers) and a single application of fluorine would be insufficient to obliterate the diameter of the dentin tubules. A larger number of sessions may have been necessary for a greater effect of fluoride on the reduction of DH.[1]
In this study, the mean age of individuals diagnosed with DH was 48.4 years. Similar results were reported by Davari et al., who demonstrated that DH is more prevalent among individuals between 20 and 50 years.[4] However, the study by Chowdhary et al. showed a higher prevalence of DH among individuals in their 20 s, suggesting that the development of secondary dentin throughout the life course may contribute to the reduction of DH and consequently, to the lower prevalence of this condition among older individuals.[22]
Gingival recession may result in DH.[18] Mechanical trauma and inflammatory processes are etiological factors associated with gingival recession. The degree of the recession is directly associated with the severity of the exposure of dentin tubules, contributing to a higher prevalence and severity of DH. In the present study, the groups evaluated were similar with respect to the degree of the gingival recession, and the total sample presented a mean size of the gingival recession of 3.5 mm.
When the DHEQ-15 is employed, one can observe how much the DH impacts the quality of life of the individuals evaluated. The impact of DH on quality of life measured by this questionnaire may be used as a parameter to justify treatment or even to evaluate the efficacy of DH treatment. In our study, the most prevalent responses were “I strongly agree” and “I agree.” Both response options were highly scored in the questionnaire and higher DHEQ-15 scores indicate a greater negative impact of DH on individuals' daily life, which demonstrates that treatment should have indeed been provided to participants.[15] This finding reinforces the assessment of therapies for DH, contributing to the success of treatment protocols, and to the improvement of their effectiveness.
The evaluation of the effects of treatments for DH is not simple, since pain is a subjective outcome, being difficult to quantify. Each individual presents a particular threshold of pain and may present great variation for the same stimulus. However, VAS is a widely used and accepted pain assessment tool.[26] Moreover, in the present study, the tactile and evaporative stimuli were used to measure pain level. Holland et al. confirmed the usefulness of tactile and evaporative stimuli (physiological and controllable stimuli) in studies evaluating DH.[27]
Our study was a randomized and single-blinded clinical trial. The assignment of the participants across the groups was concealed, precluding selection bias. However, the study also has shortcomings, such as the limited number of participants and losses over the study period, restricting the 7-day evaluation to a few individuals. Further studies with larger samples and longer follow-up are necessary to provide a better evaluation of the effects of DH treatment on the long term. Studies assessing other therapies available for DH are also warranted.
CONCLUSION
DH is an oral condition that has a significant impact on the quality of life of individuals. Therapy with diode laser was more effective in reducing DH than therapy with fluoride. However, further research is encouraged to confirm the results presented herein and to improve treatment protocols for DH.
Financial support and sponsorship
The study was financially supported by PRPq-UFMG.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Marto CM, Baptista Paula A, Nunes T, Pimenta M, Abrantes AM, Pires AS, et al. Evaluation of the efficacy of dentin hypersensitivity treatments-a systematic review and follow-up analysis. J Oral Rehabil. 2019;46:952–90. doi: 10.1111/joor.12842. [DOI] [PubMed] [Google Scholar]
- 2.Favaro Zeola L, Soares PV, Cunha-Cruz J. Prevalence of dentin hypersensitivity: Systematic review and meta-analysis. J Dent. 2019;81:1–6. doi: 10.1016/j.jdent.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Lima TC, Vieira-Barbosa NM, Grasielle de Sá Azevedo C, de Matos FR, Douglas de Oliveira DW, de Oliveira ES, et al. Oral health-related quality of life before and after treatment of dentin hypersensitivity with cyanoacrylate and laser. J Periodontol. 2017;88:166–72. doi: 10.1902/jop.2016.160216. [DOI] [PubMed] [Google Scholar]
- 4.Davari A, Ataei E, Assarzadeh H. Dentin hypersensitivity: Etiology, diagnosis and treatment; a literature review. J Dent (Shiraz) 2013;14:136–45. [PMC free article] [PubMed] [Google Scholar]
- 5.Umberto R, Claudia R, Gaspare P, Gianluca T, Alessandro del V. Treatment of dentine hypersensitivity by diode laser: A clinical study. Int J Dent. 2012;2012:858950. doi: 10.1155/2012/858950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillam DG. A new perspective on dentine hypersensitivity – Guidelines for general dental practice. Dent Update. 2017;44:33. doi: 10.12968/denu.2017.44.1.33. [DOI] [PubMed] [Google Scholar]
- 7.West NX, Seong J, Davies M. Management of dentine hypersensitivity: Efficacy of professionally and self-administered agents. J Clin Periodontol. 2015;42(Suppl 16):S256–302. doi: 10.1111/jcpe.12336. [DOI] [PubMed] [Google Scholar]
