Abstract
Background:
Various clinical trials provide evidence about the safety, effectiveness, and therapeutic success of percutaneous left atrial appendage closure (LAAC) using various occlusion devices. These devices are foreign materials implanted into the left atrium and may deteriorate left atrial (LA) function. The aim of this study was to evaluate the change in transesophageal echocardiography (TEE)-derived LA strain after LAAC.
Methods and Results:
The study included 95 patients (age: 75 ± 6.7 years, 67% male) who underwent percutaneous LAAC. LA strain was evaluated at three different time intervals by TEE (baseline, 45 days, and 180 days after the procedure). All data were analyzed using the software Image-Arena (TomTec®). Seventy patients had atrial fibrillation, whereas 25 were in sinus rhythm at baseline and during follow-up. Analysis was performed for peak atrial longitudinal strain (PALS) and peak atrial contraction strain (PACS) from segments of the lateral wall in mid-esophageal four-chamber view. PACS was obtained in patients with sinus rhythm during examinations. Compared to baseline, PALS increased at 45 days after the procedure (12.4% ± 8.4% at baseline vs. 16.0% ± 10.6% after 45 days, P = 0.001) and remained stable from 45 days to 180 days after procedure (13.8% ± 9.1% after 45 days vs. 17.2% ± 12.6% after 180 days, P = 0.092). Similarly, PACS increased at 45 days after the procedure (5.8% ± 3.9% at baseline vs. 10.6% ± 7.6% after 45 days, P = 0.001) and remained stable from 45 days to 180 days after the procedure (7.6% ± 4.5% after 45 days vs. 7.9% ± 3.1% after 180 days, P = 0.876).
Conclusions:
Our study demonstrated for the first time the improvement in TEE-derived LA strain following LAAC within 45 days of implantation. The findings suggest improved LA function following LAAC.
Keywords: Peak atrial contraction strain, peak atrial longitudinal strain, percutaneous left atrial appendage closure, transesophageal echocardiography
INTRODUCTION
Atrial fibrillation (AF) is the most common chronic arrhythmia diagnosed in clinical practice. The prevalence has been increasing worldwide so far and appears to further grow in future.[1] The greatest burden of patients with AF is the risk of stroke and death. Healey et al. reported that the prevalence of stroke 1 year after a diagnosis of AF was 4% and mortality was 11%.[2]
Oral anticoagulation is the standard therapy to prevent thromboembolic events including ischemic stroke. On the other hand, bleeding is a side effect of anticoagulation and represents an inseparable issue. In recent years, the development of novel oral anticoagulant (OAC) agents has lowered bleeding risks and increased preventive safety and efficacy. Nevertheless, various clinical constellations carry increased risk of bleeding including dialysis, pregnancy, liver dysfunction, and the use of dual antiplatelet therapy among others.[3,4]
Several studies have reported that 90% of thrombus formation occurs in the left atrial appendage (LAA) in patients with nonvalvular AF.[5] Therefore, surgical LAA exclusion or occlusion has been performed for many decades in patients with AF.[6] Nevertheless, these techniques have mostly been limited to open-heart surgery.
Most recently, percutaneous LAA closure has been reported in observational studies and registries.[7,8,9,10,11] In particular, implantations of the WATCHMAN device have been performed with a high degree of safety and efficacy.[6,7,8,9,10] Further, the noninferiority of WATCHMAN device implantation compared to oral anticoagulation using warfarin therapy for stroke prevention was demonstrated in patients with nonvalvular AF.[7,8]
The function of the LAA and contribution to cardiac hemodynamics is well established.[5,12,13] It is more compliant than the left atrial (LA) main chamber and function as a reservoir to prevent elevation in intra-atrial pressure.[12] There have been few reports that show changes in LA function after surgical LAA exclusion.[14,15] Isobe et al. indicated a reduction in atrial flow with the MAZE procedure according to transmitral flow analysis.[14] Similarly, Kamohara et al. reported that LAA exclusion reduced LA reservoir function by reducing the systolic components of pulmonary venous flow.[15] On the other hand, the influence of the percutaneous LAA closure on LA performance has not yet been clarified.
Two-dimensional speckle-tracking echocardiography (2D-STE) is an excellent modality to evaluate the myocardium through angle-independent calculation of myocardial velocities and deformation parameters such as strain and strain rate. These indices can indicate early and subclinical cardiac dysfunction. For example, it was reported that left ventricular global longitudinal strain (GLS) is superior to left ventricular ejection fraction for predicting cardiac events.[16,17] Recently, LA longitudinal strain has been shown to represent a particularly useful parameter for analyzing LA function in several conditions including AF.[18]
Finally, we assumed that the LA function might deteriorate after percutaneous LAA closure considering its physiological characteristics. In our institution, transesophageal echocardiography (TEE) is routinely performed for follow-up after percutaneous LAA closure. Consequently, we sought to investigate LA function after LAA closure quantitatively with LA GLS obtained by 2D-STE derived TEE.
