Abstract
Background: There are distinct differences specific to gender in the susceptibility, prevalence, and progression of kidney injuries. We aimed to investigate whether there is a correlation between acute kidney injury (AKI) developing in patients monitored in the intensive care unit (ICU) with regards to gender.
Methods: The current study retrospectively screened the electronic records of patients monitored in the adult ICU between 2015 and 2018. The patients’ age, gender, duration of their stay in the ICU, mortality rate, and need for hemodialysis were recorded and analyzed. The diagnosis of AKI was defined according to the Acute Kidney Injury Network (AKIN) criteria. Patients with AKIN stage 2 and stage 3 were accepted as having an AKI. Patients were separated into two groups: those who developed an AKI and those who did not. The patients were classified into age groups: those aged 18–65 years and those older than 65 years. The demographic data and gender distribution of the groups were then compared.
Results: Of the patients who developed AKI, the mean age (p =0.0001), the number of days they stayed at the ICU (p =0.006), the mortality rate (p =0.0001), and the need for hemodialysis were significantly higher than the non-AKI group. There was no statistically significant difference between the groups with regards to gender distribution (p =0.612).
Acute kidney injury was found to be statistically significantly higher in both the male and female groups over 65 years when compared to the group aged 18–65-years (male p =0.004, female p =0.002, respectively).
Conclusions: When surveying the complete patient sample, AKI in the ICU was more prevalent in adult males under 65 than their female counterparts. However, there were more incidences of AKI in women over 65 than in men over 65 years. This may be due to structural changes and comorbidities in the kidney due to advanced age, as well as a decrease in estrogen levels. HIPPOKRATIA 2019, 23(3): 126-130.
Keywords: Acute kidney injury, estrogen, androgen, gender, intensive care unit
Introduction
Studies on both humans and animals have revealed distinct differences specific to gender in the susceptibility, prevalence, and progression of kidney diseases1,2. These gender differences are observed in both acute and chronic kidney diseases like membranous nephropathy and immunoglobulin, nephropathic, and polycystic kidney diseases, respectively. There are differences in glomerular structure linked to gender3. Additionally, sex hormones have significant effects on the renin-angiotensin system. These effects may cause changes in the functions and structure of the kidneys2,4. Estrogens may affect many processes included in the pathogenesis of kidney disease, like cell proliferation, as well as collagen and proteoglycan synthesis.
Estrogens may regulate the synthesis and secretion of vasoactive agents, cytokines, and other growth factors by indirect routes. This situation may change collagen destruction modification and mesenchymal cell functions. Estrogens may regulate genes with a role in extracellular matrix metabolism by a mechanism linked to the receptor5,6. Additionally, estrogens increase antioxidant and nitric oxide synthase enzyme activity. All these mechanisms contribute to a protective effect in the progression of kidney diseases in the female5,7. There is only one study that has investigated the correlation of acute kidney injury (AKI) regarding gender among hospital patients8. However, there is no study examining whether there is a difference in AKI in terms of gender among patients monitored in the intensive care unit (ICU). In the current study, we aimed to investigate whether there is a correlation between AKI development and gender in patients monitored in the ICU.
Methods
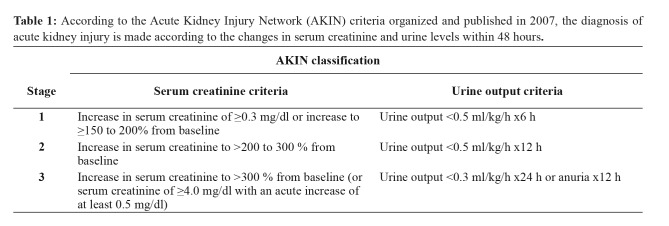
We initially obtained permission from the Non-interventional Ethics Committee of Bezmialem Vakıf University Medical Faculty (decision No: 23/282, date: 18/12/2018). The study was performed retrospectively on retrieved electronic patient records from 1,989 patients who were followed in Bezmialem Vakıf University Medical Faculty adult ICU between 01/01/2015 and 01/10/2018. The exclusion criteria were i) patients under the age of 18, ii) patients with a diagnosis of chronic renal failure, iii) patients with a high creatinine value on admission, and iv) postoperative outpatients. We examined the records of all patients who did not fall into the exclusion criteria and who had had their creatinine serum values measured. We then studied the patients’ ages, gender, duration of stay in the ICU, mortality rate, and need for hemodialysis. An AKI diagnosis was made according to the Acute Kidney Injury Network (AKIN) criteria. The AKIN criteria were organized and published by the AKIN working group in 2007. A diagnosis of AKI is made according to the changes in the serum creatinine and urine levels within 48 hours (Table 1)9,10. According to the AKIN criteria, patients in stage 2 and stage 3 were accepted as having an AKI. Patients were classified into two groups: those developing an AKI and those not developing an AKI, and according to age to those aged 18–65 years and those older than 65 years. The demographic data and gender distribution of the groups were compared.
