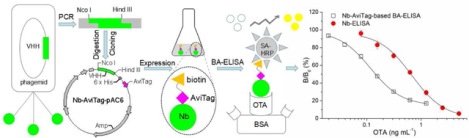
Abstract
Ochratoxin A (OTA) is a common food contaminant that threatens the consumers’ safety and health. A sensitive and selective biotin-streptavidin-amplified enzyme-linked immunosorbent assay (BA-ELISA) for OTA using a nanobody-AviTag fusion protein (Nb-AviTag) was developed in this study. The prokaryotic expression vector Nb28-AviTag-pAC6 for Nb-AviTag was constructed, followed by transformation to the AVB101 cells for antibody expression and in vivo biotinylation. The purified Nb28-AviTag was used to establish the BA-ELISA and the procedures for this Nb-AviTag-based BA-ELISA were optimized. The Nb-AviTag-based BA-ELISA exhibited the half maximal inhibitory concentration (IC50) of 0.14 ng mL−1 and the limit of detection (LOD = IC10) of 0.028 ng mL−1 for OTA basing on the optimized experiment parameters. The assay sensitivity was improved 4.6 times and 4.3 times compared to Nb-based ELISA, respectively. This method had LODs of 1.4 μg kg−1 in barley, 0.56 μg kg−1 in oats, and 0.84 μg kg−1 in rice for OTA. The average recovery percent was in a range of 84–137%, and the relative standard derivation percent ranged from 0.64% to 7.8%. The content of OTA in contaminated cereal samples was determined by both the developed Nb-AviTag-based method and liquid chromatography-tandem mass spectrometry (LC-MS/MS). The results demonstrated that the Nb-AviTag was a robust and promising bioreceptor in highly sensitive detection of OTA and other low molecular weight compounds using BA system.
Keywords: Mycotoxin, nanobody, AviTag, biotin-streptavidin amplification, immunoassay
Graphical Abstract
1. Introduction
Ochratoxin A (OTA) is a toxic molecule from the secondary metabolism of some species of Aspergillus and Penicillium. OTA contamination is commonly distrbuted in cereal, coffee beans, grape wine, and spices.1–3 OTA has the highest toxicity among OTB, OTC and other analogues. Numerous studies have indicated hepatotoxicity, nephrotoxicity, and carcinogenicity of OTA on animals and humans.4,5 In addition, the International Agency for Research on Cancer categorized OTA as a possible human carcinogen.6 Therefore, OTA contamination of food is a serious concern for consumers in any country due to its multiple toxic effects. To reduce the exposure of OTA intake from food, strict maximum limits for OTA has been set in the European Union, such as 3 μg kg−1 of cereal products and 5 μg kg−1 of cereal.7 Meanwhile, the OTA regulation is in urgent need of detection techniques with high sensitivity and selectivity.
