Abstract
Background
Early studies suggest that coronavirus disease 2019 (COVID-19) is associated with a high incidence of cardiac arrhythmias. Severe acute respiratory syndrome coronavirus 2 infection may cause injury to cardiac myocytes and increase arrhythmia risk.
Objectives
The purpose of this study was to evaluate the risk of cardiac arrest and arrhythmias including incident atrial fibrillation (AF), bradyarrhythmias, and nonsustained ventricular tachycardia (NSVT) in a large urban population hospitalized for COVID-19. We also evaluated correlations between the presence of these arrhythmias and mortality.
Methods
We reviewed the characteristics of all patients with COVID-19 admitted to our center over a 9-week period. Throughout hospitalization, we evaluated the incidence of cardiac arrests, arrhythmias, and inpatient mortality. We also used logistic regression to evaluate age, sex, race, body mass index, prevalent cardiovascular disease, diabetes, hypertension, chronic kidney disease, and intensive care unit (ICU) status as potential risk factors for each arrhythmia.
Results
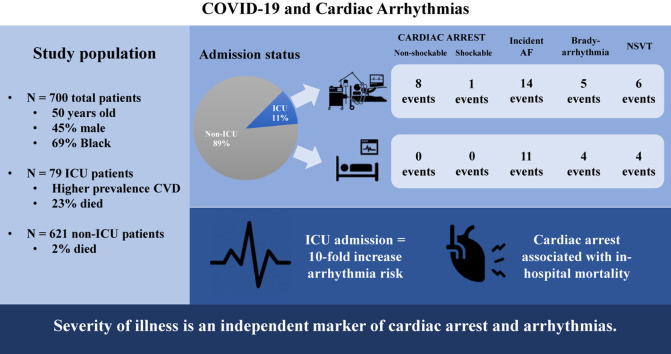
Among 700 patients (mean age 50 ± 18 years; 45% men; 71% African American; 11% received ICU care), there were 9 cardiac arrests, 25 incident AF events, 9 clinically significant bradyarrhythmias, and 10 NSVTs. All cardiac arrests occurred in patients admitted to the ICU. In addition, admission to the ICU was associated with incident AF (odds ratio [OR] 4.68; 95% confidence interval [CI] 1.66–13.18) and NSVT (OR 8.92; 95% CI 1.73–46.06) after multivariable adjustment. Also, age and incident AF (OR 1.05; 95% CI 1.02–1.09) and prevalent heart failure and bradyarrhythmias (OR 9.75; 95% CI 1.95–48.65) were independently associated. Only cardiac arrests were associated with acute in-hospital mortality.
Conclusion
Cardiac arrests and arrhythmias are likely the consequence of systemic illness and not solely the direct effects of COVID-19 infection.
Keywords: Atrial fibrillation, Arrhythmia, Cardiac arrest, COVID-19, Mortality, Nonsustained ventricular tachycardia
Graphical abstract
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emerged as a global pandemic, infecting more than 1 million patients in the United States as of early May 2020.1 Early reports from China suggested an overall cardiac arrhythmia incidence of 17% in patients hospitalized for COVID-19. A higher arrhythmia rate (44%) was observed in patients with COVID-19 admitted to the intensive care unit (ICU).2 However, details of the type and burden of arrhythmias in this population have not been elucidated. Similarly, in another observational report from China that included 187 hospitalized patients, Guo et al3 reported an overall ventricular tachycardia (VT)/ventricular fibrillation (VF) incidence rate of 7% during hospitalization. In addition, reports from Italy and New York City have described a concomitant increase in out-of-hospital cardiac arrests that are associated with the cumulative incidence of COVID-19.4 , 5 These findings raise concerns that SARS-CoV-2 infection and secondary cardiac injury may increase the arrhythmia risk.
We sought to systematically evaluate the risk of cardiac arrest and arrhythmias including incident atrial fibrillation (AF), bradyarrhythmias, and nonsustained ventricular tachycardia (NSVT) in a large urban patient population hospitalized for COVID-19. We also evaluated correlations between the presence of these arrhythmias and acute in-hospital mortality.
Methods
Study population
Our inpatient cohort included all patients with COVID-19 admitted at the Hospital of the University of Pennsylvania between March 6, 2020, and May 19, 2020. In total, 700 patients had confirmed SARS-CoV-2 infection by polymerase chain reaction testing of a nasopharyngeal sample. The study received expedited approval by the Institutional Review Board at the University of Pennsylvania.
