Abstract
Curcumin displays anticancer properties; however, some issues with the drug delivery mode limit its therapeutic use. Although reformulation and derivatization of curcumin have improved its bioavailability, curcumin derivatives may not retain the same anticancer properties as the parent compound. The present study investigated the anticancer properties of two curcumin complexes, the iron-curcumin [Fe(Cur)3] and boron-curcumin [B(Cur)2] complexes, in the MDA-MB-231 breast cancer cell line. The cellular localization of curcumin, B(Cur)2 and Fe(Cur)3 was determined by fluorescence microscopy. Cell proliferation, migration and invasion were also analysed. Furthermore, apoptosis-associated proteins were detected by using a proteome profiler array, and ion channel gene expression was analysed by reverse transcription-quantitative PCR. The results demonstrated that the three compounds were localized in the perinuclear and cytoplasmic regions of the cell, and displayed cytotoxicity with IC50 values of 25, 35 and 8 µM for curcumin, B(Cur)2 and Fe(Cur)3, respectively. In addition, the three compounds inhibited cell invasion, whereas only curcumin and B(Cur)2 inhibited cell migration. Furthermore, cell exposure to curcumin resulted in an increase in the relative expression of the two key proapoptotic proteins, cytochrome c and cleaved caspase-3, as well as the antiapoptotic protein haem oxygenase-1. In addition, curcumin increased the expression levels of the voltage-gated potassium channels Kv2.1 and Kv3.2. Similarly, the expression levels of the chloride channel bestrophin-1 and the calcium channel coding gene calcium voltage-gated channel auxiliary subunit γ4 were increased following exposure to curcumin. Taken together, these results indicated that Fe(Cur)3 and B(Cur)2 may display similar anticancer properties as curcumin, suggesting that chemical complexation may be considered as a strategy for improving the potency of curcumin in the treatment of breast cancer.
Keywords: breast cancer, curcumin, potassium channels, haem oxygenase-1
Introduction
Cancer was responsible for 9.6 million deaths in 2018 worldwide, according to the World Health Organization Global Cancer Observatory (1). Breast cancer is the most common cancer in women with 2.1 million newly diagnosed cases worldwide in 2018 (1). Breast cancer is a heterogeneous disease with several subtypes that are classified based upon a variety of clinical and histopathological features (2-4). The lack of expression of oestrogen receptor (ER) and progesterone receptor (PR) and the absence of human epidermal growth factor Receptor-2 (HER2) overexpression assessed by immunohistochemistry defines the triple-negative breast cancer (TNBC) subtype. Poor long-term outcomes are associated with TNBC compared with other breast cancer subtypes (5-8). Mammography screening accounted for a decrease in breast cancer mortality in the recent years (9,10). However, despite the numerous therapeutic options, including chemotherapy, hormonal therapy and radiotherapy, about 30% of patients who are treated at early-stages relapse (11). In most cases, treatment is effective at first, but resistance to therapy and disease progression eventually occur. Because treatment resistance has become common (11), it is crucial to provide novel treatment strategies. Alternative treatments may emerge from the development of either novel/unconventional therapeutic compounds or novel pharmacological approaches. The natural phytochemical compound curcumin exerts some effects on several biochemical pathways and drug targets. At present, curcumin is being widely investigated for its potential therapeutic use in various diseases, including Alzheimer's disease, cystic fibrosis and several types of cancer (12-19).
Curcumin has been reported to display antioxidant, anti-inflammatory, antiproliferative, proapoptotic and anticancer properties (20). The antioxidant effects of curcumin have been used in the treatment of pancreatitis (21), spinal cord injury (22) and complications associated with diabetes (23). The anti-inflammatory effects of curcumin have been demonstrated in diabetes (23) and in the treatment of cancer therapy-induced oral mucositis (24). Curcumin also displays anticancer properties that are applicable to the clinic (25-27). The clinical use of curcumin has been limited by its low aqueous solubility and stability, resulting in low oral bioavailability (28,29). Structural modification and encapsulation of curcumin have been used to improve its pharmacokinetic profile (30). Furthermore, reformulations of curcumin have been investigated in order to improve its delivery directly to the tumour mass within the breast tissue (31-33). Improved curcumin stability has been achieved by structural modifications, including replacement of the β-diketone moiety with metal ions (34,35); however, the effects of these modifications on the cytotoxic properties of curcumin have not yet been investigated. In addition, whether these novel complexes can enter the cell and localize in a similar manner to curcumin remain unknown. A suitable breast cancer cell line may be employed to investigate the localization and movement of curcumin-derived compounds into cells. The MDA-MB-231 cell line (36) is an aggressive and invasive breast cancer cell line, which lacks ER, PR and HER2 expression. This cell line is therefore referred to as a triple-negative breast cancer cell line (37). The absence of ER expression results in MDA-MB-231 cell insensitivity to antihormone-based therapies, including tamoxifen (38). Clinically, triple-negative breast cancer has limited treatment options. The MDA-MB-231 cell line is thus commonly used to investigate the molecular basis of this type of breast cancer and for the development of novel therapeutic approaches.
Resistance to apoptosis is a key characteristic of cancer cells (39). Curcumin has been reported to inhibit proliferation by inducing apoptosis in MDA-MB-231 breast cancer cells via increasing the Bax/Bcl-2 ratio or upregulating p21 expression (40,41). It was also reported that curcumin can decrease MDA-MB-231 cell migration and invasion without affecting apoptosis (42). However, the anticancer activity of curcumin in relation to apoptosis, and the expression of proapoptotic and antiapoptotic proteins have not been fully described.
Ion channels directly or indirectly influence most basic cellular processes. Subsequently, ion channels also affect most malignant processes in cancer cells, including sustained proliferation (43-45), tissue invasion (43), metastasis (46,47) and programmed cell death (48,49). A previous study demonstrated that molecular, biological and pharmacological inhibition of voltage-gated potassium channels (Kv) reduces cancer cell proliferation, whereas overexpression of certain Kv channels can stimulate cell proliferation (50). Furthermore, when cells receive a death stimuli, they decreases in size during a process called apoptotic volume decrease (AVD). AVD occurs before the cascade of biochemical events that induces apoptosis (51) and is primarily induced by potassium efflux across the plasma membrane. Numerous potassium channels are essential for controlling and regulating the flow of potassium into and out of the cell. Because of the pivotal role of potassium channels during AVD, cancer cells may evade apoptosis by downregulating potassium channel expression. For example, Kv1.3 and Kv1.5 are expressed at reduced levels in many types of human cancer cells compared with normal cells (52). Other potassium channels, including voltage-gated potassium channels Kv1.1, Kv1.3, Kv1.5, Kv2.1 and Kv11.1, serve crucial roles during apoptosis (53). In addition to AVD associated with a decrease in cytoplasmic potassium concentration, this potassium movement promotes various cellular events that are critical for programmed cell death. These events include mitochondrial depolarization and cytochrome c release from mitochondria as well as cleavage of procaspase 3 and enhanced endonuclease activity (53). Furthermore, Bax is known to inhibit mitochondrial potassium channels downstream of pro-apoptotic signals. The activation of the mitochondrial pathway of apoptosis by the pro-apoptotic protein Bax is mediated via the direct inhibition of mitochondrial Kv1.3 channels by BAX in the outer mitochondrial membrane (54). It has been reported that curcumin displays inhibitory effects on potassium channels, mediating the curcumin-induced anticancer and anti-inflammatory properties in monocytic leukaemia and effector memory T cells (55,56). In addition, curcumin decreases potassium channel currents by inactivating the gating of Kv2.1 in 293 cells (57).
It has been demonstrated that the solubility and stability of curcumin can be increased by complexation with boron and iron (34). The aim of this study was to assess the anti-cancer properties of these curcumin complexes. The present study examined the effects of soluble structurally modified curcumin-based compounds on the MDA-MB-231 cell line. Furthermore, the inhibitory effects of curcumin, boron-curcumin [B(Cur)2] and iron-curcumin [Fe(Cur)3] on MDA-MB-231 cell proliferation, migration and invasion were investigated. In addition, the effect of the curcumin compounds on the apoptosis-proteome and gene expression of certain ion-channels was assessed.
Materials and methods
Cell lines
The oestrogen negative MDA-MB-231 cell line was obtained from the American Type Culture Collection. Cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.; cat. no. 12491015) supplemented with 5% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA; cat. no. F2442), 100 U/ml penicillin, 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.; cat. no. 15070063), 600 µg/ml L-glutamine (Sigma-Aldrich; Merck KGaA; cat. no. G7513) and 6/500 ml 100X non-essential amino acids (Gibco; Thermo Fisher Scientific, Inc.; cat. no. 11140050) and placed at 37°C in a humidified incubator containing 5% CO2.
Drugs and reagents
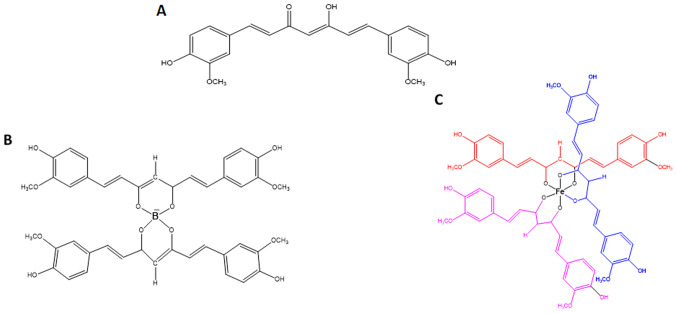
Curcumin was purchased from Sigma-Aldrich: Merck KGaA (cat. no. C7727). B(Cur)2 and Fe(Cur)3 were synthesized as previously described by Khalil et al (58). The improved photostability of B(Cur)2 and Fe(Cur)3 relative to curcumin has been previously validated (34). Curcumin, B(Cur)2 and Fe(Cur)3 were initially dissolved in DMSO into a 10 mM stock solution that was stored at -20°C. Stock solutions were later diluted in DMEM to the desired final concentrations. The structures of curcumin, B(Cur)2 and Fe(Cur)3 are presented in Fig. 1.
