Abstract
Graves' disease is an autoimmune thyroid disorder that mainly presents as hyperthyroidism and is caused by thyrotropin receptor antibodies (TRAbs) that stimulate thyroid-stimulating hormone receptors. We previously reported that Graves' disease patients and healthy controls both had Epstein–Barr virus (EBV)-infected TRAb-positive B cells and the EBV-reactivated induction of these B cells in cultures may induce the production of TRAbs. In the present study, we quantified serum TRAb-IgG and TRAb-IgM levels in 34 Graves' disease patients and 15 controls using ELISA to elucidate the mechanisms underlying EBV-related antibody production. As expected, TRAb-IgG and TRAb-IgM levels were higher in Graves' disease patients than in controls; however, TRAb-IgM levels were significantly higher than those of TRAb-IgG levels, whereas total IgM levels were lower than total IgG levels. On the other hand, the enhanced production of TRAb-IgM was frequently observed in patients with EBV reactivation. These results are consistent with the fact that the percentage of autoreactive IgM B cells are higher than that of autoreactive IgG B cells, and support the EBV-related polyclonal B cell activation. It is necessary to clarify the biological characteristics of TRAb-IgM and the relationship between TRAb isotypes and the biology of Graves' disease.
Introduction
Graves' disease is an autoimmune thyroid disorder that mainly causes hyperthyroidism. Other well-known symptoms of Graves' disease are infiltrative ophthalmopathy and diffuse goiter. Thyrotropin receptor antibodies (TRAbs) in Graves' disease patients are circulating autoantibodies against the thyroid-stimulating hormone receptor (TSHR). TRAbs generally function as stimulating autoantibodies because they compete with thyroid stimulating hormone (TSH) for the specific binding sites of TSHR and activate signaling pathways to release thyroid hormone (7,21).
Several potential risk factors have been identified for Graves' disease, particularly heredity. Graves' disease patients have specific mutations in susceptibility genes, including human leukocyte antigen (HLA) and cytotoxic T lymphocyte-associated antigen 4 (CTLA4). These genes have been reported to contribute to disease development, but only yield a risk ratio less than two- to four-folds (7,17,18,20,21). Therefore, we cannot explain the development of Graves' disease by genetic susceptibility alone. Infection, stress, gender, and pregnancy have been suggested as environmental risk factors (7,17,21).
Epstein–Barr virus (EBV) is a human γ-herpesvirus. After primary infection, EBV latently persists as resting B cells and is sometimes reactivated (6). The reactivation of EBV may be related to host antibody production, and EBV reactivation and plasma cell differentiation in the host have been reported to occur simultaneously (5,6). This may be the process responsible for the various autoimmune diseases related to EBV (3,6,16,22). A previous study demonstrated that systemic lupus erythematosus patients had an abnormally high EBV load in the peripheral blood (3).
We also found a correlation between serum TRAbs levels and EBV early antigen (EA) antibody (Ab) levels in Graves' disease patients (9). We showed that both Graves' disease patients and healthy controls had B cells that were EBV-infected and also expressed TRAbs on their surfaces (10). Furthermore, these B cells in culture produced TRAbs by EBV-reactivated induction (11).
In the present study, we quantified serum TRAb-IgG and TRAb-IgM levels in Graves' disease patients and healthy controls using applied ELISA system. As expected, TRAb-IgG and TRAb-IgM levels were higher in Graves' disease patients than in controls; however, TRAb-IgM levels were significantly higher than TRAb-IgG levels. On the other hand, patients with high EBV Ab levels also had higher TRAb-IgM levels. We will present the detailed data and discuss these unexpected findings.
Materials and Methods
Patients and controls
Serum samples were obtained from 34 Graves' disease patients and 15 healthy controls.
Graves' disease patients comprised 3 males and 31 females and the age range was 14–71. Controls are 5 males and 10 females and the age range was 20–55.
