Abstract
Background/objectives
During the 2019 coronavirus disease (COVID-19) outbreak, obesity may contribute to COVID-19 transmission and deterioration. In addition, many patients with COVID-19 infection have suffered liver damage which might contribute to a worse prognosis. We conducted a clinical epidemiological analysis to investigate the association of overweight/obesity and abnormal liver function (ALF) with hospitalized duration in patients infected with COVID-19.
Subjects/methods
Fifty-eight patients with diagnosed COVID-19 (22 women & 36 men; average age: 49.2 ± 13.1 yr) were included, and their clinical data were collected at The Second Affiliated and Yuying Children’s Hospital of Wenzhou Medical University, Zhejiang. Overweight/obesity was determined as body mass index (BMI) ≥24 kg/m2, ALF was determined as alanine aminotransferase >40 U/L, and prolonged hospitalization was lasting more than the median value of the hospitalized days (19 days) in this population.
Results
The proportions of prolonged hospitalization were elevated in patients with overweight/obesity and ALF compared with those without overweight/obesity (62.1% versus 26.1%, P = 0.010) and those without ALF (70.6% versus 41.5%, P = 0.043). Kaplan–Meier analysis showed that the hospitalized duration was increased from the patients with neither overweight/obesity nor ALF to those with either overweight/obesity or ALF, and to those with both of overweight/obesity and ALF (mean with 95% confidence interval: 16.4 [14.5–18.3] versus 25.3 [21.6–29.1] versus 28.3 [24.6–32.0], P for trend = 0.001). Being discharged from hospital in time was inversely and independently associated with BMI (hazard ratio [HR] = 0.75, 95% CI: 0.63–0.90, P for trend = 0.002) and ALT (HR = 0.95, 95% CI: 0.92–0.99, P for trend = 0.007).
Conclusions
Present findings suggested that overweight/obesity and/or ALF contributed to predicting a probability of prolonged hospitalization in patients with COVID-19 infection, to whom extra attentions and precautions should be paid during clinical treatments.
Subject terms: Risk factors, Obesity
Introduction
Since coronavirus disease 2019 (COVID-19) emerged at the end of 2019, it rapidly became a global pandemic, involving nearly 3 million individuals infected [1]. As a global threat to human health, the outbreak of COVID-19 is bringing public health to the forefront.
As the American COVID-19-associated hospitalization surveillance network reported, obesity is one of the most common underlying conditions in patients hospitalized with COVID-19 [2]. By analogy to H1N1 infection, which obesity predisposed to its severe pulmonary form [3], obesity might play a critical role in COVID-19 transmission and deterioration. Recently, clinical researchers discovered that for COVID-19, obesity was associated with hospital admission, severity, intensive medical intervention, and mortality [4–6]. Given the evidence supporting that obesity increases the duration of virus shedding of influenza A [7], the influence of obesity on the disease course and recovery of COVID-19, which remained to be unclear, is of importance to investigate.
The main clinical symptoms of patients with COVID-19 are nonspecific, similar to other viral infections targeting the respiratory system, included fever, dry cough, weakness, and breathing difficulty [8]. Clinical evidence marked the presence of extra-pulmonary manifestations of COVID-19, like digestive symptoms (diarrhea, nausea, and vomiting) [9]. Notably, abnormal liver function (ALF) has been reported in 16.1–76.3% of patients with COVID-19 [10]. Liver damage in patients with coronavirus infections might be directly caused by the viral infection of liver cells. Previously, liver damage has been reported as an important risk factor for severe outcome and death in severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus [11]. Similarly, recent clinical studies have reported that severity of COVID-19 was associated with the incidence of liver injury [12], suggesting clinically significant liver dysfunction, an important characteristic of coronavirus infections, may be able to predict the prognosis of COVID-19. However, there are limited studies exploring the effect of existing liver-related comorbidities on the disease course and recovery of COVID-19 [12].
According to the report from the Chinese Center for Disease Control and Prevention, 81% of confirmed cases were classified as mild in spectrum of COVID-19 [13]. The disease course and recovery of this part of patients determined the health and economic burden COVID-19 caused, which could be reflected by the hospitalized duration to some extent. Therefore, enrolling patients with mild disease of COVID-19, the present study conducted a clinical epidemiological analysis to investigate the association of overweight/obesity and ALF with hospitalized duration to provide clinical evidence for prevention and treatment of COVID-19.