To date, various sophisticated instruments-based methods have been applied to the sensitive and accurate detection of OTA, mainly including high performance liquid chromatography, gas chromatography, liquid chromatography-mass spectrometry, and combined methods.8–11 Nevertheless, these methods of instrument are labor-intensive, time-consuming, and costly for sample pretreatment and detection. Thus, they can not be used for routine detection of numerous samples simultaneously. Alternatively, immunoassays, especially the enzyme-linked immunosorbent assay (ELISA), have been widely applied to the detection of hazards in food because of their easy operation, low cost, and high sensitivity.12,13 Moreover, the detection sensitivity of conventional ELISA can be further improved using various signal amplification strategies, including biotin-streptavidin-amplified (BA) system, chemiluminescence, and fluorescence, and combined methods.14–20 The BA system has continued to attract interest of researchers because of the high specificity and affinity between streptavidin and biotin.21 Compared to the conventional ELISA, the BA-ELISA facilitates the attachment of more horseradish peroxidase, alkaline phosphatase, or other reporter molecules for catalytic reaction of substrates and thus improve the detection sensitivity.22
Most of the previously reported BA-ELISAs involve the preparation of a conventional antibodies chemically labeled with biotin molecules, including monoclonal antibodies and polyclonal antibodies.14,23 Nevertheless, the chemical coupling procedure may lead to the loss of antibody activity and variability from batch to batch, and thus influence the sensitivity of BA-ELISA.24,25 Advancement in gene engineering technique has provided a green biosynthesis method to replace the chemical coupling reactions for labeling antibodies, such as single-chain variable fragment-alkaline phosphatase fusions (scFv-AP) and scFv-green fluorescence protein fusions.25–28 A recently reported construct of scFv-AviTag fusion protein still retains good antibody activity and is effective in vivo biotinylation during protein production, indicating its potential as a novel immunodiagnostic reagent.29 However, the drawbacks of scFv, such as low solubility and stability, limit its extensive application.30 It has been reported that two novel antibody molecules naturally lacking of light chains, heavy chain antibody (HCAb) and immunoglobulin new antigen receptor (IgNAR), were found in the camelids and sharks, respectively.31,32 Cloning and recombinant expression of the variable domain of the HCAb (VHH) and IgNAR can produce a single domain antibody retaining antigen-binding capacity, which is also known as a nanobody (Nb) for its nanometer size.33 As an alternative to conventional antibodies, Nb’s are more attractive than scFv and provide several unique features. They have high thermal stability, high solubility in water, and they can be genetically manipulated in various expression systems.33 There are numerous recent reports on the production of Nb’s and their derivates for environmental and food analysis.16,34–44 However, few reports have been published on the construction and application of Nb-AviTag fusion protein in detection of toxic low molecular weight compounds.
This study describes construction of an Nb-AviTag fusion protein and development of a BA-ELISA for OTA. Information on the construction of the prokaryotic expression vector of Nb-AviTag, expression and purification of the Nb-AviTag, and establishment of a BA-ELISA using the Nb-AviTag for OTA detection in cereal is detailed. Various parameters were used to characterize the developed Nb-AviTag-based BA-ELISA, including sensitivity, selectivity, accuracy, and precision. To validate the developed method, both of the Nb-AviTag-based BA-ELISA and LC-MS/MS were conducted to detect the OTA in contaminated cereal samples. The Nb-AviTag-based BA-ELISA selectively and sensitively detected OTA in different cereal matrices showing that it will be helpful in food analysis.
2. Material and methods
2.1. Reagent and chemicals
Standards of fumonisin B1 (FB1), ochratoxin A, and ochratoxin C (OTC) were obtained from Pribolab Pte. Ltd. (Singapore). Standards of aflatoxin B1 (AFB1) and zearalenone (ZEN) were from Fermentek Ltd. (Jerusalem, Israel). Ochratoxin B (OTB) standard was procured from Bioaustralis (Smithfield, NSW, Australia). Deoxynivalenol (DON) standard was from Sigma-Aldrich (CA, USA). Horseradish peroxidase-labeled streptavidin (SA-HRP) was purchased from CWBIO (Beijing, China). Primers, non-fat milk powder, 3,3′,5,5′-teramethylbenzidine (TMB), microplates, d-biotin, and affinity chromatography columns (3 mL, 8.9 × 63 mm) were obtained from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). Nickel-Nitilotriacetic acid (Ni-NTA) sepharose 6 fast flow (FF) suspension and Tween 20 were purchased from Solarbio (Beijing, China). Nco I and Hind III were procured from NEB (Beijing) Ltd. (Beijing, China). The coating antigen OTA-BSA and the phagemid vector pHEN1-VHH28 were prepared in our previous studies.34,36 All other reagents were of analytical grade.