METHODS
Study patients
Two hundred and twenty-three patients with nonvalvular AF who underwent percutaneous LAA closure at the University Hospital Jena between September 2012 and November 2018 were enrolled in this retrospective study. All patients had nonvalvular AF with high risk for stroke according to the CHA2 DS2-VASc score and high bleeding risk according to the HAS-BLED score with a contraindication to long-term OAC therapy either with Vitamin K antagonists or nonvitamin K antagonist OACs (NOACs). Prior to the procedure, all patients underwent TEE examinations to detect the existence of thrombi and to measure LAA size or to exclude patients with contraindication to LA appendage closure (LAAC) due to mitral stenosis. All patients were informed about the procedure and gave written informed consent.
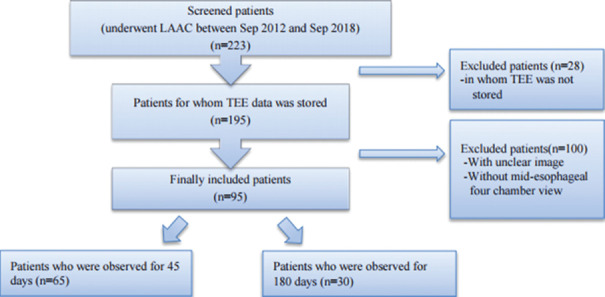
The evaluation by TEE was routinely performed before the implantation (baseline) and 45 and 180 days after the implantation. The purpose of the follow-up was to evaluate device position, device-related thrombus, and the presence of residual peri device leaks with Doppler technique. Of all the 223 patients, 28 patients were excluded due to lack of adequate echocardiographic evaluation before or after the procedure [Figure 1]. Second, 104 patients were excluded because of poor echocardiographic window for the evaluation of LA mechanics. Finally, 95 patients were included in our study. Of those, 65 patients were followed up only once (45 days) and 30 were followed up twice (45 days and 180 days). Patients with changes in heart rhythm between baseline and at postimplantation follow-up were excluded from the study.
Figure 1.
Exclusion and inclusion criteria
Left atrial appendage closure procedure
The implantation of LAAC devices was performed using a standard procedure.[19] The LAA closure device was implanted under conscious sedation and TEE control in the catheterization laboratory. The device used was a WATCHMANN® device (Boston Scientific Corporation, Natick, MA, USA) and delivered via the right femoral vein. Deployment and positioning of the device were controlled by fluoroscopy and periprocedural TEE. LAA was reached through a transseptal puncture. An arterial access sheath was used for pressure measurement, marking the aortic root during transseptal puncture and quick complication management. Decision on device size was made upon anatomical morphology and measurements in echocardiography and fluoroscopy. During the procedure, heparin was administered at a dosage of 100 U/kg body weight with an activated clotting time of about 250 s. After implantation, patients remained on aspirin 100 mg and either NOACs or Vitamin K antagonists (internal normalized ratio 2.0–3.0) until 45 days postimplant. At 45 days, TEE was performed to assess the device placement to assess whether the LAA was closed completely and thrombus on the device was ruled out. When flow was noted around the device >5 mm, consideration was given to keep the patient on anticoagulation until it had decreased to <5 mm. Patients ceasing anticoagulant began clopidogrel 75 mg and aspirin daily through 3–6 months postimplant and continued taking aspirin daily. In some cases, aspirin was finished at 12 months at the discretion of the physician. In patients with impaired renal function, enoxaparine was prescribed for 4 weeks without using OAC and replaced by aspirin and clopidogrel for 3 months and aspirin was continued at 12 months.
Echocardiographic assessment
Transthoracic echocardiography (TTE) and TEE examinations were performed using an ultrasound system (IE33 or EPIQ, Philips Healthcare, Andover, MA, USA). The recordings were processed using an acoustic-tracking dedicated software (Image-Arena™ Version 4.6; TomTec Imaging Systems, Unterschleissheim, Germany), which allowed for an off-line semi-automated analysis of speckle-based strain. We used the ventricular cycle as the reference point to calculate LA strain in TEE examination.[17] We traced LA endocardial border at the end diastole and end systole except for pulmonary vein and LAA from mid-esophageal four-chamber view, delineating the region of interest that then was subdivided into three segments (interatrial, lateral, and roof). Two consecutive cardiac cycles were recorded and averaged. Therefore, we defined the onset of the QRS complex (at ventricular end-diastole) as the zero strain, and all longitudinal LA strain values were positive.
