Abstract
Transthoracic echocardiography is a useful diagnostic technique for the identification of intracardiac and extracardiac masses, which can evaluate morphologic properties of the masses such as their location, attachment, shape, size, mobility, and possible hemodynamic-related implications. Apart from physiological variants and structural normal mimickers, echocardiography can detect principal intracardiac masses such as neoplasms, thrombi, vegetation, and extracardiac masses such as metastatic lesions. Moreover, transesophageal echocardiography can provide further details and provide higher accuracy in case a deeper examination of the mass is needed. This review will focus on the systematic evaluation of intra-/extracardiac masses including epidemiology and morphological and echocardiographic features, providing practical and technical tips to health-care professionals to achieve correct identification of the masses. General data on cardiac masses were extracted via PubMed/MEDLINE search engine from indexed reviews, original studies, and clinical case reports. The echocardiographic features of cardiac masses were reviewed according to the most relevant international cardiology and echocardiography scientific societies' position statements.
Keywords: Cardiac masses, echocardiography, neoplasia, thrombus, vegetation
INTRODUCTION
Transthoracic echocardiography (TTE) can provide quality, dynamic imaging assessment of intracardiac and extracardiac masses. TTE can be performed bedside and is a well-known widespread diagnostic technique useful for morphological and functional assessment of the heart. However, basic premises need to be stressed:expert and certified ultrasound skill of health-care professional who performs the exam is mandatory; this is not a redundant statement as interobserver variability due to unskillfulness is clearly related to diagnostic pitfalls;[1,2,3] and a thorough understanding of normal anatomy and normal variants considering embryologic residues is crucial in cardiac imaging which will further avoid potential misdiagnosis. Clearly, echocardiographic findings have to be related to patient's history and clinical data. Moreover, TTE may be limited in obese patients and those with poor acoustic windows. Complete diagnostic achievement of any cardiac mass obviously derives from multimodality imaging approach and definitive diagnostic histologic examination. Both transthoracic and transesophageal echocardiography (TOE) associated with three-dimensional (3D) echocardiography can be a value-added tool in identifying masses and differential diagnosis.[4]
METHODS
We considered and classified the most important and frequently scanned cardiac masses in echocardiography analyzing their epidemiology and main echocardiographic features. We included some exemplary cases, reviewing the scientific literature via PubMed/MEDLINE database, searching for the most relevant case reports, reviews, and original articles. Echocardiographic pictures presented in this review were extracted from an archive of clinical cases actually diagnosed and managed in the cardiology department of a single-center, community, public health hospital located in Rome – Italy. All the examinations were performed by the European Society of Cardiology/European Association of Cardiovascular Imaging (ESC/EACVI), formerly accredited cardiology consultant in high-volume performance echocardiography laboratory.
Both TTE and TOE pictures presented in this review were acquired using an IE33 Philips ultrasound equipment (Philips Medical Systems”, 22100 Bothell-Everett Highway, Bothell, WA 98021-8431, USA). We analyzed and classified main echocardiographic findings related to cardiac masses, providing specific clinical and echocardiographic details, showing exemplary pictures, and correlating specific echocardiographic features with recommendations acquired from echocardiographic position statements extracted from the most relevant international scientific societies of cardiology and cardiovascular imaging field in order to underline and stress which features to search for, in every specific case.
Our aim is to provide a practical tool that can be useful in daily practice for health-care professionals who perform echocardiographic examinations. This review will not analyze anecdotal, extraordinary cases but will focus on more frequently encountered daily practice routine cases providing a useful, pragmatic tool to improve correct analysis of ultrasound images, leading to correct diagnosis.
Normal variants, mimickers, foreign bodies, and echocardiographic artifacts
A solid knowledge of morphological anatomy and embryological development of the heart is mandatory for any health-care professional performing echocardiography. Any health-care professional working as a sonographer or imaging consultant needs to know about normal variants and nonpathological, or pathological, but not cardiac mass-related echo findings.[5,6,7] Ultrasound artifacts need to be considered as well: they can potentially mislead to incorrect identification of nonexisting intracardiac masses.[8]
The main echocardiographic findings that can mimic cardiac masses are reported in Table 1.
Table 1.
Structures potentially mimicking cardiac masses in echocardiography
| Normal variants or congenital remnants | Pathological variants not related to specific cardiac masses | Iatrogenic foreign bodies or ectopic structures | Artifacts |
|---|---|---|---|
| LV webs, heartstrings, and chords | Apical form of hypertrophic cardiomyopathy | Pacemaker wires | Near-field clutter |
| Apical trabeculations | Interatrial septal aneurysm | AICD leads | Beam-width artifact |
| LA chords | Venous varices (clumps of veins) | Occluder interatrial devices | Enhancement |
| Dilated coronary sinus (PLAX view) | Lambl’s excrescences | Hiatal hernia | Reverberation |
| Prominent descending aorta (A4Ch view) | Nodules of Arantius | Heterotopic thyroid tissue | Refraction |
| Multiple lobes of the LA appendage | Fibrinous pericardial patch | Central venous lines | Mirror image |
| Pectinate muscles in LA or RA appendages | Dystrophic calcification of the mitral annulus | Mitral and tricuspid interventional | |
| Prominent moderator band | Endomyocardial fibrosis/Loeffler endocarditis | devices (e.g., MitraClipTM, | |
| Prominent crista terminalis | Hematic cyst (childhood) | MitralixTM, TriClipTM) | |
| Prominent Eustachian valve | Pericardial cyst | ||
| Chiari network | Mesothelial/monocytic incidental cardiac excrescences | ||
| Redundant mitral chordae apparatus and redundant leaflet tissue of the mitral valve | |||
| Bronchogenic cyst | |||
| Prominent epicardial fat | Other infective masses (echinococcus cyst, aspergilloma, tuberculoma) Noncompaction cardiomyopathy Prominent or calcified papillary muscles Dense mitral annular calcification Lipomatous hypertrophy of interatrial septum |
LV=Left ventricular, LA=Left atrium, RA=Right atrium, PLAX=Parasternal long axis, A4Ch=Apical four chamber, AICD=Automated implantable cardioverter defibrillator
All these structures and anatomical variants have to be considered in the differential diagnosis before labeling echocardiographic findings as pathological cardiac masses.
Figure 1 shows an example of prominent crista terminalis. The crista terminalis is a muscular band that origins between the right sides of superior and inferior vena cava orifices and extends until reaching the opening of right atrial (RA) appendage.[9]
Figure 1.
