Abstract
Objective
To determine whether comorbidity presence, frequency or type is associated with Physical Activity (PA) levels in people with Osteoarthritis (OA).
Design
Secondary data analysis of adults aged ≥45, with OA related pain recruited to the BEEP trial (knee pain, n = 514) (ISRCTN93634563) and the MOSAICS trial (peripheral joint pain, n = 525) (ISRCTN06984617). Comorbidities considered were respiratory, cardiovascular diseases (CVD), depression, type 2 diabetes and obesity. Self-report PA was measured using the Physical Activity Scale for the Elderly (PASE). Linear regression models were used to estimate the mean change (β) in PA with comorbidity presence, frequency and type adjusting for potential confounding covariates.
Results
In the BEEP trial comorbidity presence was associated with a decrease in PASE score (β = -32.25 [95% confidence interval (95% CI) −48.57, −15.93]). Each additional comorbidity was associated with an incrementally lower PASE score, one comorbidity (β = −24.42 [-42.45, −6.38]), two comorbidities β = −34.76 [-56.05, −13.48]), and three or more comorbidities β = −73.71 [-106.84, −40.58]) compared to those with no comorbidity. This pattern was similar in MOSAICS, but with a plateau in association from two comorbidities onward. In BEEP and MOSAICS, respiratory (β = −40.60 [-60.50, −20.35]; β = −11.82 [-34.95, 11.31]) and CVD (β = −27.15 [-53.25, −1.05]; β = −30.84 [-51.89, −9.80]) comorbidities were associated with the largest reduction in PASE scores respectively.
Conclusion
Comorbidity presence and frequency is associated with lower PA levels and respiratory and CVD comorbidities have the greatest impact. Future exploratory work needs to be done to understand how and why comorbidity is associated with PA levels in people with OA.
Keywords: Osteoarthritis, Comorbidity, Physical activity, Exercise, Trials, Secondary analysis
1. Introduction
Osteoarthritis (OA) frequently co-occurs with other long-term conditions such as respiratory, cardiovascular diseases (CVD), depression, type 2 diabetes and obesity [[1], [2], [3]]. Compared with age and gender matched controls, those with OA are significantly more likely to experience comorbidities [4]. It is estimated that four out of five people with OA have at least one other long-term condition [5], with the proportion of people with OA reporting comorbidity ranging from 68 to 85% [6]. With an ageing population, the burden of OA and comorbidity is likely to rise in the future [1,3].
Current interventions for OA focus on alleviating pain and inflammation as well as improving function and quality of life (QOL) [7]. International clinical guidelines recommend physical activity (PA) in the form of strengthening and aerobic exercise for everyone with OA irrespective of condition severity and comorbidity [7,8]. PA programs have been shown to generate similar positive effects on pain and function compared to simple analgesics and oral non-steroidal anti-inflammatory drugs (NSAIDs) [[9], [10], [11]] in people with OA but with lower risks of serious adverse events [12]. PA provides numerous health benefits, including reduced risk for many long-term conditions (e.g. type 2 diabetes and hypertension), improved psycho-physiological health and an enhanced QOL[[7], [8], [9], [10]]. However, PA interventions are underused for patients with OA [13] and carrying out PA may become more complex in those with comorbidities. The existence of comorbidity creates challenges for the management of OA and other long-term conditions which are largely managed according to single condition guidelines [14]. Physiotherapists and other exercise professionals may also find it difficult to manage individuals with several coexisting long-term conditions due to lack of information and a lack of confidence of how to appropriately tailor PA [15].
People with OA have lower levels of PA than aged matched controls and PA levels may be further compounded by comorbidities [10,[16], [17], [18]]. Associations between comorbidity and levels of PA have been found in people with OA [10,[16], [17], [18]], however, such relationships have not been thoroughly investigated. Further, it is unknown if specific frequencies of comorbidity or specific types of comorbidity have more limiting effects on PA level in people with OA and there is a dearth of evidence that investigates OA phenotypes and comorbidity other than knee OA. This is the first in depth focussed investigation of comorbidity aiming to, first, investigate the cross-sectional association between comorbidity presence and PA in people with OA. Second, investigate whether different frequencies of comorbidity and third, different types of comorbidity are associated with PA level. This information could inform future treatment strategies and recommendations for OA and comorbidity adapted PA interventions.
2. Methods
This secondary analysis study used data from two randomised controlled trials (RCTs); The Benefits of Effective Exercise for knee Pain trial (BEEP) and The Management of OsteoArthritis In Consultations trial (MOSAICS). These two large RCTs recruited adults with peripheral joint pain attributed to OA, and collected self-reported PA levels, comorbidity data, similar baseline demographics and clinical measures (further details below). Using two separate datasets allowed for comparison of findings between different samples that include different OA phenotypes and different methods of measuring comorbidity.
2.1. BEEP trial and participants
The BEEP trial was a multicentre, pragmatic, parallel group RCT undertaken between 2009 and 2014 (see Foster et al; 2014 [19] for full details). The primary aim of this trial was to determine the clinical and cost effectiveness of two enhanced physiotherapy-led exercise interventions compared with usual care. The trial included 514 adults with pain or stiffness attributed to OA in one or both knees. The participants were recruited from 65 general practices in England in three ways; (1) examining medical records to identify patients consulting for knee pain in the previous 12 months, (2) identifying patients referred to physiotherapy for knee pain and (3) conducting a population survey of adults (aged 45 years and over) which included a chronic pain grade to ensure those recruited had a mean level of pain and functional difficulty similar to those recruited through methods 1 and 2 [19].
2.2. MOSAICS trial and participants
The MOSAICS study was a mixed methods study with a nested cluster RCT, undertaken between May 2011 and December 2013 [20]. The primary aim of MOSAICS was to determine the clinical and cost effectiveness of a model OA consultation (implementing the core recommendations from the NICE OA guidelines in primary care) (see Dziedzic et al, 2014 [20] for full details). 525 participants with OA, completed the trial and agreed to primary care Medical Record Review (MRR) with data available up to 3 years prior to index consultation for OA.