Table 1. According to the Acute Kidney Injury Network (AKIN) criteria organized and published in 2007, the diagnosis of acute kidney injury is made according to the changes in serum creatinine and urine levels within 48 hours.
Statistical Analysis
The statistical analyses in this study were performed with NCSS (Number Cruncher Statistical System) 2007 Statistical Software (Utah, USA). In the evaluation of data, besides the descriptive statistical methods (mean, standard deviation, median, interquartile range), other methods used include the Shapiro-Wilk normality test for the distribution of variables, an independent t-test for the comparison of two groups with normal distribution variables, the Mann-Whitney U test to compare two groups with non-normally distributed variables. A Chi-square test was used to compare qualitative data. Results were assessed at a significance level of p <0.05.
Results
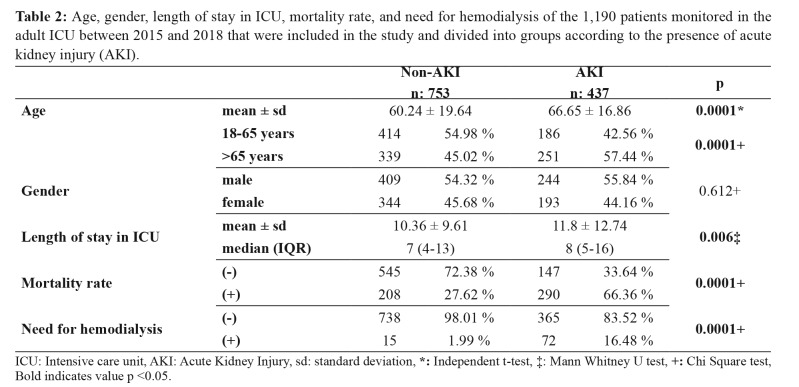
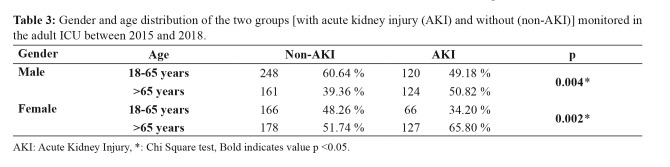
A total of 1,190 patients who met our criteria were included in the study. Of these patients, 653 were female, and 527 were male (Figure 1). The AKI group had a significantly higher mean age than the non-AKI group (p =0.0001). The number of patients over 65 years of age in the AKI group was significantly higher than in the non-AKI group (p =0.0001). However, no statistically significant difference was observed in the gender distributions between the groups (p =0.612). The patients who had AKI in the ICU spent significantly more time in the hospital than the non-AKI group (p =0.006). The mortality rate was significantly higher in the AKI group than in the non-AKI group (p =0.0001). The need for hemodialysis was also significantly higher in the AKI group compared to the non-AKI group (p =0.0001) (Table 2). Both males and females over 65 years of age had significantly higher rates of acute kidney injury than in the group aged 18–65-year (male p =0.004, female p =0.002, respectively) (Table 3).
Figure 1. Patient selection algorithm of the 1,190 patients monitored in the adult ICU between 2015 and 2018 who met the inclusion and exclusion criteria and were included in the current study. ICU: Intensive care unit, AKI: Acute kidney injury.