2.2. Construction of the expression vector Nb-AviTag-pAC6
The expression vector Nb28-AviTag-pAC6 was constructed as follows. Briefly, the Nb28 fragment was amplified from the pHEN1-VHH28 phagemid vector by using the forward primer 5′-CTG CAG CCA TGG CAT GAA AAA GAC AGC TAT CGC GAT TGC-3′ (Nco I site underlined) and reverse primer 5′-ATG CAG AAG CTT ATG GTG ATG GTG ATG GTG CTG GCC G-3′ (Hind III site underlined). The purified PCR product of Nb28 fragment was digested with Nco I and Hind III, followed by inserting into the similarly digested vector pAC6 at a mole ratio of 3:1. After heat shock transformation of DNA and ampicillin resistance screening, positive clones of E. coli DH5α containing Nb28-AviTag-pAC6 were randomly picked and amplified for plasmid extraction, followed by DNA sequencing using primer 5′-CCG GCT CGT ATA ATG TGT GG-3′.
2.3. Preparation and identification of the Nb28-AviTag
The recombinant vector Nb28-AviTag-pAC6 was transformed into the AVB101 chemical competent cells by heat shock in 42°C water bath for 90 s. The transformation products were then spread on Tryptone-Yeast extract-HEPES (TYH) agar plate with 10 μg mL−1 chloramphenicol and 100 μg mL−1 ampicillin, and cultured overnight at 37°C. A randomly picked positive clone was inoculated into TYH medium 10 μg mL−1 chloramphenicol and 100 μg mL−1 ampicillin and shaken overnight at 37°C. Subsequently, 2 mL of the overnight culture was transferred into 200 mL of TYH medium with 100 μg mL−1 ampicillin and 5 mg mL−1 glucose. The mixture was shaken vigorously at 37°C until the OD600 reached 0.7, followed by induction expression with 1.5 mM IPTG and biotinylation with 50 μM d-biotin (37°C, 3 h). The bacteria was collected by centrifugation (10000 g, 10 min), followed by resuspension in PBS (0.01 M, pH 7.4) and ultrasonication. The supernatant was separated by centrifugation and filtered through a 0.22 μm membrane filter. Then, the Nb-AviTag in filtrate was purified using the Ni-NTA affinity chromatography method according to our previous work.38 After quantification with a NanoDrop Lite, the purified Nb28-AviTag was stored at −20°C for later use.
Purity identification of the purified Nb28-AviTag was conducted using the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis according to our previous study.38 To identify the effectiveness of the in vivo biotinylation system for Nb28 and the antigen binding activity of Nb28-AviTag, a dot immunoassay was conducted as described before, except that the HRP-labeled rabbit anti-6× histidinetag polyclonal antibody was changed to the SA-HRP solution (0.3 μg mL−1 in PBS).37
2.4. Procedures of the Nb-AviTag-based BA-ELISA
The Nb-AviTag-based BA-ELISA for indirect competitive detection of OTA was conducted as follows. Briefly, 100 μL/well of the OTA-BSA (1.0 μg mL−1 in PBS) was added into the microplate and incubated (37°C, 2 h). After 3 washes with PBS −0.05% Tween 20 solution (PBST), the plate was incubated with 300 μL/well of the 3% (w/v) non-fat milk powder-PBS solution to block the non-specific binding sites (37°C, 1 h). The plate was washed and incubated with the competitive reaction system, which was consist of 50 μL of OTA standard solution with various concentrations and equal volume of Nb28-AviTag solution (37°C, 45 min). After 3 washes with PBST, 100 μL/well of the SA-HRP solution (0.5 μg mL−1 in PBS) was transferred into the plate and incubated for another 45 min. The plate was rinsed with PBST and incubated with 100 μL/well of TMB solution (37°C, 10 min), followed by terminating the color reaction with 100 μL/well of 2 M sulfuric acid. The optical density at 450 nm (OD450) of wells were measured with a microplate reader, and the standard competitive inhibition curves for OTA were constructed as described before.36
2.5. Selectivity of the Nb-AviTag-based BA-ELISA
To evaluate the OTA selectivity of the BA-ELISA using Nb28-AviTag, the cross reactivity (CR) of Nb28-AviTag with analytes including OTA analogues (OTB and OTC) and several other mycotoxins (AFB1, DON, FB1, and ZEN) was determined. The CR was equal to the value of (IC50 of OTA/IC50 of the determined analytes)] × 100.