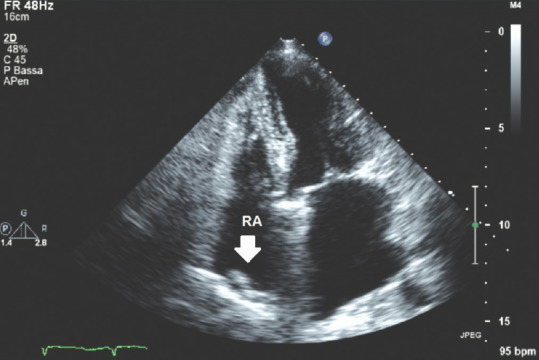
Transthoracic echocardiography apical four-chamber view; white arrow: Crista terminalis. RA = Right atrium
Plain understanding of current clinical conditions and historical clinical record of the patients needs to be acquired by the health-care professional before performing an echocardiogram: the knowledge of remote clinical history will simplify potential misdiagnosis, allowing an easier recognition of foreign iatrogenic bodies such as pacemaker leads in right atrial sections, interatrial closure devices, or even already-diagnosed anatomical and normal variants.
Cardiac masses: Classification, epidemiology, and echocardiographic findings
Cardiac masses, most often encountered in echocardiography, can be briefly classified into the following three groups: thrombi,[10,11] vegetation,[12,13] and neoplasms.[14]
Thrombi
We need to distinguish between left section thrombi and right section thrombi according to different pathophysiology. The former are generally related to atrial fibrillation, prosthetic valves, and ischemic heart disease, whereas the latter are usually related to deep-vein inferior limb thromboembolic disease, foreign iatrogenic body-attached thrombus, and right heart side myocardial infarction-related thrombus.
The size, shape, protrusion, and location of the thrombi can vary depending on the specific etiology, pathophysiology, or underlying cardiac disease.
Left section thrombi
Atrial fibrillation has a prevalence of about 2%–3% in the general population and is well known to relate to left atrium (LA) thrombus formation, and the risk of systemic embolic events ranges from 5% to 7% without adequate anticoagulation.[15,16]
LA thrombi can be located in the LA appendage in case of atrial fibrillation and can protrude or not into the LA lumen or be included within the auricola, and they are usually detected using TOE. Although further studies are needed, whenever data are available taking advantage of multimodality radiological imaging approach, anatomy of the left atrial appendage (LAA) has to be considered as well. LAA can be classified according to morphology into four types: “cactus,” “chicken wing,” “Windsock,” and “Cauliflower.” Patients with chicken wing LAA morphology are less likely to have an embolic event.[17] Two-dimensional and 3D TOE echocardiography can add useful indirect information about LAA morphology. Pulsed wave Doppler (PW) flows in correspondence of LAA possibly need to be measured during TOE examinations as atrial fibrillation patients with nonchicken wing LAA morphologies show a reduced LAA emptying flow velocity (maximum peak of mean emptying velocity at TOE lower than 30–40 cm/s.)[18] and an increased prevalence of spontaneous echo contrast inside the LA when compared with patients with chicken wing morphology.[19] Thus, whenever reduced LAA emptying velocity PW flows and spontaneous echo contrast are detected, a high suspicion of LA thrombus needs to be considered.
Figure 2 in a 19° multiplanar probe TOE picture focusing on LAA shows an example of an LAA remarkably large, isoechoic thrombus partially protruding into the LA lumen in a patient affected by atrial fibrillation: position of the thrombus (interauricular), its isoechoic or hyperechoic brightness, spontaneous echo contrast, and known current atrial fibrillation support the echocardiographic diagnosis of LAA thrombus.
Figure 2.
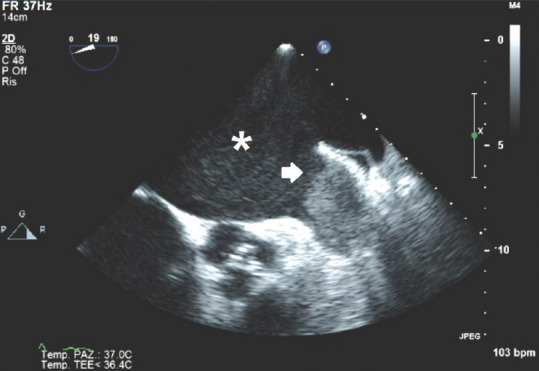
Transoesophageal echocardiography 19° short-axis view, pointing left atrial appendage; white arrow: Interauricular thrombus; *Spontaneous echo contrast into the LA. LA = Left atrium
Prosthetic aortic and mitral valves can predispose to the formation of thrombi adherent to prosthetic parts in case of lack of therapeutic anticoagulation, and atrial septal aneurysm can also predispose to atrial thrombus formation. TTE may evidence an incongruous transvalvular flow in case of prosthetic thrombosis both to color Doppler and continuous-wave Doppler analysis, with the former showing turbulence and aliasing effect and the latter showing increased transvalvular flow mean pressure gradients due to reduced effective orifice area. TOE can be more accurate in identifying the position and morphology of intravalvular or periprosthetic thrombus, showing restricted leaflets or disc motion, abnormal regurgitation, loss of physiological regurgitant jets in mechanical valves, or direct visualization of the thrombus or pannus formation adherent to prosthetic structures.[20]
Figure 3 shows a dysfunctional, mechanical, prosthetic mitral valve with an isoechoic intravalvular mass consisting in a thrombus due to lack of therapeutic anticoagulation; here the nondefinite margins of the thrombus and the intravalvular mass that interferes with normal mechanical leaflet motion reducing the intravalvular blood flow can be seen and an hyperechoic effect beyond the level of the mitral valve due to interfering thrombotic body and on color Doppler analysis an abnormal regurgitant flow can also be clearly seen related to the prosthetic incontinence due to the presence of the thrombotic mass.
Figure 3.
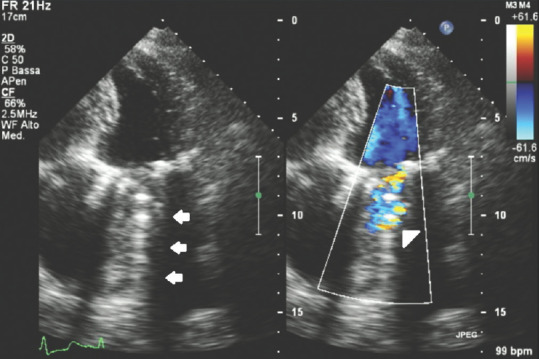
Transthoracic echocardiography apical four-chamber view of a dysfunctional mechanical, mitral prosthetic valve with intravalvular thrombus; white arrows: Hyperechoic effect beyond the level of the prosthetic thrombotic valve; white head arrow: Aliasing effect on color Doppler box due to blood flow turbulence related to prosthetic dysfunction because of intravalvular mass
The incidence of left ventricular (LV) thrombi is approximately 7/10,000 patients. Eighty percent of patients who develop intraventricular thrombi are affected by ischemic heart disease, while the rest is due to dilated cardiomyopathy and stress-induced cardiomyopathy.[21]
In case of systolic dysfunction and in particular of apical akinesia, with or without evidence of LV apical aneurysm, it is sometimes possible that a thrombus is generated close to LV apex.[15] LV thrombi are usually identified as discrete echo-dense (isoechoic or hyperechoic) intraluminal masses with defined margins that are distinct from the endocardium and seen throughout systole and diastole, have mural attachment, are sessile, and are usually located in the apex; one of the most useful discriminating features is the coexistence of the underlying abnormalities of regional or global LV wall motion.[10,19] TTE has a sensitivity of 95% and a specificity of 86% in the detection of LV thrombus. Echocardiographic contrast agent can be used to allow for a more accurate assessment of LV volumes and eventually thrombus detection in case of doubt.[22]
Figure 4 shows an apical thrombus: It shows the thrombotic nature of the mass as it is isoechoic, has a mural attachment, with defined contours distinguished from endocardial edge, and is clearly related to apical akinesia.