All subjects provided written informed consent for participation in the study, and the study protocol was approved by the Medical Ethics Committee for Human Subject Research at the Faculty of Medicine, Tottori University, Yonago, Japan (No. 707. 707-1-12). At the time of diagnosis, all patients had symptoms, including at least one of the following: (1) signs of thyrotoxicosis such as tachycardia, weight loss, finger tremors, and sweating; (2) diffuse enlargement of the thyroid gland; (3) exophthalmos and/or specific ophthalmopathy. All patients met the following criteria: (1) elevated serum levels of free T4 and/or free T3; (2) suppression of serum TSH (<0.1 μU/mL); (3) positive for TRAbs or thyroid-stimulating antibody.
Three out of the 38 patients had received no treatment, whereas 35 were undergoing treatments with antithyroid drugs. Healthy controls comprised of 14 healthy volunteers. Their thyroid function was normal, and subjects that had any familial history of thyroid disease were excluded.
ELISA of total serum Ig and anti-EBV Ab concentrations
Serum immunoglobulin (Igs) and anti-EBV Ab concentrations were measured by ELISA. The total IgG and IgM concentrations of 38 patients and 11 healthy controls were measured using a Human IgG/IgM ELISA Quantitation Set (Bethyl Laboratories, Montgomery, TX). The anti-EBV Ab concentrations of all subjects, comprising EBV-EA-IgG and EBV-VCA-IgG, were measured by ELISA (EA; Vircell, Granada, Spain, VCA; Abcam, Cambridge, United Kingdom) according to the manufacturer's instructions. Serum levels of total IgG/IgM, EBV-EA-IgG, and EBV-VCA-IgG are shown in Table 1.
Table 1.
Mean Levels of Serum TRAb-IgG and TRAb-IgM and the Anti-EBV Antibody Index (EA-IgG and VCA-IgG) Between Patients and Controls
| Mean Igs (mg/mL) | Mean TRAbs (ng/mL) | Mean anti-EBV Ab index | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Case group | Case numbers | IgG | IgM | M/G | TRAb-IgG | TRAb-IgM | TRAb M/G | EA-IgG | VCA-IgG |
| Patients | 34 | 12.07 | 1.22 | 0.10 | 32.82 | 246.89 | 10.53 | 21.57 | 50.03 |
| Controls | 15 | 16.69 | 1.60 | 0.09 | 14.70 | 210.89 | 16.30 | 18.17 | 45.37 |
| p | <0.001a | 0.040a | 0.039a | 0.029a | |||||
Serum levels of TRAbs in Graves' disease patients and controls were measured by TRAb-isotype ELISA. Antibody index: sample absorbance/cut off absorbance × 100. These results indicated that none of the subjects had EBV primary infection. Significant differences between groups were evaluated using the Mann–Whitney U test for TRAb-IgG and VCA-IgG; the two-sample t-test for TRAb-IgM, EA-IgG.
Significantly different.
Ab, antibody; EA, early antigen; EBV, Epstein–Barr virus; Ig, immunoglobulin; M/G, IgM/IgG; TRAb, thyrotropin receptor antibody; TRAb M/G, TRAb-IgM/TRAb-IgG; VCA, virus capsid antigen.
ELISA of the TRAb isotype
Serum TRAb-IgG and TRAb-IgM levels were determined by the applied ELISA system. We used full-length recombinant human TSHR (Abnova, Taipei, Taiwan) and goat anti-TSHR IgG against the human THSR C terminus (Santa Cruz Biotechnology, Santa Cruz, CA) to coat ELISA plate wells. Binding specification between this human TSHR and anti-TSHR IgG was previously confirmed in our study (10).
