Abstract
Context:
Nanoparticles, owing to their smaller size, penetrate regions inaccessible to other delivery systems, such as periodontal pockets. Thus, the present study aimed to comparatively evaluate efficacy of 2% curcumin with nanocarrier and 1% chlorhexidine gel as a local drug delivery (LDD) in the treatment of periodontal pockets.
Materials and Methods:
Forty-five chronic periodontitis patients with pocket depth 5–7 mm in two or more teeth were selected. Full-mouth scaling and root planing (SRP) was done for all patients followed by random allocation to the three treatment groups, namely SRP group (Group 1), 2% curcumin with nanogel (Group 2), and 1% chlorhexidine gel (Group 3). Clinical parameter assessment and microbiological analysis of subgingival plaque samples for Aggregatibacter actinomycetemcomitans (Aa), Porphyromonas gingivalis (Pg), and Tannerella forsythia (Tf) was done at baseline, 21st day, and 45th day.
Results:
The results showed that when the two LDD agents were used as an adjunct to SRP in chronic periodontitis, there was an improvement in all clinical parameters. Evaluation of microbiological parameters also showed a significant reduction in Aa, Pg, and Tf levels. Comparison of 2% turmeric extract with a nanocarrier system with 1% chlorhexidine gel showed that both the agents had a comparable antibacterial effect on the three selected periodontopathic bacteria.
Conclusion:
The present study showed that both the LDD agents showed an effective improvement of clinical and microbiologic parameters. 2% curcumin delivered with a nanocarrier system showed results comparable to chlorhexidine gel and hence shows promising future as an LDD agent in the treatment of periodontal pockets.
Keywords: Chlorhexidine, chronic periodontitis, curcumin, local drug delivery, Pluronic F127
INTRODUCTION
Etiology of periodontal disease is complex and treatment invariably involves mechanical debridement of tooth surfaces and proper oral hygiene maintenance. However, complete mechanical debridement is difficult to accomplish specially in sites with deep periodontal pockets due to the presence of tissue invasive pathogenic microflora within gingival tissues and root surface complexities. Hence, adjunctive administration of systemic or local antibiotics is needed, but it has drawbacks such as repeated intakes required to obtain optimal drug concentration, bacterial resistance, and disruption of commensal flora.[1] Hence, there is a need for devising better strategies to deliver drugs in controlled manner directly into the periodontal pocket to be treated, thus decreasing adverse effects such as eliminating systemic uptake of drug and reducing bacterial resistance.[2]
Various devices have been used for local drug delivery (LDD) such as fibers, strips, films, gels, microparticles, and more recently nanoparticles. Recent years have seen advances in the field of pharmaceutical nanotechnology grow by leaps and bounds. This has resulted in formulation of miniaturize devices for controlled drug delivery which improve bioavailability of therapeutic agents, prolong drug effect at site of delivery, and enhance stability against biodegradation.[3]
A family of nanoscale particulate materials, hydrogel nanoparticles, otherwise called nanogels, are now gaining popularity.[4] Various nanoparticles are in use such as Pluronic F127, Pluronic F68, P123, and P85. Pluronic F127 has advantages of thermoreversible gelation and high capacity to solubilize in water forming crystalline mesophases, thus improving stability and prolonging drug release.[5]
Lately, treatment of diseases using more naturally available antibiotic products, namely, turmeric, tulasi, aloe vera, and pomegranate, has gained popularity.[6] Of them, turmeric whose active component is curcumin with properties such as antibiotic, anticarcinogenic, analgesic, anti-inflammatory, antiarthritic, and blood purifier is widely used.[7] Anti-inflammatory properties of curcumin involve inhibition of prostaglandin biosynthesis from arachidonic acid pathway during inflammation.[8]
Studies have shown that turmeric can be used in the treatment of gingivitis and periodontitis.[9,10,11] Studies using 2% whole turmeric gel as an adjunct to scaling and root planing (SRP) proved to be more effective in the treatment of periodontal pockets and showed a greater reduction in clinical parameters and activity of “red complex” microorganisms as against sites receiving SRP alone.[12,13]
A primary concern in developing curcumin for clinical use is low oral bioavailability attributed to poor absorption. Hence, nanotechnology-based strategies are being aggressively explored worldwide to enhance curcumin's bioavailability.[14,15] Hence, the present study was envisioned to combine advantages of Pluronic nanogel to deliver curcumin in periodontal pockets and evaluate its efficacy in the treatment of periodontal pockets. The present study aimed to comparatively evaluate the efficacy of 2% curcumin with nanocarrier and 1% chlorhexidine gel as an adjunct to SRP in patients with chronic periodontitis using clinical and microbiological parameters.
MATERIALS AND METHODS
Patients between 25 and 50 years, who agreed to comply with the study protocol, were selected from the outpatient section of the department of periodontics. The study was approved by the institutional ethical board, and written informed consent was obtained from all patients. The study has been registered in the Clinical Trials Registry-India with registry No. CTRI/2017/09/009730. The study was conducted from January 2015 to November 2016. Patients clinically diagnosed as localized or generalized mild-to-moderate chronic periodontitis (AAP 1999 classification) with probing pocket depth (PPD) of 5–7 mm with two or more teeth and no periodontal treatment rendered within the past 6 months were included in the study. All third molars and patients with endodontic-periodontal lesions, using tobacco or tobacco-related products, history of antibiotic therapy within the past 3 months, with known allergy to turmeric and chlorhexidine, any systemic diseases, pregnant women, and lactating mothers were excluded.
Materials used
2% curcumin nanogel
It was freshly prepared at chairside by adding 2% curcumin powder to 20% Pluronic nanogel before delivering to the selected site.
Preparation of 20% Pluronic nanogel
It was done using “cold method” as it is more appropriate for thermolabile drugs. Preweighed, i.e., 20 g of Pluronic F127 (Sigma Aldrich, Bengaluru, Karnataka, India) was gradually added over 2 or 3 min to 80 g (1 g = 1 ml) of cold water (5°C–10°C) in a 250 ml beaker containing a magnetic stirring bar with gentle mixing to enhance the surface hydration of Pluronic F127, thus increasing dissolution. The container was left dormant in the refrigerator at 4°C (39.2°F) overnight to achieve complete solution of Pluronic F127 in water, following which formulation was allowed to thaw to room temperature, thus forming nanogel with 20% concentration of Pluronic.
Preparation of 2% curcumin nanogel
2% curcumin powder (Konark Herbals and Health Care, Mumbai, Maharashtra, India) was added gradually to 20% Pluronic nanogel and triturated well to get 2% curcumin nanogel which was refrigerated. Nanogel thus prepared was delivered into the selected sites.
