Abstract
Purpose/Objective(s):
Recurrent and/or previously irradiated head and neck cancers (HNC) are therapeutically challenging and may benefit from high-dose, highly accurate radiation techniques, such as stereotactic ablative radiation therapy (SABR). Here, we compare set-up and positioning accuracy across HNC subsites to further optimize the treatment process and PTV margin recommendations for HN-SABR.
Materials/Methods:
We prospectively collected data on 405 treatment fractions across 79 patients treated with SABR for recurrent/previously irradiated HNC. First, interfractional error was determined by comparing ExacTrac x-ray to the treatment plan. Patients were then shifted and residual error measured with repeat x-ray. Next, cone-beam-CT (CBCT) was compared to ExacTrac for positioning agreement and final shifts were applied. Lastly, intrafractional error was measured with x-ray before each arc. Results were stratified by treatment site into skull base, neck/parotid, and mucosal.
Results:
Most patients (66.7%) were treated to 45Gy/5 fractions (range: 21–47.5Gy, 3–5 fractions). The initial mean±SD interfractional errors were −0.2±1.4mm (A/P), 0.2±1.8mm (C/C) and −0.1±1.7mm (L/R). Interfractional 3D-vector error was 2.48±1.44, with skull base significantly lower than other sites (2.22 vs. 2.77, P=0.0016). All interfractional errors were corrected to within 1.3mm and 1.8°. CBCT agreed with ExacTrac to within 3.6mm and 3.4°. CBCT disagreements and intrafractional errors of >1mm or >1° occurred at significantly lower rates in skull base sites (CBCT: 16.4% vs. 50.0% neck; 52.0% mucosal; P<0.0001; Intrafractional: 22.0% vs. 48.7% all others; P<0.0001). Final PTVs were 1.5mm (skull base), 2.0mm (neck/parotid), and 1.8mm (mucosal).
Conclusion:
HN-SABR PTV margins should be optimized by target site. PTV margins of 1.5–2mm may be sufficient in the skull base, while 2–2.5mm may be necessary for neck and mucosal targets. When using ExacTrac, skull base sites show significantly fewer uncertainties throughout the treatment process, while neck/mucosal targets may require the addition of CBCT to account for positioning errors and internal organ motion.
INTRODUCTION
Although multimodality treatment has improved clinical outcomes in cancers of the head and neck (HNC), more than one-third of patients still experience local failure.1–2 These locally recurrent tumors are often challenging to treat given their elaborate anatomical locations and invasive nature. While surgery remains the optimal modality for local control, many cases may also require adjuvant radiation due to adverse pathologic features such as positive margins, perineural invasion (PNI), or extracapsular extension (ECE).3–4 An additional subset of patients may not be surgical candidates at all, leaving radiation as the only remaining local treatment option. Standard fractionated radiation has been used with some success, however many patients have already received radiation during their initial treatment, leading to an increased risk of serious toxicity.5-6
These limitations led to the consideration of stereotactic ablative radiotherapy (SABR). SABR relies on modern image guidance and advanced treatment planning to deliver ablative doses of highly conformal radiation treatments in a limited number of fractions. These high doses also dictate a steeper dose gradient in the planning process which has the potential benefit of limiting the volume of normal tissue irradiated.7 A growing preponderance of data suggest that HN-SABR may lead to better outcomes compared to standard fractionated reirradiation.8–11 However, the high doses and tight margins with SABR necessitate an increased level of confidence in the treatment set-up and delivery process. Even minute shifts in patient position, when unaccounted for, can result in insufficient dose to the tumor and/or excessive dose to the adjacent critical structures. To minimize and correct for any potential movement, SABR relies on a combination of robust immobilization and image-guidance. Additionally, because uncertainty cannot be completely eliminated, expansions called planning target volumes (PTV) are also built into radiation planning. There is currently no formal consensus as to the ideal planning and set-up process for HN-SABR, with most recommendations extrapolated from the conventionally fractionated IMRT setting.
To this end, we quantified the magnitude of set-up and positioning errors at each step of the treatment process in patients with recurrent and/or previously irradiated HNC treated with SABR using our custom immobilization system. We hypothesized that the degree of error could differ among head and neck (H&N) subsites and performed the analyses with the goal of providing site-specific recommendations for immobilization, image-guidance, and optimal treatment planning volumes.
MATERIALS AND METHODS
Patient Selection
An IRB-approved study (IRB # PA14–0198) was conducted on 79 patients undergoing HN-SABR treatment for recurrent and/or previously irradiated HNC from 2013–2018. All patients had either a recurrent tumor with history of prior irradiation or a second primary within a previously irradiated field. All data was collected prospectively.
Immobilization and Simulation
The immobilization system used was a customized adaptation of our existing thermoplastic mask system used for conventionally fractioned IMRT, and has previously been described in detail.12 In summary, all patients were simulated in the supine position using non-contrast CT with 1mm slices. Immobilization was performed using a custom-moldable Klarity AccuCushion (Klarity Medical Products, Newark, OH) to immobilize the head and shoulder region. A pre-heated bite block (Precise Bite, Civico, Coralville, IA) or custom oral stent with bite block attachment was inserted into the mouth and indexed to the maxillary teeth before hardening into a reproducible molded position. Finally, a thermoplastic mask (Orfit Industries America, Wijnegem, Belgium) extending from the vertex to the lateral aspect of the shoulders and across the chest was indexed to the Klarity and bite block and allowed to harden into a rigid position. Six IR passive reflection spheres were placed on the mask in different axes. Patients were instructed not to swallow during simulation and treatment to minimize internal organ motion.