Figure 1.
Chemical structures of (A) curcumin, (B) boron-curcumin and (C) iron-curcumin.
Fluorescence microscopy
M DA-M B-231 cells (350×103 cell/ml) were seeded in 8-chamber slides (Eppendorf; cat no. 30742036) and incubated for 24 h at 37°C with 5% CO2. Subsequently, cells were treated with curcumin (25 µM), B(Cur)2 (30 µM) and Fe(Cur)3 (8 µM) or vehicle (control group) for 5 min and immediately fixed with 3.7% formaldehyde at room temperature for 10 min. Cells were counterstained with DAPI at room temperature for 5 min to visualize the nucleus. Stained cells were observed using a BX43 fluorescence microscope (Olympus Corporation; magnification, ×100) to determine the cellular localization of curcumin and its metal derivatives. Images were analysed using CellSens Standard software (version 1.9; Olympus Corporation).
Sulforhodamine B (SRB) assay
The in vitro effect of curcumin, B(Cur)2 and Fe(Cur)3 on breast cancer cell proliferation was evaluated by using the SRB assay as previously described by Skehan et al (59). Briefly, MDA-MB-231 cells were seeded in triplicate at the density of 1×104 cells/well into a 96-well plate and incubated overnight at 37°C with 5% CO2. Cells were treated with vehicle or various concentrations (5, 10, 20, 30, 40, 50 and 100 µM) of curcumin, B(Cur)2 and Fe(Cur)3 for 48 h. Subsequently, cells were fixed overnight with cold 10% trichloroacetic acid (Sigma-Aldrich; Merck KGaA; cat. no. 91228) at room temperature, washed with distilled H2O and air-dried. Cells were stained with 100 µl of 0.4% SRB stain (Sigma-Aldrich; Merck KGaA; cat. no. S1402) (diluted in 1% acetic acid) for 30 min at room temperature, washed with acetic acid (Sigma-Aldrich; Merck KGaA; cat no. 33209) and air-dried. SRB stain was solubilized in 10 mM unbuffered Tris base solution. Absorbance was measured at a wavelength of 540 nm with reference wavelength of 650 nm using a micro-plate reader. Cell proliferation was calculated according to the following formula: Cell proliferation=100-[(absorbance of treated cells/absorbance of untreated cells) x100]. IC50 values were calculated using GraphPad Prism software version 5 (GraphPad Software, Inc.) to compare the cytotoxicity of the two complexes. For subsequent cell migration and invasion assays, the IC50 dose, one dose above the IC50 and one dose below the IC50 were used. For cell localization, mRNA and protein expression assays, cells were treated with the IC50 dose.
Cell migration by wound healing assay
MDA-MB-231 cells were seeded in 24-well plates and incubated at 37°C with 5% CO2 until they reach 80-90% confluence. A 100-µl pipette tip was used to make a single scratch in the cell monolayer and image of the scratch was captured at 0 h with a light microscope (magnification, ×4). Subsequently, cells were treated with various concentrations of curcumin, B(Cur)2 and Fe(Cur)3 or vehicle (control group) and incubated for 24 h at 37°C with 5% CO2. The concentrations used were 5, 10, 15, 20, 25 and 30 µM for curcumin and B(Cur)2, and 2, 4, 5, 6 and 8 µM for Fe(Cur)3. On day 2, the width of the scratch was photographed and measured. Wound closure was calculated according to the following formula: Wound closure=100-[(scratch width at 24 h/scratch width at 0 h) x100]. Cells were not serum-starved during the assay.
Agarose invasion assay
The under-agarose assay was performed to determine the random invasion of treated and untreated cells towards serum components found in the agarose gel. Ultra-pure agarose (0.9%; Invitrogen; Thermo Fisher Scientific, Inc.) was dissolved in PBS supplemented with minimum essential medium (Gibco; Thermo Fisher Scientific, Inc.) containing 5% FBS and incubated at room temperature in 6-well plates until it solidifies. Subsequently, 2-mm wells were formed in the agarose gel as previously described (60) and cells (4×104 cells/well) were pre-treated with 5, 10, 15, 20, 25 and 30 µM curcumin and B(Cur)2 and 2, 4, 5, 6 and 8 µM Fe(Cur)3 (or vehicle for control) before being loaded into the wells. Following 24 h incubation, the number of cells that had invaded the agarose gel were manually counted under a light microscope (magnification, ×4).
Profiling of apoptosis-associated proteins
The effect of curcumin, B(Cur)2 and Fe(Cur)3 on the expression of apoptosis-associated proteins was determined using the Proteome Profiler™ Array Human Apoptosis Array kit (R&D Systems, Inc.; cat no. ARY009) according to the manufacturer's instructions. Briefly, MDA-MB-231 cells were cultured in a 75 cm2 flask and treated for 48 h with curcumin (25 µM), B(Cur)2 (35 µM), Fe(Cur)3 (8 µM) or vehicle. Cells (80% confluent) were lysed and total proteins of treated and untreated cells were extracted and quantified using a Bradford Assay Protein Quantitation kit (Abcam). Extraction was performed using the solution provided in the kit 'Lysis Buffer 17' (Part no. 895943). The extraction solution contained aprotinin (10 µg/ml), leupeptin (10 µg/ml) and pepstatin (10 µg/ml). Protein arrays (nitrocellulose membranes spotted with 35 apoptotic proteins) were blocked with the array buffer 1 for 1 h at room temperature. Subsequently, proteins (280 µg) were transferred onto the arrays and incubated overnight at 4°C. After being washed, the arrays were incubated at room temperature with antibody detection cocktail for 1 h followed by the addition of Streptavidin-HRP for 30 min. The signal was finally obtained by adding Chemi Reagent Mix on the membrane. Protein spots were visualized using LI-COR detection system and the pixel density of each spot was quantified using Image J software (National Institutes of Health, version 1.46r). The relative expression of proteins in the treated cells were normalized to those in the untreated cells. The relative changes were based on differences in the pixel density of each spot displayed on images of the membrane array. The assay was performed in duplicate and the mean of the pixel density value was used for calculations.
RNA extraction
Total RNA was extracted from MDA-MB-231 cells following treatment with curcumin, B(Cur)2, Fe(Cur)3 or vehicle by using the RNeasy kit (Qiagen; cat. no. 74104) according to the manufacturer's protocol. The concentration of RNA was determined using a NanoDrop 1,000 spectrophotometer (Thermo Fisher Scientific, Inc.).
Ion-channel gene expression analysis by reverse transcription-quantitative (RT-q) PCR
Total RNA (1 µg) from cells treated with curcumin, B(Cur)2, Fe(Cur)3 or vehicle was reverse transcribed into cDNA using the RT2 First Strand kit (Qiagen; cat. no. 330401) according to the manufacturers' protocol. cDNA was then subjected to RT-qPCR array analysis for a panel of 84 neuronal ion channel genes. RT-qPCR was performed on a 96-well Human Neuronal Ion Channels RT2 profiler PCR array (cat. no. PAHS-036ZA; Qiagen) using RT2 SYBR Green/ROX PCR Master Mix (Qiagen) and an ABI 7500 real time PCR machine (Applied Biosystems; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The reaction conditions were as follows: 10 min at 95°C followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C. Each sample was run in biological triplicates. The gene expression data were uploaded and analyzed using an online analysis software package (Qiagen, Inc., processed via the Qiagen portal at http://www.qiagen.com/geneglobe, July 2018). The array included five housekeeping genes as internal standards for data normalization, ACTB, B2M, GAPDH, HPRT1 and RPLP0. Relative gene expression was represented by fold change which was calculated by RT2-analysis software using the threshold cycle (Ct) and based upon the 2−ΔΔCt method (61). Fold change was defined by the normalised gene expression (2−ΔCt) in the Test Sample divided by the normalized gene expression (2−ΔCt) in the Control Sample (vehicle).