We added 100 μL of 0.1 mg/L anti-TSHR IgG diluted by coating buffer (0.05 M carbonate–bicarbonate) followed by the method of Bolton et al. (1,15) to each well and incubated ELISA plates overnight at 4°C. After washing with ELISA wash solution (50 mM Tris, 0.14 M NaCl, 0.05% Tween 20), we added 200 μL of blocking solution (50 mM Tris, 0.14 M NaCl, 1% BSA) and incubated plates at 37°C for 60 min. We washed the plates and added recombinant human TSHR diluted to 4 ng/mL with assay buffer (50 mM Tris, 0.14 M NaCl, 1% BSA, 0.05% Tween 20). After shaking at room temperature for 30 min, we incubated ELISA plates at 37°C for 60 min and washed again.
Fifty microliters of assay buffer was added to each ELISA plate well, followed by 50 μL of sample serum. ELISA plates were incubated at 37°C for 60 min and washed again. We added 100 μL of a horseradish peroxidase (HRP)-conjugated goat anti-human IgG-Fc detection antibody (for TRAb-IgG assay) or an HRP-conjugated goat anti-human IgM-Fc detection antibody (for TRAb-IgM assay), which was used in the ELISA for total Ig described above, to each well.
After washing, we added 100 μL of TMB substrate solution to each well and placed plates in the dark at room temperature for 20 min. One hundred microliters of stop solution (0.18 M H2SO4) was added. We measured the absorbance of each well at 450 nm. We calculated TRAb-IgG or TRAb-IgM concentrations in samples using a standard curve that was generated at the same time using human reference serum in the ELISA of total Ig described above.
The intra-assay coefficient of variation (CV) was 8.06% and inter-assay CV was 4.96% for TRAb-IgG measurements. Intra-assay CV was 7.82% and inter-assay CV was 3.09% for TRAb-IgM measurements.
Statistical analyses
We used SPSS software for Windows 15.0J for statistical analyses (SPSS, Inc., Chicago, IL). Two-sample t-test was used for normally distributed samples; Mann–Whitney U test or Wilcoxon rank-sum test was adopted for the samples, which did not follow normal distribution.
Results
Serum TRAb-IgM levels were significantly higher than TRAb-IgG levels in contrast to the ratio of serum total IgM to IgG levels
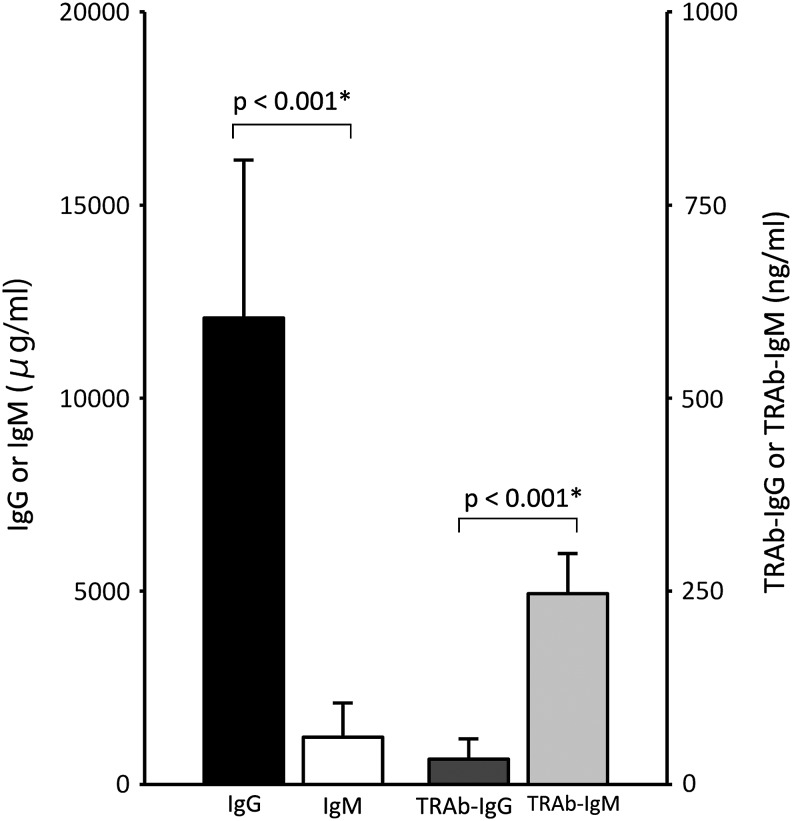
In Graves' disease patients and controls, serum total IgG levels were significantly higher than total IgM levels (Table 1). This typical ratio and amount of IgG to IgM appear to indicate that no subjects had any marked immunodeficiency. On the other hand, serum TRAb-IgG levels were significantly lower than TRAb-IgM levels (Table 1). In both Graves' disease patients and healthy individuals, the total IgG levels were higher than the total IgM levels, whereas the TRAb-IgM levels were higher than the TRAb-IgG levels. The proportion of TRAb-IgG to TRAb-IgM in patients was higher than that of controls (TRAb-IgM/TRAb-IgG of patients was lower than that of controls) (Table 1 and Fig. 1).