2.6. Sample analysis
For Nb-AviTag-based BA-ELISA analysis, cereal samples (barley, oats, and rice) were pretreated as follows. Briefly, 5 g of ground cereal sample was immersed in 10 mL of 50% methanol solution. After 20 min of ultrasonic treatment and 10 min of centrifugation (4°C, 10000 g), the supernatant was separated from the mixture and diluted for analysis. For validation study using LC-MS/MS, sample pretreatment and detection were performed as described previously.35
3. Results and discussion
3.1. Preparation, identification, and characterization of the Nb28-AviTag
The recombinant expression vector Nb28-AviTag-pAC6 was constructed by subcloning the VHH28 gene into the pAC6 vector system (Figure 1A). The C-terminal AviTag contained in the pAC6 vector was used for in vivo biotinylation during expression. The expression strain AVB101 of the vector Nb28-AviTag-pAC6 has an IPTG inducible vector pBirAcm, which contains the BirA gene engineered into pACYC184. The BirA gene encodes the biotin-protein ligase which can activate the biotin to form biotinyl 5’-adenylate for biotinylation. The Nb28-AviTag was expressed by IPTG induction and purified on a Ni-NTA resin column. The results of expression and purification of the Nb28-AviTag were characterized by SDS-PAGE (Figure 1B). A target protein band of approximately 19 kDa was separated from the induced cells (Lane 1). However, it was not found in the non-induced cells (Lane 2). In addition, high purity of the Nb28-AviTag was obtained since only one band of target protein was observed in lane 3. A dot immunoassay was conducted to identify the existence of Nb28-AviTag (Figure 1C). The purple dots appeared on the PVDF membrane, indicating the successful expression of Nb28-AviTag retaining OTA-binding activity.
Figure 1.
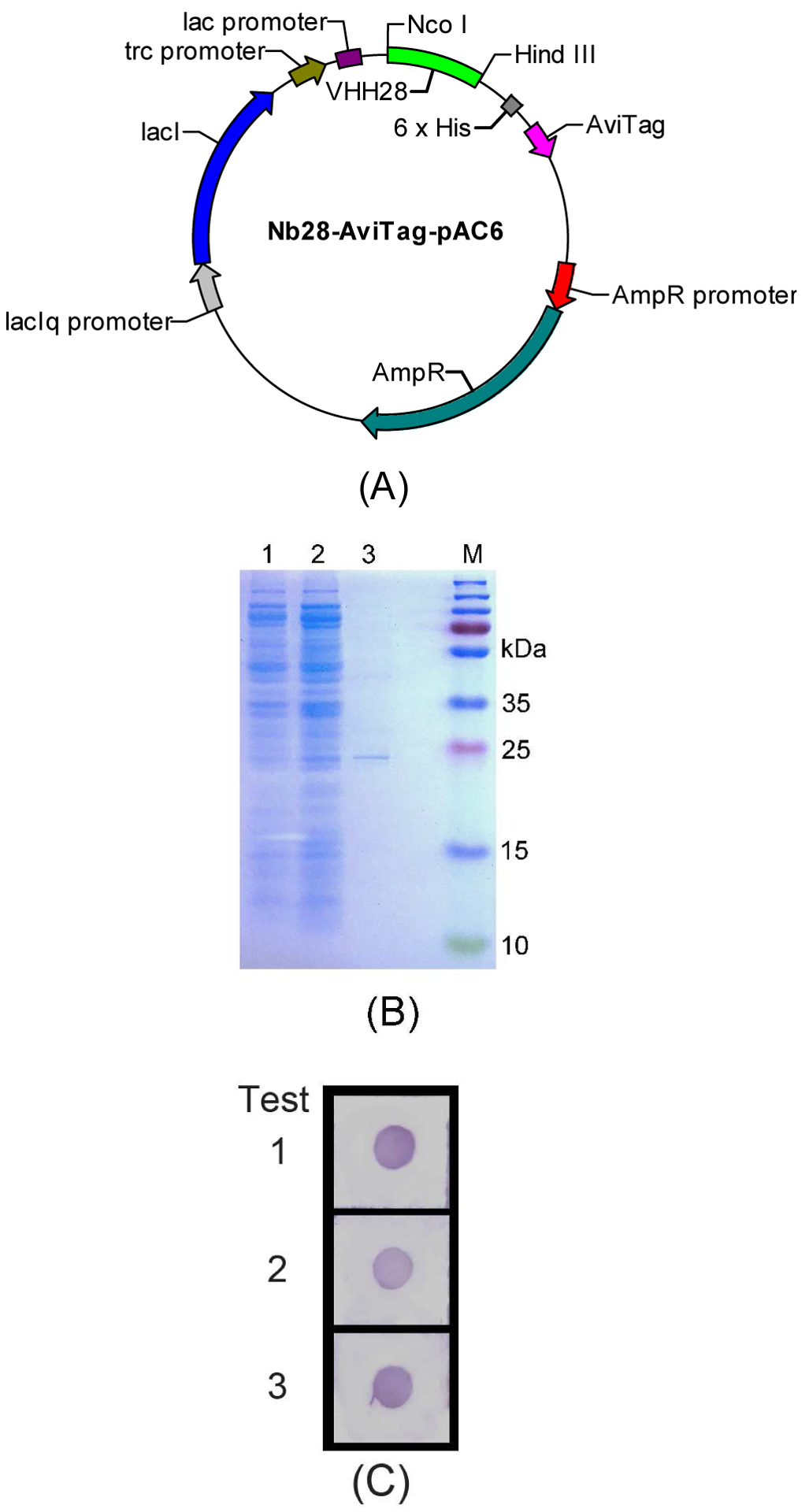
Preparation and identification of the Nb28-AviTag. (A) Plasmid map of Nb28-AviTag-pAC6. (B) SDS-PAGE and coomassie brilliant blue staining analysis of Nb28-AviTag. Lane M: Prestained protein marker. Lane 1: Whole cell protein under non-induced condition. Lane 2: Whole cell protein under induced condition. Lane 3: Ni-NTA sepharose-purified Nb28-AviTag. (C) Identification of Nb28-AviTag by dot immunoassay. The assay was performed in triplicate.