Treatment planning was performed using the Pinnacle TPS (Version 9.8 or 9.10, Philips Radiation Oncology Systems, Andover, MA). Target volumes and organs at risk were contoured based on simulation planning CT images which were co-registered to treatment position MRI, PET-CT and/or dual-energy CT (DECT) image datasets. A 2mm PTV margin was added for skull base targets and 3mm for non-skull base targets. Treatment plans were created using volumetric-modulated arc therapy (VMAT) with 2–3 arcs per patient and were evaluated with the goal of the target volume (PTV) receiving at least 95% of the prescription dose (e.g. PTV V100 >95%). Mucosal targets were normalized to the 95% isodose line (IDL) as dose homogeneity was preferred to reduce risk of mucosal ulceration, whereas skull base/nodal targets were prescribed to 90% or lower IDL to create a sharper dose fall-off towards critical structures and preferentially allow hot spots of >110–120% within the GTV or PTV. Stereotactic treatments were all delivered on the same linear accelerator (Varian TrueBeam STx, Varian Medical Systems, Palo Alto, CA) in the same treatment vault with image-guidance using the Brainlab ExacTrac stereoscopic X-ray 6D system (Brainlab AG, Feldkirchen, Germany) and Varian on-board CBCT. The entire set-up and treatment delivery time was generally 20–30 minutes in total duration.
Determination of Set-up and Positioning Errors
Initial Set-up Error (INTERfractional)
Following patient immobilization in the treatment devices, initial alignment was performed using ExacTrac IR tracking. An ExacTrac stereoscopic kV x-ray was taken and bony alignment was compared to the planning CT to determine the magnitude of error between treatment fractions, called the “initial set-up error” (i.e. “interfractional error”). Errors were quantified in each of the six ExacTrac axes. Translational axes were defined as vertical (anterior/posterior, “A/P”), longitudinal (cranial/caudal, “C/C”), and lateral (left/right, “L/R”), and rotational axes included yaw, roll, and pitch.
Residual Error
After determining the initial set-up error as above, patients were shifted to the correct alignment using the 6D-couch. ExacTrac x-ray was then repeated to determine “residual error”, which was defined as the remaining difference between this second x-ray and the planning scan, after initial shifts using the ExacTrac system. This was again quantified in the translational and rotational axes.
CBCT-Positioning Agreement
Because ExacTrac alignment utilizes a combination of IR surface beads and bony x-ray, soft tissue alignment was performed using CBCT with 1mm slice thickness. The CBCT images were volumetrically matched to the planning CT using the Varian 4D Integrated Treatment Console in the six axes by prioritizing match to GTV, but also taking soft tissue and bony alignment into account (Figure 1). Differences in alignment were used to compare the ExacTrac bony x-ray alignment to the CBCT positioning to determine agreement between the two modalities (“CBCT-positioning agreement”). Physicians performed manual shifts in the translational directions when necessary. While verification was performed in all axes, CBCT-based rotational shifts were not available given limitations of the 6D-couch treatment console. Once CBCT shifts had been applied, an additional ExacTrac x-ray was performed and the final position was recorded to serve as the baseline for intrafractional error measurement, effectively indexing the ExacTrac to the final CBCT-shifted position.
Figure 1:
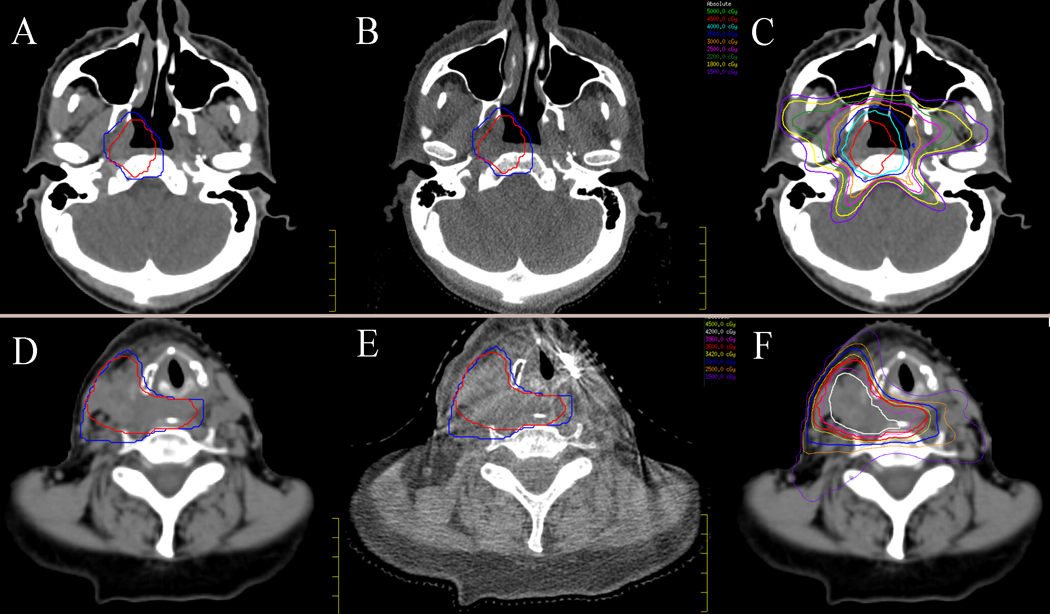
Treatment planning and set-up images for a skull base target (A-C) and neck target (D-F). A: Planning CT for skull base target with PTV_4500 (red) and PTV_3500 (blue); B: Corresponding cone-beam CT at the time of treatment; C: Skull base planning CT with isodose lines; D: Planning CT for neck target with PTV_3600 (red) and PTV_3000 (blue); E: Corresponding CBCT; F: Neck target planning CT with isodose lines
Intrafractional Error
After final positioning had been verified, treatment was initiated. ExacTrac x-ray was taken immediately prior to the delivery of each arc. These x-rays were compared to the final pre-treatment verification x-ray by calculating the difference between the new position and the final post-CBCT ExacTrac position. This difference was used to quantify “intrafractional error”, which was defined as error that may occur during the “beam-on” phases of the treatment fraction. Correctional shifts were generally only applied for >2mm discrepancies. Intrafractional error was calculated using the mean error of the 2–3 pre-arc x-rays in each axis for each patient.