Covariates
We systematically evaluated all clinical records to obtain demographic characteristics and medical comorbidities. In addition, we recorded the admission profile that included vitals, laboratory tests, and antiviral medications received during hospitalization. Demographic characteristics included age, sex, and race. Comorbid conditions included any history of coronary heart disease, which is defined as coronary artery disease or myocardial infarction, heart failure, hypertension, history of AF, diabetes, obstructive sleep apnea, chronic obstructive pulmonary disease, liver disease, and chronic kidney disease (CKD). We also evaluated any procedural history of implantable cardioverter-defibrillator or permanent pacemaker placement.
Admission data included temperature, oxygen saturation on presentation, and body mass index (BMI). Basic laboratory measures included white blood cell count and electrolyte (potassium and magnesium) concentrations. In addition, we recorded troponin, B-type natriuretic peptide, D-dimer, procalcitonin, and high-sensitivity C-reactive protein concentrations.
Outcomes
We determined clinical outcomes (deceased, hospitalized, or discharged) for each patient by the time of censoring on May 24, 2020. Each patient’s medical chart was also reviewed for the presence of cardiac arrest or arrhythmias. Specifically, for all patients, we reviewed telemetry logs, nursing records, and physician notes for cardiac arrests and arrhythmias including incident AF, bradyarrhythmias, and NSVT. Patients with a history of AF were excluded from the incident AF analysis. We defined bradyarrhythmias as clinically significant episodes of bradycardia that were associated with hypotension. For these cases, we also confirmed that a medical intervention was performed. For cardiac arrests, we documented whether the initial rhythm recorded was VF, VT, sinus bradycardia, heart block, asystole, or pulseless electrical activity.
Statistical analysis
All data were included as study variables to characterize admitted patients. Baseline characteristics were compared between patients in the ICU and those admitted to a non-ICU setting by using χ2, Student t, Fisher exact, or Mann-Whitney U test. We then calculated the incidence of cardiac arrests and arrhythmic events: incident AF, bradyarrhythmias, and NSVT. We used logistic regression to evaluate the association between selected clinical characteristics including age, sex, race, BMI, history of heart failure, CHD, diabetes, hypertension, CKD, and ICU status and each arrhythmia in univariate and multivariable models. In exploratory analysis, we also used logistic regression analysis to evaluate the association between each arrhythmia type and in-hospital mortality in both unadjusted and adjusted analyses. Multivariable models adjusted for age, sex, race, BMI, history of heart failure, CHD, diabetes, hypertension, CKD, ICU status on admission, and hydroxychloroquine use. SAS version 9.4 (SAS Institute Inc, Cary, NC) was used for these analyses, and a P value of less than .05 was considered statistically significant.
Results
Our hospitalized cohort of 700 patients with COVID-19 had a mean age of 50 ± 18 years (Table 1 ); 314 (45%) were male; and 486 (69%) were African American. The majority of patients were admitted to a non-ICU setting that included cardiac telemetry. Only 79 (11%) of patients were admitted to the ICU. Compared with those admitted to a non-ICU setting, patients in the ICU were older and had a higher prevalence of cardiovascular disease, hypertension, diabetes, pulmonary disease including obstructive sleep apnea and chronic obstructive pulmonary disease, liver disease, and CKD. Patients in the ICU also had lower oxygen saturation on presentation than did those admitted to a non-ICU setting. In terms of the biomarker profile, patients in the ICU were more likely to have an elevated troponin concentration and higher concentrations of B-type natriuretic peptide, D-dimer, procalcitonin, and high-sensitivity C-reactive protein on admission than patients admitted to a non-ICU setting. Furthermore, patients in the ICU were more likely to be administered hydroxychloroquine or remdesivir than those admitted to a non-ICU setting. We did not observe any differences in sex or race according to ICU status. Only 39 (6%) of patients in our cohort had a history of AF, and 20 (3%) had a cardiac implantable electronic device that included either an implantable cardioverter-defibrillator or a permanent pacemaker. No differences were observed in any of these arrhythmic measures according to ICU status.
Table 1.