2.3. Outcomes of interest
2.3.1. Socio-demographics
Both the BEEP and MOSAICS trials datasets included participants’ socio-demographics; age, gender and partner status (yes or no).
2.4. Physical activity
PA level was measured by the self-report Physical Activity Scale for the Elderly (PASE) in both trial datasets [21]. The scale measures leisure, household and occupational PA with weighting specific to the frequency and duration of each activity carried out over the previous week. The PASE scale gives a continuous score from 0 up to 400+, with higher scores indicating higher PA levels. Washburn et al (1999) [21] validated the PASE as a measure of PA suitable for use when measuring the association of PA, health and physical function in older adults. PASE has acceptable retest reliability (ICC = 0.77), and moderate correlation with the IPAQ (international physical activity questionnaire) scale (r = 0.61) [22]. The PASE has been shown to have positive correlation with other PA measures such as the 6-min walk test (r = 0.35) and knee strength (r = 0.41) in adults with knee pain [23].
2.5. Comorbidity
Comorbidities were the primary independent variable of interest. Comorbidity was captured through self-report (BEEP) and MRR (MOSAICS). For the MRR the read code definitions for identifying comorbidities were derived from previous studies (for example; type 2 diabetes [24], heart disease, asthma and Chronic Obstructive Pulmonary Disease (COPD) [26]). In order to have consistency across the two datasets for future comparison, the comorbidities were grouped based on type of comorbidity.
Individual comorbidities from the two datasets were collapsed into five overarching categories, respiratory (asthma, bronchitis, COPD), CVD (angina, heart failure, stroke, heart attack), depression, type 2 diabetes and obesity. Obesity was calculated from Body Mass Index (BMI) (calculated using height and weight) and used as a dichotomous variable (obese (BMI ≥30) yes/no) as well as categorical (underweight/normal (BMI < 25), overweight (BMI 25–29.9), obese BMI ≥ 30). An a priori decision was made not to include hypertension due to its high prevalence and high association as a risk factor for other investigated comorbidities.
These comorbidity categories were then used to create other variables to answer the 3 aims of this study. These were “comorbidity presence”, which categorised participants into either having or not having any of the five comorbidity categories (respiratory, CVD, depression, type 2 diabetes, obesity); “comorbidity frequency”, which counted the number of comorbidities each individual participant had (0, 1, 2, 3+ (the frequency of 4 and 5 comorbidities were too small to use in separate analyses, therefore a 3+ group was formed)); “types of comorbidity” (presence of respiratory, CVD, depression type 2 diabetes or obesity comorbidity).
2.6. Clinical variables
Physical function (as measured by Western Ontario and McMaster Universities Arthritis Index (WOMAC) (different versions in the separate datasets; BEEP 0–68, MOSAICS 0–32) [26]), pain (WOMAC), anxiety (Generalised Anxiety Disorder (GAD7)) [27], quality of life (EuroQOL 5 Dimensions (EQ5D) [28] were collected. These measures are widely used in OA clinical research and have shown to be reliable and valid and may confound the association between comorbidity and PA [29,30].
2.7. Statistical analysis
All data analyses for this study were carried out using complete case analysis for the BEEP and MOSAICS datasets separately using SPSS version 24 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp). Descriptive statistics described participant characteristics, independent and dependent variables.
The association between comorbidity and PA levels was estimated using linear regression. The dependent variable for all three objectives was PA score (PASE). The mean change in PA level (β) with 95% confidence interval (CI) was estimated in participants with at least one comorbidity compared to participants with no comorbidities (objective 1); in participants with 1, 2 and 3+ comorbidities compared to participants with no comorbidities (objective 2) and; in the presence of a particular comorbidity type compared to absence of that comorbidity (objective 3). Results were then adjusted for age, gender, partner, physical function and anxiety which were selected a priori. P value < 0.05 was deemed to be statistically significant. The assumptions of linear regression and relevant tests that were carried out to ensure these were satisfied are as follows; a linear relationship (scatter plots of the independent variables against the dependent variable), univariate normality and normal distribution of residuals (histograms and P–P plots), little collinearity between independent variables (Pearson's correlations and the Variance Inflation Factor), no autocorrelation of residuals (Durbin-Watson statistic) and homoscedasticity (scatter plots of the residuals against the predicted values) [31].
Independent variables available in both datasets considered as confounding variables were selected based on results of past research [[32], [33], [34]], theoretical and clinical importance and collinearity checks [35]. Pairs of the independent variables were checked for collinearity using Pearson's correlations [36]. Correlations with any r value > 0.7 dealt with by removing the variable which were considered to have less clinical importance whilst maintaining homogeneity between datasets [30]. The EQ5D-3L was correlated with other variables (e.g. WOMAC function r = 0.664) and considered less clinically important. WOMAC pain and WOMAC function were highly correlated in the BEEP dataset (r = 0.798), however, MOSAICS only measured WOMAC function data. Therefore, the EQ5D-3L and WOMAC pain were removed from the analyses.
