Abstract
The impact of the local inhibition of soluble epoxide hydrolase (sEH), which metabolizes vasodilator and anti-inflammatory epoxyeicosanoids, on diabetic skin microvascular dysfunction was assessed. In diabetic db/db mice, basal skin blood flow assessed using laser Doppler imaging was similar to that of control mice, but thermal hyperemia was markedly reduced. Two hours after the topical administration of an aqueous gel containing the sEH inhibitor t-AUCB (400 mg/L) the peak concentration of t-AUCB was detected in the skin of diabetic mice, which quickly decreased thereafter. In parallel, 2 hours after application t-AUCB treatment thermal hyperemia was increased compared to the control gel. Quantification of t-AUCB in plasma of treated animals showed no or low systemic diffusion. Furthermore, HES histological staining of skin biopsies showed that skin integrity was preserved in t-AUCB-treated mice. Finally, for pig ear skin, a surrogate for human skin, using Franz diffusion cells we observed a continuous diffusion of t-AUCB from 2 hours after application to beyond 24h. A single topical administration of a sEH inhibitor improves microcirculatory function in the skin of db/db mice and might represent a new therapeutic approach to preventing the development of skin complications in diabetic patients.
Keywords: diabetes, skin microvascular dysfunction, soluble epoxide hydrolase, topical form
BACKGROUND
Diabetic foot ulcers (DFUs) are a common and serious complication of diabetes mellitus, and associated with major morbidity. Indeed, diabetes is the primary cause of non-traumatic lower-limb amputation [1]. The skin’s microcirculation, by maintaining perfusion and delivering oxygen and nutrients, plays a key role in tissue survival. Skin microvascular dysfunction occurs early in the pathophysiology of diabetes and contributes to poor wound healing and the development of foot complications in diabetic patients [2–4]. Endothelial dysfunction as well as sensory and autonomic neuropathies, are thought to contribute to a reduction in the functional capacity of the microvasculature [2–4], and topical treatments targeting this microvascular dysfunction may help to improve wound healing while minimizing potential systemic side effects [5].
Increasing evidence suggests that alterations in the endothelium-derived epoxyeicosatrienoic acids (EETs) pathway are involved in the pathophysiology of the endothelial dysfunction associated with type 2 diabetes [6,7]. One interesting approach would thus be to increase the bioavailability of EETs, which are formed by the action of cytochrome P450 and display powerful vasodilating, anti-inflammatory and angiogenic properties [6,7]. In vivo, EETs are rapidly converted to the less active dihydroxyeicosatrienoic acids by soluble epoxide hydrolase (sEH), which is the target of a new class of pharmacological inhibitors [8,9]. Interestingly, exogenous EET administration as well as genetic or pharmacological inhibition of sEH has been shown to accelerate wound epithelialization and neovascularization in ob/ob mice, and in the hairless mouse-ear wound model [8–10]. However, to date, no study has evaluated their impact on skin microcirculatory function. Since in animals, the systemic administration of sEH inhibitors has been successfully used to improve endothelial function of aorta and coronary arteries [11,12], we hypothesized that local cutaneous sEH inhibition may improve endothelium-dependent microvascular reactivity.
In this context, the aim of the present study was to assess the effect of a topical formulation containing a sEH inhibitor on skin microcirculation in diabetic mice.
METHODS
Animals and treatments
The protocol was approved by the local institutional review committee (decision number: C 38 516 10 006, n°2017011312598602-V5#8531) and conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Nine-week-old male wild-type C57BL/6J and db/db (BKS(D)-Leprdb/JOrlRj) mice, a genetic model of type 2 diabetes, were acquired from Janvier Labs (Le Genest-Saint-Isle, France). These mice were allowed to acclimate to the photoperiod (12-hour light/12-hour dark) and temperature conditions (22±1°C) for one week prior to the start of the study. A 2-hour topical administration (20 μL) of a newly developed gel-like, aqueous pharmaceutical preparation containing the sEH inhibitor trans-4-(4-(3-adamantan-1-yl-ureido)-cyclohexyloxy)-benzoic acid (t-AUCB: 400 mg/L) dissolved in dimethyl sulfoxide (DMSO) or a vehicle control gel was applied to the dorsal skin of db/db mice, depilated two days before the experiments. Assessment of microvascular function, skin biopsies (50 mm2) and intra-cardiac blood sampling were performed at 2 and 24 hours after gel application. Animals were anaesthetized with isoflurane (induction at 3% during 3 minutes, and then maintained at 2%) and placed on a heated pad to maintain stable core body temperature (37.5±0.5°C).