1% chlorhexidine gel (Chlosite)
Commercially available chlorhexidine gel, i.e., Chlosite (Ghimas Service Providers, Delhi, India) was used. Single-dose syringe of Chlosite used contains 1 ml of xanthan gel (chlorhexidine digluconate and chlorhexidine dihydrochloride, 1:2 ratio).
Method of data collection
A total of 45 sites in 45 patients who fulfilled the inclusion criteria were enrolled into the double–blinded, parallel design, randomized controlled trial by an examiner (SG). A computer-generated randomization sequence was obtained to randomly allocate the enrolled patients into three groups by the examiner SG. Patients were allocated to the following interventions.
Group 1: 15 patients treated with SRP
Group 2: 15 patients treated with SRP and 1% chlorhexidine gel (Chlosite) as an LDD
Group 3: 15 patients treated with SRP and 2% curcumin gel with nanocarrier as an LDD.
The study protocol is summarized in Figure 1.
Figure 1.
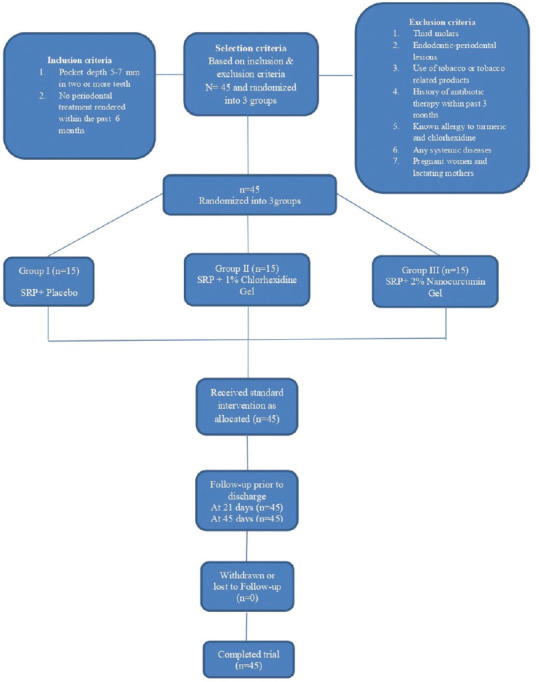
Consort flowchart
Preclinical protocol
At baseline examination, impressions were taken, and diagnostic cast was fabricated for each patient. A customized acrylic occlusal stent was fabricated using cold cure acrylic resin on the study cast to fit over the teeth selected for the study. A groove was cut in the acrylic stent to standardize point of entry to the pocket preoperatively and in successive appointments postoperatively. All measurements were made with UNC 15 periodontal probe and recorded to the nearest millimeter by the same calibrated examiner SG.
Clinical protocol
The clinical parameters, namely plaque index (PI), gingival index (GI), PPD, and clinical attachment level (CAL), were evaluated at baseline, 21st day, and 45th day by the examiner SG who was blinded to the type of intervention received. After the 45th day sample collection, all patients with persistent pockets were further subjected to standard treatment protocol for chronic periodontitis.
Sample collection
In each patient, one site with the deepest PPD in the posterior teeth was selected for the study. Subgingival plaque samples were collected from these sites using a Gracey curette (Hu-FriedyTM, Chicago, USA) by another operator (AR). Curette was inserted subgingivally parallel to the long axis of the tooth into the deepest portion of the periodontal pocket and then moved coronally by scraping along the root surface. Samples thus collected were stored in sterile saline vials at a temperature of −40°C and transferred to the laboratory where they were subjected to multiplex polymerase chain reaction (PCR) for microbial analysis.
Treatment protocol
All patients underwent full-mouth SRP by means of ultrasonic instruments (Acteon Satelec Suprasson, Merignac, France) performed by the operator (AR) followed by intervention by the same operator. A sealed envelope containing the intervention-type assignment for each individual patient was opened immediately following SRP. Treatment groups, namely SRP group (Group 1), chlorhexidine gel group (Group 2), and curcumin with nanogel group (Group 3), were thus determined. Experimental sites received a single subgingival application of curcumin-containing nanogel or chlorhexidine gel, whereas control sites did not receive any further treatment.
Placement of gel
The curcumin-containing nanogel was delivered to the bottom of the periodontal pocket using a disposable syringe with a 23-gauge blunt needle, whereas chlorhexidine gel was delivered using commercially provided cannula. All sites were covered with Coe-Pak. Patients were advised to follow their regular oral hygiene methods. Patients were recalled after 7 days for the removal of pack and clinical evaluation. Patients were recalled again on 21st and 45th day after placement of LDD for plaque sample collection and measurement of clinical parameters.
Microbiological analysis
Microbiological analysis of subgingival plaque sample was done using multiplex PCR at baseline, 21st and 45th day for the following tissue invading bacteria: Aggregatibacter actinomycetemcomitans (Aa), Porphyromonas gingivalis (Pg), and Tannerella forsythia (Tf).
Primary outcome measures
The primary outcome measures for the study were PI, GI, PPD, and CAL and changes in microbiological parameters.
Laboratory procedure
Samples collected from the selected sites were subjected to multiplex PCR analysis. Samples were processed for RNA extraction using trizol method immediately upon receiving. The extracted RNA was treated with DNase enzyme to remove any traces of DNA contamination. The RNA quantification was performed using a spectrophotometer (Sartorius India Pvt. Ltd., Bengaluru, Karnataka, India). After quantification, cDNA complementary to the isolated RNAs was prepared (Sigma Aldrich, Bengaluru, Karnataka, India). The finalized primer sequences were synthesized and purified by high-purity salt-free method.
A gradient PCR was performed to standardize the optimum annealing temperature of the designed primer using 50 ng of synthesized cDNA keeping the annealing temperature at a range of 50°C–60°C and amplified at 55°C. This was followed by semiquantitative PCR done by optimizing the concentration of cDNA to 50 ng across the sample.
Agarose gel electrophoresis
To visualize the gene expression levels in terms of the band intensity, the PCR product was analyzed in 1.5% agarose gel running at 50 volts till 2/3rd of the gel. The amplified bands were observed under ultraviolet light transilluminator and the image was captured using gel documentation unit (Biobee Tech, Bengaluru, Karnataka, India). The captured image was analyzed and intensity of the bands was calculated using the gel analyzer software Gel Analyzer 2010a [Figure 2].
Figure 2.
Gel imaging and semi quantitative analysis after agarose gel electrophoresis
Statistical analysis
Power calculation was done prior to initiation of the study. The sample size was estimated using the software GPower v. 3.1.9.2 software (Heinrich-Heine-University, Düsseldorf, Germany). Considering effect size measured (f) at 50%, power of the study at 80%, and the margin of error at 5%, the total sample size needed for the study was 42. The final sample size was rounded off to 45, and thereby, each study group comprised 15 patients.