3. Results
3.1. Baseline characteristics
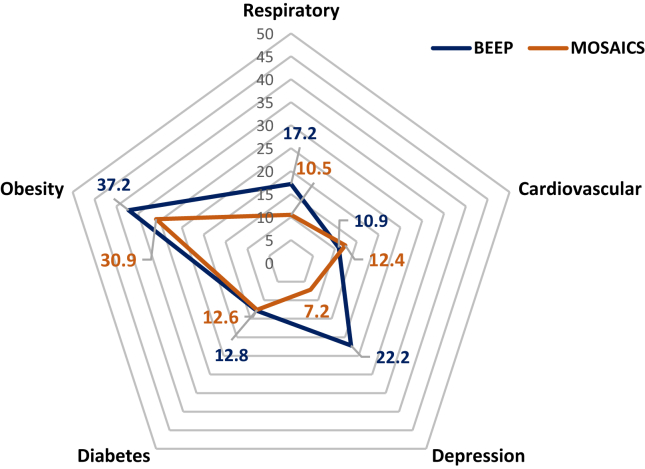
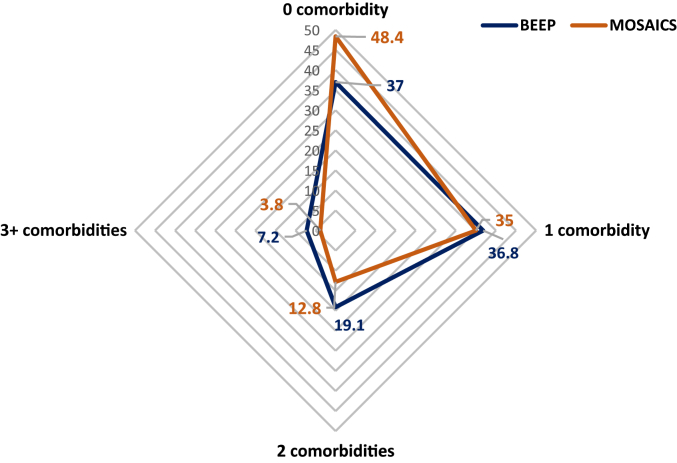
Table 1 provides a summary of the descriptive statistics of participants from the BEEP and MOSAICS datasets. The BEEP and MOSAICS trials were similar in size (n = 514; 525, respectively). BEEP had slightly more males than MOSAICS and had a slightly higher mean PASE score. BEEP had more participants reporting presence of comorbidity. The most common comorbidity was obesity in both datasets (see Figure 2.). The proportion with individual comorbidity types was similar between datasets except depression (22.2% in BEEP and 7.2% in MOSAICS) (Figure 1, Figure 2).
Table 1.
Summary of participants characteristics from the BEEP and MOSAICS trials.
| BEEP | MOSAICS | |
|---|---|---|
| Total number | 514 | 525 |
| Age | 62.85 ± 9.79 | 67.34 ± 10.47 |
| Gender (male) | 252 (49.0%) | 212 (40.4%) |
| Partner (yes) | 395 (76.8%) | 373 (71.0%) |
| GAD7 score | 3.29 ± 4.55 | 3.48 ± 4.65 |
| WOMAC Function score# | 28.08 ± 12.26 | 12.19 ± 7.28 |
| Comorbidity presence | 324 (63%) | 271 (51.6%) |
| Respiratory | 88 (17.2%) | 55 (10.5%) |
| Cardiovascular | 56 (10.9%) | 65 (12.4%) |
| Depression | 114 (22.2%) | 38 (7.2%) |
| Type 2 diabetes | 66 (12.8%) | 66 (12.6%) |
| Obesity | 191 (37.2%) | 162 (30.9%) |
| BMI | 29.63 ± 5.67 | 28.26 ± 4.96 |
| Underweight/normal | 97 (18.9%) | 135 (25.7%) |
| Overweight | 209 (40.7%) | 206 (39.2%) |
| Obese | 191 (37.2%) | 162 (30.9%) |
| One comorbidity | 189 (36.8%) | 184 (35%) |
| Two comorbidities | 98 (19.1%) | 67 (12.8%) |
| 3 + comorbidities | 37 (7.2%) | 20 (3.8%) |
| PASE | 176.90 ± 83.51 | 142.67 ± 80.28 |
All values are mean and standard deviation (±) or number and percentage (%).
BEEP = Benefits of Effective Exercise for knee Pain, MOSAICS = the Management of Osteoarthritis In Consultations, OA = Osteoarthritis, PA = Physical activity, PASE = PA Scale for the Elderly: 0–400+ (higher number = higher PA level), WOMAC = Western Ontario and McMaster Universities Arthritis Index: #BEEP 0–68/MOSAICS 0–32 (higher = worse function), GAD 7 = Generalized Anxiety Disorder 7: 0–21 (21 = worst anxiety), BMI = Body Mass Index, Underweight/normal (<25), overweight (25–29.9), obese (BMI ≥30).
Figure 2.
Proportion (%) of the BEEP and MOSAICS trial participants with different comorbidity types.
Figure 1.
Proportion (%) of the BEEP and MOSAICS trial participants with different comorbidity frequencies (0, 1, 2, 3+).
3.2. Objective 1: To investigate whether comorbidity presence is associated with PA level in adults with OA
Table 2 shows both unadjusted and adjusted associations between PASE and comorbidity presence. Results for each objective are presented as; beta-coefficient (lower, upper 95% confidence interval limits). Unadjusted comorbidity presence was significantly associated with a decrease in PASE score in BEEP β = −27.93 (−44.10, −11.75) and MOSAICS β = −20.86 (−36.02, −5.70).
Table 2.
Objective 1: Unadjusted and adjusted models for objective 1: PASE score with comorbidity presence.
| Independent variable | BEEP |
MOSAICS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| (N) | β coefficient | 95% CI Lower | Upper | (N) | β coefficient | 95% CI Lower | Upper | ||
| Unadjusted | Comorbidity presence | 445 | −27.93∗ | −44.10 | −11.75 | 429 | −20.86∗ | −36.02 | −5.70 |
| Adjusted | Comorbidity presence | 390 | −32.25∗ | −48.57 | −15.93 | 405 | −6.50 | −21.11 | 8.11 |
| Age | −2.49∗ | −3.36 | −1.63 | −2.48∗ | −3.20 | −1.76 | |||
| Gender (female) | −18.59∗ | −34.21 | −2.97 | −25.32∗ | −39.57 | −11.06 | |||
| Partner (yes) | 30.22∗ | 7.73 | 52.70 | 14.89 | −1.54 | 31.31 | |||
| WOMAC function# | -0.24 | -0.92 | 0.43 | −1.58∗ | −2.69 | −0.46 | |||
| GAD7 | −1.57 | −3.34 | 0.21 | −2.08∗ | −3.75 | −0.41 | |||
Unadjusted and adjusted models for objective 1 in BEEP and MOSAICS datasets.
∗denotes statistically significant at p < 0.05.
Footnotes: complete case data, multiple linear regression adjusted for age, gender, partner, WOMAC function and GAD7. β coefficient represents mean change in PASE score. Higher PASE scores indicate higher levels of PA. Age (increase in one year); Gender (reference: male); Partner (reference: no partner); Higher WOMAC function indicates worse function (one increase unit in WOMAC function score, # BEEP 0–68/MOSAICS 0–32); Higher GAD7 indicates worse anxiety (one increase unit in GAD7 score).