FIG. 1.
Immunoglobulin isotype difference in serum total Ig and TRAb levels in Graves' disease patients. The ratio of IgG to IgM between total Ig and TRAb was contrastive. *p < 0.001 is significant by Wilcoxon Signed-Rank Test. TRAb, thyrotropin receptor antibody.
TRAb-IgG and TRAb-IgM levels were significantly higher in Graves' disease patients than in controls
TRAb-IgG and TRAb-IgM levels were both significantly higher in Graves' disease patients than in controls (TRAb-IgG, p < 0.001; TRAb-IgM, p = 0.040; Table 1 and Fig. 2). Four healthy individuals had more TRAb-IgM than the average of TRAb-IgM in Graves' disease patients.
FIG. 2.
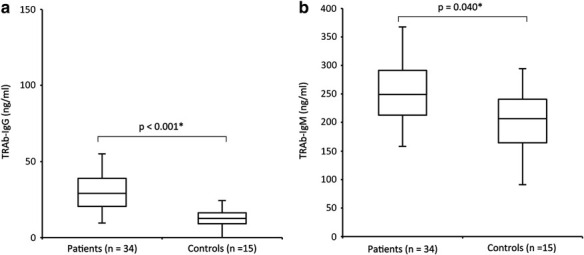
Difference in serum TRAb levels between Graves' disease patients and controls. (a) Serum TRAb-IgG levels are significantly higher in Graves' disease patients than in controls (healthy subjects). (b) Serum TRAb-IgM levels are also significantly higher in Graves' disease patients than in controls. (a) p < 0.001*, the Mann–Whitney U Test. (b) p = 0.040*, the two-sample t-test.
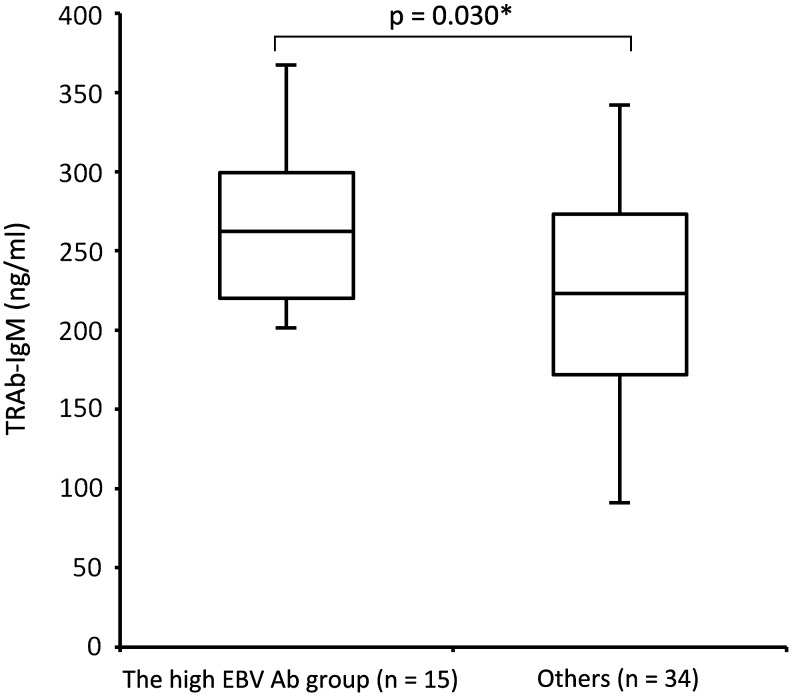
Higher TRAb-IgM production was observed in high anti-EBV Ab group
All subjects were divided into a high EBV Ab group and others based on the serum anti-EBV Ab index (Table 2). The high EBV Ab group that constituted 15 samples presented high Ab index for EA-IgG (>21.32) and VCA-IgG (>49.56), whereas others that comprised of 34 cases did not. TRAb-IgM levels were significantly higher in the high EBV Ab group than in others (p = 0.030; Fig. 3), whereas no significant difference was observed in TRAb-IgG levels between the high EBV Ab group and others (p = 0.461).