Figure 4.
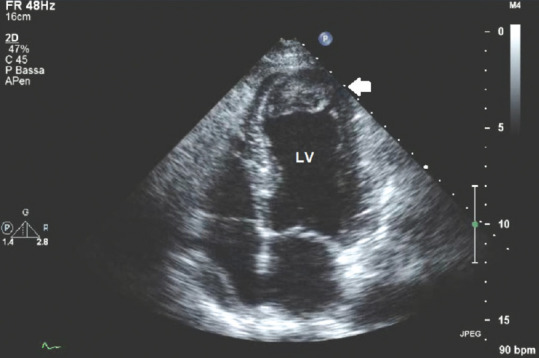
Transthoracic echocardiography apical four-chamber view with the presence of an apical thrombus; white arrow: Apical, intraventricular isoechoic thrombotic mass with defined margins distinct from the endocardial edge
Figure 5 shows a thrombotic mass in a zoomed, live 3D TTE apical 4-chamber scan showing the mobility and the size and the shape of the pedunculated mass attached to LV akinetic apex.
Figure 5.
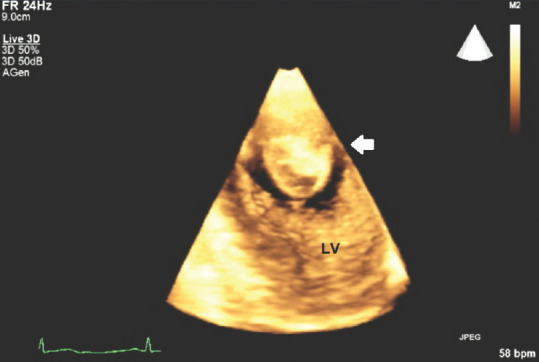
Transthoracic echocardiography three-dimensional apical four-chamber view showing an apical pedunculated thrombus; white arrow: Apical left ventricular, intraluminal thrombus
3D TTE and TEE can provide remarkable added value in echocardiographic assessment of cardiac masses, and its use has to be considered especially if surgical planning is necessary. 3D evaluation allows unlimited slicing and cropping, favors anatomic detail recognition, and allows visualization of 3D structures in motion, evaluation of the size of the cardiac mass, visualization of the true apex and accurate calculation of LV volumes and ejection fraction in case of good acoustic windows and favorable anatomic alignment of heart structures.[4]
The echocardiographic features and main clinical correlations of left sections thrombi are summarized in Table 2.
Table 2.
Clinical and echocardiographic features of left section thrombotic masses
| Left section mass | Clinical/echocardiographic findings |
|---|---|
| Left atrial thrombus | Atrial fibrillation/flutter |
| Isoechoic/hyperechoic intra-atrial, sessile, or mobile mass | |
| LAA echo-dense mass location | |
| Prosthetic valve thrombus | Isoechoic/hyperechoic peri-/intravalvular sessile or mobile mass |
| Lack of proper anticoagulation | |
| Prosthetic dysfunction (increased transvalvular mean CW Doppler gradient, restricted leaflet/disc motion, color Doppler aliasing effect, etc.) | |
| LV thrombus | Isoechoic/hyperechoic mass with defined margins distinct from endocardial edge and mural attachment |
| Apical location | |
| Wall motion-related abnormalities (hypokinesia and akinesia) | |
| Ischemic heart disease |
LAA=Left atrial appendage, CW=Continuous wave, LV=Left ventricular
Right section thrombi
In patients with confirmed diagnosis of pulmonary embolism (PE), right heart located or migrating thrombi are visualized by echocardiography in only ~4% of cases.[23,24]
Therapeutic management needs to be tailored on every specific patient in these cases. Clearly, echocardiographic detection of a right-sided migrating thrombus in a patient with suspected PE actually confirms the diagnosis; however, all the diagnostic and therapeutic steps to validate the diagnosis and coherently treat the patient are mandatory.[25]
Figure 6 shows a case of migrating thrombus in a patient with a diagnosis of PE: here, a bilobed hyperechoic/isoechoic mass with definite edges wandering into the right atrium and protruding into the LV as well is shown.
Figure 6.
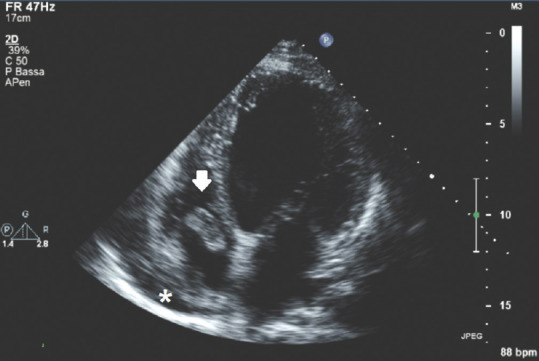
Transthoracic echocardiography apical 4-chamber view showing a right intra-atrial thrombus; white arrow: bilobated intra-atrial thrombus; *Small, anechoic amount of pericardial effusion
Any foreign iatrogenic body inserted into the heart can provide support to the formation of thrombotic masses; we then have to consider specific conditions such as the presence of central vein catheters or pacemaker leads as possible concurrent substrates for thrombotic formation and raise a high suspicion of thrombus whenever a hyperechoic/isoechoic in plus image attached to this device is encountered in TTE.[26,27] Differential diagnosis obviously includes the possibility of vegetation presence versus thrombus: clinical data associated with ultrasound findings will lead to correct diagnostic conclusive considerations.
Right ventricular thrombi can be associated with right myocardial infarction as well as its directly related complication;[28] in this case, already suggested recommendations for LV thrombotic mass identification in ischemic heart disease are valid.
The echocardiographic features and main clinical correlations of right section thrombi are summarized in Table 3.
Table 3.