Abbreviations: β: unstandardized beta coefficient; CI: Confidence Interval; WOMAC: Western Ontario and McMaster Osteoarthritis Index; GAD7: Generalised Anxiety Disorder Questionnaire.
After adjusting for confounding variables (variables that may influence both the dependent variable and independent variable: age, gender, partner status, WOMAC function and GAD7), comorbidity presence remained significantly associated with a decrease in PASE score in BEEP β = −32.25 (−48.57, −15.93) and the strength of association increased. In MOSAICS, however, the association between comorbidity presence and PASE attenuated after adjustment and was no longer statistically significant β = −6.50 (−21.11, 8.11). Increasing age and female gender were significantly associated with lower PASE score in both datasets. In the BEEP dataset having a partner was associated with an increased PASE score. In the MOSACIS dataset, worse function (WOMAC) and worse anxiety (GAD) was associated with lower PASE score.
3.3. Objective 2: To investigate whether comorbidity frequency is associated with PA level in adults with OA
In BEEP, the unadjusted associations found each additional comorbidity was significantly associated with a lower PASE score; one comorbidity β = −19.85 (−38.11, −1.59), two comorbidities β = −32.72 (−53.91, −11.52), and three or more comorbidities β = −52.68 (−83.42, −21.94) compared to those with no comorbidity. After adjusting for confounding variables, comorbidity frequency remained significant and grew in association with lower PASE scores across all frequencies of comorbidity; one comorbidity β = −24.42 (−42.45, −6.38); two comorbidities β = −34.76 (−56.05, −13.48) and three or more comorbidities β = −73.71 (−106.84, −40.58).
The unadjusted and adjusted models for MOSAICS found having one comorbidity was not significantly associated with PASE score; 0; β = −8.51 (−25.11, 8.08) compared to no comorbidity. However, having two comorbidities β = −45.06 (−68.45, −21.66) and three or more comorbidities β = −52.25 (−91.39, −13.11) were significantly associated with an incremental decrease in PASE scores compared to no comorbidity. In adjusted analyses associations attenuated. Having one comorbidity remained non-significant β = 1.39 (−14.30, 17.08). Having three or more comorbidities was no longer significantly associated with PASE score β = −24.70 (−62.15, 12.76). However, presence of two comorbidities remained significantly associated with a decrease in PASE score β = −26.84 (−49.30, −4.38) (Table 3).
Table 3.
Objective 2: Unadjusted and adjusted models for BEEP and MOSAICS PASE score and comorbidity frequency.
| Independent variable | BEEP |
MOSAICS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| (N) | β coefficient | 95% CI Lower | Upper | (N) | β coefficient | 95% CI Lower | Upper | ||
| Unadjusted | 1 comorbidity | 445 | −19.85∗ | −38.11 | −1.59 | 429 | −8.51 | −25.11 | 8.08 |
| 2 comorbidities | 445 | −32.72∗ | −53.91 | −11.52 | 429 | −45.06∗ | −68.45 | −21.66 | |
| 3+ comorbidities | 445 | −52.68∗ | −83.42 | −21.94 | 429 | −52.25∗ | −91.39 | −13.11 | |
| Adjusted | 1 comorbidity | 390 | −24.42∗ | −42.45 | −6.38 | 405 | 1.39 | −14.30 | 17.08 |
| 2 comorbidities | 390 | −34.76∗ | −56.05 | −13.48 | 405 | −26.84∗ | −49.30 | −4.38 | |
| 3+ comorbidities | 390 | −73.71∗ | −106.84 | −40.58 | 405 | −24.70 | −62.15 | 12.76 | |
PASE score for each comorbidity category variable (reference 0 comorbidity).
∗denotes statistically significant at p < 0.05.
Footnotes: complete case data, multiple linear regression adjusted models adjusted for age, gender, partner, WOMAC and GAD7. β coefficient represents change in PASE score. Higher PASE scores indicate higher levels of PA.
Abbreviations: β = unstandardized beta coefficient; CI= Confidence Interval; WOMAC: Western Ontario and McMaster Osteoarthritis Index; GAD7 = Generalised Anxiety Disorder Questionnaire.
3.4. Objective 3: To investigate whether different types of comorbidity are associated with PA level in adults with OA
In BEEP, respiratory, type 2 diabetes and obesity comorbidities were all significantly associated with a decrease in PASE score (Table 4). Respiratory had the strongest association with lower PASE scores β = −40.11 (−60.07, −20.14), followed by type 2 diabetes β = −26.68 (−48.82, −4.55) and obesity β = −25.78 (−41.06, −9.50). CVD (β = −25.75 (−48.04, 0.54)) and depression (β = 3.12 (−15.53, 21.76)) were not significantly associated with PASE score.
Table 4.
Objective 3: Unadjusted and adjusted models for BEEP and MOSAICS PASE score and comorbidity type.
| Independent variable | BEEP |
MOSAICS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| (N) | β coefficient | 95% CI Lower | Upper | (N) | β coefficient | 95% CI Lower | Upper | ||
| Unadjusted | Respiratory | 463 | −40.11∗ | −60.07 | −20.14 | 432 | −22.43 | −46.98 | 2.12 |
| Cardiovascular | 463 | −23.75 | −48.04 | 0.54 | 432 | −43.00∗ | −65.08 | −20.91 | |
| Depression | 462 | 3.12 | −15.53 | 21.76 | 432 | −23.24 | −53.06 | 6.58 | |
| Type 2 diabetes | 463 | −26.68∗ | −48.82 | −4.55 | 432 | −34.42∗ | −56.64 | −12.20 | |
| Obesity | 448 | −25.28∗ | −41.06 | −9.50 | 430 | −7.62 | −24.12 | 8.88 | |
| BMI Categorical (reference <25) | |||||||||
| Overweight (25–29.9) | 448 | 21.84∗ | 0.25 | 43.44 | 413 | 3.11 | −16.21 | 22.42 | |
| Obese (30+) | 448 | −9.83 | −31.75 | 12.09 | 413 | −5.62 | −26.08 | 14.84 | |
| Adjusted | Respiratory | 403 | −40.60∗ | −60.50 | −20.35 | 408 | −11.82 | −34.95 | 11.31 |
| Cardiovascular | 403 | −27.15∗ | −53.25 | −1.05 | 408 | −30.84∗ | −51.89 | −9.80 | |
| Depression | 402 | −4.93 | −23.62 | 13.76 | 408 | −10.27 | −38.45 | 17.92 | |
| Type 2 diabetes | 403 | −30.19∗ | −54.25 | −6.12 | 408 | −17.03 | −38.11 | 4.05 | |
| Obesity | 393 | −27.72∗ | −44.08 | −11.36 | 406 | 1.71 | −13.94 | 17.35 | |
| BMI Categorical (reference <25) | |||||||||
| Overweight (25–29.9) | 393 | 12.17 | −9.68 | 34.02 | 392 | −3.37 | −21.57 | 14.83 | |
| Obese (30+) | 393 | −18.65 | −41.73 | 4.42 | 392 | -0.46 | −20.20 | 19.28 | |
PASE score for each comorbidity and BMI category variable (reference: underweight/normal).