Statistical Software Package SPSS version 22 (IBM SPSS Statistics for Windows, Version 22.0, IBM Corp., released in 2013, Armonk, New York, USA) was used. The difference in mean scores of various study variables at baseline and follow-up periods between three study groups was compared using one-way ANOVA test followed by bonferroni post hoc Analysis and Greenhouse–Geisser method.
The difference in the mean scores of various study variables between different time intervals within each study group was compared using repeated measures of ANOVA followed by bonferroni post hoc analysis and Greenhouse–Geisser method.
The level of significance (P value) was set at P < 0.05.
RESULTS
A total of 45 patients (i.e., one site per patient) with 15 patients in each group completed the study and there was no loss of sample in follow-up [Figure 1].
The demographic characteristics of patients at baseline are presented in Table 1. Intergroup comparison for gender and age did not show any statistical significance.
Table 1.
Comparison of demographic characteristics among three study groups
| Variables | Categories | Group 1, n (%) | Group 2, n (%) | Group 3, n (%) | P |
|---|---|---|---|---|---|
| Gender | Males | 11 (73.3) | 12 (80.0) | 13 (86.7) | 0.66† |
| Females | 4 (26.7) | 3 (20.0) | 2 (13.3) | ||
| Age | Mean | 44.5 | 38.1 | 35.9 | 0.05‡ |
| SD | 10.1 | 9.1 | 9.4 | ||
| Range | 29-59 | 26-61 | 21-51 |
†Chi-square test; ‡One-way ANOVA test; *Statistically significant (P<0.05). Group 1 – SRP; Group 2 – SRP + 1% chlorhexidine gel; Group 3 – SRP + 2% nanocurcumin gel; SD – Standard deviation; SRP – Scaling and root planing; P – P value; n – No of subjects
Table 2 shows comparison of all clinical and microbial parameters done using one-way ANOVA test for all the three groups. At baseline, the mean values of the different study variables did not show any statistical significance.
Table 2.
Comparison of mean scores of different study variables at baseline between three groups
| Variables | Group | n | Mean | SD | SE | Minimum | Maximum | P |
|---|---|---|---|---|---|---|---|---|
| GI | Group 1 | 15 | 1.00 | 0.34 | 0.09 | 0.6 | 1.9 | 0.46 |
| Group 2 | 15 | 0.93 | 0.23 | 0.06 | 0.5 | 1.3 | ||
| Group 3 | 15 | 0.88 | 0.20 | 0.05 | 0.5 | 1.2 | ||
| PI | Group 1 | 15 | 1.07 | 0.18 | 0.05 | 0.8 | 1.3 | 0.34 |
| Group 2 | 15 | 1.09 | 0.26 | 0.07 | 0.3 | 1.4 | ||
| Group 3 | 15 | 0.97 | 0.23 | 0.06 | 0.5 | 1.3 | ||
| PPD | Group 1 | 15 | 4.78 | 0.98 | 0.25 | 3.15 | 6.63 | 0.26 |
| Group 2 | 15 | 4.55 | 0.87 | 0.22 | 3.13 | 5.89 | ||
| Group 3 | 15 | 4.24 | 0.78 | 0.20 | 3.02 | 5.67 | ||
| CAL | Group 1 | 15 | 4.54 | 1.16 | 0.30 | 2.16 | 5.96 | 0.91 |
| Group 2 | 15 | 4.55 | 0.79 | 0.20 | 3.34 | 5.98 | ||
| Group 3 | 15 | 4.41 | 0.98 | 0.25 | 2.88 | 6.39 | ||
| Aa | Group 1 | 15 | 980.3 | 155.0 | 40.0 | 782 | 1325 | 0.20 |
| Group 2 | 15 | 1064.0 | 160.4 | 41.4 | 827 | 1378 | ||
| Group 3 | 15 | 964.4 | 161.8 | 41.8 | 697 | 1239 | ||
| Tf | Group 1 | 15 | 1511.7 | 260.6 | 67.3 | 1015 | 1998 | 0.10 |
| Group 2 | 15 | 1346.3 | 518.3 | 133.8 | 736 | 2589 | ||
| Group 3 | 15 | 1193.3 | 365.1 | 94.3 | 449 | 1874 | ||
| Pg | Group 1 | 15 | 521.9 | 139.9 | 36.1 | 236 | 712 | 0.30 |
| Group 2 | 15 | 570.9 | 185.2 | 47.8 | 301 | 903 | ||
| Group 3 | 15 | 473.1 | 180.6 | 46.6 | 120 | 789 |
*Statistically significant (P<0.05). Group 1 – SRP; Group 2 – SRP + 1% chlorhexidine gel; Group 3 – SRP + 2% nanocurcumin gel; CFU/ml – Colony-forming units/ml; SRP – Scaling and root planing; GI – Gingival index; PI – Plaque index; PPD – Probing pocket depth; CAL – Clinical attachment level; Aa – Aggregatibacter actinomycetemcomitans (CFU/ml); Tf – Tannerella forsythia (CFU/ml); Pg – Porphyromonas gingivalis (CFU/ml); SD – Standard deviation; SE – Standard error; P – P value; n – No of subjects
Table 3 shows comparison of mean GI, PI, PPD, and CAL scores between case and control groups at baseline and 21 and 45 days. The mean scores for Group 1, 2, and 3 from baseline to 21 days and baseline to 45 days were statistically significant. However, there was no statistically significant difference observed between 21 and 45 days follow-up evaluation for all three groups, except for PPD scores in Group 1, which showed significant difference even between 21 and 45 days follow-up evaluation. The intragroup comparison showed statistically significant difference in all three groups at baseline and 21 and 45 days.
Table 3.