To characterize the solvent tolerance of Nb28-AviTag, the performance of Nb28-AviTag dissolved in different concentrations of methanol, ethanol, dimethyl sulfoxide (DMSO), and dimethyl formamide (DMF) was investigated (Figure S-1 in SI). The retained binding activity percent was used to evaluate the solvent effect on the performance of Nb28-AviTag in immunoassay, and was calculated as [OD450 (antibody diluted by PBS buffers containing various concentrations of organic solvent)/OD450 (antibody diluted by PBS)] × 100%. Nb28-AviTag was diluted in each solution and its binding activity with antigen OTA-BSA was tested. The retained binding activity percent generally decreased as the concentration of organic solvent was increased, since the organic solvent could influence the interaction between antibody and antigen. As the solvent concentration was increased from 0% to 2.5%, an obvious reduction of the binding activity was observed except for acetonitrile. The binding activity of Nb28-AviTag increased from 63% to 83% as the methanol concentration was increased from 2.5% to 10%, while it decreased to 22% as the methanol concentration was up to 60% (Figure S-1A in SI). Similar result was observed for acetonitrile except that the binding activity was no less than 115% with the acetonitrile concentration ranging from 2.5% to 20% (Figure S-1E in SI). Ethanol, DMSO, and DMF had considerable and comparable effects on Nb28-AviTag, but a better tolerance of Nb28-AviTag to DMF was observed as the solvent concentration did not exceed 40% (Figure S-1B, S-1C, and S-1D in SI). The Nb28-AviTag showed the best tolerance to acetone among these tested solvents, and maintained 103% of its binding activity at 60% of acetone (Figure S-1F in SI). Thus the effects of various organic reagents on the activity of Nb28-AviTag were evaluated. The results indicated the better tolerance of Nb28-AviTag to methanol, acetonitrile, and acetone than to ethanol, DMF, and DMSO, since no less than 55% of its binding activity were retained at 40% of methanol, acetonitrile, and acetone. In addition, a better tolerance of antibody to organic solvents could lead to a less dilution of sample extract and high sensitivity of detection when the matrix effect is ignorable.45
3.2. Optimization of the Nb-AviTag-based BA-ELISA