∗denotes statistically significant at p < 0.05.
Footnotes: complete case data, multiple linear regression adjusted models adjusted for age, gender, partner, WOMAC and GAD7. β coefficient represents change in PASE score. Higher PASE scores indicate higher levels of PA.
Abbreviations: β = unstandardized beta coefficient; CI= Confidence Interval; WOMAC: Western Ontario and McMaster Osteoarthritis Index; GAD7 = Generalised Anxiety Disorder Questionnaire.
Respiratory β = −40.6 (−60.5, −20.35), type 2 diabetes β = −30.19 (−54.25, −6.12) and obesity β = −27.72 (−44.08, −11.36) remained associated with lower PASE score in the adjusted models (Table 4) and CVD (β = −27.15 (−53.25, −1.05) was now significant. Depression remained non-significant; β = −4.93 (−23.62, 13.76).
In MOSAICS, the unadjusted models (Table 4), showed CVD β = −43.00 (−65.08, −20.91) and type 2 diabetes β = −34.42 (−56.64, −12.20) were significantly associated with a decrease in PASE score. Respiratory and depression were non-significantly associated with PASE scores, but estimates suggest a negative association; β = −22.43 (−46.98, 2.12), β = −23.24 (−53.06, 6.58); respectively. Obesity had the smallest magnitude of association and was non-significant; β = −7.62 (−24.12, 8.88).
After adjusting for confounding variables, only CVD conditions remained significant and had the strongest magnitude of association with PASE scores; β = −30.84 (−51.89, −9.80). Respiratory β = −11.82 (−34.95, 11.31), depression β = −10.27 (−38.45, 17.92) and type 2 diabetes β = −17.03 (−38.11, 4.05) were no longer significantly associated with PASE scores, with a weaker but still negative association whilst obesity β = 1.71 (−13.94, 17.35) was not significantly associated.
The relationship between BMI as a categorical variable and PA is also included at the bottom of both the BEEP and MOSAICS models in Table 4.
4. Discussion
This is the first study to investigate and describe in detail the association between comorbidity and PA in people with OA. Comorbidity presence was associated with lower PA level. Our findings suggest a potential dose-response relationship with activity levels decreasing as the number of comorbidities increase in people with OA. This relationship was more pronounced in the BEEP trial than in the MOSAICS trial. Distinct comorbidity types had different magnitudes of association with lower PA levels across both datasets.
People with OA commonly also have other comorbidities [1,2]. In the current study samples, of the comorbidities under investigation, obesity had the highest prevalence; 37.2% and 30.9% in BEEP and MOSAICS trials, respectively. Reeuwijk et al (2010) [37] found obesity prevalence to be lower at 23.9% in a cohort of people with hip and knee OA in the Netherlands. Other comorbidity rates in their OA sample were similar to our findings, respiratory: 15.6%, CVD (cardiac event, coronary and stroke): 10.1% and type 2 diabetes: 9.7% [37].
Within the BEEP and MOSAICS trials, the reported levels of conditions such as obesity, type 2 diabetes and CVD disease, that have clear diagnostic features, appear to be similar for both self-report and MRR. However, there was some inter-dataset variation in terms of sample proportion with other comorbidity types. Depression appears to be under reported in MOSAICS compared to BEEP. This may be due to general practitioners using a variety of codes for recording symptoms associated with depression, or not all cases of depression being discussed or recorded [38]. Research has shown that the stigma of reporting depression may make people reluctant to seek professional help; although 20–40% of community dwelling older people show signs of depression, fewer than 10% consult a general practitioner [39].
Previous research on community dwelling older adults also showed PASE scores to be higher for healthy participants: 155 (males = 172; females = 139) compared to those with health conditions, ranging from 118 to 139 [40]. Our findings of lower levels of PA in people with OA and comorbidity presence are similar to those reported in a systematic review of correlates of PA in people with knee and hip OA by Stubbs et al (2015) [16]. Stubbs and colleagues found PA level was reduced with higher BMI and comorbidity presence [16]. Similar to our multivariable models, increased age, worse OA symptoms, female gender have been found to be associated with lower levels of PA [16].
In our cross-sectional analyses, comorbidity presence was significantly associated with PA level in the BEEP adjusted model. This supports previous studies demonstrating that OA combined with another long-term condition, increased the likelihood of lower PA levels [[16], [17], [18]]. In a study by Cook et al (2018) [41], the presence of comorbidity was associated with reduced PA in the general population and to a greater extent in those with inflammatory rheumatic or musculoskeletal disease (IRMD). Specifically, myocardial and vascular comorbidities and depression were associated with reduced odds of moderate or high level of PA in those with IRMD [41]. These results support the findings of our study as both CVD and depression reduced PA levels in the models.
Addressing the second aim, in the current study, as comorbidity frequency increased, PASE score declined. In BEEP, the addition of one, two and three or more comorbidities was incrementally associated with a greater magnitude of difference in PASE score. A similar pattern was observed in the MOSAICS dataset, but with a plateau in the affect from 2 comorbidities upwards. Similarly, increased comorbidity burden has previously been shown to be associated with reduced odds of reporting moderate to high levels of PA in participants with IRMD [41]. Low levels of PA and more sedentary time are key risk factors for multiple long-term conditions such as type 2 diabetes, obesity and CVD [42].