Local and systemic quantification of t-AUCB
Plasma and skin levels of t-AUCB were quantified by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) [13]. Briefly, skin tissues were mixed with 1 mL of methanol-water (50:50 v/v) and ultrasonicated for 10 min, or 100 μL of plasma were mixed with 300 μL of methanol, allowing protein precipitation. Then, skin and plasma samples were thoroughly vortexed for 10 sec and centrifuged at 16,100 g for 5 min. The resulting supernatants were collected and analyzed by LC-MS/MS. Chromatographic separation was performed on a Kinetex C18 column (2.6-μm particle size, 50-mm length × 3-mm inner diameter). The autosampler temperature was set at 8 °C, the column oven at 30 °C, the injected volume was 20 μL and the flow rate was 400 μL/min. The mobile phase was 0.2% formic acid in methanol (solvent A) and 2 mM ammonium formate with 0.2% formic acid in water (solvent B). The elution started with 95% B (0–2 min), 95–5% B (2–5 min), 5% B (5–10 min), 5–95% B (10–11 min), 95% B (11–12 min). The following multiple reaction monitoring (MRM) transitions: m/z 412.9 to m/z 135.1 and m/z 412.9 to m/z 93.0 in positive ion mode were used to detect t-AUCB (quantification and confirmation transitions respectively). Skin levels were normalized to tissue weight.
Assessment of skin microvascular function
Skin microvascular reactivity to local heating was used as an index of endothelium-dependent function [14]. Dorsal skin blood flow was measured by laser Doppler imaging (LDI; PeriScan PIM, Perimed, Järfälla, Sweden) over 10 minutes before heating (baseline flow). The skin was then heated at 41°C during 20 minutes using a 0.5 cm2 heating probe regulated with an internal thermometer. Skin blood flow was then recorded during the following 15 minutes.
Data were digitized, stored on a computer, and analysed offline with signal processing software (PimSoft v1.5.4.8078, Perimed, Järfälla, Sweden). Baseline and peak hyperemia were expressed as arbitrary perfusion units (A.P.U.), averaged over 3 minutes immediately before, and one minute immediately after heating, respectively. Thermal hyperemia was subsequently calculated as the difference between peak hyperemia and baseline skin blood flow.
Skin integrity
Skin biopsies were carefully sampled and immediately fixed in a 4% formalin solution for 24 hours. After proper fixation, tissue samples were embedded in paraffin and stored at room temperature until analysis when 4μm sections were deparaffinized and stained with standard Hematoxylin-Eosin (H&E). Slides were analyzed by an experienced pathologist (JMP).
Transdermal passage of t-AUCB across pig ear skin
The percutaneous absorption of t-AUCB was studied using Frantz diffusion cells [15]. The skin from pig’s ears was chosen for the experiments as it is very similar to that of human skin and closer than mouse skin [16,17]. The Franz’s cells had a contact area of 2 cm2 and the experiments were conducted at 32°C. The donor compartment was filled with 20 μL of the t-AUCB-containing gel (400 mg/L). The receptor compartment contained 4.5 mL of PBS and was under magnetic stirring. Samples from the receptor compartment were collected at different time points over 24 hours to determine the percutaneous flow of t-AUCB. The t-AUCB quantification was performed by LC-MS/MS.