Statistical analysis of positioning errors and PTV determination
The mean and standard deviation of the above errors were calculated. The results were analyzed for all patients cumulatively and then stratified into treatment sites, which were categorized into four groups by anatomical location:
Skull base, which consisted of anterior skull base (ethmoid, maxillary sinus, orbital structures), nasopharynx, retropharyngeal nodes, sphenoid, cavernous sinus, posterior skull base (C1–2, occiput, jugular foramen, hypoglossal region), and lateral skull base (petrous apex, cochlea)
Neck/Parotid, which consisted of cervical and supraclavicular nodes, the parotid gland, and associated structures
Mucosal, which consisted of the oropharynx, oral cavity, and larynx
Other, which was any subsite not fitting groups 1–3.
For each subsite, systematic error (Σ), random positioning errors (σ), and 3D-vector displacement errors were determined. The number of clinically significant errors, defined as >1–2mm or >1–2°, depending on step of the process, were also compared across groups. Two-sample t-tests with unequal variance were used to compare means. Chi-squared tests were used to evaluate proportional rates of clinically significant errors between groups.
PTV margins (m) for confidence level (p) to achieve dose level (d) were determined using van Herk’s formula13 and the modified formula by Gordon and Siebers 14, 15 which accounts for hypofractionated regimens:
where α is a function of confidence level (p), β is a function of dose level (d), and n is the number of fractions. Systematic error (Σ) was calculated by using the standard deviation of the mean error. Random positioning error (σ) was calculated using the root mean square of the standard deviations. PTVs were calculated to the 95% confidence level (CI) using α = 2.79, and to the 90% CI using α = 2.5, both assuming n=5 fractions. To specify the 95% dose level, β = 0.7 was used. For example, if the mPTV(95%, 95%) was calculated as 3mm, this would imply that a PTV margin of 3mm was required to ensure a minimum dose of 95% (β = 0.7) to the CTV for 95% of patients (α = 2.79). Suggested PTV margins were determined at each step of the treatment process and stratified by subsite. Results were reported for the overall largest PTV (assuming uniform expansion) and by each translational direction (assuming non-uniform expansion). Because interfractional and positioning errors were corrected prior to treatment, final PTV margins were based on intrafractional error and were inclusive of any intrafractional shifts, when necessary. It is important to note that the classic van Herk and Gordon and Siebers formulas do not account for rotational errors in the final margin calculation.
Additionally, the van Herk margin formula was originally designed for 3D plans with σp (penumbra) of 3.2mm.13 With IMRT, including the 2–3 arc VMAT plans for our SABR cases, there are beam overlapping effects that tend to enlarge the dose penumbra around the targets. Because the majority of HN-SABR cases are in the reirradiation setting, we have much stricter dose constraints on all organs-at-risk that are adjacent to the target. To achieve these constraints, a typical treatment plan will often have dose gradients of 10% per mm at the target edge that is adjacent to the critical structure(s), which equates to 60% dose fall off over 6mm. This is similar to the penumbra width of 6.4mm when σp = 3.2mm.15 Based on these factors, we chose to use the linearized van Herk formula (0.7 * σm), which assumes σp = 3.2mm, to estimate PTV margins in our study. Of note, in the full version of the van Herk margin formula, the random error (at the 95% dose level) is 1.64(σ – σp), where. The Gordon and Seibers formula builds upon the linearized van Herk estimation and thus this was used for both margin formula calculations.
RESULTS
Patient/Treatment Characteristics
The 79 patients studied had a total of 84 HN-SABR treatment courses. Four hundred five individual fractions were available for analysis. Treated subsites included: skull base (n=45), neck/parotid (n=22), and mucosal (n=15). Two sites did not fit a predefined category (1 scalp/cranium and 1 post-auricular), and therefore this “other” group was included in summary statistics but was not separately stratified for subsite analysis given the small size. The mean patient age was 62.7 years. The only significant difference between groups was age (58.4 vs. 67.6 years, skull base vs. others; P = 0.0017)
All courses ranged from 3–5 fractions and the most common dose/fractionation scheme was 45Gy in 5 fractions (n=56, 66.7%). The median target volume was 17.6 cm3 (range 1.5–178.1), and 75% of targets were between 5–30 cm3.