Table 2.
Mean Levels of Serum TRAb-IgG and TRAb-IgM and Anti-EBV Antibody Levels (EA-IgG and VCA-IgG) Between the High EBV Ab Group and Others
| Mean TRAbs (ng/mL) | |||
|---|---|---|---|
| Case group | Case numbers | TRAb-IgG | TRAb-IgM |
| The high EBV Ab (EBV reactivation) | 15 | 26.98 | 262.20 |
| Others | 34 | 27.40 | 224.25 |
| p | 0.461 | 0.030a | |
The high EBV Ab group comprised 15 samples that presented with a high antibody index in EA-IgG (>21.32) and VCA-IgG (>49.56), suggesting EBV reactivation. Others comprised 34 samples, excluding the 15 patients in the high EBV Ab group. Significant differences between groups were evaluated using the Mann–Whitney U test for TRAb-IgG and two-sample t-test for TRAb-IgM.
Significantly different.
Ab, antibodies.
FIG. 3.
Difference in serum TRAb-IgM levels between the high EBV Ab group and others. Serum TRAb-IgM levels are significantly higher in the high EBV Ab group (EBV reactivation) than in others (p = 0.030*, the two-sample t-test). High EBV Ab represents samples with a high antibody index in EA-IgG and VCA-IgG, suggesting EBV reactivation. Others are constituted samples, except for cases with high EBV Ab levels. Ab, antibody; EA, early antigen; EBV, Epstein–Barr virus; VCA, virus capsid antigen.
In this study, the sample numbers were not enough, where high EBV Ab group was comprised of 14 Graves' disease patients and 1 control, and the other group was comprised of 20 Graves' disease patients and 14 controls.
In a previous study, we reported that peripheral blood mononuclear cell (PBMC) derived from patients and healthy controls both could secrete TRAb by the EBV reactivation induction (10,11).
TRAb that stimulates thyroid follicular cells are stated to be IgG class (7), but we consider that the secretion of TRAb-IgM has the different meaning, and TRAb-IgM could be secreted in EBV reactivated condition, regardless of patients or controls.
Therefore, we adopted all subjects and divided them into EBV-reactivated group and others, to examine the difference in autoantibody production between them.
Discussion
Mature naive B cells that encounter specific antigens are activated by CD4(+) T cells. Activated B cells have the ability to enter the germinal center in which they undergo isotype switching and somatic hyper mutation (SHM), and ultimately become plasma cells and produce high-affinity, class-switched IgG antibodies in the bone marrow for >120 days (8,13,14). Accordingly, the IgG class antibody is dominant in the blood. Therefore, we expected TRAb-IgG levels to be higher than TRAb-IgM levels in the serum; however, the result obtained was opposite in that TRAb-IgM levels were significantly higher than TRAb-IgG levels.