Statistical analysis
All values are expressed as mean ± SEM. The Shapiro-Wilk test was used to assess normality. Analyses of the differences between diabetic and control mice for basal skin blood flow and thermal hyperemia were performed using an unpaired t-test or the nonparametric Mann-Whitney rank-sum test. Analyses of the variation in basal skin blood flow and thermal hyperemia induced by the t-AUCB-containing gel were performed using mixed effects models with time as fixed effect and the mouse as a random effect followed, in case of significance, by Bonferroni post-hoc tests to compare the baseline value to other time points after application. Analyses of the differences in the effect of the t-AUCB-containing gel and the vehicle gel on basal skin blood flow and thermal hyperemia were performed by repeated-measures ANOVA, and we assessed the influence of the group, of time and the time*group interaction. Statistical analyses were performed with NCSS software (version 07.1.14). A two-sided P<0.05 was considered as statistically significant.
RESULTS
At untreated skin sites, cutaneous blood flow was slightly but significantly lower in db/db mice compared to control mice (Figure 1A). In contrast, there was a more marked reduction in thermal hyperemia in db/db mice compared to controls (Figure 1B), showing the presence of diabetic skin microcirculatory dysfunction.
Figure 1.
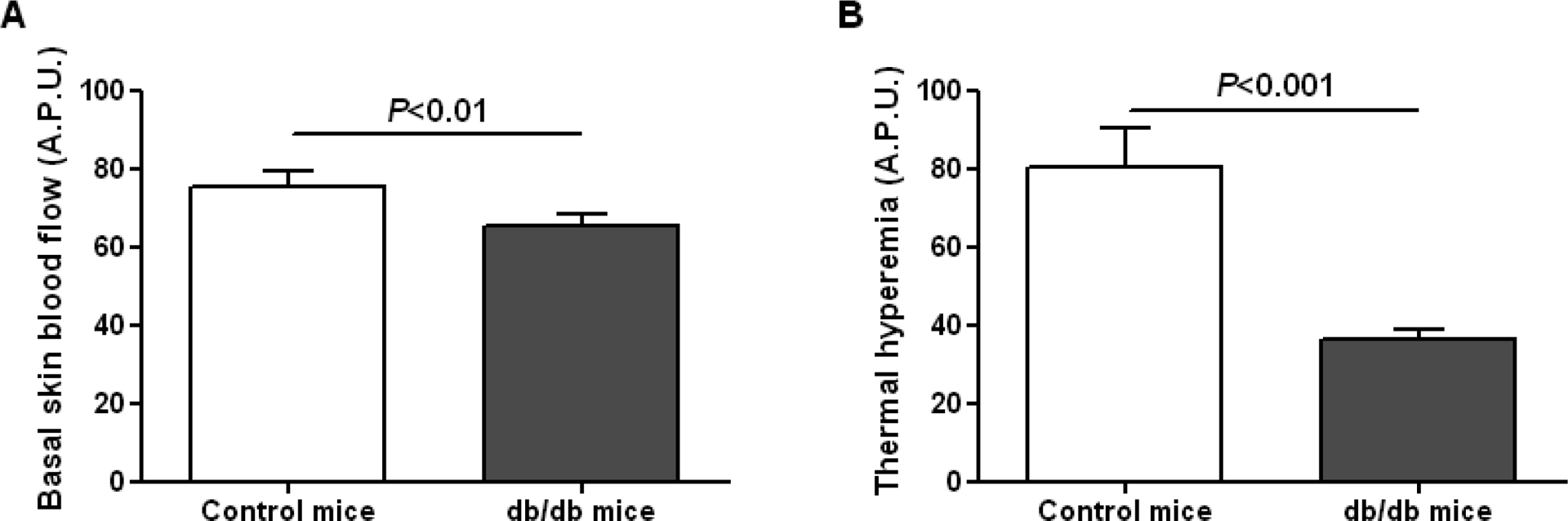
Basal skin blood flow (A) and thermal hyperemia (B) measured by laser Doppler imaging in control (n=7) and db/db mice (n=31). A.P.U.: arbitrary perfusion unit.