Initial Setup Error
Mean±SD initial set-up errors were −0.2±1.4mm (A/P), 0.2±1.8mm (C/C) and −0.1±1.7mm (L/R) in the translational axes and 0.0±1.1° (yaw), 0.0±0.9° (roll) and −0.5±0.9° (pitch) in the rotational axes. The resulting PTV margins (95% CI, GSH formula) were 4.1mm A/P, 5.0mm C/C, and 4.4mm L/R. A total of 190 of 405 treatment fractions (46.9%) had a translation shift of >2mm or a rotational shift of >2° in one or more axes on initial set-up, with at least one occurrence in 64/84 treatment courses (76.2%). Ten fractions showed a >5mm or >5° difference, seven of which were in the neck/mucosal groups. Of the 1142 total rotational measurements, only 56 had an absolute error >2°. Interfractional 3D-vector error showed a mean±SD of 2.48±1.44, with SB errors significantly smaller than all other sites (2.22 vs. 2.77, P=0.0016). Table 1 provides a summary of all errors, PTV margins, and 3D-vectors across subsites.
Table 1:
Mean ± standard deviation for set-up and positioning errors in six axes across different head and neck subsites with calculated PTV margins for 90% and 95% confidence intervals. AP: Anterior/Poster; CC: Cranial/Caudal; LR: Left/Right; PTV: Planning Target Volume; GSH: Gordon and Siebers Hypofractionation formula; CVH: Classic van Herk formula
| AP (mm) | CC (mm) | LR (mm) | Yaw (°) | Roll (°) | Pitch (°) | 3D Vector | PTV (90% GSH) | PTV (95% GSH) | PTV (95% CVH) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Interfractional Error | All | −0.2 ± 1.4 | 0.2 ± 1.8 | −0.1 ± 1.7 | 0 ± 1.1 | 0 ± 0.9 | −0.5 ± 0.9 | 2.5 ± 1.5 | 4.5 mm | 5.0 mm | 4.9 mm |
| Skull Base | −0.2 ± 1.2 | 0.2 ± 1.7 | −0.3 ± 1.4 | −0.1 ± 1.0 | 0 ± 0.7 | −0.4 ± .09 | 2.2 ± 1.3 | 4.5 mm | 5.0 mm | 4.9 mm | |
| Neck | −0.6 ± 1.7 | 0.3 ± 2.1 | 0 ± 1.6 | 0 ± 1.1 | 0.1 ± 1.0 | −0.7 ± 1.1 | 2.8 ± 1.4 | 5.2 mm | 5.8 mm | 5.7 mm | |
| Mucosal | −0.5 ± 1.7 | 0.1 ± 1.4 | 0.5 ± 2.0 | 0 ± 1.2 | −0.2 ± 0.8 | −0.3 ± 0.8 | 2.5 ± 1.7 | 5.1 mm | 5.6 mm | 5.6 mm | |
| Residual Error | All | 0.0 ± 0.3 | 0 ± 0.4 | 0 ± 0.3 | 0 ± 0.2 | 0 ± 0.3 | 0 ± 0.3 | 0.5 ± 0.5 | 1.0 mm | 1.1 mm | 0.8 mm |
| Skull Base | 0 ± 0.2 | 0 ± 0.3 | 0 ± 0.2 | 0 ± 0.2 | 0 ± 0.2 | 0 ± 0.2 | 0.4 ± 0.2 | 0.9 mm | 1.0 mm | 0.7 mm | |
| Neck | −0.1 ± 0.4 | 0 ± 0.4 | −0.1 ± 0.4 | 0 ± 0.3 | 0 ± 0.3 | −0.1 ± 0.4 | 0.6 ± 0.4 | 1.2 mm | 1.3 mm | 1.1 mm | |
| Mucosal | 0 ± 0.3 | 0 ± 0.3 | −0.1 ± 0.3 | 0 ± 0.2 | 0 ± 0.3 | 0 ± 0.3 | 0.5 ± 0.3 | 0.9 mm | 1.0 mm | 0.7 mm | |
| CBCT Positioning Agreement | All | −0.1 ± 0.6 | −0.1 ± 0.6 | 0.1 ± 0.4 | 0.1 ± 0.6 | 0 ± 0.5 | 0.1 ± 0.6 | 0.8 ± 0.6 | 1.6 mm | 1.7 mm | 1.5 mm |
| Skull Base | −0.1 ± 0.4 | −0.2 ± 0.5 | 0.1 ± 0.3 | 0.2 ± 0.4 | 0 ± 0.4 | 0.2 ± 0.4 | 0.6 ± 0.4 | 1.3 mm | 1.4 mm | 1.2 mm | |
| Neck | −0.2 ± 0.7 | −0.1 ± 0.7 | 0.2 ± 0.6 | 0 ± 0.8 | 0.0 ± 0.7 | 0.2 ± 0.8 | 1.0 ± 0.7 | 1.8 mm | 1.9 mm | 1.6 mm | |
| Mucosal | 0 ± 0.9 | −0.1 ± 0.9 | 0.2 ± 0.5 | 0 ± 0.7 | 0 ± 0.6 | 0 ± 0.9 | 1.1 ± 0.8 | 2.4 mm | 2.6 mm | 2.4 mm | |
| Intrafractional Error | All | 0.0 ± 0.7 | −0.1 ± 0.8 | 0 ± 0.5 | 0 ± 0.4 | 0 ± 0.5 | −0.1 ± 0.5 | 0.8 ± 0.5 | 1.5 mm | 1.6 mm | 1.4 mm |
| Skull Base | −0.1 ± 0.4 | −0.1 ± 0.5 | 0 ± 0.4 | 0 ± 0.4 | 0 ± 0.4 | −0.1 ± 0.4 | 0.6 ± 0.4 | 1.4 mm | 1.5 mm | 1.3 mm | |
| Neck | 0.0 ± 0.8 | −0.2 ± 0.7 | 0.0 ± 0.8 | −0.1 ± 0.5 | −0.1 ± 0.6 | −0.1 ± 0.6 | 1.1 ± 0.6 | 1.8 mm | 2.0 mm | 1.7 mm | |
| Mucosal | 0.1 ± 1.0 | 0.3 ± 1.4 | 0 ± 0.6 | 0.1 ± 0.4 | 0.1 ± 0.5 | 0.1 ± 0.6 | 0.9 ± 0.6 | 1.7 mm | 1.8 mm | 1.5 mm | |