A checkboard titration was firstly implemented to obtain the optimal concentration of the OTA-BSA (0.5 μg mL−1) and the corresponding antibody Nb28-AviTag (40 ng mL−1) as shown in Table S-1 in SI. To achieve the optimal performance of the Nb-AviTag-based BA-ELISA, various experimental conditions were optimized (Figure S-2 in SI). The OD450 of the negative control (ODmax) and IC50 were applied to evaluate assay performance. Methanol is commonly used as the solvent of OTA for standard solution and sample pretreatment. However, the antibody-antigen interaction can be influenced by methanol.36 Effect of the assay buffer containing various methanol concentrations on the assay performance was assessed (Figure S-2A in SI). As the methanol concentration was increased, both the IC50 and ODmax changed significantly. The lowest IC50 and the highest ODmax was achieved when the final concentration of methanol was 2.5%. Reaction time (15, 30, or 45 min) for competition binding to Nb28-AviTag between OTA-BSA and OTA was optimized (Figure S-2B in SI). The IC50 increased with increasing time and ODmax changed slightly. Reaction time of 15 min was chosen as the optimal time because of the lowest IC50 (0.39 ng mL−1). The incubation time and dilution factor for SA-HRP were optimized similarly (Figure S-2C and S-2D in SI), and 15 min of incubation time and 4000 of dilution factor were selected. Ionic strength and pH of the assay buffer are another two key factors that can influence the interaction of antibody and antigen.36 Effects of assay buffer containing various ionic strength on the assay performance are shown in Figure S-2E in SI. Similar values of IC50 (0.094 and 0.090 ng mL−1) were obtained as the ionic strength was 0.5×PBS and 2.5×PBS. Since the higher ODmax (1.4) was obtained at 0.5×PBS, ionic strength of 0.5×PBS was selected for further optimization. The effects of the buffer with various pH values (5.5 to 9.5) on the assay were assessed. The IC50 declined when the pH increased from 5.5 to 8.5, followed by increasing slightly from 0.11 to 0.14 as the pH ranged from 8.5 to 9.5. The ODmax varied slightly from 0.82 to 0.88 as the pH did not exceed 7.4, while it sharply decreased as the pH reached 8.5 and 9.5. The optimal pH was 7.4 considering the IC50 and ODmax. Thus the conditions for assay performance were optimized not only for sensitivity but also to yield an assay giving results that are not sensitive to minor alternations in assay conditions.