Subjects and methods
Study design and participants
The present single-centered, retrospective study was conducted in The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University. Data were derived from 58 confirmed patients who were admitted to the hospital between February 5, 2020 and February 20, 2020 (six patients of them lack the data of height). All of the patients were diagnosed as mild COVID-19 according to the standards for “Diagnosis and Treatment Scheme of New Coronavirus Infected Pneumonia” (trial version 6) [14]. The criteria of hospital discharge were as followings: (1) body temperature was kept normal for more than 3 consecutive days; (2) significant reduction of respiratory symptoms; (3) substantial improvement determined by conventional chest radiography detection; (4) at least two consecutively negative results of RT-PCR testing separated by an interval of ≥24 h. Prolonged hospitalization was defined as lasting more than the median value of the hospitalized days (19 days) in this population (discharging from hospital in time as ≤19 days).
This study was approved by the Institutional Review Board of The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University. Due to the retrospective nature of the study, informed consent was waived.
Anthropometric and laboratory measurements
Every patient underwent a physical examination after admission, which included anthropometric measurements (body height and weight) and blood pressure (BP). Body height and weight were used to calculate body mass index (BMI) as follows: BMI = weight (kg)/height2 (m2). Systolic BP (SBP) and diastolic BP (DBP) were assessed with a mercury sphygmomanometer in triplicate with a 3-min interval after 10 min of rest. Overweight/obesity were defined as BMI ≥ 24 kg/m2 [15].
Laboratory measurements were performed standardly. Venous blood samples were harvested from all confirmed patients after an overnight fast. All laboratory data were obtained from the first serum collection during hospitalization. The peripheral absolute value of white blood cell, including neutrophils, monocytes, and lymphocyte, fasting plasma glucose (FPG), alanine aminotransferase (ALT), albumin (Alb), serum creatinine (Scr), blood urea nitrogen (BUN), and C-reactive protein (CRP) were measured using standard methods. The present study defined ALF as ALT more than the upper limit of normal value (40 U/L).
Statistical analysis
The statistical analyses were performed with the statistical software package version 16.0 (SPSS Inc., Chicago, IL, USA). The normality of data distribution was accessed by the one-sample Kolmogorov–Smirnov test. Continuous variables were expressed as mean ± standard deviation or median with interquartile range according to normal or skewed distribution. Intergroup comparisons of normally and skewed distributed data were carried out by the unpaired student’s t test and Mann–Whitney U test, respectively. The chi-square test was used for intergroup comparisons of categorical variables. Kaplan–Meier curves reflected and compared the hospitalized duration and possibility to be discharged from hospital in time between different groups. Cox regression analysis identified the independent factors associated with being discharged from hospital in time. All reported P values were two-tailed, and P < 0.05 was considered statistically significant.
Results
Clinical characteristics of the study participants
A total of 58 participants with an average age of 49.2 ± 13.1 years (age range: 20–77 years) were enrolled in present study, including 36 men (62.1%) and 22 women (37.9%). Compared with men, women showed lower levels of peripheral absolute value of monocytes and Scr, as well as a shorter hospitalized duration (all P < 0.05). There was no gender difference in other variables (Table 1).
Table 1.
Characteristics of the study participants.
| Variable | Total N = 58 | Men N = 36 | Women N = 22 |
|---|---|---|---|
| Age (years) | 49.2 ± 13.1 | 48.7 ± 14.4 | 50.0 ± 10.9 |
| BMI (kg/m2) | 23.3 ± 3.1 | 23.8 ± 2.8 | 22.6 ± 3.4 |
| SBP (mmHg) | 129.8 ± 14.8 | 132.7 ± 13.4 | 125.1 ± 16.2 |
| DBP (mmHg) | 83.9 ± 12.5 | 86.0 ± 12.6 | 80.6 ± 11.8 |
| WBC (×109/L) | 4.8 ± 1.2 | 5.0 ± 1.0 | 4.5 ± 1.4 |
| Neutrophils | 3.0 ± 1.0 | 3.1 ± 0.9 | 2.8 ± 1.1 |
| Monocytes | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1* |
| Lymphocyte | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.5 |
| FPG (mmol/L) | 5.2 (4.8–6.0) | 5.3 (4.7–6.6) | 5.1 (4.8–5.8) |
| Alb (g/L) | 38.8 ± 3.5 | 39.0 ± 3.8 | 38.6 ± 2.9 |
| ALT (U/L) | 35.9 ± 18.0 | 38.5 ± 14.7 | 31.6 ± 22.2 |
| Scr (μmol/L) | 66.7 ± 18.2 | 74.0 ± 16.7 | 66.7 ± 18.2* |
| BUN (mmol/L) | 4.1 ± 1.4 | 4.3 ± 1.2 | 3.7 ± 1.7 |
| CRP (mg/L) | 8.9 (3.3–36.6) | 9.9 (2.8–36.8) | 5.1 (3.3–28.7) |
| Hospitalized duration (days) | 19.2 ± 5.5 | 20.2 ± 6.1 | 17.5 ± 3.6* |
Data are expressed as means ± SD and median (interquartile range).