Residual Error
All initial set-up errors were corrected to within 1.3mm and 1.8°. Twenty-four out of 405 fractions (5.9%) had residual errors >1mm or >1°. Only 4 of these occurred in skull base sites, compared to 14 in neck/parotid (1.9% vs 13.2%, P<0.0001) and 2 (2.7%) in mucosal sites. There were no significant differences in residual error across any of the six axes between groups. Residual 3D-vector error showed a mean±SD of 0.47±0.33. Similar to set-up error, mean residual 3D-vector was significantly smaller for skull base vs. all other sites (0.36 vs. 0.59, P<0.00001)
CBCT-Positioning Agreement
CBCT agreed with ExacTrac to within 3.6mm and 3.4°. Disagreements of >1mm or >1° occurred at a significantly lower rate in skull base sites (16.4%), compared to 50.0% of fractions in neck/parotid (P<0.0001) and 52.0% in mucosal sites (P<0.0001). 3D-vector error showed a mean±SD of 0.81±0.58. Similar to set-up and residual error, mean 3D-vector here was significantly smaller for skull base vs. all other sites (0.61 vs. 1.05, P<0.00001).
One hundred forty-three fractions (35.3%) required shifts based on CBCT imaging (mean shift 1.0mm). Only 65 fractions (16.0%) required shifts >1mm. Any shift (19.6% for skull base vs. 53.8% for neck/parotid; 53.3% for mucosal, P<0.0001) and shifts >1mm (6.1% for skull base vs. 26.7% for all others, P<0.0001) occurred at significantly lower rates in the skull base group.
Intrafractional Error
Mean±SD intrafractional errors are listed in Table 1. The largest intrafractional errors were 3.6mm and 3.9°, however translational errors over the correction threshold (>2mm) in one or more axes only occurred 25 times (7 of which were in one challenging patient) in the 1046 total pre-beam x-rays for a rate of 2.4%. There were also significantly fewer >1mm or >1° errors in the skull base group (22.0% skull base vs 48.7% all others, P<0.0001), and mean 3D-vector errors were significantly smaller for skull base targets (0.63 skull base vs. 1.06 neck/parotid vs. 0.91 mucosal; skull base vs. others: P<0.000001). Intrafractional rotational errors >2° only occurred in 11 of 2982 total rotational measurements.
The resulting calculated PTV margin (95% CI, GSH formula, n=5 fractions) to account for intrafractional uncertainty was 1.6mm (1.6mm A/P, 1.6mm C/C, 1.4mm L/R) for the entire sample. PTV margins by subsite were 1.5mm for skull base, 2.0mm for neck/parotid, and 1.8mm for mucosal. For skull base subsites, PTV margins by axis were 1.3mm A/P, 1.5mm C/C, and 1.2mm L/R. Similarly for neck/parotid sites, margins were 2.0mm A/P, 1.9mm C/C, and 1.6mm L/R. Finally, margins for mucosal sites were 1.8mm A/P, 1.4mm C/C, and 1.1mm L/R. Moving to a 90% confidence interval decreased these values by 0.1–0.2mm, as listed in Table 1. Similarly, using the classic van Herk formula resulted in slightly smaller recommended margins. The calculated PTV margins for both recipes were within 0.1mm when using fewer (n=3 or 4) or greater numbers of fractions (n=6) compared to the five fraction regimen used for reporting all other results.
Regarding σp (penumbra), the intrafractional σm (motion error standard deviation) was measured as 0.4 – 0.8mm. Therefore, assuming σp = 3.2mm and σm = 0.4mm, the random error will be 0.04mm using the full Van Herk formula. Using the linearized format of 0.7* σm would equal 0.28mm. Thus, our final calculated PTV margins using the linearized format are more conservative in practice.