LA deformation is a cyclic process, which can be subdivided into three phases: reservoir, conduit, and contraction.[20] The echocardiography software gives the longitudinal strain curve. During the atrial reservoir phase, LA wall stretches and at the result, its strain curve increases, reaching a first positive peak at the end of atrial filling. The peak value is peak atrial longitudinal strain (PALS), representing atrial compliance [Figure 2]. After the reservoir phase, LA empties quickly, its volume reduces, and the curve initially decreases, up to a plateau corresponding to the conduit phase. Additionally, in patients in sinus rhythm, the plateau is followed by a second positive peak due to atrial contraction and finally reaching a negative peak. The second positive peak value is peak atrial contraction strain (PACS) representing atrial contractile function.
Figure 2.
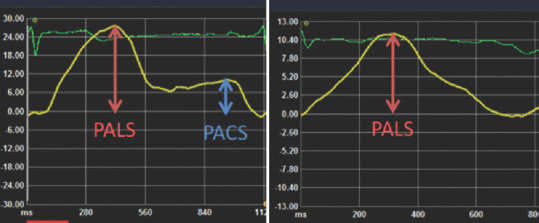
Left atrial strain curve. Left panel: a case in sinus rhythm at the time of echocardiographic exam, right panel: a case with atrial fibrillation at the time of echocardiographic exam. The reservoir function of the left atrium is assessed with the first peak positive strain (peak atrial longitudinal strain) and the contractile function by the second peak positive strain (peak atrial contraction strain). Peak atrial contraction strain is not observed in case s with atrial fibrillation because of loss of atrial contraction
All data were analyzed by one observer (SI). In our retrospective data, in some cases, it was hard to trace LA septum and roof wall. Therefore, we measured PALS and PACS from LA lateral strain curve by TEE. In patients who were in AF at the time of echocardiography, PACS was not obtained. Additionally, we obtained the values of LA emptying fraction (LAEF) and LA fractional area change (LAFAC) from the mid-esophageal four-chamber view.
Statistical analysis
All quantitative data were expressed as mean ± standard deviation. Differences between baseline and postimplantation of the LAA closure device were analyzed by the Wilcoxon Signed-rank test. PALS and hemodynamic data were compared using the Pearson's correlation. Statistical analysis was carried out with SPSS® version 21 (Japan IBM, Tokyo, Japan). P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
The mean age of our patients was 75 ± 6.7 years, with male predominance (67%). At the time of echocardiographic exam, 70 patients (74%) had AF, whereas 25 (26%) were in sinus rhythm. The mean CHA2 DS2-VASc score was 4.5 ± 1.4, and the mean HAS-BLED score was 4.1 ± 0.9, indicating a high risk for stroke as well as bleeding complications. The common reason for the procedure was major bleeding[21] (30%) and minor bleeding[21] (31%), followed by stroke in spite of anticoagulation (14%) and coagulation defects in liver disease (8%). All procedures were performed successfully without any major complications. Characteristics of the study patients are summarized in Table 1. Hemodynamics data were taken from measurements during device implantation in the catheterization laboratory. The mean LA pressure in ten patients and mean right atrial pressure in 71 patients were not recorded.
Table 1.