About 70–90% of circulating B cells have IgM as their surface globulin (2,4,12,14), and some of them contain autoreactive antibodies that have escaped from negative selection in central immune tolerance. It is difficult for these autoreactive B cells to encounter their specific antigen because the autoantigens, such as the nucleus, DNAs, or cellular organs, are generally packed inside the cells. Therefore, most of them may never be activated, leave the lymphoid tissue, and die (14). However, autoreactive B cells with persistent EBV infection can survive.
Nakamura and Casali showed that the percentage of autoreactive IgM B cells are far higher than that of autoreactive IgG B cells, and that patients with autoimmune disease have higher autoreactive IgG B cells than control subjects, and they suggested that autoimmune B cell repertoire are constituted by polyclonal B cell activation and antigen-driven responses (2,12).
Consistent to their report, in our results, serum TRAb-IgM levels were higher than TRAb-IgG levels, and Graves' disease patients had more TRAb-IgG than controls (TRAb-IgM/TRAb-IgG of patients and controls were 10.53 and 16.30, respectively; Table 1).
Higher percentage of TRAb-IgM than TRAb-IgG was thought to be the influence of higher percentage of circulating total IgM-B cells than that of total IgG-B cells, and this higher percentage of TRAb-IgM was more apparent in EBV reactivation state, which suggests the polyclonal B cell activation by the reactivation of persistent EBV.
EBV latent membrane protein 1 (LMP1) is expressed in some phases of latency, whereas during EBV reactivation, numerous infectious progeny are produced, and most of the newly infected cells become latent III state with various viral antigens, including LMP1 (6). These latent III cells are the cells that could be differentiated to produce antibodies.
LMP1 induces the activation of B cells without antigen stimulation by mimicking the CD40 signal (19), and protects them against apoptosis by upregulating the expression of bcl-2 (6). Furthermore, EBV reactivation causes B cell differentiation into plasma cells and the production of antibody (5,10,11). We presume that EBV-LMP1 expression enables the polyclonal B cell activation, and rescue autoreactive IgM B cells, which forced out from the lymphoid tissue, and EBV reactivation makes them produce autoantibodies.
Four healthy individuals had more TRAb-IgM than the average of TRAb-IgM in Graves' disease patients. The mechanism for polyclonal B cell activation by EBV would be common to all subjects, and healthy controls as well as patients have TRAb(+)EBV(+) cells (10,11). Therefore, the healthy controls that were influenced by potent effect of EBV reactivation may have high TRAb-IgM. Although the TRAb-IgG of these four controls were lower than the mean TRAb-IgG of patients, they require careful observation.
In conclusion, we measured serum TRAb-isotype levels, and demonstrated that TRAb-IgM levels are higher than TRAb-IgG levels, and TRAb-IgM levels were significantly higher in the high EBV Ab (EBV reactivation) group than in others.
These results suggest that, in EBV reactivation-related antibody production, B cells are activated polyclonally; thus, the autoreactive B cells excluded from the lymphoid tissue due to the difficulties in their antigen-dependent activation are also activated and produce autoantibodies.
It will be important to clarify the characteristics of TRAb-IgM and the relationship between TRAb isotypes and biology of Graves' disease.