Clinical and echocardiographic features of right section thrombotic masses
| Right section mass | Clinical/echocardiographic features |
|---|---|
| Right atrial thrombus | Suspicion or already-validated diagnosis of PE |
| Isoechoic/hyperechoic intra-atrial, mobile mass with definite edges and variable size or shape | |
| Right ventricular thrombus | Isoechoic/hyperechoic intraventricular mobile mass with definite edges and variable size or shape |
| Migrating thrombus into RV in suspected or already-validated diagnosis of PE | |
| Myocardial infarction of the right ventricle (wall motion-related abnormalities, isoechoic/hyperechoic mass with defined margins distinct from endocardial edge and mural attachment) |
PE=Pulmonary embolism, RV=Right ventricle
Vegetation
Endocarditis is defined as “an inflammation of the endocardium” that can be related to different etiologies, though large part is caused by infectious agents. The incidence of infective endocarditis is between 2 and 10 cases/100,000 person/year.[29,30] IE is more commonly associated with invasive medical procedures, injection drug use, and older age.[31,32] About 75% of patients who develop IE have underlying structural heart disease.[33] In the past, rheumatic heart disease with mitral stenosis was the most common valvular defect in patients with IE. Recently, the most common predisposing lesions are mitral regurgitation, aortic valve disease, and congenital heart disease.[34,35] Mitral valve prolapse associated with moderate or severe regurgitation is another risk factor for IE.[36] The presence of a prosthetic cardiac valve is a remarkable risk factor for IE.[37] European data report a IE prevalence ranging from 0.8% to 3% among intensive care unit patients.[38,39] Vegetation consists in a mass of platelets, fibrin, microorganisms, and inflammatory cells related to infectious active process originating on a preexisting structural substrate; those lesions can be identified by echocardiography and represent the active bacterial focus of infection.
TTE and TOE must be performed as soon as a concrete suspicion of endocarditis occurs, according to clinical complete evaluation and considering modified Duke diagnostic criteria,[40] in order to achieve earlier and proper therapeutic approach.
The echocardiographic findings related to IE are reported in Table 4 as reported in an ESC/EACVI consensus paper.[41,42]
Table 4.
Echocardiographic findings in infective endocarditis
| Echocardiographic findings in infective endocarditis | |
|---|---|
| Infectious finding | Echocardiographic findings |
| Vegetation | Oscillating or nonoscillating intracardiac mass on valve or other endocardial structures, or on implanted intracardiac material |
| Abscess | Thickened, nonhomogeneous perivalvular area with echodense or echolucent appearance |
| Pseudoaneurysm | Pulsatile perivalvular echo-free space, with color Doppler flow detected |
| Perforation | Interruption of endocardial tissue continuity traversed by color Doppler flow |
| Fistula | Color Doppler communication between two neighboring cavities through a perforation |
| Valve aneurysm | Saccular bulging of valvular tissue |
| Dehiscence of a prosthetic valve | Paravalvular regurgitation identified by TTE/TOE, with or without rocking motion of the prosthesis |
TTE=Transthoracic echocardiography, TOE=Transesophageal echocardiography
Figure 7 shows a multiplanar probe 124° oriented, long-axis TOE image at the level of a native aortic valve that spots an isoechoic, indented, intravalvular aortic mass which oscillates throughout complete cardiac cycle mainly protruding on the LV outflow tract and partially jutting through the aortic orifice into the aorta during systole. This mass clearly represents a vegetation.
Figure 7.
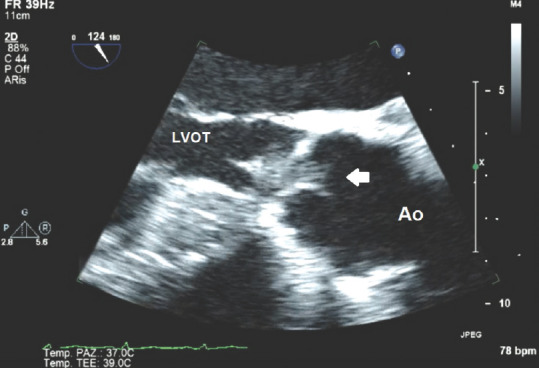
Transesophageal echocardiography 124° view at the level of aortic valve showing aortic vegetation; white arrow: Vegetation; LVOT = Left ventricular outflow tract; Ao = Ascending aorta
Figure 8 shows a multiplanar probe 41° oriented TOE image identifying a round-shaped mass attached to an implantable cardioverter defibrillator lead oscillating into the right ventricle. In this case, the pedunculated, round, isoechoic mass is not clearly distinguishable from a thrombus if based only on echocardiographic findings, and differential diagnosis becomes hard without related clinical data.
Figure 8.
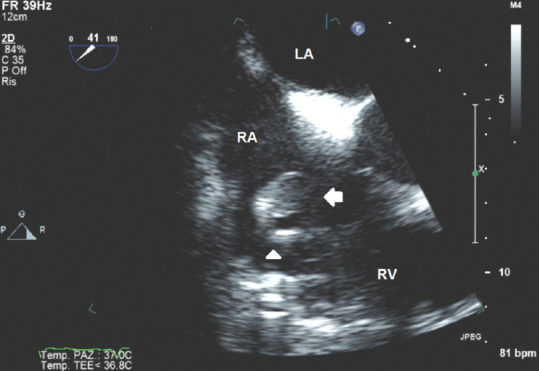
Transesophageal echocardiography 41° view showing the right sections of the heart and a vegetation attached to an implantable cardioverter lead; white arrow: Vegetation; white head arrow: Implantable cardioverter lead. RA = Right atrium; LA = Left atrium; RV = Right ventricle
In general, controversies origin when trying to discriminate vegetation from thrombus, but concurrent clinical findings, laboratory tests, and complete clinical evaluation of the patient will lead to correct diagnosis.
Neoplasms
The prevalence of primary cardiac tumors is lower than 0.1% in large autoptic studies.[43] The incidence of cardiac metastatic masses involving cardiac tissues ranges from 2.3% to 18.3% of patients with extracardiac malignant neoplasms.[44] Among the benign cardiac tumors, which represent approximately 90% of all diagnosed cardiac neoplasms, the majority are myxomas, followed by papillary fibroelastomas.[13] The most common primary malignant cardiac tumors are sarcoma, angiosarcoma, and leiomyosarcoma. In younger patients (<18 years old), fibroma and rhabdomyoma are the most frequent lesions. Primary malignant tumors in children are mostly represented by rhabdomyosarcoma and variants of malignant teratoma.[13,45]
Cardiac neoplasms are statistically associated with specific localization in the heart: usually, myxomas and lipomas are mainly related to atrial localization, whereas other kinds of neoplasms such as rhabdomyomas and lymphomas are more often localized in ventricles. Fibroelastomas are typically attached to valvular tissues.[46]
Echocardiographic contrast agents can be useful to confirm the presence of an intracardiac mass. Contrast hyperenhancement of scanned masses can raise the suspicion of malignant and highly vascularized tumors as higher contrast enhancement is related to higher vascularization and help in differential diagnosis as thrombi are not affected by contrast agents' use; myxomas generally tend to be only partially enhanced by contrast agents.[47,48]
To evaluate a cardiac mass in echocardiography, we then need to consider some of the following key factors if we already have excluded the diagnostic suspicion of thrombus or vegetation: age of the patient, localization of the mass, clinical general context related to the patient, and ultrasound features of the mass.