Patient characteristics
| Characteristics | n=95 |
|---|---|
| Age, mean±SD | 75±6.8 |
| Male, n (%) | 64 (67) |
| BMI (kg/m2) | 27±7.1 |
| AF, n (%) | |
| Permanent | 62 (65) |
| Paroxysmal | 33 (34) |
| CHA2DS2-VASc score, mean±SD | 4.4±1.4 |
| HAS-BLED score, mean±SD | 4.1±0.9 |
| Heart failure, n (%) | 81 (85) |
| Diabetes, n (%) | 38 (40) |
| Hypertension, n (%) | 92 (97) |
| Smoking, n (%) | 24 (25) |
| Dyslipidemia, n (%) | 54 (57) |
| Chronic renal failure, n (%) | 56 (60) |
| Chronic lung disease, n (%) | 28 (30) |
| Previous CVA, n (%) | 16 (17) |
| Ischemic heart disease, n (%) | 38 (40) |
| Previous PCI, n (%) | 25 (26) |
| Previous CABG, n (%) | 4 (4) |
| Previous MI, n (%) | 14 (15) |
| Previous TAVI, n (%) | 2 (2) |
| Previous MitraClip repair | 2 (2) |
| PM/ICD/CRTD, n (%) | 18 (19) |
| NYHA, n (%) | |
| I | 37 (39) |
| II | 40 (42) |
| III | 13 (14) |
| IV | 5 (5) |
| Indication for LAAC, n (%) | |
| Major bleeding | 28 (30) |
| Minor bleeding | 29 (31) |
| Stroke in spite of anticoagulation | 13 (14) |
| Coagulation defects in liver disease | 8 (8) |
| Others | 17 (18) |
| Laboratory data | |
| Hemoglobin (mmol/l) | 7.5±1.4 |
| eGFR (ml/min/1.73m2) | 57.0±23.7 |
| BNP (pg/ml) | 371.04±473.6 |
| Echocardiographic parameters (TTE) | |
| LVEF, n (%) | 58.1±14.9 |
| Left atrial diameter (mm) | 48.5±9.3 |
| TRPG (mmHg) | 36.3±11.5 |
| Hemodynamics in catheterization (mmHg) | |
| Systolic arterial pressure | 138±30 |
| Diastolic arterial pressure | 69±15 |
| Mean arterial pressure | 96±18 |
| LA pressure (n=85) | 19±6.0 |
| Right atrial pressure (n=24) | 12±6.2 |
LA=Left atrial, SD=Standard deviation, BMI=Body mass index, CVA=Cerebrovascular accident, PCI=Percutaneous coronary intervention, CABG=Coronary artery bypass surgery, MI=Myocardial infarction, TAVI=Transcatherter aortic valve implantation, eGFR=Estimated glomerular filtration rate, LVEF=Left ventricular ejection fraction, TRPG=Tricuspid regurgitation peak gradient, TTE=Transthoracic echocardiography, NYHA=New York Heart Association, BNP=Brain natriuretic peptide, PM/ICD/CRTD=Pacemaker/implantable cardioverter defibrillator/cardiac resynchronization therapy device, LAAC=LA appendage closure, AF=Atrial fibrillation
Transesophageal echocardiography derived peak atrial longitudinal strain and peak atrial contraction strain
Our data were divided in three cohorts: Patients who had TEE at baseline and after 45 days (cohort 1: n = 95), patients who had TEE at baseline and after 180 days (cohort 2: n = 30) and patients who had TEE at baseline, after 45 and 180 days (cohort 3: n = 28).
Compared to baseline, PALS increased after 45 and 180 days (cohort 1:12.4% ± 8.4% at baseline vs. 16.0% ±10.7% after 45 days, cohort 2: P =0.001; 10.1% ± 6.7% at baseline vs. 17.1% ± 12.4% after 180 days P = 0.002). In patients with TEE at three time points, PALS after 45 days increased compared to baseline (cohort 3:10.1% ± 6.8% at baseline vs. 13.8% ± 9.1% after 45 days, P = 0.009) and it remained stable between 45 days and 180 days (cohort 3:13.8% ± 9.1% after 45 days vs. 17.2% ±12.6% after 180 days, P = 0.092) [Figure 3].
Figure 3.
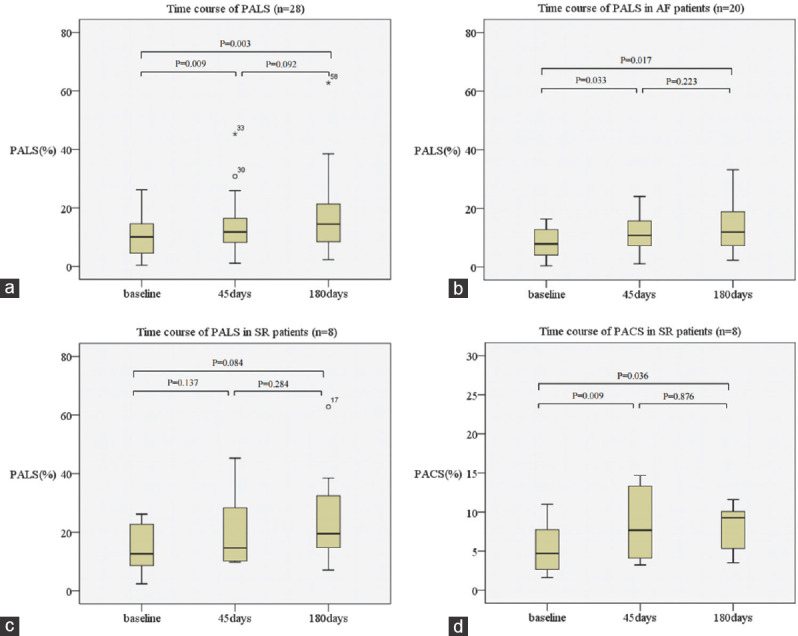
Time course of data in patients who had transesophageal echocardiography data at baseline, 45 days and 180 days (cohort 3). (a) Time course of peak atrial longitudinal strain. (b) Time course of peak atrial longitudinal strain in patients with atrial fibrillation. (c) Time course of peak atrial longitudinal strain in patients in sinus rhythm. (d) Time course of peak atrial contraction strain in patients in sinus rhythm
In patients with AF (n = 70), PALS after 45 and 180 days was increased compared to baseline (cohort 1: 10.8% ± 7.7% at baseline vs. 13.4% ± 7.1% after 45 days, P = 0.012; cohort 2: 8.5% ± 5.1% at baseline vs. 13.9% ± 8.1% after 180 days, P = 0.014). In patients in sinus rhythm (n = 25), PALS after 45 days was increased (cohort 1:16.6% ± 9.0% at baseline vs. 23.2% ± 15.2% after 45 days, P = 0.032), whereas PALS after 180 days was not changed (cohort 2:14.6% ± 9.0% at baseline vs. 25.4% ± 17.7% after 45 days, P = 0.084).