Higher frequency of comorbidities may lead to more barriers to carrying out regular PA. For example, living with multiple long-term conditions can add complexity to carrying out PA by potentially reducing physical performance and influencing psychological factors [43,44], such as self-efficacy for exercise [45]. Barriers to the uptake and maintenance of PA in those with OA have been investigated previously and include a lack of knowledge or belief in the benefits of exercise and low self-efficacy [45,46]. Low self-efficacy for exercise is also associated with lower levels of PA in people with other conditions such as type 2 diabetes [47] and it is possible that living with multiple long term conditions may have a cumulative negative effect on self-efficacy for exercise which in turn may contribute to lower levels of PA.
In the models investigating specific types of comorbidity, a greater number of comorbidity types were found to be associated with PASE score in BEEP than in MOSAICS. This could be explained by the different methods for measuring comorbidity or the different types of OA included in the MOSAICS sample possibly being less associated with PA (e.g. hand OA). CVD comorbidity was consistently significantly associated with a reduction in PASE scores in both datasets. CVD comorbidity existed in just over 10% of both trial datasets. In primary care, patients with OA and CVD have greater baseline OA pain, symptom burden and functional limitations, specifically walking disability [48] which may in part explain their lower levels of PA.
Physical health comorbidities such as respiratory conditions and type 2 diabetes had a negative association with PASE scores in the current study. People with type 2 diabetes or respiratory conditions may be excluded from PA treatment or be recommended PA that is suboptimal. This could be due to concerns from health care practitioners regarding comorbidity specific physiological factors (e.g. hypoglycaemia in type 2 diabetes), symptoms (e.g. breathlessness in respiratory conditions) and adverse events during PA [49].
4.1. Strengths and limitations
A strength of this study was the use of two large RCT datasets, different OA joint phenotypes and two methods for the measurement of comorbidity (self-report and MRR) which makes the results more generalisable to different OA populations. The range of comorbidities investigated within this study are those that most commonly exist alongside OA and are likely to influence PA behaviour [7]. The use of multiple linear regression meant that we could adjust for known confounders in the models.
A limitation of our secondary data analysis is that it was not possible to investigate all comorbidities that may influence PA or adjust for confounding variables not captured on the original datasets. We were unable to investigate the role of comorbidity severity which is of interest as it may influence the relationship between comorbidity presence or frequency and PA level. Also, medication use for specific comorbidities could act as either confounders or effect modifiers. The cross-sectional nature of our study prevents us inferring causation in our findings and it is possible that a reciprocal relationship exists where comorbidities may be both risk factors for, or a result of, reduced PA in the samples.
The use of a self-reported PA measure in both trials could be criticised as objectively measured PA is seen as the preferred method of measurement. Although there is some evidence for the validity of PASE for use in OA populations, in people with hip OA the PASE has a relatively large standard error of measurement (SEM) of 31, also it may still be prone to self-report bias including recall bias, misclassification and the over/underestimation of PA level [50]. There is no accepted clinically important change in PASE in the literature which limits our ability to interpret whether the reductions of PA associated with different comorbidities are of clinical importance. It is acknowledged that there was some missing data which may influence the findings by reducing the statistical power or producing biased estimates. Levels of missing data were generally low in both datasets with levels of outcome PASE missing data 10% in BEEP and 18% in MOSAICS. Finally, although the BEEP and MOSAICS trial samples were similar to people with OA in primary care and community settings in the UK, generalisability is limited to the eligibility of both study protocols. For example, these datasets excluded people with joint replacements, those unable to access physiotherapy or General Practice and those residing in nursing homes who may have more severe comorbidities or be of an older age.
4.2. Future research and clinical recommendations
Although our findings showed comorbidity to be associated with self-report PA in people with OA, it did not investigate how and why this might be the case. Hence, qualitative explanatory research exploring attitudes and beliefs and experiences of the impact that comorbidities have on PA in people with OA is indicated. Further work could also identify ways to overcome barriers to PA in the presence of OA and comorbidity. Our findings suggest that interventions tailored to increasing PA in people with OA and comorbidity are required since this group is at high risk of low PA levels due to the existence of their comorbidity.
5. Conclusion
In conclusion, in patients with OA, comorbidity is associated with lower PA levels and this association grows in magnitude with increasing comorbidity frequency. People with OA and certain types of comorbidity such as respiratory and CVD disease may be important to subgroup in terms of PA interventions. Future exploratory work needs to be done to understand how and why comorbidity is associated with PA levels in people with OA.
Author contributions
SM, EH, CJ, JQ designed the study. SM performed data analysis and interpreted the data with advisory input from TRM and JQ. SM was lead author and all authors contributed to writing, revising and approval of the final version of this manuscript.
Role of funding source
SM is funded by a Keele University Acorn PhD studentship. JQ is part funded by a NIHR Clinical Research Network West Midlands, Research Scholar Fellowship. CJ and EH are part funded by the National Institute for Health Research (NIHR) Applied Research Collaboration (ARC) West Midlands. Supported by National Institute for Health Research (NIHR) Programme grant RP-PG-0407-10,386 and in part by Arthritis Research UK Centre in Primary Care grant 18,139. The views expressed are those of the author(s) and not necessarily those of the NHS, NIHR or the Department of Health and Social Care OR Public Health England.
Declaration of Competing Interest
All authors have none to declare.
Acknowledgements
The authors thank all participants and staff involved with the BEEP and MOSAICS trials. We would like to personally acknowledge Professor Kelvin Jordan for his help and work with access and clustering of the MOSAICS MRR data. SM also thanks Keele University for its support of this work through an Acorn PhD Studentship.