Figure 9 in apical 5-chamber view shows an isoechoic round mass jutting into the LA attached by a small pedunculated trait to the basal wall of the LA. Figure 10 in a subcostal view of the same case shows that the isoechoic mass seems partially attached to the basal interatrial septum as well, with the latter showing a type 1R aneurysm according to Olivares–Reyes classification of interatrial septal aneurisms.[49]
Figure 9.
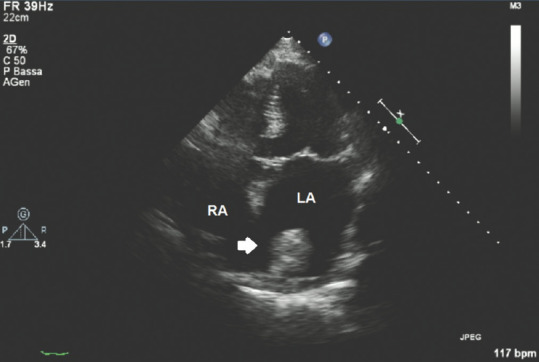
Transthoracic echocardiography apical five-chamber view showing a myxoma into the left atrium; white arrow: Myxoma; RA = Right atrium; LA = Left atrium
Figure 10.
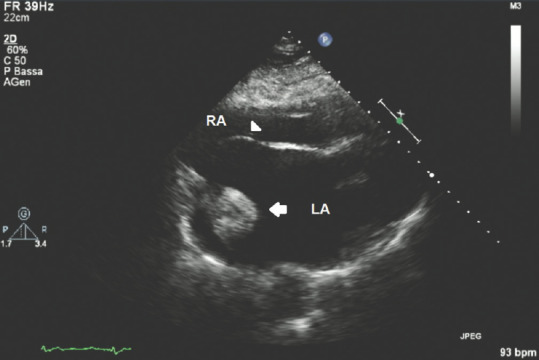
Transthoracic echocardiography subcostal view showing a myxoma into the left atrium attached to the basal part of the interatrial septum; white arrow: Myxoma; white head arrow: Interatrial septum; RA = Right atrium; LA = Left atrium
Figures 11 and 12 show interesting 3D TEE and TOE pictures of a fibroelastoma attached to the aortic left coronary aortic cusp; here, the isoechoic dense nature of the rounded pedunculated mass can be clearly identified, attached to the left coronary aortic cusp, and can be measured and accurately evaluated in its shape using 3D TTE and TOE scans.
Figure 11.
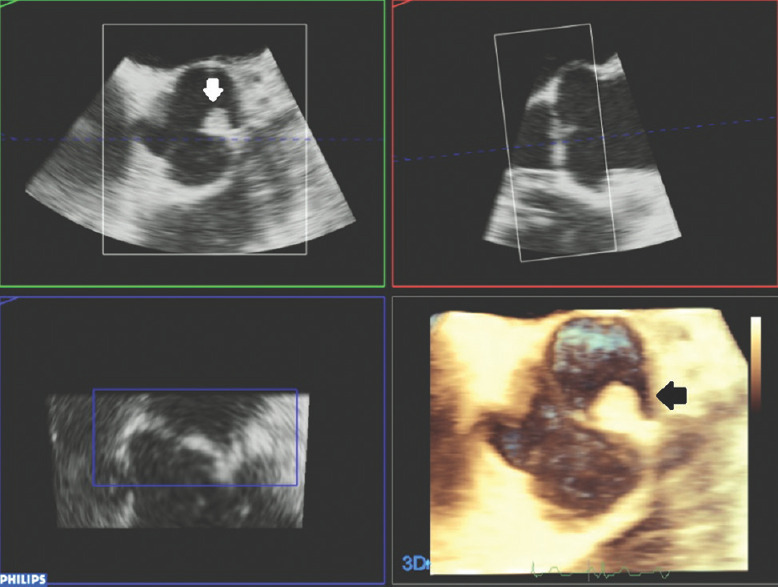
Three-dimensional transesophageal echocardiography reconstruction and multiplanar views of fibroelastoma attached to the left coronary aortic cusp; white and black arrows: Fibroelastoma mass
Figure 12.
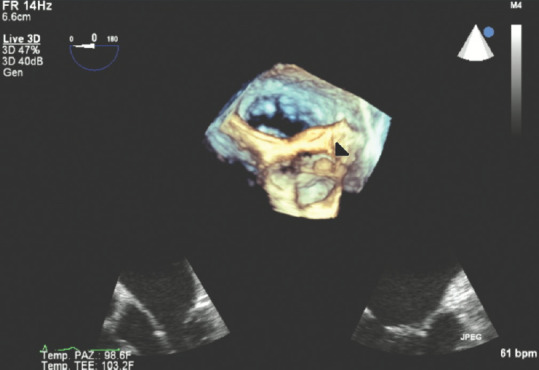
Three-dimensional transesophageal echocardiography image of fibroelastoma attached on the left coronary aortic cusp; white arrow: fibroelastoma mass
Figure 13 shows a dramatic picture of a pericardial, remarkably large, isoechoic/hyperechoic, heterogeneous mass adherent and partially infiltrating LV wall, compatible with a secondary, metastatic lesion of a primary melanoma; here, a small amount of pericardial effusion located posteriorly to the right atrium can also be seen.
Figure 13.
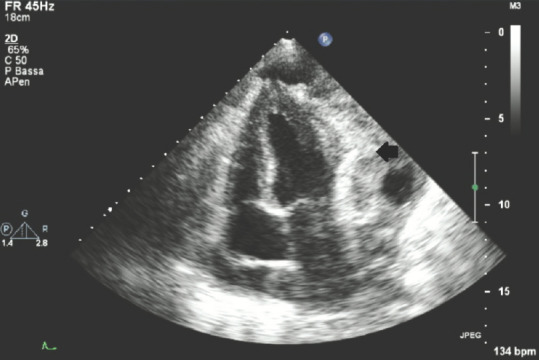
Transthoracic echocardiography apical four-chamber view showing a pericardial metastatic lesion of a primary melanoma; black arrow: Metastatic pericardial mass
The main clinical and echocardiographic features of neoplasms are summarized in Table 5.