3.3. Comparison of the Nb-AviTag-based BA-ELISA and Nb-based ELISA
The BA system is known for having one of the strongest noncovalent interactions. It has become one of the most extensively applied affinity pairs in biological assays because of the strength and specificity of the interaction. Using the optimal working conditions, the standard inhibition curve of Nb28-AviTag-based BA-ELISA was established with a linear detection range (IC20–80) of 0.051–0.70 ng mL−1 (Figure 2). Meanwhile, the standard inhibition curve of unfused Nb28-based ELISA (Nb-ELISA) was also created with a linear detection range of 0.23–1.7 ng mL−1 as described previously (Figure 2).36 The Nb-ELISA had IC50 of 0.65 ng mL−1 and LOD of 0.12 ng mL−1 for OTA. Thus, the Nb-AviTag-based BA-ELISA showed approximately 4.6 times and 4.3 times improvement in both the IC50 (0.14 ng mL−1) and LOD (0.028 ng mL−1) compared with the Nb-ELISA.36
Figure 2.
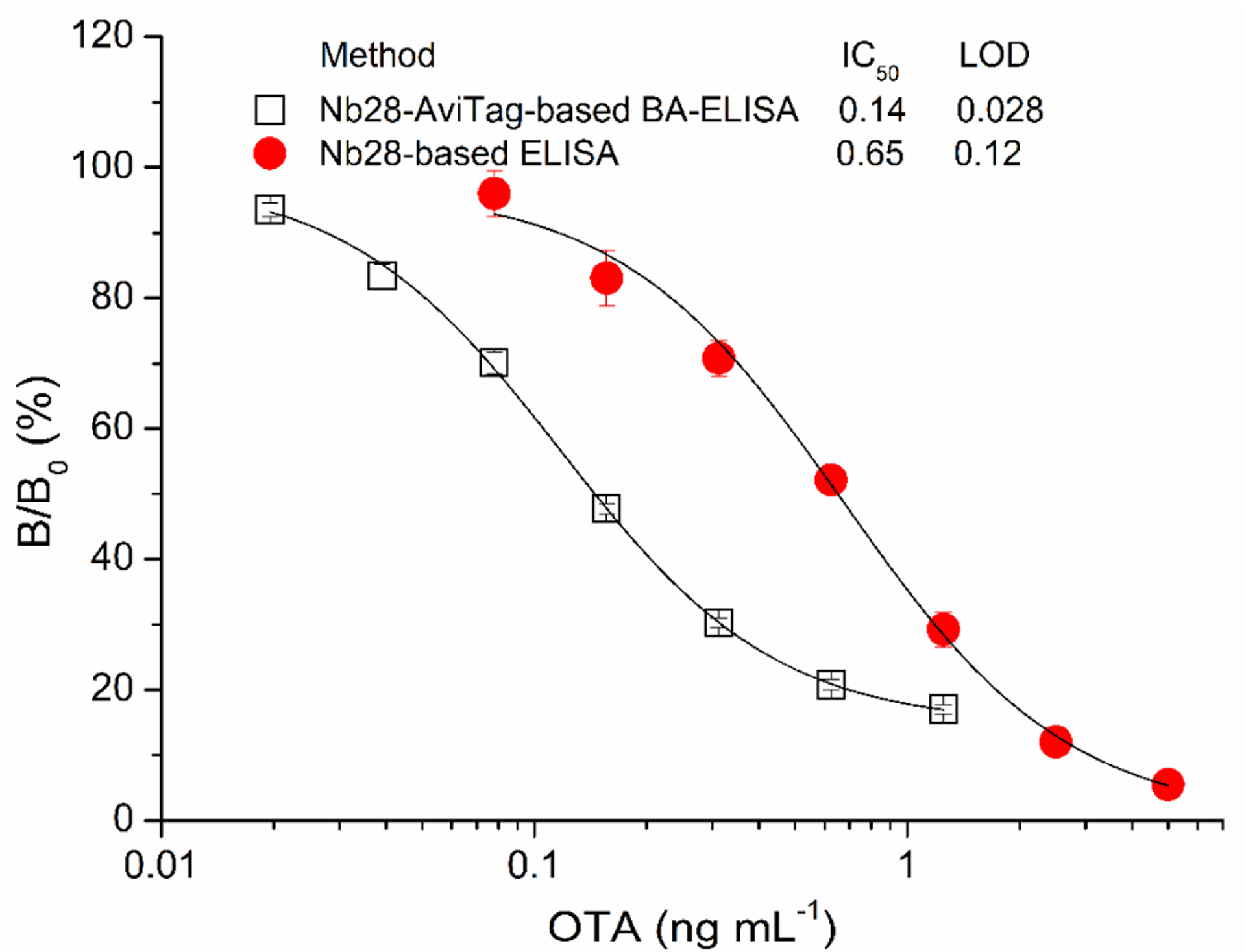
Comparison of the standard inhibition curves of the Nb28-based ELISA and the Nb28-AviTag-based BA-ELISA. The error bars represent the standard deviation of triplicate tests.