Baseline characteristics of patients with COVID-19 at the time of admission
| Characteristic | Overall | Patients in the ICU | Patients in the non-ICU ward | P∗ |
|---|---|---|---|---|
| No. of patients | 700 | 79 | 621 | – |
| Demographic characteristics | ||||
| Age (y) | 50 ± 18 | 63 ± 16 | 48 ± 18 | <.0001 |
| Male | 314 (45) | 40 (51) | 274 (44) | .27 |
| African American | 486 (69) | 51 (65) | 435 (70) | .52 |
| Comorbidities | ||||
| Coronary heart disease | 76 (11) | 21 (27) | 55 (9) | <.0001 |
| Heart failure | 88 (13) | 22 (28) | 66 (11) | <.0001 |
| Hypertension | 347 (50) | 62 (78) | 285 (46) | <.0001 |
| Atrial fibrillation history | 39 (6) | 5 (6) | 34 (5) | .79 |
| ICD/PPM | 20 (3) | 5 (6) | 15 (2) | .064 |
| Diabetes mellitus | 182 (26) | 35 (44) | 147 (24) | <.0001 |
| Obstructive sleep apnea | 124 (18) | 23 (29) | 101 (16) | .0048 |
| COPD | 63 (9) | 14 (18) | 49 (8) | .0040 |
| Liver disease | 67 (10) | 14 (18) | 53 (9) | .0089 |
| Chronic kidney disease | 80 (11) | 16 (20) | 64 (10) | .0089 |
| Current tobacco | 51 (7) | 4 (5) | 47 (8) | .49 |
| Admission profile | ||||
| Temperature (°F) | 98.6 ± 1.0 | 98.9 ± 1.6 | 98.6 ± 0.9 | .14 |
| Oxygen saturation on presentation (%) | 92.2 ± 11.7 | 89.4 ± 10.3 | 92.5 ± 12.0 | .0006 |
| BMI (kg/m2) | 31 ± 9 | 33 ± 12 | 31 ± 8 | .14 |
| Baseline laboratory values | ||||
| WBC count (cells/μL) | 7.3 ± 3.9 | 9.9 ± 6.3 | 6.9 ± 3.2 | <.0001 |
| Potassium concentration (mmol/L) | 4.1 ± 0.5 | 4.3 ± 0.8 | 4.0 ± 0.5 | .0178 |
| Magnesium concentration (mmol/L) | 1.9 ± 0.4 | 2.1 ± 0.5 | 1.9 ± 0.3 | .0992 |
| Nonelevated troponin concentration† | 291 (78) | 44 (62) | 247 (82) | .0003 |
| BNP concentration (pg/mL) | 2940 ± 7962 | 5347 ± 10381 | 2214 ± 6950 | <.0001 |
| D-dimer concentration (ng/mL) | 3.3 ± 10.9 | 7.2 ± 21.1 | 2.2 ± 5.1 | .0005 |
| Procalcitonin concentration (ng/mL) | 1.7 ± 9.9 | 2.8 ± 10.8 | 1.4 ± 9.6 | <.0001 |
| High-sensitivity CRP concentration (mg/L) | 85.3 ± 55.3 | 112.3 ± 52.1 | 75.1 ± 53.1 | <.0001 |
| Medications during hospitalization | ||||
| Hydroxychloroquine | 172 (25) | 53 (67) | 119 (19) | <.0001 |
| Remdesivir | 57 (8) | 20 (25) | 37 (6) | <.0001 |
Values are presented as mean ± SD or as n (%).
BMI = body mass index; BNP = B-type natriuretic peptide; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease 2019; CRP = C-reactive protein; ICD = implantable cardioverter-defibrillator; ICU = intensive care unit; PPM = permanent pacemaker; WBC = white blood cell.
P-value comparisons for patients in the ICU vs those in the non-ICU ward.
Nonelevated troponin concentration on admission is defined as <0.010 ng/mL. There were 373 patients, who had troponin measured at admission.