Table 5.
Structural and echocardiographic features of most common neoplasia
| Neoplasms | Localization | Enhancement with contrast agents | Age of the patient | Echo features |
|---|---|---|---|---|
| Mixoma | LA (majority of cases) RA | Mild enhancement | Any age | Sessile, pedunculated, isoechoic, definite margins |
| Fibroelastoma | Valve structures | Depending on vascularization | Any age | Small size, endocardial attachment |
| Lipoma | Subendocardium, or pericardium or on cardiac valve structures | No enhancement | Any age | Broad based, immobile, no pedicle, well circumscribed |
| Rhabdomyoma | Ventricular walls, atrioventricular valves | Remarkable enhancement | <1 year old | Small, well-circumscribed isolated, or multiple nodules or a pedunculated mass |
| Sarcomas Angiosarcomas | LARA | Variable, not always significant enhancement | Any age | Broad-based heterogeneous echogenicity; hypoechogenic areas may indicate tumor necrosis |
| Lymphoma | Especially in the RA | Variable | Any age | Homogeneous, infiltrating masses leading to “wall thickening” or nodular masses |
LA=Left atrium, RA=Right atrium
Extracardiac masses
A brief mention has to be spent about extracardiac masses encountered in echocardiography. Although not frequent, in these cases, the masses are not related to myocardial tissue and are visible during echocardiographic examination in patients affected by diseases that are not cardiac in etiology.
Figure 14 depicts an apical four-chamber view with color Doppler double image showing an isoechoic mass compressing the LA that is an already well-known diagnosed pulmonary neoplasia in a young patient; careful attention has to be paid in these cases and complete clinical context of the patients can lead to correct interpretation of the images.
Figure 14.
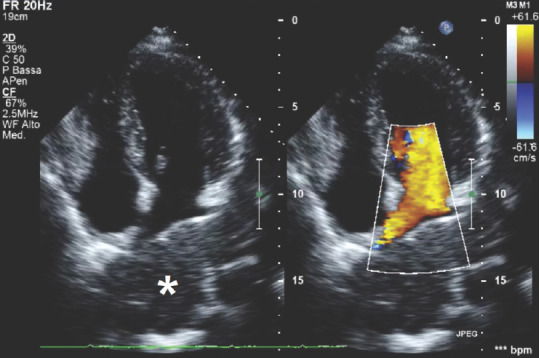
Transthoracic echocardiography apical four-chamber view showing an extracardiac, isoechoic mass represented by pulmonary neoplasia; *Extracardiac neoplasia
Figure 15 shows an off-axis subcostal view pointing to the ectopic mass of an infiltrating renal neoplasia invading the inferior vena cava. Here, an isoechoic mass protruding into the lumen of the inferior vena cava that clearly shows its dense nature on color Doppler analysis as well can be seen; color Doppler box clearly shows blood turbulence around the solid mass that on the contrary does not show color enhancement at all.
Figure 15.
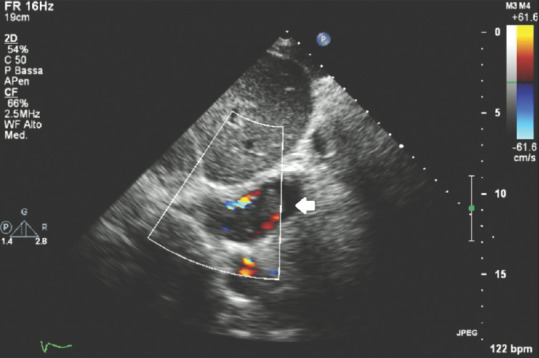
Transthoracic echocardiography subcostal off-axis view showing a neoplastic renal mass protruding into the lumen on inferior vena cava; white arrow: Renal echo-dense mass neoplasia
Figure 16 shows a parasternal long-axis view of a patient affected by a large hiatal hernia: this condition can simulate a left atrial intracardiac/extracardiac mass showing an isoechoic-hyperechoic, dense, mass-mimicking-area located posteriorly to the LA. Sonographers must be aware of this rare ultrasound finding in order to achieve correct differential diagnosis: the history and physical examination of the patient and properly performed slightly “off-axis” parasternal long-axis and apical views, together with possible, respiratory fluctuation of the hernia and intraluminal swirling of gastroesophageal reflux matter in subcostal views, help making the correct diagnosis.
Figure 16.
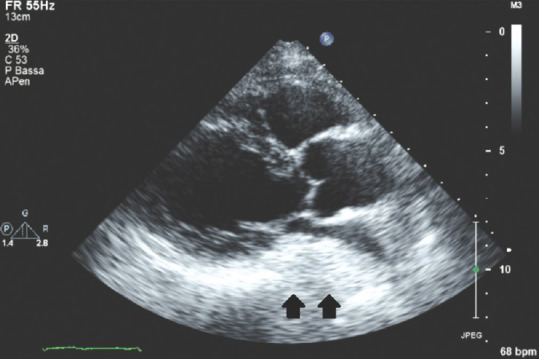
Transthoracic echocardiography parasternal long-axis view showing a large isoechoic/hyperechoic hiatal hernia close to the posterior wall of the left atrium; black arrows: Hiatal hernia
Useful additional tips in ultrasound/morphological evaluation of cardiac masses
When scanning an identified thrombus, we need to remember some key echographic features: the mural portion of thrombus is acoustically brighter than the distal portion as the latter mostly consists of red blood cells held together by a delicate fibrin mesh;[50] clots that show less echo-brightness are more likely to embolize as they are softer and fresher in their structure;[51] mobile, protruding, pedunculated, and more recently organized thrombi are more likely to origin emboli;[52] and sessile and pedunculated large thrombi may show an ipoechoic/anechoic central core related to colliquation.[53] Thrombi do not acquire enhancement after administration of ultrasound contrast agents.[48]
CONCLUSIONS
Echocardiography is a useful technique to diagnose intracardiac and extracardiac masses. Shape, size, location, and ultrasound features of the scanned mass associated with clinical and other instrumental data can lead to correct diagnosis of the lesions. Main cardiac masses consist of thrombi, vegetation, and neoplasms. Expert echocardiographic evaluation of the masses can help in the differential diagnosis, leading to specific diagnostic suspicion and proper treatment. 3D TEE and TOE together with multimodality imaging approach usually optimally define the nature of the mass.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Popescu BA, Andrade MJ, Badano LP, Fox KF, Flachskampf FA, Lancellotti P, et al. European Association of Echocardiography recommendations for training, competence, and quality improvement in echocardiography. Eur J Echocardiogr. 2009;10:893–905. doi: 10.1093/ejechocard/jep151. [DOI] [PubMed] [Google Scholar]
- 2.Evangelista A, Flachskampf F, Lancellotti P, Badano L, Aguilar R, Monaghan M, et al. European Association of Echocardiography recommendations for standardization of performance, digital storage and reporting of echocardiographic studies. Eur J Echocardiogr. 2008;9:438–48. doi: 10.1093/ejechocard/jen174. [DOI] [PubMed] [Google Scholar]