Comparison of the mean gingival index, plaque index, probing pocket depth, clinical attachment level scores at baseline, 21 days and 45 days within each study group
| Variables | Group | Time | Mean | SD | SE | P† | Difference | P |
|---|---|---|---|---|---|---|---|---|
| GI | Group 1 | Baseline | 1.00 | 0.34 | 0.09 | 0.001* | T1 versus T2 | 0.004* |
| 21 days | 0.75 | 0.17 | 0.05 | T1 versus T3 | 0.002* | |||
| 45 days | 0.85 | 0.27 | 0.07 | T2 versus T3 | 0.11 | |||
| Group 2 | Baseline | 0.93 | 0.23 | 0.06 | <0.001* | T1 versus T2 | <0.001* | |
| 21 days | 0.77 | 0.18 | 0.05 | T1 versus T3 | <0.001* | |||
| 45 days | 0.79 | 0.21 | 0.05 | T2 versus T3 | 1.00 | |||
| Group 3 | Baseline | 0.88 | 0.20 | 0.05 | <0.001* | T1 versus T2 | <0.001* | |
| 21 days | 0.71 | 0.16 | 0.04 | T1 versus T3 | 0.001* | |||
| 45 days | 0.74 | 0.16 | 0.04 | T2 versus T3 | 0.90 | |||
| PI | Group 1 | Baseline | 1.07 | 0.18 | 0.05 | <0.001* | T1 versus T2 | <0.001* |
| 21 days | 0.78 | 0.11 | 0.03 | T1 versus T3 | <0.001* | |||
| 45 days | 0.86 | 0.17 | 0.04 | T2 versus T3 | 0.05 | |||
| Group 2 | Baseline | 1.09 | 0.26 | 0.07 | <0.001* | T1 versus T2 | <0.001* | |
| 21 days | 0.73 | 0.15 | 0.04 | T1 versus T3 | <0.001* | |||
| 45 days | 0.79 | 0.16 | 0.04 | T2 versus T3 | 0.06 | |||
| Group 3 | Baseline | 0.97 | 0.23 | 0.06 | 0.002* | T1 versus T2 | 0.01* | |
| 21 days | 0.75 | 0.11 | 0.03 | T1 versus T3 | 0.009* | |||
| 45 days | 0.79 | 0.13 | 0.03 | T2 versus T3 | 0.49 | |||
| PPD | Group 1 | Baseline | 4.78 | 0.98 | 0.25 | <0.001* | T1 versus T2 | <0.001* |
| 21 days | 4.23 | 0.95 | 0.25 | T1 versus T3 | 0.04* | |||
| 45 days | 4.47 | 0.97 | 0.25 | T2 versus T3 | 0.02* | |||
| Group 2 | Baseline | 4.55 | 0.87 | 0.23 | <0.001* | T1 versus T2 | <0.001* | |
| 21 days | 4.02 | 0.93 | 0.24 | T1 versus T3 | <0.001* | |||
| 45 days | 4.11 | 0.93 | 0.24 | T2 versus T3 | 0.09 | |||
| Group 3 | Baseline | 4.24 | 0.78 | 0.20 | <0.001* | T1 versus T2 | <0.001* | |
| 21 days | 3.87 | 0.74 | 0.19 | T1 versus T3 | <0.001* | |||
| 45 days | 4.01 | 0.76 | 0.20 | T2 versus T3 | 0.08 | |||
| CAL | Group 1 | Baseline | 4.54 | 1.16 | 0.30 | <0.001* | T1 versus T2 | <0.001* |
| 21 days | 4.25 | 1.11 | 0.29 | T1 versus T3 | 0.01* | |||
| 45 days | 4.31 | 1.12 | 0.29 | T2 versus T3 | 0.98 | |||
| Group 2 | Baseline | 4.55 | 0.79 | 0.20 | <0.001* | T1 versus T2 | <0.001* | |
| 21 days | 4.15 | 0.70 | 0.18 | T1 versus T3 | <0.001* | |||
| 45 days | 4.26 | 0.72 | 0.19 | T2 versus T3 | 0.01 | |||
| Group 3 | Baseline | 4.41 | 0.98 | 0.25 | <0.001* | T1 versus T2 | <0.001* | |
| 21 days | 4.13 | 0.96 | 0.25 | T1 versus T3 | <0.001* | |||
| 45 days | 4.22 | 0.96 | 0.25 | T2 versus T3 | 0.09 |
†Greenhouse Geisser method; *Statistically significant (P<0.001). T1 – Baseline; T2 – 21 days; T3 – 45 days; Group 1 – SRP; Group 2 – SRP + 1% chlorhexidine gel; Group 3 – SRP + 2% nanocurcumin gel; GI – Gingival index; PI – Plaque index; PPD – Probing pocket depth (mm); CAL – Clinical attachment level (mm); SD – Standard deviation; SRP – Scaling and root planing; SE – Standard error; P – P value
Table 4 shows comparison of mean scores of different clinical parameters at 21 and 45 days follow-up period between the three groups, which was not statistically significant.
Table 4.
Comparison of mean scores of different clinical parameters at 21 days and 45 days follow-up period between 3 groups
| Variables | Group | n | Mean | SD | SE | Minimum | Maximum | P | Difference | P |
|---|---|---|---|---|---|---|---|---|---|---|
| 21 days follow-up period | ||||||||||
| GI | Group 1 | 15 | 0.75 | 0.17 | 0.04 | 0.5 | 1.1 | 0.69 | - | - |
| Group 2 | 15 | 0.77 | 0.18 | 0.05 | 0.4 | 1.1 | ||||
| Group 3 | 15 | 0.71 | 0.16 | 0.04 | 0.4 | 0.9 | ||||
| PI | Group 1 | 15 | 0.78 | 0.11 | 0.03 | 0.6 | 0.9 | 0.53 | - | - |
| Group 2 | 15 | 0.73 | 0.15 | 0.04 | 0.3 | 0.9 | ||||
| Group 3 | 15 | 0.75 | 0.11 | 0.03 | 0.6 | 0.9 | ||||
| PPD | Group 1 | 15 | 4.23 | 0.95 | 0.24 | 2.95 | 5.98 | 0.54 | - | - |
| Group 2 | 15 | 4.02 | 0.93 | 0.24 | 2.45 | 5.65 | ||||
| Group 3 | 15 | 3.87 | 0.74 | 0.19 | 2.76 | 5.22 | ||||
| CAL | Group 1 | 15 | 4.25 | 1.11 | 0.29 | 2.09 | 5.56 | 0.93 | - | - |
| Group 2 | 15 | 4.15 | 0.70 | 0.18 | 2.97 | 5.34 | ||||
| Group 3 | 15 | 4.13 | 0.96 | 0.25 | 2.53 | 6.12 | ||||
| 45 days follow-up period | ||||||||||
| GI | Group 1 | 15 | 0.85 | 0.27 | 0.07 | 0.5 | 1.5 | 0.37 | - | - |
| Group 2 | 15 | 0.79 | 0.21 | 0.05 | 0.4 | 1.1 | ||||
| Group 3 | 15 | 0.74 | 0.16 | 0.04 | 0.4 | 0.9 | ||||
| PI | Group 1 | 15 | 0.86 | 0.17 | 0.04 | 0.7 | 1.2 | 0.41 | - | - |
| Group 2 | 15 | 0.79 | 0.16 | 0.04 | 0.4 | 1.0 | ||||
| Group 3 | 15 | 0.79 | 0.13 | 0.03 | 0.5 | 1.1 | ||||
| PPD | Group 1 | 15 | 4.47 | 0.97 | 0.25 | 2.88 | 6.15 | 0.34 | - | - |
| Group 2 | 15 | 4.11 | 0.93 | 0.24 | 2.64 | 5.54 | ||||
| Group 3 | 15 | 4.01 | 0.76 | 0.20 | 2.84 | 5.34 | ||||
| CAL | Group 1 | 15 | 4.31 | 1.12 | 0.29 | 2.11 | 5.8 | 0.97 | - | - |
| Group 2 | 15 | 4.26 | 0.72 | 0.18 | 3.05 | 5.46 | ||||
| Group 3 | 15 | 4.22 | 0.96 | 0.25 | 2.67 | 6.18 | ||||
*Statistically significant (P<0.05). Group 1 –SRP; Group 2 – SRP + 1% chlorhexidine gel; Group 3 – SRP + 2% nanocurcumin gel; GI – Gingival index; PI – Plaque index; PPD – Probing pocket depth (mm); CAL – Clinical attachment level (mm); SD – Standard deviation; SE – Standard error; P – P value; n – No of subjects
Table 5 evaluates the microbial assessment by multiplex PCR analysis of microbial samples for Aa, Pg, and Tf between case and control group at baseline and 21 and 45 days. The mean Aa, Pg, and Tf scores from baseline to 21 days, baseline to 45 days, and 21–45 days were statistically significant in all the three groups, except in Group 1 where no significant difference was observed between baseline to 21 days for Aa and Pg and baseline to 45 days for Pg. There was also no significant difference between 21 and 45 days for Pg in Group 2 and 3. The intragroup comparison showed statistical significant difference in all three groups at baseline and 21 and 45 days follow-up evaluation except in Group 1 for Tf.