DISCUSSION:
SABR is increasingly used to treat recurrent and previously irradiated HNC, with early clinical outcomes suggesting favorable local control rates and manageable acute/late toxicity.8–11 As adoption of SABR is expanded, refinements in the set-up, planning, and treatment processes will be increasingly important to ensure safe and reliable delivery. This study demonstrated a relatively high degree of immobility using our cushion-mask-bite block system, but also highlighted the importance of correcting positioning errors with image-guidance prior to treatment, especially in the neck and mucosal groups. The data ultimately suggested that overall PTV margins of ≤2mm were adequate to ensure that 90–95% of patients received the prescription dose to at least 95% of the target volume.
However, not all H&N subsites were equal, with skull base targets showing significantly lower error magnitudes at nearly all steps of the set-up and treatment processes. These differences equated to tighter final PTV margin recommendations for skull base sites, where 1.5–2mm may be adequate for target coverage. In contrast, the greater degree of positioning error for neck and mucosal targets may necessitate margins of 2–2.5mm. It is important to emphasize that the van Herk or Gordon and Siebers margin recipes of calculating PTV expansions do not account for rotational errors or uncertainties in image registration, treatment planning, and machine calibration, and thus in practice, clinicians may need to incorporate additional margin to ensure target volumes are fully encompassed. However, the very low rate of significant intrafractional rotational errors (>2°) in this study (0.4%) suggests that rotation was largely inconsequential for most treatments using our immobilization system. Additionally, the importance of using the appropriate formula was demonstrated by the classic van Herk formula underestimating margins by 0.1–0.3mm.
A plausible explanation for the differences in error rates is that the central location of the skull base acts as the fulcrum around which other H&N sites rotate. Thus, small movements are increasingly amplified in targets further from this central axis. Another likely contributor is that skull base targets are inherently aligned better with rigid cranial bony anatomy and have less internal organ motion than neck and mucosal sites. This concept has not been fully explored in SABR but has been supported across conventionally fractionated IMRT set-up analyses, where 2.2–4.0mm margins have been recommended for clivus-level targets vs. 4.0–6.9mm in the neck and c-spine.16-20
The clinical implications of differential PTV margins are of particular importance in SABR given the high doses and sharp fall-off. For example, skull base reirradiation sites are often in close proximity to the optic apparatus, intracranial carotids, brainstem, and cranial nerves, where there is increased risk of significant treatment-related morbidity or mortality. The importance of limiting dose to nearby structures is reflected in early reports on clinical outcomes for HN-SABR showing 4–36% rates of G3–4 toxicity and up to 8–17% rates of fatal carotid blowout events.9, 11, 21–23 Thus, reducing PTV margins may allow better sparing of these critical structures to decrease toxicity. Supporting this concept is a study that compared 3mm and 5mm PTV expansions with conventionally fractionated HN-IMRT, finding no difference in control rates or marginal failures but significantly lower rates of feeding-tube dependency and esophageal stricture on long-term follow-up.23
In contrast to skull base targets, mucosal and neck sites may require slightly larger PTVs to mitigate the risk of geographical misses. The data suggest this was due to an increased reliance on soft tissue alignment with CBCT, likely attributed to comparatively less rigid association with bony landmarks. Even small daily set-up variations can result in significant cold spots, with data demonstrating that underdosing a 1% volume of the target by 20% of the prescription dose can reduce local control probability by 11% in the conventionally fractionated setting.24 This may play a role in the 11–59% local failure rates seen in the HN-SABR outcomes data, although these recurrent tumors are inherently difficult to control and often not as responsive to radiation as their upfront counterparts.8,11,21–22 In light of this, using a one-size-fits-all PTV margin may not be optimal for HN-SABR when there appear to be differences between skull base and non-skull base subsites, thereby risking undertreatment of target sites and/or overtreatment of critical organs. Additionally, analyses on the impact of tumor volume and dose for HN-SABR have shown that large volume tumors required higher doses to achieve the same response rates, and that higher doses overall were associated with better LRC.8 Thus, smaller PTVs may not only allow for sparing of the nearby tissue but potential dose escalation to meet the same constraints as larger PTVs. The differences in PTV margins by individual translational axes also present an opportunity to further optimize treatment volumes, where non-uniform expansions might be used to further spare reirradiated tissues in a particular direction.
The rate of CBCT to ExacTrac kV x-ray agreement provided insightful data into the role of image-guidance in HN-SABR. Overall, 16% of SABR fractions required a shift >1mm using CBCT, highlighting the importance of soft-tissue alignment in the set-up process. However, subsite analysis again revealed key differences. The data showed that 80% of skull base treatments required no shift at all and only 6% required a significant shift (>1mm). However, neck and mucosal targets required a shift in more than 50% of the treatments and a significant shift in over 25%. Thus, the workflow and optimal image-guidance modality may differ by H&N subsite, where skull base targets may be sufficiently aligned using bony anatomy with ExacTrac kV x-ray, but neck and mucosal sites may see a greater benefit from the addition of soft-tissue alignment using CBCT. This point is emphasized by the PTV margins for CBCT agreement seen in Table 1. Because CBCT shifts were applied prior to treatment, we could not provide an estimate of total overall margins in the absence of CBCT. However, the calculated PTV expansions at the CBCT step of at least 2.6mm in mucosal sites compared to only 1.4mm for skull base targets would likely grow even further apart with the addition of intrafractional errors. Similar comparisons of ExacTrac kV and CBCT have been performed for conventionally fractionated HN-IMRT and intracranial stereotactic treatments, demonstrating that CBCT is preferential for soft-tissue and c-spine sites, while ExacTrac performs comparably to CBCT for intracranial sites.26–30 While using both forms of image-guidance provides increased confidence and allows for correctional adjustments, it is possible that both are not needed in every SABR case.