Statistical analysis
Data were presented as the means ± standard error of the mean of at least three independent experiments. Statistical analyses were performed using SPSS version 25 (IBM Corp.) and GraphPad Prism version 5 (GraphPad Software, Inc.). Data were compared using Student's t-test and two-way ANOVA followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Curcumin, B(Cur)2 and Fe(Cur)3 localize in the cell cyto- plasm and around the nucleus
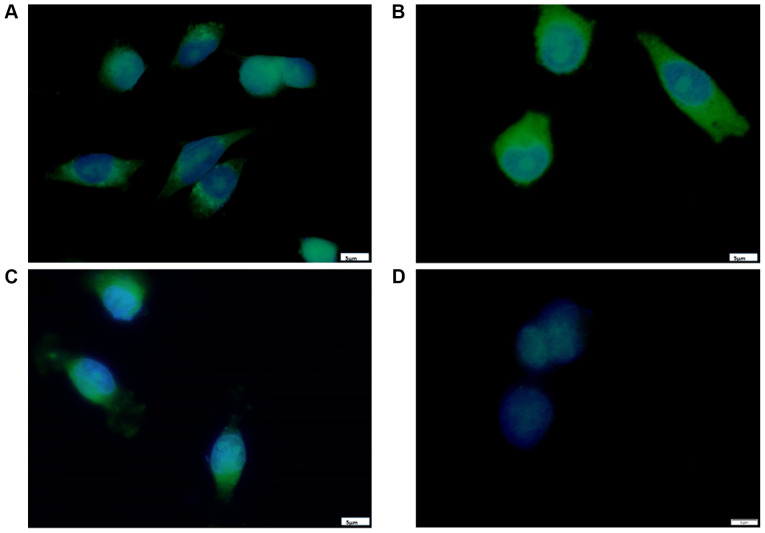
The results from fluorescence microscopy displayed a cytoplasmic localization of curcumin B(Cur)2 and Fe(Cur)3 in MDA-MB-231 cells (Fig. 2); however, the three compounds displayed differences in their exact localization after a short incubation period. As presented in Fig. 2A, the curcumin-treated cells displayed fluorescence mainly around the perinuclear region with a punctate pattern. In Fig. 2B, cells incubated with B(Cur)2 displayed homogenous fluorescence in the cytoplasm, and a few cells displayed a distinct halo around the nucleus, presumably within the nuclear membrane. As presented in Fig. 2C, Fe(Cur)3-treated cells displayed a nuclear-centric pattern of localization with no obvious fluorescence in the nuclear membrane or nucleus. Furthermore, the three compounds altered the morphology of MDA-MB-231 cells from the typical spindle-shape to a more spherical shape.
Figure 2.
Localization of (A) curcumin, (B) B(Cur)2, (C) Fe(Cur)3 and (D) DMSO vehicle in MDA-MB-231 cells. Cells were seeded at subconflu-ency and left to attach overnight at 37°C with 5% CO2. The following day, cells were treated with IC50 doses of curcumin, B(Cur)2 or Fe(Cur)3 (green) for 5 min and subsequently fixed. Cells were counterstained with DAPI as a nuclear stain (blue). Magnification, ×100. Scale bar, 5 µm. B(Cur)2, boron-curcumin; Fe(Cur)3, iron-curcumin.
Curcumin, B(Cur)2 and Fe(Cur)3 are cytotoxic for MDA-MB-231 cells
The effect of various concentrations (5-100 µM) of curcumin, B(Cur)2 and Fe(Cur)3 on cell proliferation was assessed using the SRB assay. The results demonstrated that curcumin displayed an inhibitory effect on MDA-MB-231 cell proliferation of 66-85%, with an effective dose starting at 30 µM curcumin (Fig. 3A). By contrast, B(Cur)2 displayed a weaker cytotoxic effect (60%) on MDA-MB-231 cells, which occurred with a higher dose (40 µM; Fig. 3B). Fe(Cur)3 displayed the most potent cytotoxic effect on MDA-MB-231 cell proliferation (20-90%), with a significant decrease in cell proliferation at the lowest dose (5 µM; Fig. 3C). In addition, the IC50 values of curcumin, B(Cur)2 and Fe(Cur)3 were 25, 35 and 8 µM, respectively.
Figure 3.
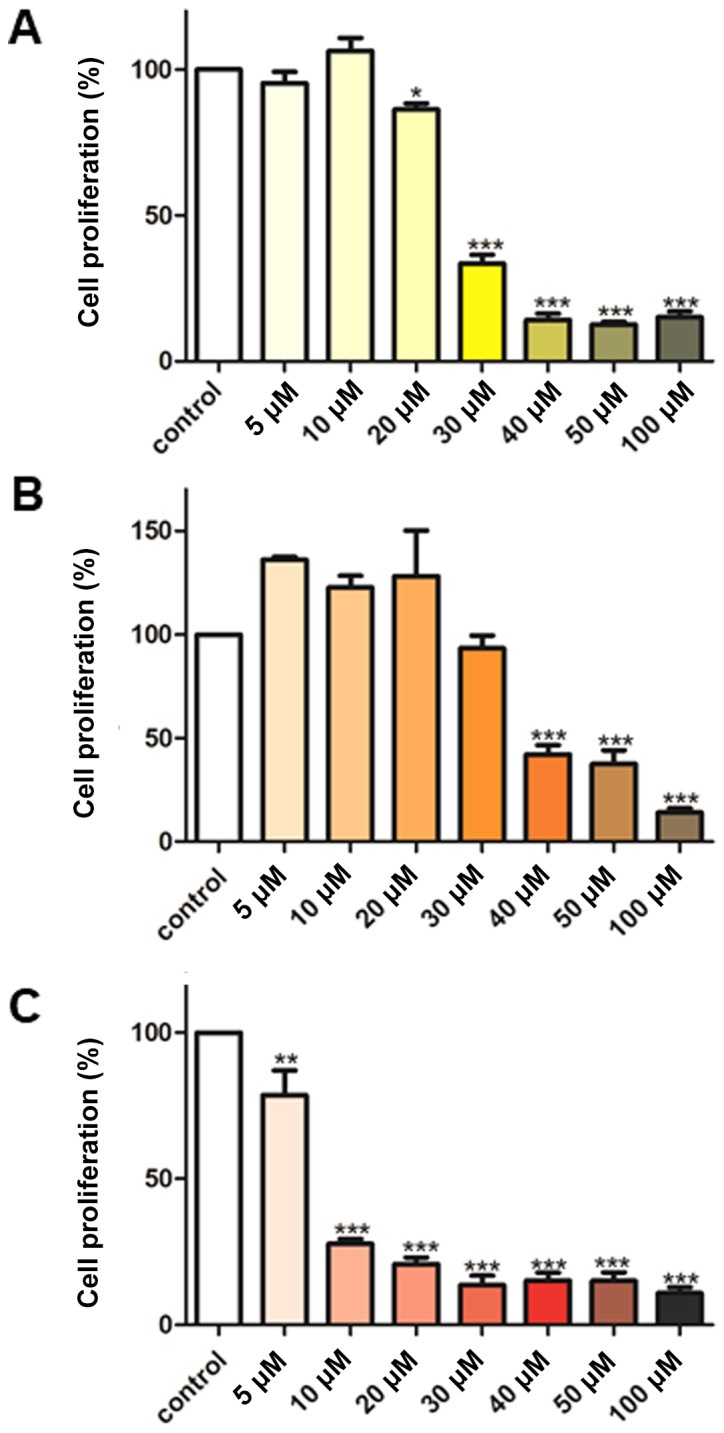
Effect of (A) curcumin, (B) B(Cur)2 and (C) Fe(Cur)3 on MDA-MB-231 cell proliferation. Cells grown in 96-well plates were untreated (control; white bars) or treated with curcumin, B(Cur)2 or Fe(Cur)3 for 48 h (5-100 µM). Cell proliferation was assessed using the Sulforhodamine B assay. Data were presented as the means ± standard error of the mean of 5-6 independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. control. B(Cur)2, boron-curcumin; Fe(Cur)3, iron-curcumin.
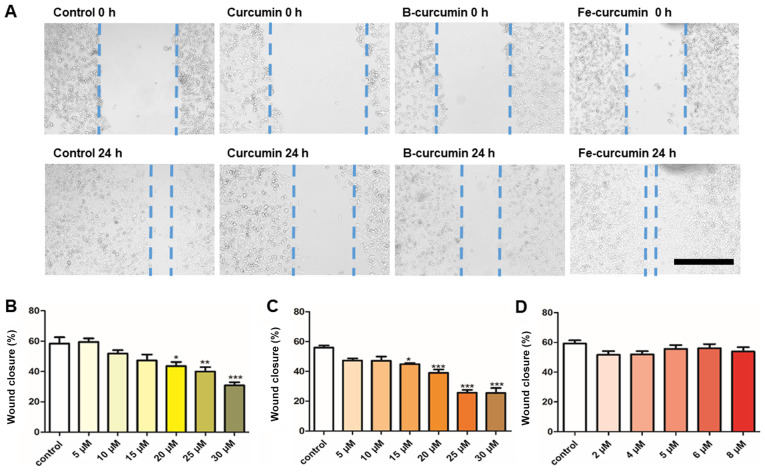
Curcumin and B(Cur)2 alter MDA-MB-231 cell migratory ability
The results from the wound healing assay demonstrated that curcumin and B(Cur)2 had an inhibitory effect on MDA-MB-231 cell migratory ability (Fig. 4A). Curcumin and B(Cur)2 inhibited cell migration by 60-70% at 20-30 µM and 15-35 µM, respectively (Fig. 4B and C). Fe(Cur)3 had no effect on cell migratory ability (Fig. 4C).
Figure 4.
Effect of curcumin and curcumin complexes on MDA-MB-231 wound healing capacity. (A) Representative images of wound healing following exposure to the three compounds at 0 and 24 h. Magnification, ×4. Scale bar, 500 µm. Curcumin and B(Cur)2 were tested at 30 µM and Fe(Cur)3 was tested at 8 µM. Effects of (B) curcumin, (C) B(Cur)2 and (D) Fe(Cur)3 on MDA-MB-231 cell migration. Cells grown in 24-well plates were untreated (control; white bars) or treated with different concentrations of curcumin, B(Cur)2 or Fe(Cur)3 for 24 h. Cell migration was assessed using a wound healing assay. Data were presented as the means ± standard error of the mean of 4-9 independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. control. B(Cur)2, boron-curcumin; Fe(Cur)3, iron-curcumin.