We carefully compared the effect of the t-AUCB-containing gel to that of a vehicle control gel in db/db mice. Both gels were applied on the same animal, with a minimal distance of 1 cm between the two application sites. Both gels increased basal skin blood flow after a 2-hour long application, but with no significant difference between groups (Figure 2A). However, the t-AUCB-containing gel significantly increased thermal hyperemia compared to the vehicle control gel (Figure 2B).
Figure 2.

Basal skin blood flow (A) and thermal hyperemia (B) measured by laser Doppler imaging before and after a 2-hour topical application of the t-AUCB-containing gel (20 μL at 400 mg/L) and the vehicle control gel on the dorsal skin of db/db mice (n=13). *P<0.05 vs. before topical application, †P<0.05 vs. vehicle control gel. A.P.U.: arbitrary perfusion unit.
After the 2-hour gel application, t-AUCB was detectable in skin biopsies and these levels had drastically decreased 24 hours after application, demonstrating transdermal permeation of t-AUCB across db/db mouse skin (Figure 3A). Consistently, thermal hyperemia returned to baseline values 24 hours after gel application (Figure 3B).
Figure 3.

A, Skin levels of t-AUCB, quantified by liquid chromatography coupled to tandem mass spectrometry, 2 (n=5) and 24 hours (n=6) after topical application of the t-AUCB-containing gel (20 μL at 400 mg/L) on the dorsal skin of db/db mice. B, Thermal hyperemia measured by laser Doppler imaging before (n=19), 2 (n=13) and 24 hours (n=6) after the topical application of the t-AUCB-containing gel on the dorsal skin of db/db mice. *P<0.05 vs. before topical application. A.P.U.: arbitrary perfusion unit.
Analysis of plasma samples showed no systemic diffusion of t-AUCB, assessed at 2 and 24 hours after application of the t-AUCB-containing gel, except for one animal (Table 1). In addition, no significant inflammatory infiltration was observed in mouse skin at either 2 or 24 hours after gel application (Figure 4).
Table 1.
Quantification of t-AUCB in plasma
| 2 hours | 24 hours | |
|---|---|---|
| Below LOQ (2.4 nM) | 4 (80%) | 4 (100%) |
| Above LOQ (2.4 nM) | 1 (20%) | 0 (0%) |
Data are n (%). LOQ: limit of quantification.
Figure 4.

Representative images of hematoxylin & eosin staining of mouse skin 8 and 24 hours after topical application of t-AUCB-containing gel (20 μL at 400 mg/L) on the dorsal skin of db/db mice.
Finally, at the same dosage, a continuous diffusion of t-AUCB was observed across pig ear skin from 2 hours up to 24h after application (Figure 5).
Figure 5.

Evolution of t-AUCB level, quantified by liquid chromatography coupled to tandem mass spectrometry, in the receptor compartment of Frantz cells from 0 to 24 hours after gel application (20 μL at 400 mg/L) to pig-ear skin (n=3 per time point).
DISCUSSION
The major finding of this preliminary study is that sEH inhibition by t-AUCB using a topical formulation increases thermal hyperemia in the skin, an index of endothelium-dependent microvascular reactivity, in a murine model of diabetes. As microvascular endothelial dysfunction is a hallmark of diabetes, and considering the role of impaired cutaneous microcirculation in poor wound healing in diabetics, such a strategy may be an interesting therapeutic approach for DFUs.
EETs are endothelium-derived vasodilating factors with powerful anti-inflammatory and pro-angiogenic properties that could be useful in the treatment of the cardiovascular complications of type 2 diabetes [6,7]. Despite increasing evidence suggesting a possible role for EETs in diabetes-related endothelial dysfunction, no study had previously focused on diabetic skin microvascular dysfunction. The use of thermal hyperemia as a reactivity test was motivated by the involvement of EETs, together with NO, in the response to local heating in humans [18].