Finally, while this analysis is strengthened by the prospective collection of a sizeable number of treatment fractions, there are several limitations to address. First, this was a single institution study using a specific set-up and treatment process which other institutions and practices may or may not be capable of implementing, including our custom immobilization system. Additionally, given that the data was collected over a 5-year period, there may be some variability among the staff involved in the set-up and positioning process. There were also unequal numbers of patients in each group, and there may also be considerable variation within the target groupings, leaving an opportunity for future studies to provide a more granular breakdown by focusing on specific subsites. Furthermore, some treatment sites may not fit our pre-defined subsite groups, and thus may need to be more carefully analyzed to determine adequate margins. CBCT also was not performed during initial set-up or after treatment, as this is not standard practice at our institution, however having this data would allow for better quantification of soft tissue motion. Finally, the design of the study does not provide clinical outcomes data to corroborate with the PTV margin recommendations, however the implications of our estimated PTV margins will be validated in the future by comparing failure rates by subsite and looking at the spatial/dosimetric relationship of local failures to various PTV expansions.
CONCLUSIONS
This study evaluated set-up and positioning accuracy in recurrent and/or previously irradiated HNC treated with SABR. The results demonstrated that a cushion-mask-bite block immobilization system combined with ExacTrac kV x-ray and CBCT allows for 1.5–2.5mm PTV margins overall. In clinical practice, additional margin may be indicated to account for institution-specific uncertainties in treatment planning, machine calibration, and other sources of systematic error. Subsite analysis revealed significant variation in the rate and magnitude of set-up and positioning errors, with skull base targets showing increased accuracy at nearly every step of the treatment process. Thus, a one-size-fits-all PTV expansion and treatment process may not be optimal for HN-SABR, and may result in undertreatment of target sites and/or overtreatment of organs at risk. Furthermore, CBCT appears to play a larger role in aligning mucosal and neck sites than it does for skull base targets when added to the ExacTrac system. Future studies might consider using smaller PTV margins for skull base sites (1.5–2mm) and larger margins for neck mucosal sites (2.0–2.5mm) to assess the clinical impact of these findings with respect to toxicity and local control.
Acknowledgments
S.M. has a consulting agreement with Oscar Healthcare. D.I.R. serves on the scientific advisory board for Merck. J.P. serves on the scientific advisor board for Cyberknife for Accuray, Inc. S.J.F. reports personal fees from Varian, grants and personal fees from C4 Imaging, grants from Eli Lilly, grants from Elekta, grants and personal fees from Hitachi, other support from Breakthrough Chronic Care, personal fees from Boston Scientific, and personal fees from the National Comprehensive Cancer Network, all of which are outside the submitted work. C.D.F. has received grants (unrelated to the present work) from GE Healthcare, Elekta AB, National Science Foundation (NSF 1557559), National Institutes of Health (NIH 1R56DE025248–01, 1R25EB025787–01, 5R01CA214825–02; 5R01CA225190–02; 5R01CA218148–02, CA088084), the Sabin Family Foundation, and an MD Anderson Institutional Research Grant; payments for lectures from the University of Texas Health Science Center San Antonio and from Elekta AB; royalties from Demos Medical Publishing; and travel/accommodations/meeting expenses from OHSU, Greater Baltimore Medical Center, University of Illinois, Elekta AB, and the Translational Research Institute (TRI) Australia.
REFERENCES
- 1.Cooper JS, Zhang Q, Pajak TF, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84(5):1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garden AS, Harris J, Trotti A, et al. Long-term results of concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: a phase II trial of the radiation therapy oncology group (RTOG 99–14). Int J Radiat Oncol Biol Phys. 2008;71(5):1351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haque S, Karivedu V, Riaz MK, et al. High-risk pathological features at the time of salvage surgery predict poor survival after definitive therapy in patients with head and neck squamous cell carcinoma. Oral Oncol. 2019;88:9–15., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SN, Cohen MA, Givi B, et al. Salvage surgery for locally recurrent oropharyngeal cancer. Head Neck. 2016;38 Suppl 1:E658–64.17764087, [DOI] [PubMed] [Google Scholar]
- 5.Langer CJ, Harris J, Horwitz EM, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Protocol 9911. J Clin Oncol. 2007;25(30):4800–5. [DOI] [PubMed] [Google Scholar]
- 6.Spencer SA, Harris J, Wheeler RH, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck. 2008;30(3):281–8. [DOI] [PubMed] [Google Scholar]
- 7.Kung SW, Wu VW, Kam MK, et al. Dosimetric comparison of intensity-modulated stereotactic radiotherapy with other stereotactic techniques for locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2011;79(1):71–9 [DOI] [PubMed] [Google Scholar]