Curcumin, B(Cur)2 and Fe(Cur)3 inhibit MDA-MB-231 cell random invasion
Curcumin, B(Cur)2 and Fe(Cur)3 significantly inhibited cell invasion (Fig. 5). Curcumin was the most effective, inhibiting cell invasion by 16-84% at concentrations between 5 and 30 µM (Fig. 5A). B(Cur)2 and Fe(Cur)3 inhibited cell invasion by 45-56 and 48-53%, respectively (Fig. 5B and C).
Figure 5.
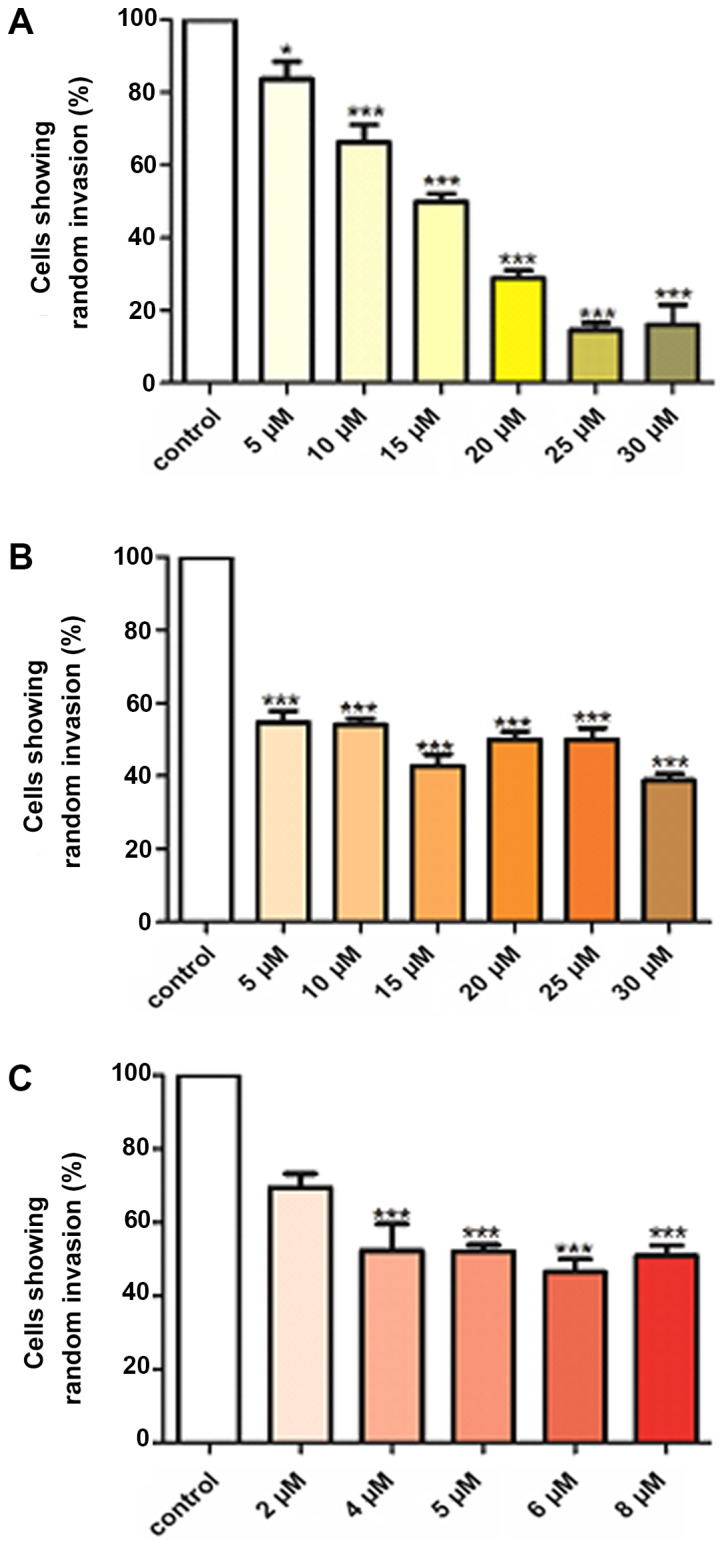
Effect of (A) curcumin, (B) B(Cur)2 and (C) Fe(Cur)3 on the random invasion of MDA-MB-231 cells towards serum components found in agarose gel. Cells loaded in agarose wells were untreated (control; open bars), or treated with different concentrations of curcumin, B(Cur)2 or Fe(Cur)3 for 24 h. Curcumin and B(Cur)2 were tested at 5-30 µM and Fe(Cur)3 was tested at 2-8 µM. Invading cells were manually counted. Data were presented as the means ± standard error of the mean of 3-9 independent experiments. *P<0.05 and ***P<0.001 vs. control. B(Cur)2, boron-curcumin; Fe(Cur)3, iron-curcumin.
Effects of curcumin, B(Cur)2 and Fe(Cur)3 on apoptosis- associated proteins
The relative expression levels of 35 apoptosis-related proteins were assessed. A total of 17 proteins were detected in the MDA-MB-231 cell extracts according to the proteome profiler assay (Table I). The relative expression of the proapoptotic proteins cytochrome c and cleaved caspase-3 was markedly increased in MDA-MB-231 cells following treatment with B(Cur)2 and Fe(Cur)3. Cell treatment with curcumin also increased the expression of cytochrome c and cleaved caspase-3, but in a more modest way. Furthermore, cell treatment with curcumin, B(Cur)2 and Fe(Cur)3 decreased the expression of the three antiapoptotic proteins cIAP-1, claspin and survivin by <10-fold. In addition, an increase in the expression of haem oxygenase-1 (HO-1) was observed in MDA-MB-231 cells following treatment with curcumin, B(Cur)2 and Fe(Cur)3, in particular with B(Cur)2 and Fe(Cur)3 higher compared with curcumin.
Table I.
Regulation of pro-apoptotic and anti-apoptotic proteins in MDA-MB-231 cell line following treatment with curcumin, B(Cur)2 and Fe(Cur)3.
| Protein | Effect on apoptosis | Curcumin | B(Cur)2 | Fe(Cur)3 |
|---|---|---|---|---|
| Bad | Pro | ↓ | ↓ | |
| Pro-caspase 3 | Pro | ↓ | ↓ | ↓ |
| Cleaved caspase 3 | Pro | ↓ | ↑↑↑ | ↑↑↑ |
| Cytochrome c | Pro | ↓ | ↑↑ | ↑↑ |
| TRAIL R2/DR5 | Pro | ↓ | ↓ | |
| FADD | Pro | ↓ | ↓ | ↓ |
| HIF-1α | Pro | ↓ | ||
| HSP70 | Pro | ↓ | ↓ | ↓ |
| HTRA2/Omi | Pro | ↓ | ↓ | ↓ |
| Phospho-p53 (S15) | Pro | ↓ | ↓ | ↓ |
| Phospho-p53 (S46) | Pro | ↓ | ↓ | ↓ |
| Phospho-p53 (S392) | Pro | ↓ | ↓ | ↓ |
| SMAC/Diablo | Pro | ↓ | ↓ | |
| cIAP-1 | Anti | ↓ | ↓ | ↓ |
| Claspin | Anti | ↓ | ↓ | ↓ |
| HO-1 | Anti | ↑↑↑↑ | ↑↑↑↑↑ | ↑↑↑↑↑ |
| Survivin | Anti | ↓ | ↓ | ↓ |
Direction of arrow indicates up- or downregulation compared with untreated control group. Number of arrows indicates the relative difference in density of pixels between control and treated sample. The fold difference in pixel density was as follows: ↑↑=10, ↑↑↑=100, ↑↑↑↑=1,000 and ↑↑↑↑↑=10,000. B(Cur)2, boron-curcumin; Fe(Cur)3, iron-curcumin.