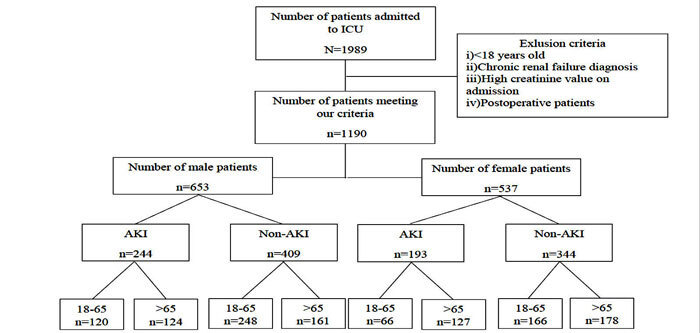
Table 2. Age, gender, length of stay in ICU, mortality rate, and need for hemodialysis of the 1,190 patients monitored in the adult ICU between 2015 and 2018 that were included in the study and divided into groups according to the presence of acute kidney injury (AKI).
ICU: Intensive care unit, AKI: Acute Kidney Injury, sd: standard deviation, *: Independent t-test, ‡: Mann Whitney U test, +: Chi Square test, Bold indicates value p <0.05.
Table 3. Gender and age distribution of the two groups [with acute kidney injury (AKI) and without (non-AKI)] monitored in the adult ICU between 2015 and 2018.
AKI: Acute Kidney Injury, *: Chi Square test, Bold indicates value p <0.05.
Discussion
Acute kidney injury is a complicated medical condition that affects the outcome of patients in ICU9. The development of AKI prolongs the duration of ICU stay and significantly increases the morbidity and mortality of these patients in both the short- and long-term10-12. This study’s results align with previous findings that the mortality rate is higher and the length of stay at the ICU longer in the group of patients who developed AKI. Prevention of AKI and the determination of risk factors are crucial for reducing morbidity and mortality. Age and gender are among the factors affecting the occurrence of AKI10,13. Recently, epidemiologic studies have shown that the presence of kidney disease is higher and progresses more rapidly in males7. Androgens are a risk factor for glomerular injury. They contribute to the disruption of hemodynamics and the progression of damage in the kidneys. Experimental animal models have confirmed that androgens are important for the greater extend of renal injury in the male sex3,7. Tunicamycin, which is an agent contributing to the endoplasmic reticulum stress in male rats, induces apoptosis more rapidly as measured with Bax and caspase 3 activation. In female rats administered with testosterone before the tunicamycin, induction of endoplasmic reticulum stress markers occurred with regression of tissue morphology and renal functions, similar to male rats9,14.
Neugarten et al collected all previously-published gender-distributed AKI data focused on patients who were monitored in hospital and concluded that the incidence of AKI developing in the males of this sample was higher than that of the females8. Chung et al15 also supported this conclusion. However, the Kidney Disease Improving Global outcomes (KDIGO) guidelines, used to predict AKI, state that females are susceptible to the development of hospital-sourced AKI16 In our study, we found that more males developed AKI in the ICU. Of the 437 patients who developed an AKI, 55.8 % (n =244) were male and 44.2 % (n =193) were female. While this difference was not statistically significant when our patients were classified by age, there was a higher difference between genders in the aged 18–65 years group, with 120 males and 66 females developing an AKI. In the group of patients over 65 years of age, 124 male patients and 127 female patients developed an AKI. Structural and physiologic changes occur in the kidneys as people age. Nephrons’ mass reduces, and vascular and glomerular degeneration develops at a microscopic level. The glomerular filtration rate reduces, as the tendency toward cellular apoptosis increases. All these changes explain the increasing occurrence of AKI as patients age17.
Moreover, increased comorbid factors and the severity of diseases present in older patients also contribute to an increase in the incidence of AKI9. Kolhe et al18 investigated factors that contribute to instances of AKI by following patients through 15 years, checking in with them once every five years. They found that the average age for AKI increased from 59.7 to 65.1 years and that the probability of developing an AKI in the male population was significantly higher in all three periods. Even during the different phases of the menstrual cycle, high estrogen levels show proliferative and antiapoptotic effects on proximal tubular cells, and short-term treatment with estrogen is known to have protective and therapeutic renal effects7. The protective effect of estrogen is limited in premenopausal females, and it is emphasized that the protective effect is lost with advancing age and menopause19,20. Our study found that younger women were significantly less likely to develop an AKI than women over 65, which confirms the previous statement regarding estrogen.