We observed a reduction in basal skin blood flow in diabetic db/db mice compared to wild-type mice that is probably mainly related to lower vascular density [19]. In addition, although no data were available in animal models of diabetes when we designed the study, we demonstrated altered microvascular reactivity to thermal hyperemia in diabetic mice. Thus, as shown in humans [20], measuring blood flow response to a standardized local heat stimulus provides a suitable model for the study of the skin microvascular dysfunction associated with diabetes in mice.
In this context, we tested the impact of a topical formulation containing t-AUCB, an inhibitor of EET degradation by sEH [11,13], on microvascular dysfunction in the skin of db/db mice. Quantification of t-AUCB in skin biopsies revealed significant transdermal permeation of the molecule 2 hours after gel application, associated with increased basal skin blood flow, compared to baseline, and thermal hyperemia. However, the vehicle control gel increased basal skin blood flow in a similar manner. This result supports previous data showing a direct vasodilating effect of the vehicle DMSO [21]. In fact, the topical administration of DMSO has even been proposed for use in humans to treat the skin complications of systemic scleroderma, which is also characterized by microvascular dysfunction and a risk of ulcers, but the results of randomized controlled trials were disappointing [22,23]. While, the DMSO vehicle had no effect on reactivity, in contrast, the t-AUCB-containing gel improved thermal hyperemia compared to the vehicle control gel, demonstrating an improvement in skin microvascular reactivity. This result shows that, as previously demonstrated in coronary and peripheral arteries [11,12], sEH plays a major role in the vascular dysfunction of the skin associated with type 2 diabetes.. Although the objective of this preliminary study was not to assess the effect of sEH inhibition on wound healing, it provides a first proof-of-principle in an animal model with prolonged wound healing [24].
Importantly for potential human use, histological analysis revealed no signs of skin toxicity with the t-AUCB-containing gel. In addition, quantification of t-AUCB in plasma from exposed animals showed low systemic diffusion of the drug, except in only one animal /5. This may be important because, although the first results obtained in the initial phases of clinical development suggest that sEH inhibitors were safe [25,26], some data show that increasing EET bioavailability might be associated with adverse effects and in particular might potentiate tumor development [6,7,27,28]. Moreover, because mouse skin is thin and the animals were shaved for the experiments, which could have led to an underestimation of the time needed for the transdermal passage of t-AUCB compared to that in humans, the pharmacokinetic study was performed on isolated, more human-like, pig ear skin. A progressive and continuous diffusion of t-AUCB was observed, suggesting that topical sEH inhibitors could be particularly useful in the prevention and/or treatment of skin complications in patients with type 2 diabetes.
CONCLUSION
These results show that the acute topical administration of a sEH inhibitor improves skin microvascular reactivity in a model of type 2 diabetes. The absence of skin toxicity, the limited systemic diffusion and the demonstration of the progressive passage of the sEH inhibitor across a more human-like animal source of skin, support the use of this therapeutic strategy in patients with type 2 diabetes with potential for the prevention of skin complications and in particular DFUs development. The next steps will be to show, using repeated dosing experiments, that this improvement in skin microcirculatory function translates into effective prevention of diabetes-associated dermatological complications and to confirm the limited systemic diffusion of the molecule so as to avoid potential long-term side effects.
ACKNOWLEDGEMENTS
The authors thank Mr. Tony Pereira (Laboratory of Pharmacokinetics, Toxicology and Pharmacogenetics, Rouen University Hospital, France), for his technical assistance in the quantification of t-AUCB, and Dr. Alison Foote (Grenoble Alpes University Hospital, France) for editing the manuscript.
FUNDING
This study was co-supported by grants from the Fondation de France (2011-20459), the French National Research Agency (ANR-16-CE17-0012) and the National Institute of Health (NIEHS/R01 ES002710).