References
- 1.Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M., et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kriegsman D.M., Deeg D.J., Stalman W.A. Comorbidity of somatic chronic diseases and decline in physical functioning: the Longitudinal Aging Study Amsterdam. J. Clin. Epidemiol. 2004;57:55–65. doi: 10.1016/S0895-4356(03)00258-0. [DOI] [PubMed] [Google Scholar]
- 3.Theis K.A., Brady T.J., Helmick C.G. No one dies of old age anymore: a coordinated approach to comorbidities and the rheumatic diseases. Arthritis Care Res. 2016;69:1–4. doi: 10.1002/acr.23114. [DOI] [PubMed] [Google Scholar]
- 4.Caporali R., Cimmino M.A., Sarzi-Puttini P., Scarpa R., Parazzini F., Zaninelli A., et al. Comorbid conditions in the AMICA study patients: effects on the quality of life and drug prescriptions by general practitioners and specialists. Semin Arthritis Rheum. 2005;35:31–37. doi: 10.1016/j.semarthrit.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 5.ARTHRITIS RESEARCH UK . 2017. Musculoskeletal Conditions and Multimorbidity. [Google Scholar]
- 6.Zambon S. 2016. Role of Osteoarthritis, Comorbidity, and Pain in Determining Functional Limitations in Older Populations: European Project on Osteoarthritis. [DOI] [PubMed] [Google Scholar]
- 7.NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE . NICE Guideline 177; London: 2014. Osteoarthritis: Care and Management in Adults. [Google Scholar]
- 8.Fernandes L., Hagen K.B., Bijlsma J.W.J., Andreassen O., Christensen P., Conaghan P.G., et al. Eular recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Dis. 2013:1–11. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 9.McAlindon T.E., Bannuru R.R., Sullivan M.C., Arden N.K., Berenbaum F., Bierma-Zeinstra S.M., et al. OARSI guidelines for the non-surgical management of knee. Osteoarthritis Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Krauss I., Mueller G., Haupt G., Steinhilber B., Janssen P., Jentner N., Martus P. Effectiveness and efficiency of an 11-week exercise intervention for patients with hip or knee osteoarthritis: a protocol for a controlled study in the context of health services research. BMC Publ. Health. 2016;16:367. doi: 10.1186/s12889-016-3030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fransen M., Mcconnell S., Hernandez-Molina G., Reichenbach S. The Cochrane Library; 2014. Exercise for Osteoarthritis of the Hip. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quicke J.G., Foster N.E., Thomas M.J., Holden M.A. Is long-term physical activity safe for older adults with knee pain?: a systematic review. Osteoarthritis Cartilage. 2015;23:1445–1456. doi: 10.1016/j.joca.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Blackburn S., Higginbottom A., Taylor R., Bird J., Østerås N., Hagen K.B., et al. Patient-reported quality indicators for osteoarthritis: a patient and public generated self-report measure for primary care. Res. Involv. Engagem. 2016;2 doi: 10.1186/s40900-016-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Rooij M., Van D.L., Avezaat E., Hakknen A., Klaver R., Maas T., et al. Development of comorbidity-adapted exercise protocols for patients with knee osteoarthritis. Clin. Interv. Aging. 2014;9:829–842. doi: 10.2147/CIA.S55705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loza E., Jover J.A., Rodriguez L., Carmona L., Episer study group . Elsevier; 2009. Multimorbidity: Prevalence, Effect on Quality of Life and Daily Functioning, and Variation of This Effect when One Condition Is a Rheumatic Disease, Seminars in Arthritis and Rheumatism; pp. 312–319. Elsevier. [DOI] [PubMed] [Google Scholar]
- 16.Stubbs B., Hurley M., Smith T. What are the factors that influence physical activity participation in adults with knee and hip osteoarthritis? A systematic review of physical activity correlates. Clin. Rehabil. 2015;29:80–94. doi: 10.1177/0269215514538069. [DOI] [PubMed] [Google Scholar]
- 17.Dunlop D., Song J., Semanik P.A., Sharma L., Chang R.W. Physical activity levels and functional performance in the osteoarthritis initiative: a graded relationship. Arthritis Rheum. 2011;63:127–136. doi: 10.1002/art.27760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbolsheimer F., Schaap L.A., Edwards M.H., Maggi S., Otero Á., Timmermans E.J., et al. Physical activity patterns among older adults with and without knee osteoarthritis in six European countries. Arthritis Care Res. 2016;68:228–236. doi: 10.1002/acr.22669. [DOI] [PubMed] [Google Scholar]
- 19.Foster N.E., Healey E.L., Holden M.A., Nicholls E., Whitehurst D.G., Jowett S., et al. A multicentre, pragmatic, parallel group, randomised controlled trial to compare the clinical and cost-effectiveness of three physiotherapy-led exercise interventions for knee osteoarthritis in older adults: the BEEP trial protocol (ISRCTN: 93634563) BMC Muscoskel. Disord. 2014;15 doi: 10.1186/1471-2474-15-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dziedzic K.S., Healey E.L., Porcheret M., Ong B.N., Main C.J., Jordan K.P., et al. Implementing the NICE osteoarthritis guidelines: a mixed methods study and cluster randomised trial of a model osteoarthritis consultation in primary care-the Management of OsteoArthritis in Consultations (MOSAICS) study protocol. Implement. Sci. 2014;9 doi: 10.1186/s13012-014-0095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Washburn R.A., Mcauley E., Katula J., Mihalko S.L., Boileau R.A. The physical activity scale for the elderly: evidence for validity. J. Clin. Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 22.Svege I., Kolle E., Risberg M.A. Reliability and validity of the physical activity scale for the elderly (PASE) in patients with hip osteoarthritis. BMC Muscoskel. Disord. 2012;13 doi: 10.1186/1471-2474-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Washburn R.A., Mcauley E., Katula J., Mihalko S.L., Boileau R.A. The physical activity scale for the elderly (PASE): development and evaluation. J. Clin. Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 24.Barnett L.A., Jordan K.P., Edwards J.J., Yu D., van der Windt D.A. Does metformin protect against osteoarthritis? An electronic health record cohort study. Prim. Health Care Res. Dev. 2017;18:623–628. doi: 10.1017/S1463423617000287. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y., Hayward R., Chew-Graham C.A., Hubbard R., Croft P., Sims K., Jordan K.P. Prognostic value of first-recorded breathlessness for future chronic respiratory and heart disease a cohort study using a UK national primary care database. Br J Gen Pract. 2020 doi: 10.3399/bjgp20X708221. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellamy N., Buchanan W.W., Goldsmith C.H., Campbell J., Stitt L.W. Validation study of the WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1998;15:1833–1840. [PubMed] [Google Scholar]
