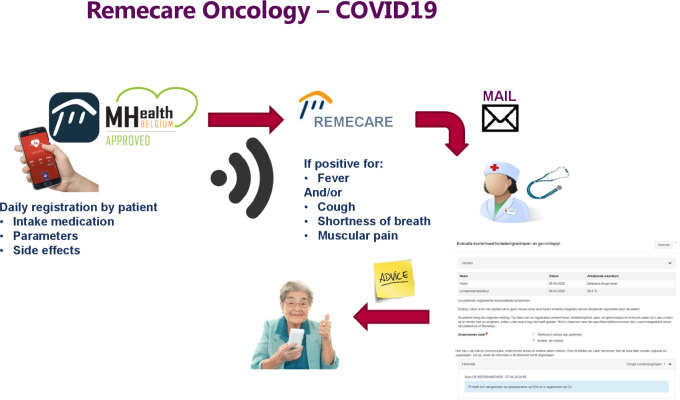
COVID-19 is an infectious pandemic disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus with varying presentations ranging from asymptomatic, sensation of a mild cold or influenza to severe bilateral pneumonia and death.1 Patients with cancer and COVID-19 are at a significantly higher likelihood of poor disease outcomes.2 3 In the absence of a vaccine or adequate treatment of COVID-19 current measures to minimise the infectious risk of SARS-CoV-2 in a cancer patient population are focused on physical distancing and protective measures. As it is clear that a hospital is a high-risk setting to contract COVID-19, one of the strategies we can use to treat patients with cancer as safe as possible is to reduce hospital visits to a strict minimum. We previously reported on AMTRA (ambulatory Monitoring of cancer Therapy using an interactive Application) which is a home-based monitoring, registration and interaction PRO (Patient Reported Outcomes) tool, developed in Belgium as an academic research project.4 The platform, RemeCare Oncology, was initially developed as a home toxicity monitoring system for oral treatment, but later expanded to all anticancer treatments and linked to an interactive home blood sampling system. It proved to be effective and reliable and patients were highly satisfied using it5. Consenting patients are equipped with a PRO application (RemeCare app) for remote interactive monitoring of toxicities. During the present COVID-19 pandemic the system was used to maximise the home care of patients with cancer (COrona REmeCare Oncology). COVID-19-related complaints are routinely questioned by the AMTRA system (fever, muscular pain, cough, shortness of breath). Via an online connection the presence and severity (from grade 0 to 3) of toxicities are registered at any time and uploaded to a web-based central platform, stored in the patients’ electronic medical record (figure 1). If the registered temperature is above 38.0°C or there is at least one symptom suspicious for COVID-19 the patient is asked to come to the hospital (emergency COVID-19 screening unit) for SARS-CoV-2 formal PCR testing on a nose/throat swab. This implicates that the App does not discriminate between COVID-19 and other causes of alarm such as neutropenic fever, bacterial infections, and so on. Over the last month we used this platform in 164 patients receiving systemic cancer treatment. A COVID-19 alarm was raised in five patients and in three of them a formal diagnosis of COVID-19 could be confirmed (table 1). One patient had a laryngitis according to his general practitioner and did not have a COVID test and one patient tested negative. We are not aware of patients in this population being admitted for COVID-19 without a RemeCare alarm signal. Although further research is needed to confirm the sensitivity and specificity of our App, the current observations show that patient-reported outcome platforms work in daily life to prescreen for COVID-19. As several cases are reported in Belgium of patients with COVID-19 collapsing and dying at home despite attempts of resuscitation as they ignored their symptoms, we hope that home patient monitoring may be helpful to alert patients with cancer to seek advice at an earlier stage.
Figure 1.
Flow of the RemeCare system.
Table 1.
Characteristics of the patients with a COVID-19-related alarm included in the COREO project
| Age | Gender | WHO | Tumour type | Date of diagnosis | Metastasis | Treatment | Alarm RemeCare | Test COVID-19 | Result | Hospitalisation | Follow-up | |
| Date | Symptom | |||||||||||
| 66 | M | 2 | Urothelial cancer | 1 October 2015 | Liver/peritoneal | Chemo: CarboTaxol once weekly | 20 March 2020 | T°39.2 | Initially considered as tumour fever | |||
| 22 March 2020 | T°38.2 | |||||||||||
| 23 March 2020 | T°39.2 | 24 March 2020 | Negative | Still hospitalised at present | ||||||||
| 5 April 2020 | T°38.7 | |||||||||||
| 15 April 2020 | Positive | 15 April 2020 | ||||||||||
| 74 | M | 1 | Glioblastoma | 2 August 2018 | None | Targeted: regorafenib | 22 March 2020 | Dyspnoea/cough/myalgia/T°38 | Netherlands | Positive | 23 March 2020 | Home isolation |
| 1 April 2020 | T°39.9 | 16 April 2020 | ||||||||||
| 54 | M | 0 | Nasopharyngeal carcinoma | 12 November 2019 | Bone/lung/pleura | Chemo: cisplatin/gemcitabine | 6 April 2020 | Cough/T°39.4 | 6 April 2020 | Positive | 6 April 2020 | Admitted to hospital, discharged 24 March 2020 |
| 7 April 2020 | T°39.4 | 21 April 2020 | Positive | |||||||||
| 62 | M | 1 | Urothelial cancer | 1 December 2019 | Lung | Chemo: paclitaxel/carboplatin | 7 April 2020 | Dyspnoea/cough/myalgia | Not tested | |||
| 20 April 2020 | T°38 | Pharyngitis according to GP | ||||||||||
| No symptoms at present | ||||||||||||
| 54 | M | 0 | Rectal adenocarcinoma | 4 October 2019 | None | Chemo: CAPOX every 3 weeks | 19 April 2020 | T°38.2 | 5 April 2020 | Negative | No retesting | |
| No symptoms at present |
CAPOX, capecitabine plus oxaliplatin; COREO, COrona REmeCare Oncology; GP, general practitioner.
Footnotes
Twitter: @HPrenen
Contributors: PvD and MP designed the project. SDK collected the data. All authors reviewed the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) – China, 2020. CCDC Weekly. 2020;2(x):1-10. Available: https://github.com/cmrivers/ncov/raw/master/COVID-19.pdf [PMC free article] [PubMed]
- 2.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? an experience in New York City. Ann Oncol 2020. 10.1016/j.annonc.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasschaert M, Helsen S, Rolfo C, et al. Feasibility of an interactive electronic self-report tool for oral cancer therapy in an outpatient setting. Support Care Cancer 2016;24:3567–71. 10.1007/s00520-016-3186-2 [DOI] [PubMed] [Google Scholar]
- 5.Rasschaert M, Vulsteke C, De Keersmaeker S, et al. AMTRA: a multicentered experience of a web-based monitoring and tailored toxicity management system for cancer patients. Supportive Care in Cancer 2020;68 10.1007/s00520-020-05550-6 [DOI] [PubMed] [Google Scholar]