- 8.Rwigema JC, Heron DE, Ferris RL, et al. The impact of tumor volume and radiotherapy dose on outcome in previously irradiated recurrent squamous cell carcinoma of the head and neck treated with stereotactic body radiation therapy. Am J Clin Oncol. 2011;34(4):372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vargo JA, Ferris RL, Ohr J, et al. A prospective phase 2 trial of reirradiation with stereotactic body radiation therapy plus cetuximab in patients with previously irradiated recurrent squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2015;91(3):480–8.30410843 [DOI] [PubMed] [Google Scholar]
- 10.Ansinelli H, Singh R, Sharma DL, et al. Salvage Stereotactic Body Radiation Therapy for Locally Recurrent Previously Irradiated Head and Neck Squamous Cell Carcinoma: An Analysis from the RSSearch® Registry. Cureus. 2018;10(8):e3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanisce L, Koshkareva Y, Xu Q, et al. Stereotactic Body Radiotherapy Treatment for Recurrent, Previously Irradiated Head and Neck Cancer. Technol Cancer Res Treat. 2018;17:1533033818780086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Wang C, Phan J, et al. Improved setup and positioning accuracy using a three-point customized cushion/mask/bite-block immobilization system for stereotactic reirradiation of head and neck cancer J. Appl Clin Med Phys, 3 (2016), pp. 180–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47(4):1121–35. [DOI] [PubMed] [Google Scholar]
- 14.Herschtal A, Foroudi F, Silva L, Gill S, Kron T. Calculating geometrical margins for hypofractionated radiotherapy. Phys Med Biol. 2013;58(2):319–33. [DOI] [PubMed] [Google Scholar]
- 15.Gordon JJ, Siebers JV. Evaluation of dosimetric margins in prostate IMRT treatment plans. Med Phys. 2008;35(2):569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giske K, Stoiber EM, Schwarz M, et al. Local setup errors in image-guided radiotherapy for head and neck cancer patients immobilized with a custom-made device. Int J Radiat Oncol Biol Phys. 2011;80(2):582–9. [DOI] [PubMed] [Google Scholar]
- 17.Anjanappa M, Rafi M, Bhasi S, et al. Setup uncertainties and PTV margins at different anatomical levels in intensity modulated radiotherapy for nasopharyngeal cancer. Rep Pract Oncol Radiother. 2017;22(5):396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheo T, Loh Y, Chen D, Lee KM, Tham I. Measuring radiotherapy setup errors at multiple neck levels in nasopharyngeal cancer (NPC): A case for differential PTV expansion. Radiother Oncol. 2015;117(3):419–24. [DOI] [PubMed] [Google Scholar]
- 19.Yin WJ, Sun Y, Chi F, et al. Evaluation of inter-fraction and intra-fraction errors during volumetric modulated arc therapy in nasopharyngeal carcinoma patients. Radiat Oncol. 2013;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Garden AS, Lo J, et al. Multiple regions-of-interest analysis of setup uncertainties for head-and-neck cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64(5):1559–69. [DOI] [PubMed] [Google Scholar]
- 21.Cengiz M, Özyiğit G, Yazici G, et al. Salvage reirradiaton with stereotactic body radiotherapy for locally recurrent head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2011;81(1):104–9. [DOI] [PubMed] [Google Scholar]
- 22.Roh KW, Jang JS, Kim MS, et al. Fractionated stereotactic radiotherapy as reirradiation for locally recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2009;74(5):1348–55. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki H, Ogita M, Himei K, et al. Reirradiation using robotic image-guided stereotactic radiotherapy of recurrent head and neck cancer. J Radiat Res. 2016;57(3):288–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen AM, Yu Y, Daly ME, Farwell DG, Benedict SH, Purdy JA. Long-term experience with reduced planning target volume margins and intensity-modulated radiotherapy with daily image-guidance for head and neck cancer. Head Neck. 2014;36(12):1766–72. [DOI] [PubMed] [Google Scholar]
- 25.Hong TS, Tomé WA, Chappell RJ, Chinnaiyan P, Mehta MP, Harari PM. The impact of daily setup variations on head-and-neck intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2005;61(3):779–88. [DOI] [PubMed] [Google Scholar]
- 26.Zeidan OA, Langen KM, Meeks SL, et al. Evaluation of image-guidance protocols in the treatment of head and neck cancers. Int J Radiat Oncol Biol Phys. 2007;67(3):670–7. [DOI] [PubMed] [Google Scholar]
- 27.Tryggestad E, Christian M, Ford E, et al. Inter- and intrafraction patient positioning uncertainties for intracranial radiotherapy: a study of four frameless, thermoplastic mask-based immobilization strategies using daily cone-beam CT. Int J Radiat Oncol Biol Phys. 2011;80(1):281–90. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Shi W, Andrews D, et al. Comparison of Online 6 Degree-of-Freedom Image Registration of Varian TrueBeam Cone-Beam CT and BrainLab ExacTrac X-Ray for Intracranial Radiosurgery. Technol Cancer Res Treat. 2017;16(3):339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clemente S, Chiumento C, Fiorentino A, et al. Is ExacTrac x-ray system an alternative to CBCT for positioning patients with head and neck cancers?. Med Phys. 2013;40(11):111725. [DOI] [PubMed] [Google Scholar]
- 30.Ma J, Chang Z, Wang Z, Jackie wu Q, Kirkpatrick JP, Yin FF. ExacTrac X-ray 6 degree-of-freedom image-guidance for intracranial non-invasive stereotactic radiotherapy: comparison with kilo-voltage cone-beam CT. Radiother Oncol. 2009;93(3):602–8. [DOI] [PubMed] [Google Scholar]