Ion channel gene expression
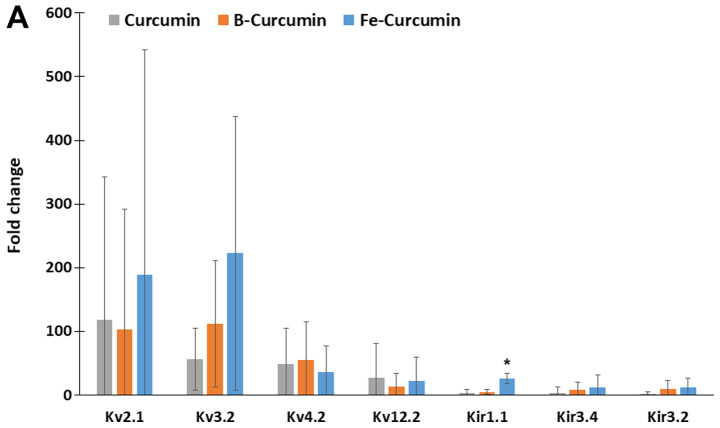
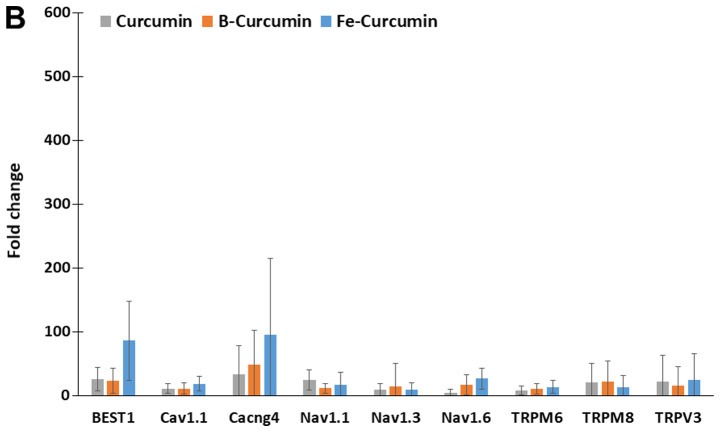
The relative expression of 84 ion-channel genes was analysed using the ion channel array profiler. The relative expression levels of 26 ion-channel genes were statistically significantly different in cells treated with curcumin, B(Cur)2 or Fe(Cur)3 compared with the untreated control cells. Furthermore, MDA-MB-231 cells treated with curcumin, B(Cur)2 and Fe(Cur)3 displayed expression levels >10-fold higher for 16 ion channel genes, including seven potassium channels and nine non-potassium channels, compared with the untreated control cells (vehicle; Fig. 6A and B). The expression levels of Kv2.1 and Kv3.2 were increased by >50-fold in MDA-MB-cells treated with curcumin, B(Cur)2 or Fe(Cur)3. Furthermore, cells treated with B(Cur)2 displayed a 50-fold increase in Kv4.2 expression level compared with the control cells. In addition, the relative expression levels of bestrophin-1 (BEST1) and calcium voltage-gated channel auxiliary subunit γ4 (CACNG4) were increased by >50-fold in cells treated with Fe(Cur)3 compared with control cells.
Figure 6.
Effects of curcumin, B(Cur)2 and Fe(Cur)3 on the relative gene expression of ion channels in MDA-MB-231 cells. MDA-MB-231 cells were untreated (control) or treated with curcumin (25 µM), B(Cur)2 (35 µM) or Fe(Cur)3 (8 µM) for 24 h. Total RNA was extracted, converted to cDNA and subjected to reverse transcription-quantitative PCR. The relative gene expression levels of (A) potassium and (B) calcium, chloride and sodium channels was evaluated. Data were presented as the means (95% confidence interval) of 3 biological replicates. The relative expression of all genes presented here was significantly different compared with untreated control (*P<0.05). B(Cur)2, boron-curcumin; Fe(Cur)3, iron-curcumin.
Discussion
The present study demonstrated that curcumin, B(Cur)2 and Fe(Cur)3 may be cytotoxic for MDA-MB-231 cells. In addition to inhibiting cell proliferation, the three compounds inhibited cell invasive ability; however, only curcumin and B(Cur)2 reduced cell migratory ability.
As B(Cur)2 and Fe(Cur)3 are new compounds, their effect on breast cancer cells requires further investigation. It has been reported that curcumin localized primarily in the perinuclear region of the cell (62). Despite structural differences, B(Cur)2 and Fe(Cur)3 displayed a similar pattern of cellular localization to curcumin in the present study. Kunwar et al (63) reported that curcumin localization was mainly at the plasma membrane, followed by the cytoplasm and the nucleus in MCF-7 breast cancer cells (63). Cytoplasmic localization of iron-containing curcumin derivative has also been reported in MCF-7 breast cancer cells (64). In the present study, curcumin, B(Cur)2 and Fe(Cur)3 induced morphological changes in MDA-MB-231 cells from a typical spindle shape to a rounded structure with no visible blebs near the plasma membrane. Ganguly et al (65) also described this feature, suggesting that curcumin may modify the cell shape and reduce cell attachment by downregulating focal adhesion kinase expression.
To further investigate the cell death mechanism, reverse transcription-quantitative PCR was performed to assess the expression of ion channels, and apoptosis proteome profiling was also conducted. The expression levels of the proapoptotic proteins cleaved caspase 3 and cytochrome c were increased in cells treated with curcumin, B(Cur)2 or Fe(Cur)3. The mitochondrial release of cytochrome c induces the activation of the caspase cascade associated with the intrinsic pathway of apoptosis (66). The increased expression of cytochrome c and cleaved caspase-3 reported in the present study suggested that the intrinsic pathway of apoptosis was associated with curcumin exposure in MDA-MB-231 cells. Furthermore, phosphorylated p53 expression levels were slightly decreased following treatment with B(Cur)2 and Fe(Cur)3. Chiu and Su (14,40) reported similar effect of curcumin on MDA-MB-231 cells, and suggested that curcumin may induce apoptosis via a p53 independent pathway or a p53-dependent Bax pathway.
In the present study, HO-1 expression level was increased in MDA-MB-231 cells in response to treatment with curcumin, B(Cur)2 and Fe(Cur)3. Previous studies also reported that curcumin treatment induces HO-1 overexpression in vitro and in vivo (67-71). In hepatoma epithelial cells, the induction of HO-1 expression occurs via a mechanism involving increased oxidative stress and p38 activation (72), whereas in renal epithelial cells, the mechanism involves activation of the protein-1 activator transcription factor (73). Although the protein expression of the antiapoptotic and proproliferative HO-1 was induced by curcumin in the present study, previous studies indicated that curcumin displays anticancer effects. It has been reported that high HO-1 expression favours cancer cell proliferation, poor prognosis and resistance to therapy (74-82). Furthermore, it was demonstrated that HO-1 is associated with antiapoptotic (83,84), proangiogenic (85) and prometastatic (86) activities. HO-1 also promotes cancer cell proliferation via a mechanism that is independent of its catalytic activity, the HO-1 nuclear translocation-induced alterations of gene transcription (87,88). At present, HO-1 is considered as a potential therapeutic target for various cancers (74,89). However, an increase in HO-1 enzyme activity has also been reported to display anticancer effects (90,91), including via the promotion of apoptosis (92,93). Furthermore, it was reported that HO-1 overexpression in breast cancer cell lines can inhibit cell proliferation and invasive ability and induce apoptosis (94-96). Similarly, Lee et al (48,69) demonstrated that curcumin can induce HO-1 protein overexpression in MDA-MB-231 cells, leading to decreased cell proliferation and invasive ability.
In the present study, curcumin, B(Cur)2 and Fe(Cur)3 had variable effects on the expression of certain ion channel genes. High gene expression levels were observed for the Kv2.1, Kv3.2 and Kv4.2 potassium channels following treatment with curcumin, B(Cur)2 or Fe(Cur)3. Furthermore, high expression levels of CACNG4, which is a calcium channel coding gene, and BEST1, which is a calcium-activated chloride channel coding gene, were observed in MDA-MB-231 cells following treatment with curcumin, B(Cur)2 and Fe(Cur)3.
Ion channel overexpression may result in a proapoptotic and antiproliferative response by increasing the movement of ions that favours a decrease in cell volume, which may be involved in initiating apoptosis (43-45,48-51). The cellular potassium efflux induces AVD and subsequent apoptotic events. By downregulating potassium channel expression, cancer cells can therefore evade apoptosis (44,53,97). Numerous voltage-gated potassium channels are known to influence AVD in cancer cells, including Kv1.1, Kv1.3, Kv1.5, Kv2.1 and Kv11.1 (53), with several studies focusing on Kv1.3 and Kv1.5 (51,52,54,98-101). In the present study, no significant alteration in Kv1.3 and Kv1.5 expression levels were observed; however, the expression level of Kv2.1, which is a potassium channel associated with apoptosis, was increased. Previous studies reported that neuronal apoptosis requires a potassium efflux via the Kv2.1 channel (97,102). Curcumin has also been reported to reduce Kv2.1 potassium currents by modulating the inactivation gating of this channel (57). Kv2.1 overexpression, which was observed in the present study, may be a cellular response to the inhibition of the function of this voltage-gates potassium channel.
Cancer cell survival is partly attributed to the evasion of apoptotic processes (39). In the present study, the results from the proteome profile and ion channel expression analyses identified two potential mechanisms underlying cancer cell apoptosis evasion. The findings suggested that curcumin may increase the expression level of HO-1 and Kv2.1 and that HO-1 may counterbalance the effects of Kv2.1. HO-1 catalyses the breakdown of haem to generate biliverdin, iron and carbon monoxide (CO), which inhibit apoptosis. Subsequently, HO-1 and its products may display some antiapoptotic effects in certain cells. CO inhibits the potassium currents generated by the proapoptotic Kv2.1 channel (49,50,103,104). The increase in Kv2.1 expression level observed in the present study may be a cellular response to the HO-1-mediated inhibition of the Kv2.1 channel activity. However, the association between HO-1 and Kv2.1 in breast cancer cells requires further investigation.
Conversely with Kv2.1, only a few studies reported an association between Kv3.2 and apoptosis. Lan et al (105) reported high relative expression of Kv1.3, Kv1.5, Kv1.6, Kv2.1 and Kv2.2 in numerous gastric cancer cell lines; however, Kv3.2 expression level was only just detectable. The increased expression level of Kv3.2 observed in the present study may therefore be specific to breast cancer cells. Some Kv channels are considered oxygen sensors, and Song et al (106) demonstrated that Kv3.1 and Kv3.4 are tumour hypoxia-related channels involved in cancer cell migratory and invasive abilities. Kv3.1 and Kv3.4 protein expression in A549 and MDA-MB-231 cells increase in a cell density-dependent manner. When Kv3.1 and Kv3.4 were inhibited using a Kv3 subfamily-specific blocker, both cell migration and invasion were inhibited (106). In the present study, cell treatment with Fe(Cur)3 induced the largest increase in Kv3.2 expression; however, Fe(Cur)3 was the only compound that failed to inhibit the breast cancer cell migratory ability in the present study. The overexpression of Kv3.2 may be linked to an attenuation in MDA-MB-231 cell ability to migrate.