Outcomes
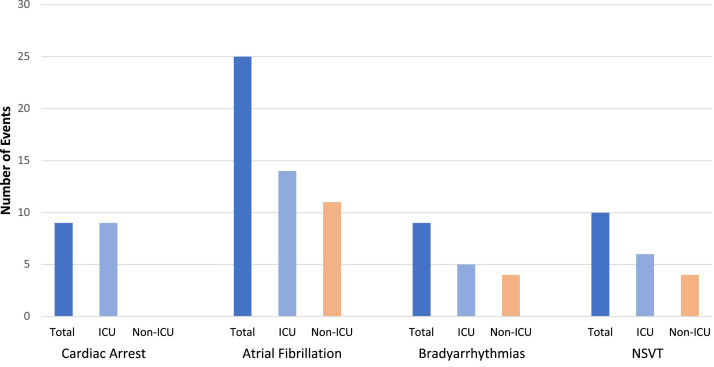
Among our cohort that included all COVID-19 inpatient admissions spanning a 74-day period, there were 30 patients (4% of the cohort) who died, 613 patients (88% of the cohort) who were discharged, and 57 (8% of the cohort) who remained hospitalized at the time of study censoring. Compared with those patients initially admitted to a non-ICU ward, patients in the ICU were more likely to die in the hospital (18 (23%) of the ICU group vs 12 (2%) of the non-ICU group; P < .001). Throughout hospitalization, there were 53 arrhythmic events. Specifically, 9 patients had a cardiac arrest, including 6 cases of pulseless electrical activity, 2 asystole events, and 1 episode of torsades de pointes (Table 2 ). In addition, there were 25 incident AF events that required pharmacological management with amiodarone and diltiazem, 9 clinically significant bradyarrhythmias, and 10 NSVT events (Figure 1 ). We did not observe any cases of heart block, sustained VT, or VF in our cohort of patients with COVID-19.
Table 2.
Characteristics of cardiac arrests in patients with COVID-19
| Patient no. | Cardiac arrest on hospital day no. | Cardiac arrest rhythm | Background/etiology | Outcome |
|---|---|---|---|---|
| 1 | 1 | Asystole | 85 yo nursing home resident presenting with respiratory distress. | ROSC; eventually WOC |
| 2 | 5 | PEA | 59 yo with a h/o systemic scleroderma and recent hospitalization for ILD presented with pneumonia and hypoxia. | ROSC; remains hospitalized |
| 3 | 2 | PEA | 35 yo who underwent elective C-section and was diagnosed with COVID-19 per routine screening. Suspected amniotic fluid embolism. | ROSC; discharged with baby |
| 4 | 18 | PEA | 41 yo with a h/o obesity, CHD, and diabetes presented with respiratory distress. | ROSC; remains hospitalized |
| 5 | 5 | PEA | 55 yo with mitral valve endocarditis and developed acute stroke. Recovering from mechanical thrombectomy and became nonresponsive. | Deceased |
| 6 | 5 | PEA | 50 yo with a h/o scleroderma after double lung transplantation 2.5 y ago presented with respiratory failure. | Deceased |
| 7 | 45 | Asystole | 74 yo presented with respiratory failure. Complicated hospitalization including multiorgan dysfunction. | Deceased |
| 8 | 1 | TdP | 42 yo presented with respiratory failure. Complicated hospitalization including left ventricular dysfunction and ECMO. | ROSC; remains hospitalized |
| 9 | 1 | PEA | 43 yo with a h/o morbid obesity presented with fever and respiratory distress. | ROSC; discharged |
CHD = coronary heart disease; COVID-19 = coronavirus disease 2019; ECMO = extracorporeal membrane oxygenation; h/o = history of; ILD = interstitial lung disease; PEA = pulseless electrical activity; ROSC = return of spontaneous circulation; TdP = torsades de pointes; WOC = withdrawal of care; yo = years old.
Figure 1.
Arrhythmic events by intensive care unit (ICU) status. The number of cardiac arrests and arrhythmias are depicted in the entire cohort of patients with coronavirus disease 2019 (dark blue), those admitted to the ICU (light blue), and those admitted to a non-ICU ward (orange). NSVT = nonsustained ventricular tachycardia.