Table 5.
Comparison of mean scores for aggregatibacter actinomycetemcomitans, porphyromonas gingivalis, tannerella forsythia at baseline, 21 days and 45 days within each group
| Group | Time | Mean | SD | SE | P† | Difference | P |
|---|---|---|---|---|---|---|---|
| Aggregatibacter actinomycetemcomitans | |||||||
| Group 1 | Baseline | 980.3 | 155.0 | 40.0 | 0.002* | T1 versus T2 | 1.00 |
| 21 days | 935.2 | 192.1 | 49.6 | T1 versus T3 | 0.02* | ||
| 45 days | 967.1 | 220.4 | 56.9 | T2 versus T3 | 0.009* | ||
| Group 2 | Baseline | 1064.0 | 160.4 | 41.4 | <0.001* | T1 versus T2 | <0.001* |
| 21 days | 474.0 | 148.7 | 38.4 | T1 versus T3 | <0.001* | ||
| 45 days | 700.0 | 134.3 | 34.7 | T2 versus T3 | 0.001* | ||
| Group 3 | Baseline | 964.4 | 161.8 | 41.8 | <0.001* | T1 versus T2 | <0.001* |
| 21 days | 391.3 | 117.3 | 30.3 | T1 versus T3 | <0.001* | ||
| 45 days | 560.1 | 133.8 | 34.5 | T2 versus T3 | <0.001* | ||
| Porphyromonas gingivalis | |||||||
| Group 1 | Baseline | 521.9 | 139.9 | 36.1 | 0.04* | T1 versus T2 | 1.00 |
| 21 days | 504.2 | 176.6 | 45.6 | T1 versus T3 | 0.25 | ||
| 45 days | 516.5 | 213.1 | 55.0 | T2 versus T3 | 0.006* | ||
| Group 2 | Baseline | 570.9 | 185.2 | 47.8 | <0.001* | T1 versus T2 | <0.001* |
| 21 days | 306.9 | 170.6 | 44.0 | T1 versus T3 | <0.001* | ||
| 45 days | 327.5 | 151.1 | 39.0 | T2 versus T3 | 0.92 | ||
| Group 3 | Baseline | 473.1 | 180.6 | 46.6 | <0.001* | T1 versus T2 | <0.001* |
| 21 days | 223.2 | 114.4 | 29.5 | T1 versus T3 | <0.001* | ||
| 45 days | 240.5 | 125.2 | 32.3 | T2 versus T3 | 0.95 | ||
| Tannerella forsythia | |||||||
| Group 1 | Baseline | 1511.7 | 260.6 | 67.3 | 0.18 | - | - |
| 21 days | 1457.1 | 373.7 | 96.5 | ||||
| 45 days | 1493.4 | 426.1 | 110.0 | ||||
| Group 2 | Baseline | 1346.3 | 518.3 | 133.8 | <0.001* | T1 versus T2 | <0.001* |
| 21 days | 691.9 | 513.3 | 132.5 | T1 versus T3 | <0.001* | ||
| 45 days | 970.8 | 550.0 | 142.0 | T2 versus T3 | <0.001* | ||
| Group 3 | Baseline | 1193.3 | 365.1 | 94.3 | <0.001* | T1 versus T2 | <0.001* |
| 21 days | 701.7 | 224.3 | 57.9 | T1 versus T3 | 0.001* | ||
| 45 days | 848.9 | 223.2 | 57.6 | T2 versus T3 | 0.01* | ||
†Greenhouse Geisser method; *Statistically significant (P<0.001). Group 1 – SRP; Group 2 – SRP + 1% chlorhexidine gel; Group 3 – SRP + 2% nanocurcumin gel; T1 – Baseline; T2 – 21 days; T3 – 45 days; SD – Standard deviation; SRP – Scaling and root planing; SD – Standard deviation; SE – Standard error; P – P value
Table 6 shows comparison of mean scores of different microbial parameters at 21 and 45 days follow-up period between the three groups. Intergroup comparison of mean Aa, Pg, and Tf values observed at 21 and 45 days between Group 1 and 2 and Group 2 and 3 showed statistical significance but not for comparison between Group 2 and 3.
Table 6.