- 8.Osmari D, Fraga S, Ferreira AC, Eduardo CP, Marquezan M, Silveira BL. In-office treatments for dentin hypersensitivity: A randomized split-mouth clinical trial. Oral Health Prev Dent. 2018;16:125–30. doi: 10.3290/j.ohpd.a40299. [DOI] [PubMed] [Google Scholar]
- 9.Ozlem K, Esad GM, Ayse A, Aslihan U. Efficiency of lasers and a desensitizer agent on dentin hypersensitivity treatment: A clinical study. Niger J Clin Pract. 2018;21:225–30. doi: 10.4103/njcp.njcp_411_16. [DOI] [PubMed] [Google Scholar]
- 10.Ayad F, Ayad N, Vazquez J, Zhang YP, Mateo LR, Cummins D. Use of a toothpaste containing 8% arginine and calcium carbonate for immediate and lasting relief of dentin hypersensitivity: A simple and effective in-office procedure. Am J Dent. 2018;31:135–40. [PubMed] [Google Scholar]
- 11.Maximiano V, Machado AC, Yoshida ML, Pannuti CM, Scaramucci T, Aranha AC. Nd: YAG laser and calcium sodium phosphosilicate prophylaxis paste in the treatment of dentin hypersensitivity: A double-blind randomized clinical study. Clin Oral Investig. 2019;23:3331–8. doi: 10.1007/s00784-018-2759-5. [DOI] [PubMed] [Google Scholar]
- 12.Machado AC, Viana ÍE, Farias-Neto AM, Braga MM, de Paula Eduardo C, de Freitas PM, et al. Is photobiomodulation (PBM) effective for the treatment of dentin hypersensitivity? A systematic review. Lasers Med Sci. 2018;33:745–53. doi: 10.1007/s10103-017-2403-7. [DOI] [PubMed] [Google Scholar]
- 13.Tabatabaei MH, Chiniforush N, Hashemi G, Valizadeh S. Efficacy comparison of Nd: YAG laser, diode laser and dentine bonding agent in dentine hypersensitivity reduction: A clinical trial. Laser Ther. 2018;27:265–70. doi: 10.5978/islsm.27_18-OR-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takamizawa T, Tsujimoto A, Ishii R, Ujiie M, Kawazu M, Hidari T, et al. Laboratory evaluation of dentin tubule occlusion after use of dentifrices containing stannous fluoride. J Oral Sci. 2019;61:276–83. doi: 10.2334/josnusd.18-0176. [DOI] [PubMed] [Google Scholar]
- 15.Douglas-de-Oliveira DW, Lages FS, Paiva SM, Cromley JG, Robinson PG, Cota LO. Cross-cultural adaptation of the Brazilian version of the dentine hypersensitivity experience questionnaire (DHEQ-15) Braz Oral Res. 2018;32:e37. doi: 10.1590/1807-3107bor-2018.vol32.0037. [DOI] [PubMed] [Google Scholar]
- 16.Douglas-de-Oliveira DW, Vitor GP, Silveira JO, Martins CC, Costa FO, Cota LO. Effect of dentin hypersensitivity treatment on oral health related quality of life-A systematic review and meta-analysis. J Dent. 2018;71:1–8. doi: 10.1016/j.jdent.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. BMJ. 2010;340:332. doi: 10.1097/AOG.0b013e3181d9d421. [DOI] [PubMed] [Google Scholar]
- 18.Holmstrup P, Plemons J, Meyle J. Non-plaque-induced gingival diseases. J Periodontol. 2018;89(Suppl 1):S28–45. doi: 10.1002/JPER.17-0163. [DOI] [PubMed] [Google Scholar]
- 19.Müller S, Huber H, Goebel G, Wimmer G, Kapferer-Seebacher I. Pain perception during debridement of hypersensitive teeth elicited by two ultrasonic scalers. Clin Oral Investig. 2017;21:1559–64. doi: 10.1007/s00784-016-1971-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schirmer C, Dos Santos GO, Rost JF, Ferreira MB, Weidlich P. Factors associated with pain and analgesic consumption following non-surgical periodontal therapy under local anaesthesia and carried out by dental students. J Clin Periodontol. 2018;45:68–77. doi: 10.1111/jcpe.12833. [DOI] [PubMed] [Google Scholar]
- 21.Suri I, Singh P, Shakir QJ, Shetty A, Bapat R, Thakur R. A comparative evaluation to assess the efficacy of 5% sodium fluoride varnish and diode laser and their combined application in the treatment of dentin hypersensitivity. J Indian Soc Periodontol. 2016;20:307–14. doi: 10.4103/0972-124X.181243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhary Z, Gupta P, Kaur J, Garg Y, Swarup N. Multifaceted assessment of dentine hypersensitivity, evaluation of demographic prevalence along with associated factors: A cross-sectional study. J Indian Soc Periodontol. 2019;23:64–8. doi: 10.4103/jisp.jisp_425_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moraschini V, da Costa LS, Dos Santos GO. Effectiveness for dentin hypersensitivity treatment of non-carious cervical lesions: A meta-analysis. Clin Oral Investig. 2018;22:617–31. doi: 10.1007/s00784-017-2330-9. [DOI] [PubMed] [Google Scholar]
- 24.Pesevska S, Nakova M, Ivanovski K, Angelov N, Kesic L, Obradovic R, et al. Dentinal hypersensitivity following scaling and root planing: Comparison of low-level laser and topical fluoride treatment. Lasers Med Sci. 2010;25:647–50. doi: 10.1007/s10103-009-0685-0. [DOI] [PubMed] [Google Scholar]
- 25.Sicilia A, Cuesta-Frechoso S, Suárez A, Angulo J, Pordomingo A, de Juan P. Immediate efficacy of diode laser application in the treatment of dentine hypersensitivity in periodontal maintenance patients: A randomized clinical trial. J Clin Periodontol. 2009;36:650–60. doi: 10.1111/j.1600-051X.2009.01433.x. [DOI] [PubMed] [Google Scholar]
- 26.Kara C, Orbak R. Comparative evaluation of Nd: YAG laser and fluoride varnish for the treatment of dentinal hypersensitivity. J Endod. 2009;35:971–4. doi: 10.1016/j.joen.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24:808–13. doi: 10.1111/j.1600-051x.1997.tb01194.x. [DOI] [PubMed] [Google Scholar]