- 3.Johri AM, Picard MH, Newell J, Marshall JE, King ME, Hung J. Can a teaching intervention reduce interobserver variability in LVEF assessment: A quality control exercise in the echocardiography lab. JACC Cardiovasc Imaging. 2011;4:821–9. doi: 10.1016/j.jcmg.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Plana JC. Added value of real-time three-dimensional echocardiography in assessing cardiac masses. Curr Cardiol Rep. 2009;11:205–9. doi: 10.1007/s11886-009-0029-5. [DOI] [PubMed] [Google Scholar]
- 5.Peters PJ, Reinhardt S. The echocardiographic evaluation of intracardiac masses: A review. J Am Soc Echocardiogr. 2006;19:230–40. doi: 10.1016/j.echo.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez DR, Bryg RJ. Normal variants in echocardiography. Curr Cardiol Rep. 2016;18:104. doi: 10.1007/s11886-016-0786-x. [DOI] [PubMed] [Google Scholar]
- 7.Miller DV, Tazelaar HD. Cardiovascular pseudoneoplasms. Arch Pathol Lab Med. 2010;134:362–8. doi: 10.5858/134.3.362. [DOI] [PubMed] [Google Scholar]
- 8.Le HT, Hangiandreou N, Timmerman R, Rice MJ, Smith WB, Deitte L, et al. Imaging artifacts in echocardiography. Anesth Analg. 2016;122:633–46. doi: 10.1213/ANE.0000000000001085. [DOI] [PubMed] [Google Scholar]
- 9.Pharr JR, West MB, Kusumoto FM, Figueredo VM. Prominent crista terminalis appearing as a right atrial mass on transthoracic echocardiogram. J Am Soc Echocardiogr. 2002;15:753–5. doi: 10.1067/mje.2002.119163. [DOI] [PubMed] [Google Scholar]
- 10.Lobo A, Lewis JF, Conti CR. Intracardiac masses detected by echocardiography: Case presentations and review of the literature. Clin Cardiol. 2000;23:702–8. doi: 10.1002/clc.4960230914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waller BF, Grider L, Rohr TM, McLaughlin T, Taliercio CP, Fetters J. Intracardiac thrombi: Frequency, location, etiology, and complications: A morphologic review – Part I. Clin Cardiol. 1995;18:477–9. doi: 10.1002/clc.4960180811. [DOI] [PubMed] [Google Scholar]
- 12.Bai AD, Steinberg M, Showler A, Burry L, Bhatia RS, Tomlinson GA, et al. Diagnostic accuracy of transthoracic echocardiography for infective endocarditis findings using transesophageal echocardiography as the reference standard: A meta-analysis. J Am Soc Echocardiogr. 2017;30:639–46. doi: 10.1016/j.echo.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization Classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10:1240–2. doi: 10.1097/JTO.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 14.Burke A, Virmani R. Atlas of Tumor Pathology: Tumors of the Heart and Great Vessels. 3rd Ser. Vol. 16. Washington DC: Armed Forces Institute of Pathology; 1996. pp. 1–11. [Google Scholar]
- 15.Bertaglia E, Anselmino M, Zorzi A, Russo V, Toso E, Peruzza F, et al. NOACs and atrial fibrillation: Incidence and predictors of left atrial thrombus in the real world. Int J Cardiol. 2017;249:179–83. doi: 10.1016/j.ijcard.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 16.Arnold AZ, Mick MJ, Mazurek RP, Loop FD, Trohman RG. Role of prophylactic anticoagulation for direct current cardioversion in patients with atrial fibrillation or atrial flutter. J Am Coll Cardiol. 1992;19:851–5. doi: 10.1016/0735-1097(92)90530-z. [DOI] [PubMed] [Google Scholar]
- 17.Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012;60:531–8. doi: 10.1016/j.jacc.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 18.Fatkin D, Kuchar DL, Thorburn CW, Feneley MP. Transesophageal echocardiography before and during direct current cardioversion of atrial fibrillation: Evidence for “atrial stunning” as a mechanism of thromboembolic complications. J Am Coll Cardiol. 1994;23:307–16. doi: 10.1016/0735-1097(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 19.Petersen M, Roehrich A, Balzer J, Shin DI, Meyer C, Kelm M, et al. Left atrial appendage morphology is closely associated with specific echocardiographic flow pattern in patients with atrial fibrillation. Europace. 2015;17:539–45. doi: 10.1093/europace/euu347. [DOI] [PubMed] [Google Scholar]
- 20.Pepi M, Evangelista A, Nihoyannopoulos P, Flachskampf FA, Athanassopoulos G, Colonna P, et al. Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism: European Association of Echocardiography (EAE) (a registered branch of the ESC) Eur J Echocardiogr. 2010;11:461–76. doi: 10.1093/ejechocard/jeq045. [DOI] [PubMed] [Google Scholar]
- 21.Lee JM, Park JJ, Jung HW, Cho YS, Oh IY, Yoon CH, et al. Left ventricular thrombus and subsequent thromboembolism, comparison of anticoagulation, surgical removal, and antiplatelet agents. J Atheroscler Thromb. 2013;20:73–93. doi: 10.5551/jat.13540. [DOI] [PubMed] [Google Scholar]
- 22.Kurt M, Shaikh KA, Peterson L, Kurrelmeyer KM, Shah G, Nagueh SF, et al. Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J Am Coll Cardiol. 2009;53:802–10. doi: 10.1016/j.jacc.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Torbicki A, Galié N, Covezzoli A, Rossi E, De Rosa M, Goldhaber SZ, et al. Right heart thrombi in pulmonary embolism: Results from the International Cooperative Pulmonary Embolism Registry. J Am Coll Cardiol. 2003;41:2245–51. doi: 10.1016/s0735-1097(03)00479-0. [DOI] [PubMed] [Google Scholar]
- 24.Barrios D, Rosa-Salazar V, Jiménez D, Morillo R, Muriel A, Del Toro J, et al. Right heart thrombi in pulmonary embolism. Eur Respir J. 2016;48:1377–85. doi: 10.1183/13993003.01044-2016. [DOI] [PubMed] [Google Scholar]
- 25.Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur Respir J. 2019;54:pii: 1901647. doi: 10.1183/13993003.01647-2019. [DOI] [PubMed] [Google Scholar]