Comparison of Mean scores of different microbial parameters at 21 days and 45 days follow-up period between 3 groups
| Variables | Group | n | Mean | SD | SE | Minimum | Maximum | P | Difference | P |
|---|---|---|---|---|---|---|---|---|---|---|
| 21 days follow-up period | ||||||||||
| Aa | Group 1 | 15 | 985.2 | 192.1 | 49.6 | 639 | 1296 | <0.001* | Group 1 versus Group 2 | <0.001* |
| Group 2 | 15 | 474.0 | 148.7 | 38.4 | 227 | 759 | Group 1 versus Group 3 | <0.001* | ||
| Group 3 | 15 | 391.3 | 117.3 | 30.3 | 221 | 655 | Group 2 versus Group 3 | 0.32 | ||
| Tf | Group 1 | 15 | 1457.1 | 373.7 | 96.5 | 1077 | 2248 | <0.001* | Group 1 versus Group 2 | <0.001* |
| Group 2 | 15 | 691.9 | 513.3 | 132.5 | 94 | 2115 | Group 1 versus Group 3 | <0.001* | ||
| Group 3 | 15 | 701.7 | 224.3 | 57.9 | 334 | 1128 | Group 2 versus Group 3 | 1.00 | ||
| Pg | Group 1 | 15 | 504.2 | 176.6 | 45.6 | 228 | 845 | <0.001* | Group 1 versus Group 2 | 0.004* |
| Group 2 | 15 | 306.9 | 170.6 | 44.0 | 74 | 652 | Group 1 versus Group 3 | <0.001* | ||
| Group 3 | 15 | 223.2 | 114.4 | 29.5 | 54 | 478 | Group 2 versus Group 3 | 0.32 | ||
| 45 days follow-up period | ||||||||||
| Aa | Group 1 | 15 | 1102.1 | 220.4 | 56.9 | 698 | 1459 | <0.001* | Group 1 versus Group 2 | <0.001* |
| Group 2 | 15 | 700.0 | 134.3 | 34.7 | 529 | 961 | Group 1 versus Group 3 | <0.001* | ||
| Group 3 | 15 | 560.1 | 133.8 | 34.5 | 314 | 756 | Group 2 versus Group 3 | 0.07 | ||
| Tf | Group 1 | 15 | 1667.4 | 426.1 | 110.0 | 1225 | 2610 | <0.001* | Group 1 versus Group 2 | <0.001* |
| Group 2 | 15 | 970.8 | 550.0 | 142.0 | 333 | 2452 | Group 1 versus Group 3 | <0.001* | ||
| Group 3 | 15 | 848.9 | 223.2 | 57.6 | 528 | 1396 | Group 2 versus Group 3 | 0.71 | ||
| Pg | Group 1 | 15 | 592.5 | 213.1 | 55.0 | 302 | 958 | <0.001* | Group 1 versus Group 2 | 0.004* |
| Group 2 | 15 | 327.5 | 151.1 | 39.0 | 124 | 697 | Group 1 versus Group 3 versus | <0.001* | ||
| Group 3 | 15 | 240.5 | 125.2 | 32.3 | 87 | 466 | Group 2 versus Group 3 | 0.34 | ||
*Statistically significant (P<0.001). Group 1 – SRP; Group 2 – SRP + 1% chlorhexidine gel; Group 3 – SRP + 2% nanocurcumin gel; Aa – Aggregatibacter actinomycetemcomitans (CFU/ml); Tf – Tannerella forsythia (CFU/ml); Pg – Porphyromonas gingivalis (CFU/ml); SD – Standard deviation; SRP – Scaling and root planing; SD – Standard deviation; SE – Standard error; P – P value; n – No of subjects
DISCUSSION
Periodontal diseases occur due to colonization of subgingival biofilm by pathogenic microorganisms.[16] Incorporation of a chemotherapeutic agent in conjunction with mechanical instrumentation provides additional antimicrobial effect for disease control. One of the advances in LDD system is use of nanoparticles.[5,17] Nanoparticles vary in size from 1 to 1000 nm. Pharmaceutical nanotechnology involves formulation of therapeutically active and biocompatible agents in various nanoforms such as nanoparticles, nanocapsules, micellar systems, and conjugates. Besides the primary task of LDD, such systems have shown to improve bioavailability and extend drug or gene effect in the target tissue, thus resulting in improved stability of these therapeutic agents against chemical/enzymatic degradation.[18] These nanoparticulate systems can dramatically increase drug absorption, thus resulting in improved bioavailability and subsequent drug dose reduction and frequency of administration.[19] Nanoparticles due to their small size have high dispersibility in aqueous medium and provide uniform distribution of the active agent and sustained activity.[18,19] All these advantages cause such particles to penetrate areas that are unapproachable by other delivery systems. However, these systems have some disadvantages such as higher production cost, problems with stability of nanoparticles, interparticular friction and sticking, higher clearance rate, and toxicity.[20]
Among various nanoparticles presently available as carriers for drug delivery, Pluronic F127 exhibits several advantages an important one being that it facilitates early attachment and enhances growth rate of human gingival fibroblasts at very low dosages helping in early wound healing.[21] A study analyzing the efficacy of local delivery of a Pluronic thermoreversible tetracycline gel along with serratiopeptidase in chronic periodontitis patients showed a significant reduction in clinical parameters.[22] Hence, with this background evidence, we chose to use Pluronic F127 as a nanocarrier in our study.
Chlosite is an agent containing 1.5% chlorhexidine of xanthan type which is a saccharide polymer and is efficient in the treatment of periodontal pockets and peri-implantitis.[23,24] Curcumin has a wide variety of applications in medicine and when used in combination with nanoparticles, its antimicrobial efficacy may increase. With this presumption, in our study, we used curcumin as an antimicrobial along with nanoparticles and compared its efficacy to chlorhexidine, which is shown to be an effective antiplaque agent in the treatment of chronic periodontitis.