In the present study, BEST1 was the only anion channel that was significantly upregulated in response to curcumin treatment. BEST1 is responsible for the production of an outward flow of chloride ions, which counterbalances transient membrane potentials induced by potassium efflux for cellular electroneutrality (51,107). Several family members of the calcium-activated chloride channels, including BEST1, have been reported to promote cell proliferation and tumorigenesis (52,53,108,109). Furthermore, it was reported that both potassium voltage-gated channel subfamily H member 1 and BEST1 are involved in the transformation of slow-growing T84 colonic carcinoma cells into fast-growing T84 cells (109).
In the present study, the effects of curcumin, B(Cur)2 and Fe(Cur)3 were evaluated in one breast cancer cell line only. Although the MDA-MB-231 cell line (36), has proven useful in laboratory investigations of genetics, molecular biology and biology of TNBC (37), this represents a limitation to this study. Further investigation in additional breast cancer cell lines, including MCF-7, is therefore required. Both MCF-7 and MDA-MB-231 cell lines are invasive ductal/breast carcinoma cells; however, these cells display significant phenotypic and genotypic differences. MCF-7 is a hormone dependent cell line due to its expression of ER and PR, whereas the MDA-MB-231 cell line lacks ER and PR. Since MDA-MB-231 cells are highly metastatic and more aggressive compared with MCF-7 cells, the MDA-MB-231 cell line was the most appropriate cell line for investigating the anticancer properties of curcumin compounds in the present study.
Previous studies reported that B(Cur)2 and Fe(Cur)3 display improved photostability and high fluorescence efficiency compared with curcumin (9,10,34,35). The present study investigated whether curcumin complexes possessed similar biological properties as curcumin. The results demonstrated that Fe(Cur)3 was more effective at inhibiting cell proliferation and random invasion at low doses compared with curcumin. Conversely, B(Cur)2 was only effective at inhibiting the cell migratory ability. B(Cur)2 and Fe(Cur)3 displayed similar anticancer properties to curcumin. Curcumin may serve as an adjuvant for chemotherapy. The increase in Kv2.1, Kv3.2 and HO-1 highlight important changes in molecular responses to curcumin induced inhibition of proliferation and invasion of breast cancer cells. In conclusion, the present study demonstrated that HO-1 and potassium channels may be considered as important therapeutic targets in breast cancer.
Acknowledgments
Not applicable.
Funding
This study was supported by the RCSI (Bahrain) internal research grants (grant no. BR00061) and the Dr Ali Al-Khalifa Research Fund (Bahrain) (grant no. AK-2017).
Availability of data and material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
FM designed the study, performed cell and molecular experiments and analysed data. FR had the original research idea, developed the experimental design, conducted data analysis and reading and revised the manuscript. ST performed cell and molecular experiments and analysed data. SC synthesised the curcumin complexes. SF was responsible for funding acquisition, data curation and manuscript writing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: A collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hugh J, Hanson J, Cheang MC, Nielsen TO, Perou CM, Dumontet C, Reed J, Krajewska M, Treilleux I, Rupin M, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: Use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27:1168–1176. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 5.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 6.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Yang J, Peng L, Sahin AA, Huo L, Ward KC, O'Regan R, Torres MA, Meisel JL. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2017;161:279–287. doi: 10.1007/s10549-016-4059-6. [DOI] [PubMed] [Google Scholar]
- 8.Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 9.Massat NJ, Dibden A, Parmar D, Cuzick J, Sasieni PD, Duffy SW. Impact of screening on breast cancer mortality: The UK program 20 years on. Cancer Epidemiol Biomarkers Prev. 2016;25:455–462. doi: 10.1158/1055-9965.EPI-15-0803. [DOI] [PubMed] [Google Scholar]
- 10.Kalager M, Zelen M, Langmark F, Adami HO. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203–1210. doi: 10.1056/NEJMoa1000727. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 12.Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem. 2005;280:20059–20068. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- 13.Pal S, Bhattacharyya S, Choudhuri T, Datta GK, Das T, Sa G. Amelioration of immune cell number depletion and potentiation of depressed detoxification system of tumor-bearing mice by curcumin. Cancer Detect Prev. 2005;29:470–478. doi: 10.1016/j.cdp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Shim JS, Kim JH, Cho HY, Yum YN, Kim SH, Park HJ, Shim BS, Choi SH, Kwon HJ. Irreversible inhibition of CD13/aminopeptidase N by the antiangiogenic agent curcumin. Chem Biol. 2003;10:695–704. doi: 10.1016/S1074-5521(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 15.Yang X, Thomas DP, Zhang X, Culver BW, Alexander BM, Murdoch WJ, Rao MN, Tulis DA, Ren J, Sreejayan N. Curcumin inhibits platelet-derived growth factor-stimulated vascular smooth muscle cell function and injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2006;26:85–90. doi: 10.1161/01.ATV.0000191635.00744.b6. [DOI] [PubMed] [Google Scholar]
- 16.Zeitlin P. Can curcumin cure cystic fibrosis? N Engl J Med. 2004;351:606–608. doi: 10.1056/NEJMcibr041584. [DOI] [PubMed] [Google Scholar]
- 17.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, Kakarala M, Carpenter PM, McLaren C, Meyskens FL, Jr, Brenner DE. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4:354–364. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011;29:208–213. doi: 10.3109/07357907.2010.550592. [DOI] [PubMed] [Google Scholar]
- 19.Hejazi J, Rastmanesh R, Taleban FA, Molana SH, Hejazi E, Ehtejab G, Hara N. Effect of curcumin supplementation during radiotherapy on oxidative status of patients with prostate cancer: A double blinded, randomized, placebo-controlled study. Nutr Cancer. 2016;68:77–85. doi: 10.1080/01635581.2016.1115527. [DOI] [PubMed] [Google Scholar]
- 20.Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Br J Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 21.Durgaprasad S, Pai CG, Vasanthkumar, Alvres JF, Namitha S. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J Med Res. 2005;122:315–318. [PubMed] [Google Scholar]
- 22.Sahin Kavaklı H, Koca C, Alıcı O. Antioxidant effects of curcumin in spinal cord injury in rats. Ulus Travma Acil Cerrahi Derg. 2011;17:14–18. doi: 10.5505/tjtes.2011.31391. [DOI] [PubMed] [Google Scholar]
- 23.Meng B, Li J, Cao H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr Pharm Des. 2013;19:2101–2113. [PubMed] [Google Scholar]
- 24.Lüer S, Troller R, Aebi C. Antibacterial and antiinflammatory kinetics of curcumin as a potential antimucositis agent in cancer patients. Nutr Cancer. 2012;64:975–981. doi: 10.1080/01635581.2012.713161. [DOI] [PubMed] [Google Scholar]
- 25.Beevers CS, Huang S. Pharmacological and clinical properties of curcumin. Botanics Targets Ther. 2011;1:5–18. [Google Scholar]
- 26.Shishodia S, Sethi G, Aggarwal BB. Curcumin: Getting back to the roots. Ann N Y Acad Sci. 2005;1056:206–217. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 28.Tønnesen HH, Karlsen J, van Henegouwen GB. Studies on curcumin and curcuminoids. VIII. Photochemical stability of curcumin. Z Lebensm Unters Forsch. 1986;183:116–122. doi: 10.1007/BF01041928. [DOI] [PubMed] [Google Scholar]
- 29.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867–1876. doi: 10.1016/S0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 30.Cartiera MS, Ferreira EC, Caputo C, Egan ME, Caplan MJ, Saltzman WM. Partial correction of cystic fibrosis defects with PLGA nanoparticles encapsulating curcumin. Mol Pharm. 2010;7:86–93. doi: 10.1021/mp900138a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang G, Shao L, Wang Y, Zhao C, Chu Y, Xiao J, Zhao Y, Li X, Yang S. Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg Med Chem. 2009;17:2623–2631. doi: 10.1016/j.bmc.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 32.Wanninger S, Lorenz V, Subhan A, Edelmann FT. Metal complexes of curcumin-synthetic strategies, structures and medicinal applications. Chem Soc Rev. 2015;44:4986–5002. doi: 10.1039/C5CS00088B. [DOI] [PubMed] [Google Scholar]