Similarly, compared to baseline, PACS was significantly increased after 45 days and 180 days (cohort 1: 5.8% ± 3.9% at baseline vs. 10.6% ± 7.6% after 45 days, P = 0.001; cohort 2: 4.5% ± 2.6% at baseline vs. 7.9% ± 3.1% after 45 days, P = 0.036). In patients with TEE at three time points, PALS after 45 days increased compared to baseline (cohort 3: 4.5% ± 2.6% at baseline vs. 7.6% ± 4.5% after 45 days, P = 0.009) and it remained stable between 45 days and 180 days (cohort 3: 7.6% ± 4.5% after 45 days vs. 17.2% ± 12.6% after 180 days, P = 0.876) [Figure 3].
Transesophageal echocardiography derived left atrial emptying fraction and left atrial fractional area change
LAEF increased after 45 and 180 days compared to baseline (cohort 1: 20.5% ± 11.8% at baseline vs. 24.7% ± 13.8% after 45 days, P < 0.001; cohort 2: 20.1% ± 11.8% at baseline vs. 25.9% ± 11.5% after 180 days, P = 0.003). In patients with TEE at three time points, LAEF after 45 days increased compared to baseline (cohort 3:19.8% ± 11.9% at baseline vs. 23.3% ± 13.4% after 45 days, P = 0.049) and it remained stable between 45 days and 180 days (cohort 3:23.3% ± 13.4% after 45 days vs. 25.8% ± 11.7% after 180 days, P = 0.212).
Similarly, LAFAC was increased after 45 days and 180 days compared to baseline (P = 0.002 in cohort 1 and P = 0.005 in cohort 2, respectively) and remained stable between 45 days and 180 days (P = 0.35 in cohort 3).
These values are summarized in Table 2.
Table 2.
Changes of left atrial strain from baseline
| Cohort 1: The data at baseline and after 45 days (n=95) | ||||||
|---|---|---|---|---|---|---|
| Baseline | After 45 days | P | ||||
| PALS (%) | ||||||
| All | 12.4±8.4 | 16.0±10.7 | 0.001 | |||
| AF (n=70) | 10.8±7.7 | 13.4±7.1 | 0.012 | |||
| SR (n=25) | 16.6±9.0 | 23.2±15.2 | 0.032 | |||
| PACS (%) | ||||||
| SR (n=25) | 5.8±3.9 | 10.6±7.6 | 0.001 | |||
| LAEF (%) | 20.5±11.8 | 24.7±13.8 | <0.001 | |||
| LAFAC (%) | 15.4±8.5 | 20.0±14.7 | 0.002 | |||
| Cohort 2: The data at baseline and after 180 days (n=30) | ||||||
| Baseline | After 180 days | P | ||||
| PALS (%) | ||||||
| All | 10.1±6.6 | 17.1±12.4 | 0.002 | |||
| AF (n=21) | 8.5±5.1 | 13.9±8.1 | 0.012 | |||
| SR (n=9) | 14.6±8.6 | 25.4±17.8 | 0.084 | |||
| PACS (%) | ||||||
| SR (n=9) | 4.5±2.6 | 7.9±3.1 | 0.036 | |||
| LAEF (%) | 20.1±11.7 | 25.9±11.5 | 0.003 | |||
| LAFAC (%) | 14.9±8.3 | 19.1±8.3 | 0.005 | |||
| Cohort 3: The data at baseline, after 45 and 180 days (n=28) | ||||||
| Baseline | After 45 days | After 180 days |
P |
|||
| Baseline versus 45 days | Baseline versus 180 days | 45 days versus 180 days | ||||
| PALS (%) | ||||||
| All | 10.1±6.8 | 13.8±9.1 | 17.2±12.6 | 0.009 | 0.003 | 0.092 |
| AF (n=20) | 8.3±5.2 | 11.2±5.8 | 13.9±8.3 | 0.033 | 0.017 | 0.223 |
| SR (n=8) | 14.6±8.6 | 20.2±12.8 | 25.4±17.8 | 0.137 | 0.084 | 0.284 |
| PACS (%) | ||||||
| SR (n=8) | 4.5±2.6 | 7.6±4.5 | 7.9±3.1 | 0.009 | 0.036 | 0.876 |
| LAEF (%) | 19.8±11.9 | 23.3±13.4 | 25.8±11.7 | 0.049 | 0.003 | 0.212 |
| LAFAC (%) | 14.7±8.4 | 17.8±9.3 | 19.0±8.4 | 0.022 | 0.004 | 0.367 |
All values are mean±SD. PALS=Peak arterial longitudinal strain, PACS=Peak arterial contraction strain, AF during exams=Patients with atrial fibrillation at the time of echocardiographic exams, SR during exams=Patients in sinus rhythm at the time of echocardiographic exams, LA=Left atrial, LAEF=LA emptying fraction, LAFAC=LA fractional area change, SD=Standard deviation
Reproducibility
Intraclass correlation coefficient (ICC) as measure of intra-observer variability for PALS and PACS were 0.98 and 0.962, respectively. Similarly, ICC for LAEF and LAFAC were 0.929 and 0.927, respectively.