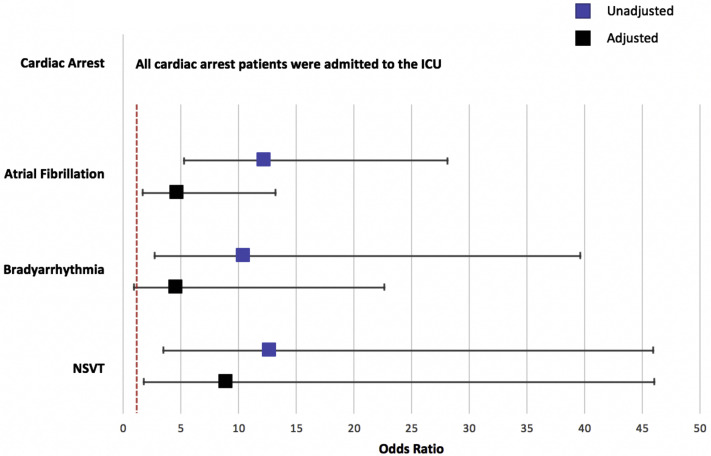
Among the assessment of selected variables that included age, sex, race, BMI, history of heart failure, CHD, diabetes, hypertension, CKD, and ICU status on admission, only ICU status emerged as having an association with each arrhythmia category. In unadjusted analysis, admission to the ICU was associated with a greater than 10-fold odds of developing each arrhythmia (Figure 2 ). All patients who had a cardiac arrest had been admitted to the ICU on initial presentation and before the development of cardiac arrest. Furthermore, after adjustment for age, sex, race, BMI, prevalent cardiovascular disease, diabetes, hypertension, CKD, and hydroxychloroquine treatment, ICU status remained independently associated with incident AF and NSVT; however, the odds for bradyarrhythmias was rendered nonsignificant (Figure 2). An increase in 1 year of age was associated with incident AF (odds ratio [OR] 1.06; 95% confidence interval [CI] 1.04–1.09), bradyarrhythmia (OR 1.03; 95% CI 1.00–1.06), and NSVT (OR 1.04; 95% CI 1.01–1.08) in univariate analysis. After multivariable adjustment, only age and incident AF remained independently associated (OR 1.05; 95% CI 1.02–1.09). Furthermore, heart failure was associated with incident AF (OR 5.61; 95% CI 2.37–13.25) and bradyarrhythmias (OR 9.16; 95% CI 2.41–34.79) in univariate analysis. After multivariable adjustment, prevalent heart failure remained independently associated with bradyarrhythmias (OR 9.75; 95% CI 1.95–48.65). No associations were observed between sex, race, BMI, diabetes, hypertension, and CKD and any of the arrhythmia categories in either univariate or multivariable analyses.
Figure 2.
Association of intensive care unit (ICU) status and cardiac arrhythmias. The odds ratios (and 95% confidence intervals) of ICU admission and specified cardiac arrhythmias are depicted. The dashed vertical red line represents an odds ratio = 1. Unadjusted models have a blue marker. Multivariable models (black marker) were adjusted for age, sex, race, body mass index, heart failure, coronary heart disease, diabetes, hypertension, chronic kidney disease, and hydroxychloroquine treatment. NSVT = nonsustained ventricular tachycardia.
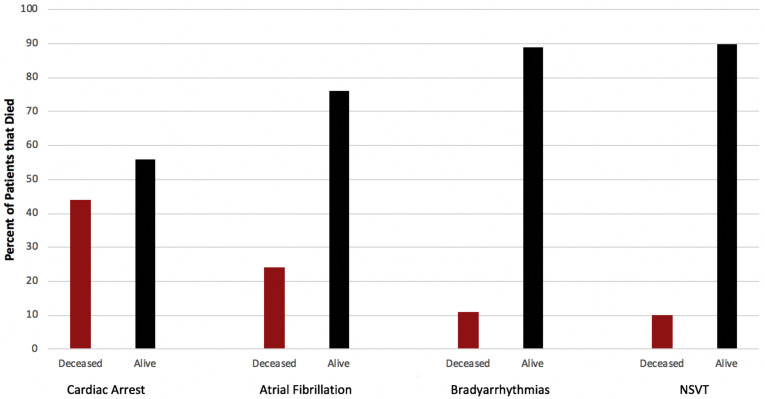
In exploratory analysis, our findings demonstrate that cardiac arrest is associated with in-hospital mortality (OR 20.47; 95% CI 5.19–80.69) even after controlling for age, sex, race, prevalent cardiovascular disease, ICU status, and hydroxychloroquine treatment (OR 34.99; 95% CI 3.49–350.69). In addition, AF was associated with in-hospital mortality (OR 6.73; 95% CI 2.52–17.98) but was attenuated to nonsignificance after multivariable analysis. No association was observed between bradyarrhythmias, NSVT, and acute mortality (Figure 3 ).
Figure 3.
Cardiac arrhythmias and death. The percentage of deceased and alive patients in each arrhythmia category is depicted. NSVT = nonsustained ventricular tachycardia.
Discussion
In our analysis of 700 patients with COVID-19 admitted over a 2.5-month period, 30 (4.3%) patients died in the hospital. The overall acute mortality was more than 10-fold higher in patients in the ICU than in those admitted to a non-ICU setting. We identified 53 arrhythmia-related events including 9 cardiac arrests, 25 incident AF cases, 9 clinically significant bradyarrhythmias, and 10 NSVTs. With the exception of the cardiac arrest cases, none of the 3 arrhythmia types were independently associated with acute mortality.