- 33.Greish K, Pittalà V, Taurin S, Taha S, Bahman F, Mathur A, Jasim A, Mohammed F, El-Deeb IM, Fredericks S, Rashid-Doubell F. Curcumin-copper complex nanoparticles for the management of triple-negative breast cancer. Nanomaterials (Basel) 2018;8:E884. doi: 10.3390/nano8110884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohammed F, Rashid-Doubell F, Cassidy S, Henari F. A comparative study of the spectral, fluorometric properties and photostability of natural curcumin, iron- and boron-complexed curcumin. Spectrochim Acta A Mol Biomol Spectrosc. 2017;183:439–450. doi: 10.1016/j.saa.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 35.Pröhl M, Schubert US, Weigand W, Gottschaldt M. Metal complexes of curcumin and curcumin derivatives for molecular imaging and anticancer therapy. Coord Chem Rev. 2016;307:32–41. doi: 10.1016/j.ccr.2015.09.001. [DOI] [Google Scholar]
- 36.Cailleau R, Olivé M, Cruciger QV. Long-term human breast carcinoma cell lines of metastatic origin: Preliminary characterization. In Vitro. 1978;14:911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- 37.Chavez KJ, Garimella SV, Lipkowitz S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010;32:35–48. doi: 10.3233/BD-2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 39.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Chiu TL, Su CC. Curcumin inhibits proliferation and migration by increasing the Bax to Bcl-2 ratio and decreasing NF-kappaBp65 expression in breast cancer MDA-MB-231 cells. Int J Mol Med. 2009;23:469–475. doi: 10.3892/ijmm_00000153. [DOI] [PubMed] [Google Scholar]
- 41.Lv ZD, Liu XP, Zhao WJ, Dong Q, Li FN, Wang HB, Kong B. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int J Clin Exp Pathol. 2014;7:2818–2824. [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HI, Huang H, Cheepala S, Huang S, Chung J. Curcumin inhibition of integrin (alpha6beta4)-dependent breast cancer cell motility and invasion. Cancer Prev Res (Phila) 2008;1:385–391. doi: 10.1158/1940-6207.CAPR-08-0087. [DOI] [PubMed] [Google Scholar]
- 43.Yee NS. Roles of TRPM8 Ion channels in cancer: Proliferation, survival, and invasion. Cancers (Basel) 2015;7:2134–2146. doi: 10.3390/cancers7040882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pardo LA, Stühmer W. The roles of K(+) channels in cancer. Nat Rev Cancer. 2014;14:39–48. doi: 10.1038/nrc3635. [DOI] [PubMed] [Google Scholar]
- 45.Urrego D, Tomczak AP, Zahed F, Stühmer W, Pardo LA. Potassium channels in cell cycle and cell proliferation. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130094. doi: 10.1098/rstb.2013.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Litan A, Langhans SA. Cancer as a channelopathy: Ion channels and pumps in tumor development and progression. Front Cell Neurosci. 2015;9:86. doi: 10.3389/fncel.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Djamgoz MB, Onkal R. Persistent current blockers of voltage-gated sodium channels: A clinical opportunity for controlling metastatic disease. Recent Pat Anticancer Drug Discov. 2013;8:66–84. doi: 10.2174/1574892811308010066. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Yang Z, Meng Z, Cao H, Zhu G, Liu T, Wang X. Knockdown of TRPM8 suppresses cancer malignancy and enhances epirubicin-induced apoptosis in human osteosarcoma cells. Int J Biol Sci. 2013;10:90–102. doi: 10.7150/ijbs.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann EK, Lambert IH. Ion channels and transporters in the development of drug resistance in cancer cells. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130109. doi: 10.1098/rstb.2013.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang M, Brackenbury WJ. Membrane potential and cancer progression. Front Physiol. 2013;4:185. doi: 10.3389/fphys.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bortner CD, Cidlowski JA. Ion channels and apoptosis in cancer. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130104. doi: 10.1098/rstb.2013.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Felipe A, Bielanska J, Comes N, Vallejo A, Roig S. Targeting the voltage-dependent K(+) channels Kv1.3 and Kv1.5 as tumor biomarkers for cancer detection and prevention. Curr Med Chem. 2012;19:661–674. doi: 10.2174/092986712798992048. [DOI] [PubMed] [Google Scholar]
- 53.Szabò I, Zoratti M, Gulbins E. Contribution of voltage-gated potassium channels to the regulation of apoptosis. FEBS Lett. 2010;584:2049–2056. doi: 10.1016/j.febslet.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 54.Szabó I, Bock J, Grassmé H, Soddemann M, Wilker B, Lang F, Zoratti M, Gulbins E. Mitochondrial potassium channel Kv1.3 mediates Bax-induced apoptosis in lymphocytes. Proc Natl Acad Sci USA. 2008;105:14861–14866. doi: 10.1073/pnas.0804236105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banderali U, Belke D, Singh A, Jayanthan A, Giles WR, Narendran A. Curcumin blocks Kv11.1 (erg) potassium current and slows proliferation in the infant acute monocytic leukemia cell line THP-1. Cell Physiol Biochem. 2011;28:1169–1180. doi: 10.1159/000335850. [DOI] [PubMed] [Google Scholar]
- 56.Lian YT, Yang XF, Wang ZH, Yang Y, Yang Y, Shu YW, Cheng LX, Liu K. Curcumin serves as a human kv1.3 blocker to inhibit effector memory T lymphocyte activities. Phytother Res. 2013;27:1321–1327. doi: 10.1002/ptr.4863. [DOI] [PubMed] [Google Scholar]
- 57.Aréchiga-Figueroa IA, Delgado-Ramirez M, Morán-Zendejas R, Rodriguez-Menchaca AA. Modulation of Kv2.1 channels inactivation by curcumin. Pharmacol Rep. 2015;67:1273–1279. doi: 10.1016/j.pharep.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 58.Khalil MI, AL-Zahem AM, Qunaibit MM. Synthesis, characterization, and antitumor activity of binuclear curcumin-metal(II) hydroxo complexes. Med Chem Res. 2014;23:1683–1689. doi: 10.1007/s00044-013-0727-9. [DOI] [Google Scholar]
- 59.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 60.Heit B, Kubes P. Measuring chemotaxis and chemokinesis: The under-agarose cell migration assay. Sci STKE. 2003;2003:PL5. doi: 10.1126/stke.2003.170.pl5. [DOI] [PubMed] [Google Scholar]
- 61.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 62.Hope-Roberts M, Horobin RW. A review of curcumin as a biological stain and as a self-visualizing pharmaceutical agent. Biotech Histochem. 2017;92:315–323. doi: 10.1080/10520295.2017.1310925. [DOI] [PubMed] [Google Scholar]
- 63.Kunwar A, Barik A, Mishra B, Rathinasamy K, Pandey R, Priyadarsini KI. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim Biophys Acta. 2008;1780:673–679. doi: 10.1016/j.bbagen.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 64.Sarkar T, Butcher RJ, Banerjee S, Mukherjee S, Hussain A. Visible light-induced cytotoxicity of a dinuclear iron(III) complex of curcumin with low-micromolar IC50 value in cancer cells. Inorganica Chimica Acta. 2016;439:8–17. doi: 10.1016/j.ica.2015.09.026. [DOI] [Google Scholar]
- 65.Ganguly KK, Sen T, Pal S, Biswas J, Chatterjee A. Studies on focal adhesion kinase in human breast cancer cell MDA-MB-231. Adv Biol Chem. 2012;2:29–42. doi: 10.4236/abc.2012.21004. [DOI] [Google Scholar]
- 66.Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 67.Xiao Y, Xia J, Wu S, Lv Z, Huang S, Huang H, Su X, Cheng J, Ke Y. Curcumin inhibits acute vascular inflammation through the activation of heme oxygenase-1. Oxid Med Cell Longev. 2018;2018:3295807. doi: 10.1155/2018/3295807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu SY, Lee YR, Huang CC, Li YZ, Chang YS, Yang CY, Wu JD, Liu YW. Curcumin-induced heme oxygenase-1 expression plays a negative role for its anti-cancer effect in bladder cancers. Food Chem Toxicol. 2012;50:3530–3536. doi: 10.1016/j.fct.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 69.Lee WY, Chen YC, Shih CM, Lin CM, Cheng CH, Chen KC, Lin CW. The induction of heme oxygenase-1 suppresses heat shock protein 90 and the proliferation of human breast cancer cells through its byproduct carbon monoxide. Toxicol Appl Pharmacol. 2014;274:55–62. doi: 10.1016/j.taap.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 70.Park J, Conteas CN. Anti-carcinogenic properties of curcumin on colorectal cancer. World J Gastrointest Oncol. 2010;2:169–175. doi: 10.4251/wjgo.v2.i4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med. 2007;19:165–172. [PubMed] [Google Scholar]
- 73.Mimche PN, Taramelli D, Vivas L. The plant-based immunomodulator curcumin as a potential candidate for the development of an adjunctive therapy for cerebral malaria. Malar J. 2011;10(Suppl 1):S10. doi: 10.1186/1475-2875-10-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nitti M, Piras S, Marinari UM, Moretta L, Pronzato MA, Furfaro AL. HO-1 induction in cancer progression: A matter of cell adaptation. Antioxidants(Basel) 2017;6:E29. doi: 10.3390/antiox6020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeon WK, Hong HY, Seo WC, Lim KH, Lee HY, Kim WJ, Song SY, Kim BC. Smad7 sensitizes A549 lung cancer cells to cisplatin-induced apoptosis through heme oxygenase-1 inhibition. Biochem Biophys Res Commun. 2012;420:288–292. doi: 10.1016/j.bbrc.2012.02.151. [DOI] [PubMed] [Google Scholar]