DISCUSSION
Percutaneous LAA closure is an interventional alternative for reduction of thromboembolic risk in patients with AF at high risk for bleeding complications on oral anticoagulation regimens. In contrast to our initial hypothesis, PALS which represents LA reservoir function was increased after the LAAC procedure. On top of that, PACS which represents LA pump function, LAEF and LAFAC also increased after the procedure.
There are few studies that investigated LA function after percutaneous LAA closure. Hanna et al.[22] assessed the effects of percutaneous LAA transcatheter occlusion on structure and function of LA and left upper pulmonary vein in 11 patients. In this study, at 6 months of follow-up, left upper pulmonary vein diameter, peak systolic and diastolic flow velocities, LA size, mitral regurgitation severity and mitral valve peak E-wave velocities were not changed from baseline. Similarly, Madeira et al.[23] assessed the influence of percutaneous LAA occlusion devices on LA function by TTE plus STE in 16 patients. They reported that, at 3 months of follow-up, no differences were found in maximum and minimum LA volume, LAEF, LA strain or strain rate in the reservoir phase before and after the procedure. Each group also demonstrated that TTE evaluation did not reveal morphological and functional changes in the LA after percutaneous LAA closure. LA end-diastolic volume and LA ejection fraction in our patients showed no significant changes from baseline as evaluated by TEE evaluation. Also, Jalal et al. showed that LA volume evaluated by contrast-enhanced cardiac computed tomography showed no statistical difference before and after percutaneous LAA closure.[24]
Contrary to the above-mentioned studies, Coisne et al.[25] demonstrated that LAA closure was associated with an improvement in LA mechanical function during a 45-day follow-up in 33 patients. With comprehensive 2D/three-dimensional TTE evaluation and 2D speckle tracking, they found increases LA volume index, 2D-LA reservoir volume and expansion index, LAEF, PALS and PACS compared to time of discharge. Our study denoted a similar tendency except for volume and expansion index.
No studies have reported on the long-term improvement of LA function after percutaneous LAA closure. In our current study, in addition to short-timed follow-up (45 days), we performed 6 months follow-up assessments after the procedure. PALS and PACS after 180 days were significantly higher than baseline, but there were no significant differences between 45 days and 180 days indicating that LA reverse remodeling occurs early after LAAC maintained during long-term follow-up.
We assume that the change in LA deformation after LAAC correlates with an increase in LA preload. Along with changes in LA functional improvement, Majunke et al.[26] showed that both atrial natriuretic peptide and brain natriuretic peptide decrease after percutaneous LAAC prior to discharge compared with baseline values. Although we could not confirm the correlation between PALS and hemodynamic data in our study, endocrine change in natriuretic peptide after excluding LAA might result in LA pressure overload, leading to increase in PALS and PACS. Another hypothesis is that decrease in LA functional volume after excluding LAA results in volume overload inside LA main chamber. Höllmer et al.[27] demonstrated that LAEF showed an inverse relation to maximal LA volume. After occluding the LAA, LA whole functional volume decreases due to the loss of LAA volume resulting in increased LAEF, PACS and LAFAC.