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, WBC white blood cell, FPG fasting plasma glucose, Alb albumin, ALT alanine aminotransferase, Scr serum creatinine, BUN blood urea nitrogen (BUN), CRP C-reactive protein.
*P < 0.05 versus men.
Comparison of hospitalized duration between different groups based on BMI and ALT
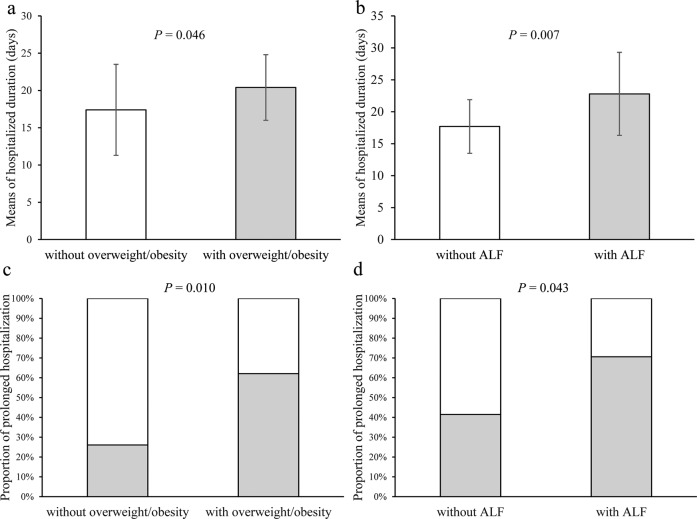
Based on BMI and ALT, patients were divided into those with or without overweight/obesity, and those with or without ALF. Twenty-nine (55.8%) patients were overweight/obesity and 17(29.3%) patients had ALF. Compared with those without overweight/obesity, patients with overweight/obesity exhibits longer hospitalized duration (17.4 ± 6.1 versus 20.4 ± 4.4 days, P = 0.046) and higher proportion of prolonged hospitalization (26.1% versus 62.1%, P = 0.010) (Fig. 1a, c). In addition, patients with ALF experienced longer hospitalized duration (22.8 ± 6.5 versus 17.7 ± 4.2 days, P = 0.007) and had higher proportion of prolonged hospitalization (70.6% versus 41.5%, P = 0.043) than those without ALF (Fig. 1b, d).
Fig. 1. Comparison of hospitalized duration between different groups based on BMI and ALT.
a Comparison of hospitalized duration between patients with and without overweight/obesity; b comparison of hospitalized duration between patients with and without ALF; c comparison of proportion of hospitalized duration between patients with and without overweight/obesity; d comparison of proportion of hospitalized duration between patients with and without ALF.
Kaplan–Meier analyses of hospitalized duration
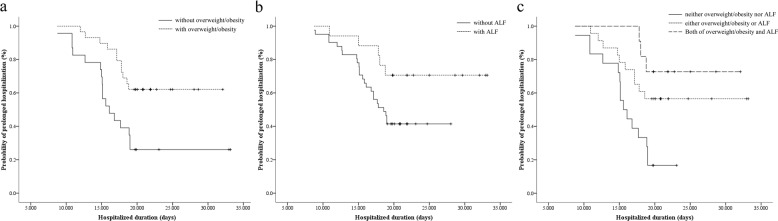
Kaplan–Meier analysis showed patients with overweight/obesity and ALF were more likely to be hospitalized longer compared with those without overweight/obesity (mean with 95% confidence interval [CI]: 26.1 [23.3–29.0] versus 19.6 [16.2–23.1], P = 0.004) (Fig. 2a), and those without ALF (mean with 95% CI: 28.1 [24.4–31.9] versus 20.4 [18.4–22.5], P = 0.046) (Fig. 2b), respectively. Further dividing the patients based on the combination of BMI and ALT, Kaplan–Meier analysis revealed that the hospitalized duration was increased from the patients with neither overweight/obesity nor ALF to those with either overweight/obesity or ALF, and to those with both of overweight/obesity and ALF (mean with 95% CI: 16.4 [14.5–18.3] versus 25.3 [21.6–29.1] versus 28.3 [24.6–32.0], P for trend = 0.001) (Fig. 2c).