- 76.Kongpetch S, Kukongviriyapan V, Prawan A, Senggunprai L, Kukongviriyapan U, Buranrat B. Crucial role of heme oxygenase-1 on the sensitivity of cholangiocarcinoma cells to chemotherapeutic agents. PLoS One. 2012;7:e34994. doi: 10.1371/journal.pone.0034994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lv X, Song DM, Niu YH, Wang BS. Inhibition of heme oxygenase-1 enhances the chemosensitivity of laryngeal squamous cell cancer Hep-2 cells to cisplatin. Apoptosis. 2016;21:489–501. doi: 10.1007/s10495-016-1216-7. [DOI] [PubMed] [Google Scholar]
- 78.Tracey N, Creedon H, Kemp AJ, Culley J, Muir M, Klinowska T, Brunton VG. HO-1 drives autophagy as a mechanism of resistance against HER2-targeted therapies. Breast Cancer Res Treat. 2020;179:543–555. doi: 10.1007/s10549-019-05489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao Z, Xu Y, Lu J, Xue J, Liu P. High expression of HO-1 predicts poor prognosis of ovarian cancer patients and promotes proliferation and aggressiveness of ovarian cancer cells. Clin Transl Oncol. 2018;20:491–499. doi: 10.1007/s12094-017-1738-7. [DOI] [PubMed] [Google Scholar]
- 80.Becker JC, Fukui H, Imai Y, Sekikawa A, Kimura T, Yamagishi H, Yoshitake N, Pohle T, Domschke W, Fujimori T. Colonic expression of heme oxygenase-1 is associated with a better long-term survival in patients with colorectal cancer. Scand J Gastroenterol. 2007;42:852–858. doi: 10.1080/00365520701192383. [DOI] [PubMed] [Google Scholar]
- 81.Loboda A, Jozkowicz A, Dulak J. HO-1/CO system in tumor growth, angiogenesis and metabolism-targeting HO-1 as an anti-tumor therapy. Vascul Pharmacol. 2015;74:11–22. doi: 10.1016/j.vph.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Maines MD, Abrahamsson PA. Expression of heme oxygenase-1 (HSP32) in human prostate: Normal, hyperplastic, and tumor tissue distribution. Urology. 1996;47:727–733. doi: 10.1016/S0090-4295(96)00010-6. [DOI] [PubMed] [Google Scholar]
- 83.Busserolles J, Megías J, Terencio MC, Alcaraz MJ. Heme oxygenase-1 inhibits apoptosis in Caco-2 cells via activation of Akt pathway. Int J Biochem Cell Biol. 2006;38:1510–1517. doi: 10.1016/j.biocel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 84.Tanaka S, Akaike T, Fang J, Beppu T, Ogawa M, Tamura F, Miyamoto Y, Maeda H. Antiapoptotic effect of haem oxygenase-1 induced by nitric oxide in experimental solid tumour. Br J Cancer. 2003;88:902–909. doi: 10.1038/sj.bjc.6600830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cherrington JM, Strawn LM, Shawver LK. New paradigms for the treatment of cancer: The role of anti-angiogenesis agents. Adv Cancer Res. 2000;79:1–38. doi: 10.1016/S0065-230X(00)79001-4. [DOI] [PubMed] [Google Scholar]
- 86.Price JT, Thompson EW. Mechanisms of tumour invasion and metastasis: Emerging targets for therapy. Expert Opin Ther Targets. 2002;6:217–233. doi: 10.1517/14728222.6.2.217. [DOI] [PubMed] [Google Scholar]
- 87.Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, Dennery PA. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 88.Biswas C, Shah N, Muthu M, La P, Fernando AP, Sengupta S, Yang G, Dennery PA. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J Biol Chem. 2014;289:26882–26894. doi: 10.1074/jbc.M114.567685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Was H, Dulak J, Jozkowicz A. Heme oxygenase-1 in tumor biology and therapy. Curr Drug Targets. 2010;11:1551–1570. doi: 10.2174/1389450111009011551. [DOI] [PubMed] [Google Scholar]
- 90.Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, Clinton Webb R, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7:693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 91.Taillé C, Almolki A, Benhamed M, Zedda C, Mégret J, Berger P, Lesèche G, Fadel E, Yamaguchi T, Marthan R, et al. Heme oxygenase inhibits human airway smooth muscle proliferation via a bilirubin-dependent modulation of ERK1/2 phosphorylation. J Biol Chem. 2003;278:27160–27168. doi: 10.1074/jbc.M300364200. [DOI] [PubMed] [Google Scholar]
- 92.Ozawa N, Goda N, Makino N, Yamaguchi T, Yoshimura Y, Suematsu M. Leydig cell-derived heme oxygenase-1 regulates apoptosis of premeiotic germ cells in response to stress. J Clin Invest. 2002;109:457–467. doi: 10.1172/JCI0213190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu XM, Chapman GB, Wang H, Durante W. Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation. 2002;105:79–84. doi: 10.1161/hc0102.101369. [DOI] [PubMed] [Google Scholar]
- 94.Hill M, Pereira V, Chauveau C, Zagani R, Remy S, Tesson L, Mazal D, Ubillos L, Brion R, Asghar K, et al. Heme oxygenase-1 inhibits rat and human breast cancer cell proliferation: Mutual cross inhibition with indoleamine 2,3-dioxygenase. FASEB J. 2005;19:1957–1968. doi: 10.1096/fj.05-3875com. [DOI] [PubMed] [Google Scholar]
- 95.Lin CW, Shen SC, Hou WC, Yang LY, Chen YC. Heme oxygenase-1 inhibits breast cancer invasion via suppressing the expression of matrix metalloproteinase-9. Mol Cancer Ther. 2008;7:1195–1206. doi: 10.1158/1535-7163.MCT-07-2199. [DOI] [PubMed] [Google Scholar]
- 96.Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxy-genase-1 by dietary polyphenols. Mol Pharmacol. 2006;69:1033–1040. doi: 10.1124/mol.105.018374. [DOI] [PubMed] [Google Scholar]
- 97.Pal SK, Takimoto K, Aizenman E, Levitan ES. Apoptotic surface delivery of K+ channels. Cell Death Differ. 2006;13:661–667. doi: 10.1038/sj.cdd.4401792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Comes N, Bielanska J, Vallejo-Gracia A, Serrano-Albarrás A, Marruecos L, Gómez D, Soler C, Condom E, Ramón Y, Cajal S, et al. The voltage-dependent K(+) channels Kv1.3 and Kv1.5 in human cancer. Front Physiol. 2013;4:283. doi: 10.3389/fphys.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lowinus T, Heidel FH, Bose T, Nimmagadda SC, Schnöder T, Cammann C, Schmitz I, Seifert U, Fischer T, Schraven B, Bommhardt U. Memantine potentiates cytarabine-induced cell death of acute leukemia correlating with inhibition of Kv1.3 potassium channels, AKT and ERK1/2 signalin. Cell Commun Signal. 2019;17:5. doi: 10.1186/s12964-018-0317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Teisseyre A, Palko-Labuz A, Sroda-Pomianek K, Michalak K. Voltage-gated potassium channel Kv1.3 as a target in therapy of cancer. Front Oncol. 2019;9:933. doi: 10.3389/fonc.2019.00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu J, Chen Z, Liu Q, Zeng W, Wu X, Lin B. Silencing of Kv1.5 gene inhibits proliferation and induces apoptosis of osteo-sarcoma cells. Int J Mol Sci. 2015;16:26914–26926. doi: 10.3390/ijms161126002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J Neurosci. 2003;23:4798–4802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Al-Owais MM, Dallas ML, Boyle JP, Scragg JL, Peers C. Heme oxygenase-1 influences apoptosis via CO-mediated inhibition of K+ channels. Adv Exp Med Biol. 2015;860:343–351. doi: 10.1007/978-3-319-18440-1_39. [DOI] [PubMed] [Google Scholar]
- 104.Al-Owais MM, Scragg JL, Dallas ML, Boycott HE, Warburton P, Chakrabarty A, Boyle JP, Peers C. Carbon monoxide mediates the anti-apoptotic effects of heme oxygenase-1 in medulloblastoma DAOY cells via K+ channel inhibition. J Biol Chem. 2012;287:24754–24764. doi: 10.1074/jbc.M112.357012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lan M, Shi Y, Han Z, Hao Z, Pan Y, Liu N, Guo C, Hong L, Wang J, Qiao T, Fan D. Expression of delayed rectifier potassium channels and their possible roles in proliferation of human gastric cancer cells. Cancer Biol Ther. 2005;4:1342–1347. doi: 10.4161/cbt.4.12.2175. [DOI] [PubMed] [Google Scholar]
- 106.Song MS, Park SM, Park JS, Byun JH, Jin HJ, Seo SH, Ryu PD, Lee SY. Kv3.1 and Kv3.4, are involved in cancer cell migration and invasion. Int J Mol Sci. 2018;19:E1061. doi: 10.3390/ijms19041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schwab A, Fabian A, Hanley PJ, Stock C. Role of ion channels and transporters in cell migration. Physiol Rev. 2012;92:1865–1913. doi: 10.1152/physrev.00018.2011. [DOI] [PubMed] [Google Scholar]
- 108.Duvvuri U, Shiwarski DJ, Xiao D, Bertrand C, Huang X, Edinger RS, Rock JR, Harfe BD, Henson BJ, Kunzelmann K, et al. TMEM16A induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 2012;72:3270–3281. doi: 10.1158/0008-5472.CAN-12-0475-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spitzner M, Martins JR, Soria RB, Ousingsawat J, Scheidt K, Schreiber R, Kunzelmann K. Eag1 and Bestrophin 1 are up-regulated in fast-growing colonic cancer cells. J Biol Chem. 2008;283:7421–7428. doi: 10.1074/jbc.M703758200. [DOI] [PubMed] [Google Scholar]