Our findings suggest that the incidence of cardiac arrests in patients with COVID-19 corresponds to the severity of illness and is not the sole consequence of the viral infection. The acute in-hospital mortality rates in both our population in the ICU and the recent studies from New York are similar and slightly more than 20%.6 , 7 The cardiac arrest rate of 11% observed in our population in the ICU approximates the 13% cardiac arrest rate observed across New York.6 The slightly higher rate in New York may be explained by combination treatment with hydroxychloroquine and azithromycin—medications that result in QT prolongation and independently increase the risk of cardiac arrest.6 None of the patients in our center were treated with azithromycin. Our findings also expand on these initial observations by specifying that nearly all the cardiac arrests in our population with COVID-19 included nonshockable rhythms such as pulseless electrical activity or asystole. Only 1 case of torsades de pointes was present. We did not observe the burden of sustained VT/VF that was reported from the early experiences in Wuhan.2 , 3 Our findings support that noncardiac causes such as systemic infection, inflammation, and illness are likely to contribute more to the etiology of cardiac arrest than direct myocardial infection or necrosis due to the viral infection. Further support is also provided by our study’s population of 621 (89%) patients admitted to a non-ICU setting, who had a much lower rate of acute mortality. No cardiac arrests were observed in this group, which comprised nearly 90% of our population with COVID-19.
Patients with more severe systemic illness as evidenced by ICU admission also had a higher likelihood of developing cardiac arrhythmias. The association with bradyarrhythmias could be explained after accounting for demographic and clinical differences such as underlying cardiovascular risk factors and disease between patients in the ICU and those admitted to a non-ICU setting. However, unmeasured factors that relate to the severity of illness likely explain the ongoing, independent association between ICU admission and incident AF and NSVT. Recent findings from the University of Alabama at Birmingham also support the higher likelihood of observing atrial arrhythmias in the population in the ICU vs population in a non-ICU setting.8 These consistent findings should highlight considerations for long-term anticoagulation therapy. COVID-19 can present with thrombotic complications including arterial and venous thrombosis.9 SARS-Cov-2 infection of endothelial cells is postulated to result in a cytokine response with release of inflammatory mediators that lead to endothelial and hemostatic activation.10 , 11 This inflammatory state may increase the risk of thromboembolic complications, especially when AF is present. Future studies will need to evaluate the most effective and safest strategies for long-term anticoagulation and rhythm management in this population.
Our study has several limitations. This analysis was from a single center serving a large urban population. As such, our findings may not be generalizable to patients with COVID-19 from across the world. In addition, some patients in the non-ICU ward were taken off telemetry during their hospitalization. As such, our ability to detect subclinical arrhythmias in these patients would be limited. Finally, our analysis was restricted to inpatient follow-up only. As such, we are unable to assess whether the presence of arrhythmic events have long-term health effects on our treated patients with COVID-19.
Conclusion
Eleven percent of patients hospitalized for COVID-19 at our center were admitted to the ICU. Cardiac arrests and arrhythmias were more likely to occur in the population in the ICU than in the population in the non-ICU ward even after controlling for underlying demographic and clinical factors. As such, cardiac arrests and arrhythmias are likely the consequence of systemic illness and not solely the direct effect of COVID-19 infection.
Footnotes
Partial support for this project was provided by the Winkelman Family Fund in Cardiovascular Innovation.
References
- 1.Coronavirus disease 2019 (COVID-19): cases in the U.S. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) [published online ahead of print March 27, 2020]. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed]
- 4.Baldi E, Sechi GM, Mare C, et al. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy [published online ahead of print April 29, 2020]. N Engl J Med. https://doi.org/10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed]
- 5.Creel-Bulos C., Hockstein M., Amin N. Acute cor pulmonale in critically ill patients with Covid-19. N Engl J Med. 2020;382:e70. doi: 10.1056/NEJMc2010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg E.S., Dufort E.M., Udo T. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colon CM, Barrios JG, Chiles JW, et al. Atrial arrhythmias in COVID-19 patients [published online ahead of print May 2020]. JACC Clin Electrophysiol. https://doi.org/10.1016/j.jacep.2020.05.015. [DOI] [PMC free article] [PubMed]
- 9.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up [published online ahead of print April 17, 2020]. J Am Coll Cardiol. https://doi.org/10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed]
- 11.Siripanthong B., Nazarian S., Muser D. Recognizing COVID-19–related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]