Study limitation
Our study has several limitations. First, this was a retrospective single-center study with a small sample size. There were few patients in sinus rhythm at the time of echocardiographic examinations and thus the number of patients with PACS was small. However, there are not many studies analyzing the impact of percutaneous LAAC in patients in sinus rhythm. Second, the observational period was limited (180 days) and future studies should acquire longer follow-up to evaluate long-term effects on LA function. Third, in our preliminary study, we analyzed only a limited area (lateral wall from mid-esophageal four-chamber view) of entire LA wall to evaluate LA strain. Nevertheless, in our population, PALS of lateral wall in patients who were followed by TTE (n = 16) was also improved after LAAC (18.0% vs. 25.8%, P = 0.002). Finally, the clinical impacts of LA strain improvement on patients are not clear. We could not find a correlation between baseline LA strain and hemodynamic parameters in catheterization [Figure 4]. Furthermore, we did not measure invasive hemodynamics data including filling pressure at follow-up and those changes after LAAC were unknown in our population. Myocardial deformation is pressure dependent and thus the relations between LA strain and clinical data before and after LAAC should be investigated in further studies.
Figure 4.
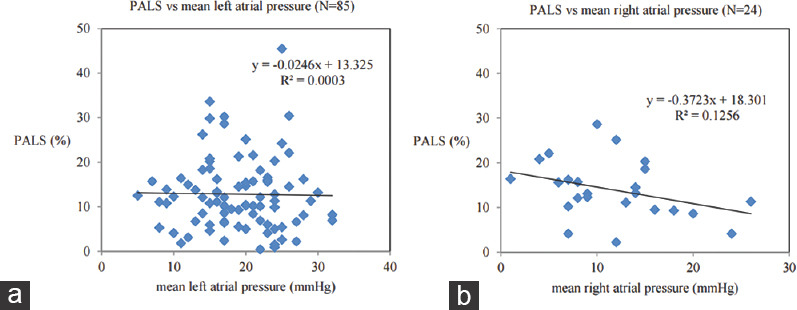
(a) Scatterplot of baseline peak atrial longitudinal strain versus mean left atrial pressure in catheterization. (b) Scatterplot of baseline peak atrial longitudinal strain versus mean right atrial pressure in catheterization
CONCLUSIONS
Our study has demonstrated for the first time the improvement in TEE-derived LA strain following LAAC within 45 days of implantation and these values were maintained at least for 6 months. These findings suggest reverse remodeling and improved function of the LA following LAAC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to express gratitude to Y. Nakajima for technical assistance with 2D-STE analysis. We also thank a scholarship from SUNRISE Lab for fundamental support to young cardiologists researching abroad.
REFERENCES
- 1.Conen D. Epidemiology of atrial fibrillation. Eur Heart J. 2018;39:1323–4. doi: 10.1093/eurheartj/ehy171. [DOI] [PubMed] [Google Scholar]
- 2.Healey JS, Oldgren J, Ezekowitz M, Zhu J, Pais P, Wang J, et al. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: A cohort study. Lancet. 2016;388:1161–9. doi: 10.1016/S0140-6736(16)30968-0. [DOI] [PubMed] [Google Scholar]
- 3.Ribic C, Crowther M. Thrombosis and anticoagulation in the setting of renal or liver disease. Hematology Am Soc Hematol Educ Program. 2016;2016:188–95. doi: 10.1182/asheducation-2016.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brener SJ, Kirtane AJ, Rinaldi MJ, Stuckey TD, Witzenbichler B, Weisz G, et al. Prediction of ischemic and bleeding events using the dual antiplatelet therapy score in an unrestricted percutaneous coronary intervention population. Circ Cardiovasc Interv. 2018;11:e006853. doi: 10.1161/CIRCINTERVENTIONS.118.006853. [DOI] [PubMed] [Google Scholar]
- 5.Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The left atrial appendage: Anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging. 2014;7:1251–65. doi: 10.1016/j.jcmg.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Squiers JJ, Edgerton JR. Surgical closure of the left atrial appendage: the past, the present, the future. J Atr Fibrillation. 2018;10:1642. doi: 10.4022/jafib.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy VY, Doshi SK, Sievert H, Buchbinder M, Neuzil P, Huber K, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation. 2013;127:720–9. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 8.Holmes DR, Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, et al. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J Am Coll Cardiol. 2014;64:1–2. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Mazzone P, D'Angelo G, Regazzoli D, Molon G, Senatore G, Saccà S, et al. Percutaneous left atrial appendage closure with WATCHMAN™ device: Peri-procedural and mid-term outcomes from the TRAPS Registry. J Interv Card Electrophysiol. 2018;52:47–52.2. doi: 10.1007/s10840-018-0351-1. [DOI] [PubMed] [Google Scholar]