In the present study, there was a significant reduction in GI, PI, PPD, and CAL scores in all three groups from baseline to 21 days and baseline to 45 days, indicating that all three treatment modalities individually were effective in reducing gingival inflammation, plaque, and pocket depth and also resulted in clinical attachment gain. However, intergroup comparison did not show a significant difference in the clinical scores between three groups when compared at baseline and 21 and 45 days, indicating that all three treatment modalities were equally effective in reducing the clinical parameters when compared with each other. These results are in accordance with other studies which showed similar results.[11,25,26,27]
The present study mainly focused on the effectiveness of the drugs curcumin and chlorhexidine in reducing the levels of periodontal pathogenic microorganisms, specifically Aa, Pg, and Tf. These bacteria were selected as there is substantial evidence that they are key periodontal pathogens and are tissue invading bacteria which may be difficult to eliminate with mechanical therapy alone.[28,29]
The results of the present study showed that Pg levels in the SRP group did not show a statistically significant reduction in occurrence when compared from baseline to 21 days and baseline to 45 days. However, when Pg levels in SRP were assessed between 21 and 45 days a significant increase was noted, indicating an active recolonization. Both CHX and CUR group showed a statistically significant reduction in Pg levels from baseline to 21 days and baseline to 45 days indicating the efficacy of both CHX and CUR in reducing the Pg levels when used as LDD agents. However, comparison of the values between 21 days and 45 days was not statistically significant, indicating slower rate of recolonization as compared to SRP group. However, comparison of CHX and CUR groups was not statistically significant, indicating the comparable efficacy of CUR and CHX in reducing bacterial level. This finding is in line with another study which investigated the inhibitory effects of Curcumin on the activity of virulence factors of Pg, namely Arg-specific proteinases (RGP) and Lys-specific proteinases (KGP), indicating its effectiveness in the treatment of periodontal disease.[29] The same study also investigated the effects of curcumin on early biofilm formation by evaluating Pg colonization and reported that curcumin inhibited biofilm formation in dose-dependent manner.[30]
A recent study showed that when a matured multispecies biofilm (formed with Streptococcus mitis, Fusobacterium nucleatum, Pg, and Aa) was treated for 24 h with minimum inhibitory concentration (MIC) of curcumin, ultrastructural changes such as significant reduction in metabolic activity were observed in the biofilm. This highlighted the antibacterial effect of curcumin on periodontal bacteria in both planktonic and biofilm modes of growth.[31] When 1% curcumin gel was used as an adjunct to SRP, substantial decrease in counts of periodontopathic bacteria (Pg, Prevotella intermedia, F. nucleatum, and Capnocytophaga) was observed after 6 months in comparison with sites which received SRP alone.[32]
In the present study, comparison of CHX group with SRP group with regard to microbiological parameters showed that SRP with CHX gel as an LDD was better than SRP alone. This could be attributed to the substantivity and antimicrobial property imparted by chlorhexidine. This finding is in accordance with the findings of another study which used 1% chlorhexidine gel in the treatment of periodontal pockets.[25]
Both CHX and CUR groups showed a statistically significant reduction in Aa and Tf levels from baseline to 21 days and baseline to 45 days, indicating the efficacy of CHX and CUR as LDD agents in reducing Aa and Tf levels in the periodontal pocket. There was a significant increase in Aa and Tf levels in both groups from 21 to 45 days; however, these levels were lower than the baseline scores. Reduction in Aa levels was similar to another study which investigated antibacterial effect of curcumin on periodontal bacteria.[31] Reduction in Tf levels was similar to another study which evaluated the effect of 0.1% turmeric mouthwash as an adjunct to SRP.[33] Studies have shown that the microbiota in the treated sites after 60 days is similar to that present at pretreatment levels due to recolonization, which could explain the increase in levels of Aa, Pg, and Tf at second follow-up visit, i.e., at 45 days.[34,35] However, the levels at 45th day were lower than the baseline levels in all the three groups. Both CHX and CUR groups showed a slower rate of recolonization as compared to SRP group, thus indicating the added advantage of both the LDD agents as compared to SRP alone. A recent literature has shown that the degree and speed of recolonization depends on the type of treatment procedure, the pattern of distribution of periodontal microorganisms in the various niches in the oral cavity, and also the quality of the patient's oral hygiene maintenance.[35] In the present study, we used 2% curcumin nanogel; however, further studies with higher concentration of the drug could be tried to check if the inhibition of the microorganisms is dose dependent.
Thus, the results of our study showed that when LDD agents, namely 2% curcumin with a nanocarrier system or 1% chlorhexidine gel, used as an adjunct to SRP in patients with chronic periodontitis, there was an improvement in all clinical parameters. Evaluation of microbiological parameters also showed that there was a significant reduction in Pg, Aa, and Tf levels, thus validating their potential as an LDD system when compared to SRP alone. Comparison of the two agents showed that both had comparable antibacterial effect on the three selected periodontopathic bacteria, thus brightening the prospects of using curcumin with nanocarrier as an LDD system in subgingival sites.
However, a study design involving larger sample size and longer follow-up period would shed more light regarding the efficacy of 2% curcumin gel as an LDD agent. Further studies incorporating assessment of MIC of curcumin, release kinetics, and assessment of substantivity of curcumin in the pocket ecosystem need to be done to provide better insight into the clinical utility of nanocurcumin as a potent LDD agent.
CONCLUSION
Results of the present study showed that when the two LDD agents were used as an adjunct to SRP in patients with chronic periodontitis, there was an improvement in all clinical and microbiological parameters, suggesting that both agents have comparable antibacterial effect.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The biochemical laboratory procedures were carried out at Credora Laboratories, Bangalore, India. I would like to express my gratitude to Dr. Rijesh, Senior Scientist, Credora Laboratories, Bangalore, India for having devoted his time throughout the duration of this study. I am thankful to Dr C Santosh Kumar, Consultant Biostatistician, Bangalore, India for helping us with the statistical analysis. I would also acknowledge all the staff members of Dept of Periodontics, Vydehi Institute of Dental Sciences and Research Center, Bangalore, India for all their support.
REFERENCES
- 1.Pallasch TJ. Pharmacokinetic principles of antimicrobial therapy. Periodontol. 2000;1996(10):5–11. doi: 10.1111/j.1600-0757.1996.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen MG, Slots J. Responsible use of antimicrobials in periodontics. J Calif Dent Assoc. 2000;28:185–93. [PubMed] [Google Scholar]