Footnotes
DECLARATION OF CONFLICTING INTERESTS
The author(s) declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
REFERENCES
- 1.Moxey PW, Hofman D, Hinchliffe RJ, Jones K, Thompson MM, Holt PJE. Epidemiological study of lower limb amputation in England between 2003 and 2008. Br J Surg. 2010;97:1348–1353. [DOI] [PubMed] [Google Scholar]
- 2.Dinh T, Tecilazich F, Kafanas A, Doupis J, Gnardellis C, Leal E, Tellechea A, Pradhan L, Lyons TE, Giurini JM, Veves A. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61:2937–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao CY, Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev. 2009;25:604–614. [DOI] [PubMed] [Google Scholar]
- 4.Jhamb S, Vangaveti VN, Malabu UH. Genetic and molecular basis of diabetic foot ulcers: Clinical review. J Tissue Viability. 2016;25:229–236. [DOI] [PubMed] [Google Scholar]
- 5.Valacchi G, Zanardi I, Sticozzi C, Bocci V, Travagli V. Emerging topics in cutaneous wound repair. Ann N Y Acad Sci. 2012;1259:136–144. [DOI] [PubMed] [Google Scholar]
- 6.Lorthioir A, Guerrot D, Joannides R, Bellien J. Diabetic cardiovascular disease - Soluble epoxide hydrolase as a target. Cardiovasc Hematol Agents Med Chem. 2012;10:212–222. [DOI] [PubMed] [Google Scholar]
- 7.Bellien J, Joannides R, Richard V, Thuillez C. Modulation of cytochrome-derived epoxyeicosatrienoic acids pathway: a promising pharmacological approach to prevent endothelial dysfunction in cardiovascular diseases? Pharmacol Ther. 2011;131:1–17. [DOI] [PubMed] [Google Scholar]
- 8.Zhao H, Chen J, Chai J, Zhang Y, Yu C, Pan Z, Gao P, Zong C, Guan Q, Fu Y, Liu Y. Cytochrome P450 (CYP) epoxygenases as potential targets in the management of impaired diabetic wound healing. Lab Invest. 2017. July;97:782–791. [DOI] [PubMed] [Google Scholar]
- 9.Sander AL, Jakob H, Sommer K, Sadler C, Fleming I, Marzi I, Frank J. Cytochrome P450-derived epoxyeicosatrienoic acids accelerate wound epithelialization and neovascularization in the hairless mouse ear wound model. Langenbecks Arch Surg. 2011;396:1245–1253. [DOI] [PubMed] [Google Scholar]
- 10.Sander AL, Sommer K, Neumayer T, Fleming I, Marzi I, Barker JH, Frank J, Jakob H. Soluble epoxide hydrolase disruption as therapeutic target for wound healing. J Surg Res. 2013;182:362–367. [DOI] [PubMed] [Google Scholar]
- 11.Roche C, Besnier M, Cassel R, Harouki N, Coquerel D, Guerrot D, Nicol L, Loizon E, Morisseau C, Remy-Jouet I, Mulder P, Ouvrard-Pascaud A, Madec AM, Richard V, Bellien J. Soluble epoxide hydrolase inhibition improves coronary endothelial function and prevents the development of cardiac alterations in obese insulin-resistant mice. Am J Physiol Heart Circ Physiol. 2015;308:H1020–H1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang LN, Vincelette J, Chen D, Gless RD, Anandan SK, Rubanyi GM, Webb HK, MacIntyre DE, Wang YX. Inhibition of soluble epoxide hydrolase attenuates endothelial dysfunction in animal models of diabetes, obesity and hypertension. Eur J Pharmacol. 2011;654:68–74. [DOI] [PubMed] [Google Scholar]
- 13.Liu JY, Tsai HJ, Hwang SH, Jones PD, Morisseau C, Hammock BD. Pharmacokinetic optimization of four soluble epoxide hydrolase inhibitors for use in a murine model of inflammation. Br J Pharmacol. 2009;156:284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roustit M, Cracowski JL. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci. 2013;34:373–384. [DOI] [PubMed] [Google Scholar]
- 15.Godin B, Touitou E. Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models. Adv Drug Deliv Rev. 2007;59:1152–1161. [DOI] [PubMed] [Google Scholar]