Fig. 2. Kaplan–Meier analyses of hospitalized duration.
a Comparison between patients with and without overweight/obesity, P = 0.004; b comparison between patients with and without ALF, P = 0.046; c comparison between patients with neither overweight/obesity nor ALF, either overweight/obesity or ALF, and both of overweight/obesity and ALF, P for trend = 0.001.
COX regression analyses of hospitalization
Defining being discharged from hospital in time as outcome, COX regression analysis showed that BMI (hazard ratio [HR] = 0.83, P for trend = 0.001) and ALT (HR = 0.96, P for trend = 0.005) were inversely associated with being discharged from hospital in time, respectively. After adjustment of age, gender, SBP, DBP, peripheral absolute value of neutrophils, monocytes, and lymphocyte, FPG, Alb, Scr, BUN, and CRP, BMI (HR = 0.75, P for trend = 0.002) and ALT (HR = 0.95, P for trend = 0.007) remained to be independently and inversely associated with being discharged from hospital in time (Table 2).
Table 2.
COX regression analyses of hospitalization.
| Model | Independent variables | Being discharged from hospital in time | ||
|---|---|---|---|---|
| HR | 95% CI | P | ||
| Model 1 | BMI | 0.83 | 0.74–0.92 | 0.001 |
| ALT | 0.96 | 0.94–0.99 | 0.005 | |
| Model 2 | BMI | 0.75 | 0.63–0.90 | 0.002 |
| ALT | 0.95 | 0.92–0.99 | 0.007 | |
Model 1: unadjusted.
Model 2: adjusted for age, gender, SBP, DBP, peripheral absolute value of neutrophils, monocytes, and lymphocyte, FPG, Alb, Scr, BUN, and CRP.
Discussion
The present study discovered that overweight/obesity and ALF predisposed patients with COVID-19 to prolonged hospitalization. BMI and ALT were independently and inversely associated with being discharged from hospital in time for these patients.
Based on the data from a large academic hospital system in New York City, American researchers found that in young and middle-aged patients with COVID-19, obesity appeared to be a potential risk factor for hospital admission and need for acute or critical care [4]. A retrospective cohort study conducted in a single French center revealed that the frequency of obesity was relatively high in patients with severe degree of COVID-19 (presence of severe acute respiratory syndrome). The requirement for invasive mechanical ventilation was associated with severe obesity. The disease was more severe with an increase in BMI, reaching maximal in patients with severe obesity [5]. A Spanish study also reported that among patients with COVID-19 admitted to intensive care unit (ICU), obesity was the most common comorbidities [16]. However, there is absence of data about the association of obesity and the disease course of COVID-19 in Chinese population, which might attribute to the lower prevalence of obesity in China than that in American and European regions [17]. In addition, the existing studies above were more concerned on the patients with obesity and severe degree of COVID-19. In consideration of the rapid increase in the prevalence of overweight and obesity worldwide, which doubled since 1980 to an extent that nearly one third of the global population is classified to be overweight or obese now [17], the role of overweight/obesity in the COVID-19 epidemic must not be ignored in patients with mild degree of COVID-19, who were the majority of infected population. Consistent with the previous findings in American and European patients, the present study uncovered that patients with overweight/obesity were more likely to be hospitalized longer, and lower level of BMI contributed to being discharged from hospital in time.
ALF has been observed in some published studies. Chinese researchers analyzed clinical characteristics of COVID-19 among the 1099 patients from 552 hospitals in 30 provinces, autonomous regions, and municipalities in mainland China and found elevated levels of ALT in 120 (19.8%) of patients with non-severe disease and 38 (28.1%) of 135 patients with severe disease. Furthermore, more patients with elevated ALT reached the primary composite end point, including admission to an ICU, the use of mechanical ventilation, or death [18]. Later, a study from south of China also reported a high prevalence of ALF (76.3%) in patients with COVID-19, and ALF became more pronounced during hospitalization within 2 weeks. Patients with ALF had higher risk to progress to severe disease [10]. Nevertheless, a study conducted in 52 critically ill adult patients did not find difference in the incidences of ALF between survivors (30%) and non-survivors (28%) [19]. Focusing on patients with mild disease, the present study revealed that ALF was associated with prolonged hospitalization, and ALT was a negative factor associated with being discharged from hospital in time.