In animal models and postmenopausal women, it is known that selective estrogen receptor modulators (raloxifene) and material mimicking most of the effects of estrogen may improve the progression of kidney disease7,21. Female rats tolerate ischemia-reperfusion injuries better than male rats, and it was shown that administering additional estrogen before such injuries protected female rats even more7. After an ischemic injury, female rats had better renal histology, lower apoptosis caspase activation, and much better survival compared to male rats3,22. However, contrary to all the data collected from animal models, females are reported to be at a higher risk for AKI after medical procedures such as cardiac surgery, aminoglycoside nephrotoxicity, and contrast nephropathy8. Female rats are more susceptible to cisplatin-sourced AKI and were observed to develop a more severe necrotic tubular injury. In nephrotoxic models, the survival of male rats is accepted as better than female rats22,23.
Limitations
In our study, included patients were heterogeneous. We faced some limitations since we only had retrospectively access to data provided to us by the electronic records. This meant that we could not access the severity scores, comorbid pathologies, and nephrotoxic medications regarding all patients. We could not obtain data about the cause of AKI development in these patients and could not discuss this with the patients themselves. Another limitation is that the data was collected from a single hospital. We acknowledge that prospective studies investigating gender differences in AKI should be performed with larger sample sizes and at the cellular and molecular levels in order to guide us further regarding the prevention and treatment of this condition.
Conclusions
In our patient sample, AKI in the ICU was more prevalent in adult males under 65 years of age than their female counterparts. However, we found more AKI in women over 65 years than in men over 65 years of age. It appears that there is a less significant difference between genders in older populations. This may be due to structural changes and comorbidities in the kidney due to advanced age, as well as a decrease in estrogen levels.
Conflict of interest
Authors have no conflicts of interest to disclose. This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Acknowledgement
The study was presented at the 22nd International Intensive Care Symposium, 2019, Istanbul.
References
- 1.Seppi T, Prajczer S, Dörler MM, Eiter O, Hekl D, Nevinny-Stickel M, et al. Sex Differences in Renal Proximal Tubular Cell Homeostasis. J Am Soc Nephrol. 2016;27:3051–3062. doi: 10.1681/ASN.2015080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki H. [Differences between men and women with chronic kidney disease] Nihon Rinsho. 2015;73 Suppl 9:629–633. [PubMed] [Google Scholar]
- 3.Silbiger SR, Neugarten J. The role of gender in the progression of renal disease. Adv Ren Replace Ther. 2003;10:3–14. doi: 10.1053/jarr.2003.50001. [DOI] [PubMed] [Google Scholar]
- 4.Haghighi M, Nematbakhsh M, Talebi A, Nasri H, Ashrafi F, Roshanaei K, et al. The role of angiotensin II receptor 1 (AT1) blockade in cisplatin-induced nephrotoxicity in rats: gender-related differences. Ren Fail. 2012;34:1046–1051. doi: 10.3109/0886022X.2012.700886. [DOI] [PubMed] [Google Scholar]
- 5.Eshraghi-Jazi F, Nematbakhsh M, Nasri H, Talebi A, Haghighi M, Pezeshki Z, et al. The protective role of endogenous nitric oxide donor (L-arginine) in cisplatin-induced nephrotoxicity: Gender related differences in rat model. J Res Med Sci. 2011;16:1389–1396. [PMC free article] [PubMed] [Google Scholar]