- 3.Lee BS, Lee CC, Wang YP, Chen HJ, Lai CH, Hsieh WL, et al. Controlled-release of tetracycline and lovastatin by poly (D, L-lactide-co-glycolide acid)-chitosan nanoparticles enhances periodontal regeneration in dogs. Int J Nanomedicine. 2016;11:285–97. doi: 10.2147/IJN.S94270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong LX, Peng Z, Li SD, Bartold PM. Nanotechnology and its role in the management of periodontal diseases. Periodontol. 2000;2006(40):184–96. doi: 10.1111/j.1600-0757.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel JG, Balaji KS, Koushik K, Navarre C, Duran SH, Rahe CH, et al. Pluronic F127 gel formulations of deslorelin and GnRH reduce drug degradation and sustain drug release and effect in cattle. J Control Release. 2002;85:51–9. doi: 10.1016/s0168-3659(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 6.Chandra Shekar BR, Nagarajappa R, Suma S, Thakur R. Herbal extracts in oral health care – A review of the current scenario and its future needs. Pharmacogn Rev. 2015;9:87–92. doi: 10.4103/0973-7847.162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. 2011;12:332–47. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu Y, Lee HM, Napolitano N, Clemens M, Zhang Y, Sorsa T, et al. 4-4. methoxycarbonyl curcumin: A unique inhibitor of both inflammatory mediators and periodontal inflammation. Mediators Inflamm. 2013;2013:329740. doi: 10.1155/2013/329740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guru SR, Kothiwale SV, Saroch N, Guru RC. Comparative evaluation of inhibitory effect of curcumin and doxycycline on matrix metalloproteinase-9 activity in chronic periodontitis. Indian J Dent Res. 2017;28:560–5. doi: 10.4103/ijdr.IJDR_461_16. [DOI] [PubMed] [Google Scholar]
- 10.Waghmare PF, Chaudhari AU, Karhadkar VM, Jamkhande AS. Comparative evaluation of turmeric and chlorhexidine gluconate mouthwash in prevention of plaque formation and gingivitis: A clinical and microbiological study. J Contemp Dent Pract. 2011;12:221–4. doi: 10.5005/jp-journals-10024-1038. [DOI] [PubMed] [Google Scholar]
- 11.Guimarães MR, Coimbra LS, de Aquino SG, Spolidorio LC, Kirkwood KL, Rossa C., Jr Potent anti-inflammatory effects of systemically administered curcumin modulate periodontal disease in vivo. J Periodontal Res. 2011;46:269–79. doi: 10.1111/j.1600-0765.2010.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaswal R, Dhawan S, Grover V, Malhotra R. Comparative evaluation of single application of 2% whole turmeric gel versus 1% chlorhexidine gel in chronic periodontitis patients: A pilot study. J Indian Soc Periodontol. 2014;18:575–80. doi: 10.4103/0972-124X.142445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behal R, Mali AM, Gilda SS, Paradkar AR. Evaluation of local drug-delivery system containing 2% whole turmeric gel used as an adjunct to scaling and root planing in chronic periodontitis: A clinical and microbiological study. J Indian Soc Periodontol. 2011;15:35–8. doi: 10.4103/0972-124X.82264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie X, Tao Q, Zou Y, Zhang F, Guo M, Wang Y, et al. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: Characterizations and mechanisms. J Agric Food Chem. 2011;59:9280–9. doi: 10.1021/jf202135j. [DOI] [PubMed] [Google Scholar]
- 15.Ratnatilaka Na Bhuket P, El-Magboub A, Haworth IS, Rojsitthisak P. Enhancement of curcumin bioavailability via the prodrug approach: Challenges and prospects. Eur J Drug Metab Pharmacokinet. 2017;42:341–53. doi: 10.1007/s13318-016-0377-7. [DOI] [PubMed] [Google Scholar]
- 16.Page RC, Kornman KS. The pathogenesis of human periodontitis: An introduction. Periodontol. 2000;1997(14):9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 17.LaVan DA, Lynn DM, Langer R. Moving smaller in drug discovery and delivery. Nat Rev Drug Discov. 2002;1:77–84. doi: 10.1038/nrd707. [DOI] [PubMed] [Google Scholar]
- 18.Rizvi SA, Saleh AM. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J. 2018;26:64–70. doi: 10.1016/j.jsps.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh R, Lillard JW., Jr Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86:215–23. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park K. Facing the truth about nanotechnology in drug delivery. ACS Nano. 2013;7:7442–7. doi: 10.1021/nn404501g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hokett SD, Cuenin MF, O'Neal RB, Brennan WA, Strong SL, Runner RR, et al. Pluronic polyol effects on human gingival fibroblast attachment and growth. J Periodontol. 2000;71:803–9. doi: 10.1902/jop.2000.71.5.803. [DOI] [PubMed] [Google Scholar]
- 22.Maheshwari M, Miglani G, Mali A, Paradkar A, Yamamura S, Kadam S. Development of tetracycline-serratiopeptidase -containing periodontal gel: Formulation and preliminary clinical study. AAPS PharmSciTech. 2006;7:76. doi: 10.1208/pt070376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain M, Dave D, Jain P, Manohar B, Yadav B, Shetty N. Efficacy of xanthan based chlorhexidine gel as an adjunct to scaling and root planing in treatment of the chronic periodontitis. J Indian Soc Periodontol. 2013;17:439–43. doi: 10.4103/0972-124X.118313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin L, Frankenthal S, Joseph L, Rozitsky D, Levi G, Machtei EE. Water jet with adjunct chlorhexidine gel for nonsurgical treatment of peri-implantitis. Quintessence Int. 2015;46:133–7. doi: 10.3290/j.qi.a32819. [DOI] [PubMed] [Google Scholar]
- 25.Vinholis AH, Figueiredo LC, Marcantonio Júnior E, Marcantonio RA, Salvador SL, Goissis G. Subgingival utilization of a 1% chlorhexidine collagen gel for the treatment of periodontal pockets. A clinical and microbiological study. Braz Dent J. 2001;12:209–13. [PubMed] [Google Scholar]
- 26.Muglikar S, Patil KC, Shivswami S, Hegde R. Efficacy of curcumin in the treatment of chronic gingivitis: A pilot study. Oral Health Prev Dent. 2013;11:81–6. doi: 10.3290/j.ohpd.a29379. [DOI] [PubMed] [Google Scholar]
- 27.Haffajee AD, Cugini MA, Dibart S, Smith C, Kent RL, Jr, Socransky SS. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol. 1997;24:324–34. doi: 10.1111/j.1600-051x.1997.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 28.Takamatsu N, Yano K, He T, Umeda M, Ishikawa I. Effect of initial periodontal therapy on the frequency of detecting Bacteroides forsythus, Porphyromonas gingivalis, and Actinobacillus actinomycetemcomitans. J Periodontol. 1999;70:574–80. doi: 10.1902/jop.1999.70.6.574. [DOI] [PubMed] [Google Scholar]
- 29.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 30.Izui S, Sekine S, Maeda K, Kuboniwa M, Takada A, Amano A, et al. Antibacterial activity of curcumin against periodontopathic bacteria. J Periodontol. 2016;87:83–90. doi: 10.1902/jop.2015.150260. [DOI] [PubMed] [Google Scholar]
- 31.Shahzad M, Millhouse E, Culshaw S, Edwards CA, Ramage G, Combet E. Selected dietary (poly) phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2015;6:719–29. doi: 10.1039/c4fo01087f. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia M, Urolagin SS, Pentyala KB, Urolagin SB, Menaka KB, Bhoi S. Novel therapeutic approach for the treatment of periodontitis by curcumin. J Clin Diagn Res. 2014;8:ZC65–9. doi: 10.7860/JCDR/2014/8231.5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mali AM, Behal R, Gilda SS. Comparative evaluation of 0.1% turmeric mouthwash with 0.2% chlorhexidine gluconate in prevention of plaque and gingivitis: A clinical and microbiological study. J Indian Soc Periodontol. 2012;16:386–91. doi: 10.4103/0972-124X.100917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sbordone L, Ramaglia L, Gulletta E, Iacono V. Recolonization of the subgingival microflora after scaling and root planing in human periodontitis. J Periodontol. 1990;61:579–84. doi: 10.1902/jop.1990.61.9.579. [DOI] [PubMed] [Google Scholar]
- 35.Mombelli A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol. 2000;2018(76):85–96. doi: 10.1111/prd.12147. [DOI] [PubMed] [Google Scholar]