- 27.Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern. Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 28.EuroQOL group EuroQol-a new facility for the measurement of health-related quality of life. Health Pol. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 29.Hawker G.A., Davis A.M., French M.R., Cibere J., Jordan J.M., March L., et al. Development and preliminary psychometric testing of a new OA pain measure–an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16:409–414. doi: 10.1016/j.joca.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skelly A.C., Dettori J.R., Brodt E.D. Assessing bias: the importance of considering confounding. Evid. Base. Spine. Care. J. 2012;3:9–12. doi: 10.1055/s-0031-1298595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marill K.A. Advanced statistics: linear regression, part II: multiple linear regression. Acad. Emerg. Med. 2004;11:94–102. doi: 10.1197/j.aem.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 32.De Rooij M., Van Der Leeden M., Cheung J., Van Der Esch M., Häkkinen A., Haverkamp D., et al. Efficacy of tailored exercise therapy on physical functioning in patients with knee osteoarthritis and comorbidity: a randomized controlled trial. Arthritis Care Res. 2017;69:807–816. doi: 10.1002/acr.23013. [DOI] [PubMed] [Google Scholar]
- 33.Messier S.P., Nicklas B.J., Legault C., Mihalko S., Miller G.D., Devita P., et al. The intensive diet and exercise for arthritis trial: 18-month clinical outcomes. Arthritis Rheum. 2011;63(10):281. [Google Scholar]
- 34.Miller G.D., Nicklas B.J., Davis C., Loser R.F., Lenchik L., Messier S.P. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity. 2006;14:1219–1230. doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- 35.Field A. Sage; 2013. Discovering Statistics Using IBM SPSS Statistics. [Google Scholar]
- 36.Tu Y.K., Kellett M., Cleregugh V., Gilthorpe M.S. Problems of correlations between explanatory variables in multiple regression analyses in the dental literature. Br. Dent. 2005;199:457–461. doi: 10.1038/sj.bdj.4812743. [DOI] [PubMed] [Google Scholar]
- 37.Reeuwijk K.G., De Rooij M., Van Dijk G.M., Veenhof C., Steultjens M.P., Dekker J. Osteoarthritis of the hip or knee: which coexisting disorders are disabling? Clin Rheumatol. 2010;29:739–747. doi: 10.1007/s10067-010-1392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barber J., Muller S., Whitehurst T., Hay E. Measuring morbidity: self-report or health care records? Fam. Pract. 2009;27:25–30. doi: 10.1093/fampra/cmp098. [DOI] [PubMed] [Google Scholar]
- 39.Barney L.J., Griffiths K.M., Jorm A.F., Christensen H. Stigma about depression and its impact on help-seeking intentions. Aust. N Z J. Psychiatr. 2006;40:51–54. doi: 10.1080/j.1440-1614.2006.01741.x. [DOI] [PubMed] [Google Scholar]
- 40.Logan S., Gottlieb B., Maitland S., Meegan D., Spriet L. The Physical Activity Scale for the Elderly (PASE) questionnaire; does it predict physical health? Int. J. Environ. Res. Publ. Health. 2013;10:3967–3986. doi: 10.3390/ijerph10093967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook M.J., Bellou E., Bowes J., Sergeant J.C., O’Neill T.W., Barton A., et al. The prevalence of co-morbidities and their impact on physical activity in people with inflammatory rheumatic diseases compared with the general population: results from the UK Biobank. Rheumatology. 2018;57:2172–2182. doi: 10.1093/rheumatology/key224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Souto Barreto P. Exercise for multimorbid patients in primary care: one prescription for all? Sports Med. 2017;47:2143–2153. doi: 10.1007/s40279-017-0725-z. [DOI] [PubMed] [Google Scholar]
- 43.Qin J., Theis K.A., Barbour K.E., Helmick C.G., Baker N.A., Brady T.J. Impact of arthritis and multiple chronic conditions on selected life domains – United States. MMWR (Morb. Mortal. Wkly. Rep.) 2015;64:578–582. [PMC free article] [PubMed] [Google Scholar]
- 44.Nur H., Sertkaya B.S., Tuncer T. Determinants of physical functioning in women with knee osteoarthritis. Aging Clin. Exp. Res. 2018;30:299–306. doi: 10.1007/s40520-017-0784-x. [DOI] [PubMed] [Google Scholar]
- 45.Quicke J.G., Foster N.E., Ogollah R.O., Croft P.R., Holden M.A. Relationship between attitudes and beliefs and physical activity in older adults with knee pain: secondary analysis of a randomized controlled trial. Arthritis Care Res. 2017;69:1192–1200. doi: 10.1002/acr.23104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobson F., Bennell K.L., French S.D., Nicolson P.J., Klaasman R.N., Holden M.A., et al. Barriers and facilitators to exercise participation in people with hip and/or knee osteoarthritis: synthesis of the literature using behavior change theory. Am. J. Phys. Med. Rehabil. 2016;1:372–389. doi: 10.1097/PHM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 47.Thomas N., Alder E., Leese G.P. Barriers to physical activity in patients with diabetes. Postgrad. Med. J. 2004 1;80:287–291. doi: 10.1136/pgmj.2003.010553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hawker G.A., Croxford R., Bierman A.S., Harvey P.J., Ravi B., Stanaitis, et al. All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: a population based cohort study. PloS One. 2014;9 doi: 10.1371/journal.pone.0091286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beckwee D., Bautmans I., Scheerlinck T., Vaes P. Exercise in knee osteoarthritis-preliminary findings: exercise-induced pain and health status differs between dropouts and retainers. Exp Gerontol. 2015;72:29–37. doi: 10.1016/j.exger.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Prince S.A., Adamo K.B., Hamel M.E., Hardt J., Gorber S.C., Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int. J. Behav. Nutr. Phys. Activ. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]