Authors' Contributions
K.K. designed the study and carried out ELISA analysis and drafted the article. K.H. and K.N. participated in the design and coordination of the study and helped to draft the article. M.M. and S.K. participated in clinical examination. M.K. and I.M. participated in the design of the study and in interpretation of the result. S.F. contributed to collect the samples. All authors read and approved the final article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bolton J, Sanders J, Oda Y, et al. Measurement of thyroid-stimulating hormone receptor autoantibodies by ELISA. Clin Chem 1999;45:2285–2287 [PubMed] [Google Scholar]
- 2.Casali P, Nakamura M, Ginsberg-Fellner F, et al. Frequency of B cells committed to the production of antibodies to insulin in newly diagnosed patients with insulin-dependent diabetes mellitus and generation of high affinity human monoclonal IgG to insulin. J Immunol 1990;144:3741–3747 [PubMed] [Google Scholar]
- 3.James JA, Kaufman KM, Farris AD, et al. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest 1997;100:3019–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein U, Küppers R, and Rajewsky K. Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood 1997;89:1288–1298 [PubMed] [Google Scholar]
- 5.Laichalk LL, and Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol 2005;79:1296–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longnecker RM, Kieff E, and Cohen J. Epstein–Barr virus. In: Knipe DM, Howley PM, eds. Fields Virology. 6 Philadelphia: Lippincott Williams & Wilkins, 2013:1898–1959 [Google Scholar]
- 7.Mandel SJ, Larsen PR, and Davies TF. Thyrotoxicosis. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology. 12 Philadelphia: Saunders, 2011:362–405 [Google Scholar]
- 8.Manz RA, Thiel A, and Radbruch A. Lifetime of plasma cells in the bone marrow. Nature 1997;388:133–134 [DOI] [PubMed] [Google Scholar]
- 9.Nagata K, Fukata S, Kanai K, et al. The influence of Epstein-Barr virus reactivation in patients with Graves' disease. Viral Immunol 2011;24:143–149 [DOI] [PubMed] [Google Scholar]
- 10.Nagata K, Higaki K, Nakayama Y, et al. Presence of Epstein-Barr virus-infected B lymphocytes with thyrotropin receptor antibodies on their surface in Graves' disease patients and in healthy individuals. Autoimmunity 2014;47:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata K, Nakayama Y, Higaki K, et al. Reactivation of persistent Epstein-Barr virus (EBV) causes secretion of thyrotropin receptor antibodies (TRAbs) in EBV-infected B lymphocytes with TRAbs on their surface. Autoimmunity 2015;48:328–335 [DOI] [PubMed] [Google Scholar]
- 12.Nakamura M, Burastero SE, Ueki Y, et al. Probing the normal and autoimmune B cell repertoire with Epstein-Barr virus. Frequency of B cells producing monoreactive high affinity autoantibodies in patients with Hashimoto's disease and systemic lupus erythematosus. J Immunol 1988;141:4165–4172 [PubMed] [Google Scholar]
- 13.Paramithiotis E, and Cooper MD. Memory B lymphocytes migrate to bone marrow in humans. Proc Natl Acad Sci U S A 1997;94:208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parham P. The Immune System, Chap 9. 3. New York: Garland Science, 2009:248–287 [Google Scholar]
- 15.Smith BR, Bolton J, Young S, et al. A new assay for thyrotropin receptor autoantibodies. Thyroid 2004;14:830–835 [DOI] [PubMed] [Google Scholar]
- 16.Sutton RN, Emond RT, Thomas DB, et al. The occurrence of autoantibodies in infectious mononucleosis. Clin Exp Immunol 1974;17:427–436 [PMC free article] [PubMed] [Google Scholar]
- 17.Tomer Y, and Huber A. The etiology of autoimmune thyroid disease: a story of genes and environment. J Autoimmun 2009;32:231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomer Y, Greenberg DA, Barbesino G, et al. CTLA-4 and not CD28 is a susceptibility gene for thyroid autoantibody production. J Clin Endocrinol Metab 2001;86:1687–1693 [DOI] [PubMed] [Google Scholar]
- 19.Uchida J, Yasui T, Takaoka-Shichijo Y, et al. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science 1999;286:300–303 [DOI] [PubMed] [Google Scholar]
- 20.Ueda H, Howson JM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 2003;423:506–511 [DOI] [PubMed] [Google Scholar]
- 21.Weetman AP, and McGregor AM. Autoimmune thyroid disease: further developments in our understanding. Endocr Rev 1994;15:788–830 [DOI] [PubMed] [Google Scholar]
- 22.Whittingham S, McNeilage J, and Mackay IR. Primary Sjögren's syndrome after infectious mononucleosis. Ann Intern Med 1985;102:490–493 [DOI] [PubMed] [Google Scholar]