Because the patients with mild disease are the majority of the patients with COVID-19 [13]. The disease course and recovery of this part of patients determined the health and economic burden and the recovery of public health system and development of society. Patients were allowed to be discharge from hospital only when they were confirmed to be not contagious [14]. Hence, the hospitalized duration could reflect not only disease course and recovery, but also the transmission of COVID-19 to some extent. Therefore, the present study findings suggested that the increasing fat accumulation (even not reaching the extent of obesity) and liver dysfunction might be involved in disease course, especially recovery, and transmission of COVID-19.
The underlying mechanism of the association of COVID-19 with overweight/obesity and ALF was unclear. With high affinity for COVID-19, human angiotensin converting enzyme 2 (ACE2), which was supposed to mediate the entry of COVID-19 into host cells [20], might be one of the common explanations. The expression of ACE2 is enriched in adipocyte [21] and cholangiocytes [22]. With large amounts of ACE2-expressing cells, people with overweight/obesity will be more vulnerable to COVID-19 and more of its pathogen, and thus they might be easy to spread the disease and hard to recover. In addition, the virus might be able to directly bind to ACE2-positive cholangiocytes, inducing systemic inflammation, and finally leading to ALF [22].
The present study has some limitations. First, this study was retrospective, and some cases had incomplete documentation for the history of present illness and the clinical data. Second, the present study did not take into consideration the medical intervention. The influence of medicine on ALF. Third, lack of the data of other indicators of liver function, such as aspartate aminotransferase and gamma-glutamyltransferase, the prevalence of ALF might be underestimated. Forth, as patients of this study were from a southeastern city in China, these findings cannot be generalized to other regions of varying epidemiological characteristics.
In conclusion, overweight/obesity and liver dysfunction contributed to prolonged hospitalization. With high risk of COVID-19, individuals with overweight/obesity and existing liver diseases required close monitoring and prevention. And for patients with COVID-19, even when the disease is mild, extra attentions and precautions should be paid for those with overweight/obesity and ALF during clinical treatments.
Funding
This research was funded by grant from National Natural Science Foundation of China, grant number 81900737; Wenzhou Science and Technology Bureau, grant number Y20190126.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiang Hu, Xiaoqiong Pan
Contributor Information
Bo Yang, Email: yb@wmu.edu.cn.
Zhen Hu, Email: huzhen998@sohu.com.
References
- 1.Johns Hopkins University & Medicine. COVID-19 map. Baltimore, MD: Johns Hopkins University; 2020. https://coronavirus.jhu.edu/map.html.
- 2.Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019-COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–64. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8:e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020. 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed]
- 5.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. Lille Intensive Care COVID-19 and Obesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020. 10.1002/oby.22831. [DOI] [PMC free article] [PubMed]
- 6.Dietz W, Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity. 2020;28:1005. 10.1002/oby.22818. [DOI] [PubMed]
- 7.Maier HE, Lopez R, Sanchez N, Ng S, Gresh L, Ojeda S, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis. 2018;218:1378–82. doi: 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68:2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han C, Duan C, Zhang S, Spiegel B, Shi H, Wang W. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115:916–23. doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: Abnormal Liver Function Tests. J Hepatol. 2020. 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed]
- 11.Parohan M, Yaghoubi S, Seraj A. Liver injury is associated with severe Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of retrospective studies. Hepatol Res. 2020. 10.1111/hepr.13510. [DOI] [PMC free article] [PubMed]
- 12.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–6. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. 10.1001/jama.2020.2648. [DOI] [PubMed]
- 14.China NHCo. Diagnosis and treatment scheme of new coronavirus infected pneumonia. 2020. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml?security_session_verify=ee9c2d723cdbcd6a4b2c0d881dcfcb2c.
- 15.Obesity group of Chinese Society of Endocrinology. Expert consensus on prevention and treatment of obesity in Chinese adults [Chinese] Chin J Endocrinol Metab. 2011;27:711–7. [Google Scholar]
- 16.Barrasa H, Rello J, Tejada S, Martín A, Balziskueta G, Vinuesa C, et al. SARS-Cov-2 in Spanish Intensive Care: early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. 2020. 10.1016/j.accpm.2020.04.001. [DOI] [PMC free article] [PubMed]
- 17.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinicalcharacteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet. Lancet Respir Med. 2020;8:475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia X, Yin C, Lu S, Chen Y, Liu Q, Bai J, et al. Two things about COVID-19 might need attention. Preprints. 2020:2020020315. 10.20944/preprints202002.0315.v1.
- 22.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020. 10.1101/2020.02.03.931766.