- 16.Herkenne C, Naik A, Kalia YN, Hadgraft J, Guy RH. Pig ear skin ex vivo as a model for in vivo dermatopharmacokinetic studies in man. Pharm Res. 2006;23:1850–1856. [DOI] [PubMed] [Google Scholar]
- 17.Abd E, Yousef SA, Pastore MN, Telaprolu K, Mohammed YH, Namjoshi S, Grice JE, Roberts MS. Skin models for the testing of transdermal drugs. Clin Pharmacol. 2016;8:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol. 2012;590:3523–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaefer C, Biermann T, Schroeder M, Fuhrhop I, Niemeier A, Rüther W, Algenstaedt P, Hansen-Algenstaedt N. Early microvascular complications of prediabetes in mice with impaired glucose tolerance and dyslipidemia. Acta Diabetol. 2010;47:19–27. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs D, Dupon PP, Schaap LA, Draijer R. The association between diabetes and dermal microvascular dysfunction non-invasively assessed by laser Doppler with local thermal hyperemia: a systematic review with meta-analysis. Cardiovasc Diabetol. 2017;16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneda T, Sasaki N, Urakawa N, Shimizu K. Endothelium-dependent and -independent vasodilator effects of dimethyl sulfoxide in rat aorta. Pharmacology. 2016;97:171–176. [DOI] [PubMed] [Google Scholar]
- 22.Scherbel AL. The effect of percutaneous dimethyl sulfoxide on cutaneous manifestations of systemic sclerosis. Ann N Y Acad Sci. 1983;411:120–30. [DOI] [PubMed] [Google Scholar]
- 23.Williams HJ, Furst DE, Dahl SL, Steen VD, Marks C, Alpert EJ, Henderson AM, Samuelson CO Jr, Dreyfus JN, Weinstein A, et al. Double-blind, multicenter controlled trial comparing topical dimethyl sulfoxide and normal saline for treatment of hand ulcers in patients with systemic sclerosis. Arthritis Rheum. 1985;28:308–314. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan SR, Underwood RA, Gibran NS, Sigle RO, Usui ML, Carter WG, Olerud JE. Validation of a model for the study of multiple wounds in the diabetic mouse (db/db). Plast Reconstr Surg. 2004;113:953–960. [DOI] [PubMed] [Google Scholar]
- 25.Chen D, Whitcomb R, MacIntyre E, Tran V, Do ZN, Sabry J, Patel DV, Anandan SK, Gless R, Webb HK. Pharmacokinetics and pharmacodynamics of AR9281, an inhibitor of soluble epoxide hydrolase, in single- and multiple-dose studies in healthy human subjects. J Clin Pharmacol. 2012;52:319–328. [DOI] [PubMed] [Google Scholar]
- 26.Lazaar AL, Yang L, Boardley RL, Goyal NS, Robertson J, Baldwin SJ, Newby DE, Wilkinson IB, Tal-Singer R, Mayer RJ, Cheriyan J. Pharmacokinetics, pharmacodynamics and adverse event profile of GSK2256294, a novel soluble epoxide hydrolase inhibitor. Br J Clin Pharmacol. 2016;81:971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, Butterfield CE, Barnés CM, Mammoto A, Mammoto T, Luria A, Benny O, Chaponis DM, Dudley AC, Greene ER, Vergilio JA, Pietramaggiori G, Scherer-Pietramaggiori SS, Short SM, Seth M, Lih FB, Tomer KB, Yang J, Schwendener RA, Hammock BD, Falck JR, Manthati VL, Ingber DE, Kaipainen A, D’Amore PA, Kieran MW, Zeldin DC. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Invest. 2012;122:178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sausville LN, Gangadhariah M, Chiusa M, Mei S, Wei S, Zent R, Luther JM, Shuey MM, Capdevila JH, Falck JR, Guengerich FP, Williams SM, Pozzi A. The cytochrome P450 slow metabolizers CYP2C9*2 and CYP2C9*3 directly regulate tumorigenesis via reduced epoxyeicosatrienoic acid production. Cancer Res. 2018. July 16 pii: canres.3977.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