- 26.Oostra CE, Chowdhury MA, Khouri SJ. Central venous catheter-associated right atrial thrombus. Am J Med Sci. 2016;351:e3. doi: 10.1016/j.amjms.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Salaun E, Deharo JC, Casalta JP, Franceschi F, Hubert S, Renard S, et al. An oscillating mass attached to a pacemaker lead: Thrombus or vegetation? A fishing story. JACC Clin Electrophysiol. 2017;3:915–6. doi: 10.1016/j.jacep.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 28.Kuno T, Imaeda S, Hashimoto K, Ryuzaki T, Saito T, Yamazaki H, et al. Recent inferior myocardial infarction complicated with a right ventricular thrombus detected by three cardiac imaging modalities. Intern Med. 2018;57:693–5. doi: 10.2169/internalmedicine.9431-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387:882–93. doi: 10.1016/S0140-6736(15)00067-7. [DOI] [PubMed] [Google Scholar]
- 30.Fatima S, Dao B, Jameel A, Sharma K, Strogatz D, Scribani M, et al. Epidemiology of infective endocarditis in rural upstate New York, 2011 – 2016. J Clin Med Res. 2017;9:754–8. doi: 10.14740/jocmr3131w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoen B, Alla F, Selton-Suty C, Béguinot I, Bouvet A, Briançon S, et al. Changing profile of infective endocarditis: Results of a 1-year survey in France. JAMA. 2002;288:75–81. doi: 10.1001/jama.288.1.75. [DOI] [PubMed] [Google Scholar]
- 32.Tleyjeh IM, Abdel-Latif A, Rahbi H, Scott CG, Bailey KR, Steckelberg JM, et al. A systematic review of population-based studies of infective endocarditis. Chest. 2007;132:1025–35. doi: 10.1378/chest.06-2048. [DOI] [PubMed] [Google Scholar]
- 33.Griffin MR, Wilson WR, Edwards WD, O'Fallon WM, Kurland LT. Infective endocarditis. Olmsted County, Minnesota, 1950 through 1981. JAMA. 1985;254:1199–202. doi: 10.1001/jama.254.9.1199. [DOI] [PubMed] [Google Scholar]
- 34.Michel PL, Acar J. Native cardiac disease predisposing to infective endocarditis. Eur Heart J. 1995;16(Suppl B):2–6. doi: 10.1093/eurheartj/16.suppl_b.2. [DOI] [PubMed] [Google Scholar]
- 35.Weinberger I, Rotenberg Z, Zacharovitch D, Fuchs J, Davidson E, Agmon J. Native valve infective endocarditis in the 1970s versus the 1980s: Underlying cardiac lesions and infecting organisms. Clin Cardiol. 1990;13:94–8. doi: 10.1002/clc.4960130206. [DOI] [PubMed] [Google Scholar]
- 36.Clemens JD, Horwitz RI, Jaffe CC, Feinstein AR, Stanton BF. A controlled evaluation of the risk of bacterial endocarditis in persons with mitral-valve prolapse. N Engl J Med. 1982;307:776–81. doi: 10.1056/NEJM198209233071302. [DOI] [PubMed] [Google Scholar]
- 37.Bashore TM, Cabell C, Fowler V., Jr Update on infective endocarditis. Curr Probl Cardiol. 2006;31:274–352. doi: 10.1016/j.cpcardiol.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Mourvillier B, Trouillet JL, Timsit JF, Baudot J, Chastre J, Régnier B, et al. Infective endocarditis in the intensive care unit: Clinical spectrum and prognostic factors in 228 consecutive patients. Intensive Care Med. 2004;30:2046–52. doi: 10.1007/s00134-004-2436-9. [DOI] [PubMed] [Google Scholar]
- 39.Karth G, Koreny M, Binder T, Knapp S, Zauner C, Valentin A, et al. Complicated infective endocarditis necessitating ICU admission: Clinical course and prognosis. Crit Care. 2002;6:149–54. doi: 10.1186/cc1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 41.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 42.Habib G, Badano L, Tribouilloy C, Vilacosta I, Zamorano JL, Galderisi M, et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr. 2010;11:202–19. doi: 10.1093/ejechocard/jeq004. [DOI] [PubMed] [Google Scholar]
- 43.Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993;117:1027–31. [PubMed] [Google Scholar]
- 44.Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases. J Clin Pathol. 2007;60:27–34. doi: 10.1136/jcp.2005.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uzun O, Wilson DG, Vujanic GM, Parsons JM, De Giovanni JV. Cardiac tumours in children. Orphanet J Rare Dis. 2007;2:11. doi: 10.1186/1750-1172-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dujardin KS, Click RL, Oh JK. The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal. J Am Soc Echocardiogr. 2000;13:1080–3. doi: 10.1067/mje.2000.107157. [DOI] [PubMed] [Google Scholar]
- 47.Kirkpatrick JN, Wong T, Bednarz JE, Spencer KT, Sugeng L, Ward RP, et al. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. J Am Coll Cardiol. 2004;43:1412–9. doi: 10.1016/j.jacc.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 48.Mansencal N, Revault-d'Allonnes L, Pelage JP, Farcot JC, Lacombe P, Dubourg O. Usefulness of contrast echocardiography for assessment of intracardiac masses. Arch Cardiovasc Dis. 2009;102:177–83. doi: 10.1016/j.acvd.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Olivares-Reyes A, Chan S, Lazar EJ, Bandlamudi K, Narla V, Ong K. Atrial septal aneurysm: A new classification in two hundred five adults. J Am Soc Echocardiogr. 1997;10:644–56. doi: 10.1016/s0894-7317(97)70027-0. [DOI] [PubMed] [Google Scholar]
- 50.Mikell FL, Asinger RW, Elsperger KJ, Anderson WR, Hodges M. Regional stasis of blood in the dysfunctional left ventricle: Echocardiographic detection and differentiation from early thrombosis. Circulation. 1982;66:755–63. doi: 10.1161/01.cir.66.4.755. [DOI] [PubMed] [Google Scholar]
- 51.Lloret RL, Cortada X, Bradford J, Metz MN, Kinney EL. Classification of left ventricular thrombi by their history of systemic embolization using pattern recognition of two-dimensional echocardiograms. Am Heart J. 1985;110:761–5. doi: 10.1016/0002-8703(85)90454-5. [DOI] [PubMed] [Google Scholar]
- 52.Kinney EL. The significance of left ventricular thrombi in patients with coronary heart disease: A retrospective analysis of pooled data. Am Heart J. 1985;109:191–4. doi: 10.1016/0002-8703(85)90443-0. [DOI] [PubMed] [Google Scholar]
- 53.Reeder GS, Lengyel M, Tajik AJ, Seward JB, Smith HC, Danielson GK. Mural thrombus in left ventricular aneurysm: incidence, role of angiography, and relation between anticoagulation and embolization. Mayo Clin Proc. 1981;56:77–81. [PubMed] [Google Scholar]