- 6.Neugarten J, Silbiger SR. Effects of sex hormones on mesangial cells. Am J Kidney Dis. 1995;26:147–151. doi: 10.1016/0272-6386(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 7.Neugarten J, Golestaneh L. Gender and the prevalence and progression of renal disease. Adv Chronic Kidney Dis. 2013;20:390–395. doi: 10.1053/j.ackd.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Neugarten J, Golestaneh L, Kolhe NV. Sex differences in acute kidney injury requiring dialysis. BMC Nephrol. 2018;19:131. doi: 10.1186/s12882-018-0937-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamerio J, Agapito Fonseca J, Jorge S, Lopes JA. Acute Kidney Injury Definition and Diagnosis: A Narrative Review. J Clin Med. 2018;7:307. doi: 10.3390/jcm7100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amini S, Najafi MN, Karrari SP, Mashhadi ME, Mirzaei S, Tashnizi MA, et al. Risk Factors and Outcome of Acute Kidney Injury After Isolated CABG Surgery: A Prospective Cohort Study. Braz J Cardiovasc Surg. 2019;34:70–75. doi: 10.21470/1678-9741-2017-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber W, Schneider J, Lahmer T, Küchle C, Jungwirth B, Schmid RM, et al. Validation of RIFLE, AKIN, and a modified AKIN definition (“backward classification”) of acute kidney injury in a general ICU: Analysis of a 1-year period. Medicine (Baltimore) 2018;97:e12465. doi: 10.1097/MD.0000000000012465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koeze J, Keus F, Dieperink W, van der Horst IC, Zijlstra JG, van Meurs M. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol. 2017;18:70. doi: 10.1186/s12882-017-0487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin SR, Kim WH, Kim DJ, Shin IW, Sohn JT. Prediction and Prevention of Acute Kidney Injury After Cardiac Surgery. Biomed Res Int. 2016;2016:2985148. doi: 10.1155/2016/2985148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodeify R, Megyesi J, Tarcsafalvi A, Mustafa HI, Hti Lar Seng NS, Price PM. Gender differences control the susceptibility to ER stress-induced acute kidney injury. Am J Physiol Renal Physiol. 2013;304:F875–F882. doi: 10.1152/ajprenal.00590.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung S, Brovman EY, Aglio LS, Beutler SS, Urman RD. Risk factors and associated complications of acute kidney injury in adult patients undergoing a craniotomy. Clin Neurol Neurosurg. 2020;190:105642. doi: 10.1016/j.clineuro.2019.105642. [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. Available at: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf, date accessed: 30/6/2019. [Google Scholar]
- 17.Chao CT, Lin YF, Tsai HB, Wu VC, Ko WJ. Acute kidney injury network staging in geriatric postoperative acute kidney injury patients: shortcomings and improvements. J Am Coll Surg. 2013;217:240–250. doi: 10.1016/j.jamcollsurg.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Kolhe NV, Muirhead AW, Wilkes SR, Fluck RJ, Taal MW. National trends in acute kidney injury requiring dialysis in England between 1998 and 2013. Kidney Int. 2015;88:1161–1169. doi: 10.1038/ki.2015.234. [DOI] [PubMed] [Google Scholar]
- 19.Eshraghi-Jazi F, Nematbakhsh M, Pezeshki Z, Nasri H, Talebi A, Safari T, et al. Sex differences in protective effect of recombinant human erythropoietin against cisplatin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2013;7:383–389. [PubMed] [Google Scholar]
- 20.Seliger SL, Davis C, Stehman-Breen C. Gender and the progression of renal disease. Curr Opin Nephrol Hypertens. 2001;10:219–225. doi: 10.1097/00041552-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Minutolo R, Gabbai FB, Chiodini P, Provenzano M, Borrelli S, Garofalo C, et al. Sex Differences in the Progression of CKD Among Older Patients: Pooled Analysis of 4 Cohort Studies. Am J Kidney Dis. 2020;75:30–38. doi: 10.1053/j.ajkd.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Wei Q, Wang MH, Dong Z. Differential gender differences in ischemic and nephrotoxic acute renal failure. Am J Nephrol. 2005;25:491–499. doi: 10.1159/000088171. [DOI] [PubMed] [Google Scholar]
- 23.Hu H, Wang G, Batteux F, Nicco C. Gender differences in the susceptibility to renal ischemia-reperfusion injury in BALB/c mice. Tohoku J Exp Med. 2009;218:325–329. doi: 10.1620/tjem.218.325. [DOI] [PubMed] [Google Scholar]