- 10.Boersma LV, Schmidt B, Betts TR, Sievert H, Tamburino C, Teiger E, et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: Peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37:2465–74. doi: 10.1093/eurheartj/ehv730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urena M, Rodés-Cabau J, Freixa X, Saw J, Webb JG, Freeman M, et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol. 2013;62:96–102. doi: 10.1016/j.jacc.2013.02.089. [DOI] [PubMed] [Google Scholar]
- 12.Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: Structure, function, and role in thromboembolism. Heart. 1999;82:547–54. doi: 10.1136/hrt.82.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regazzoli D, Ancona F, Trevisi N, Guarracini F, Radinovic A, Oppizzi M, et al. Left atrial appendage: Physiology, pathology, and role as a therapeutic target. Biomed Res Int. 2015;2015:205013. doi: 10.1155/2015/205013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isobe F, Kumano H, Ishikawa T, Sasaki Y, Kinugasa S, Nagamachi K, et al. A new procedure for chronic atrial fibrillation: Bilateral appendage-preserving maze procedure. Ann Thorac Surg. 2001;72:1473–8. doi: 10.1016/s0003-4975(01)03038-7. [DOI] [PubMed] [Google Scholar]
- 15.Kamohara K, Popović ZB, Daimon M, Martin M, Ootaki Y, Akiyama M, et al. Impact of left atrial appendage exclusion on left atrial function. J Thorac Cardiovasc Surg. 2007;133:174–81. doi: 10.1016/j.jtcvs.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 16.Sengeløv M, Jørgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, et al. Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging. 2015;8:1351–9. doi: 10.1016/j.jcmg.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–80. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 18.Cameli M, Mandoli GE, Loiacono F, Sparla S, Iardino E, Mondillo S. Left atrial strain: A useful index in atrial fibrillation. Int J Cardiol. 2016;220:208–13. doi: 10.1016/j.ijcard.2016.06.197. [DOI] [PubMed] [Google Scholar]
- 19.Möbius-Winkler S, Sandri M, Mangner N, Lurz P, Dähnert I, Schuler G. The WATCHMAN left atrial appendage closure device for atrial fibrillation. J Vis Exp. 2012:pii: 3671. doi: 10.3791/3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19:591–600. doi: 10.1093/ehjci/jey042. [DOI] [PubMed] [Google Scholar]
- 21.Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: An analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123:2363–72. doi: 10.1161/CIRCULATIONAHA.110.004747. [DOI] [PubMed] [Google Scholar]
- 22.Hanna IR, Kolm P, Martin R, Reisman M, Gray W, Block PC. Left atrial structure and function after percutaneous left atrial appendage transcatheter occlusion (PLAATO): Six-month echocardiographic follow-up. J Am Coll Cardiol. 2004;43:1868–72. doi: 10.1016/j.jacc.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 23.Madeira M, Teixeira R, Reis L, Dinis P, Paiva L, Botelho A, et al. Does percutaneous left atrial appendage closure affect left atrial performance? Int J Cardiovasc Sci. 2018;31:569–77. [Google Scholar]
- 24.Jalal Z, Iriart X, Dinet ML, Corneloup O, Pillois X, Cochet H, et al. Evaluation of left atrial remodelling following percutaneous left atrial appendage closure. J Geriatr Cardiol. 2017;14:496–500. doi: 10.11909/j.issn.1671-5411.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coisne A, Pilato R, Brigadeau F, Klug D, Marquie C, Souissi Z, et al. Percutaneous left atrial appendage closure improves left atrial mechanical function through Frank-Starling mechanism. Heart Rhythm. 2017;14:710–6. doi: 10.1016/j.hrthm.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 26.Majunke N, Sandri M, Adams V, Daehnert I, Mangner N, Schuler G, et al. Atrial and brain natriuretic peptide secretion after percutaneous closure of the left atrial appendage with the watchman device. J Invasive Cardiol. 2015;27:448–52. [PubMed] [Google Scholar]
- 27.Höllmer M, Willesen JL, Tolver A, Koch J. Left atrial volume and function in dogs with naturally occurring myxomatous mitral valve disease. J Vet Cardiol. 2017;19:24–34. doi: 10.1016/j.jvc.2016.08.006. [DOI] [PubMed] [Google Scholar]