3.4. Selectivity of the Nb-AviTag-based BA-ELISA
The selectivity study of Nb-AviTag-based BA-ELISA for OTA was performed by determining the CR of Nb28-AviTag with several compounds (OTB, OTC, AFB1, ZEN, FB1, and DON) (Figure S-3 in SI). The Nb28-AviTag was specific in its recognition of OTA and exhibited a low CR with OTB (0.48%) and OTC (3.2%) (Table 1). In addition, negligible CR (less than 0.10%) was obtained with AFB1, ZEN, FB1, and DON, which was parallel to that of the unfused Nb28 in our previous study.36 However, a higher CR (10%) with OTB was detected for unfused Nb28.36 We inferred that the steric hindrance of biotin labeled on the Nb28 might contribute to the low CR with OTB for the Nb28-AviTag. Hence, these results indicated a good selectivity of Nb-AviTag-based BA-ELISA for OTA.
Table 1.
CR of Nb28-AviTag with OTA analogues and common mycotoxins
| Analyte | IC50 (ng mL−1) | CR (%) |
|---|---|---|
| OTA | 0.14 | 100 |
| OTB | 29 | 0.48 |
| OTC | 4.3 | 3.2 |
| AFB1 | > 200 | < 0.10 |
| ZEN | > 200 | < 0.10 |
| FB1 | > 200 | < 0.10 |
| DON | > 200 | < 0.10 |
3.5. Matrix effect
The matrix effect concerns the accuracy and sensitivity of immunoassays for food analysis since it can interfere the interaction between antibody and antigen, and cause false positive or negative results. Fortunately from a screening or regulatory perspective, false positive results are far more likely. The food matrix effect can be eliminated by various ways, such as diluting the sample extract, cleaning up the sample extract using solid phase extraction, and masking the matrix effect with BSA.3,36,46 The dilution method is commonly used for the elimination of food matrix effect, while a high dilution might result in low sensitivity. To evaluate the matrix effect, a standard inhibition curve generated in 5% methanol-0.5×PBS (MeOH-PBS) was compared with those using the diluted OTA-free cereal (barley, oats, and rice) extract. The variations in the ODmax and IC50 of the assays with serially diluted sample matrix extracts were shown in Figure 3. The cereal matrix effects were elimiated as the cereal extract was diluted 50 times for barley (Figure 3A), 20 times for oats (Figure 3B), and 30 times for rice (Figure 3C), and the corresponding standard inhibition curves coincided well with the one generated in MeOH-PBS. Therefore, the sample matrix extract was diluted 50 times for barley, 20 times for oats, and 30 times for rice to overcome the matrix effect for sample analysis. Considering the dilution factor, the developed Nb-AviTag-based BA-ELISA has LODs of 1.4 μg kg−1 in barley with a linear detection range of 2.5–35 μg kg−1, 0.56 μg kg−1 in oats with a linear detection range of 1.0–14 μg kg−1, and 0.84 μg kg−1 in rice with a linear detection range of 1.5–21 μg kg−1 for OTA, respectively. These results well meet the EU requirement of regulatory limits for OTA in cereal (5 μg kg−1) and cereal products (3 μg kg−1).
Figure 3.
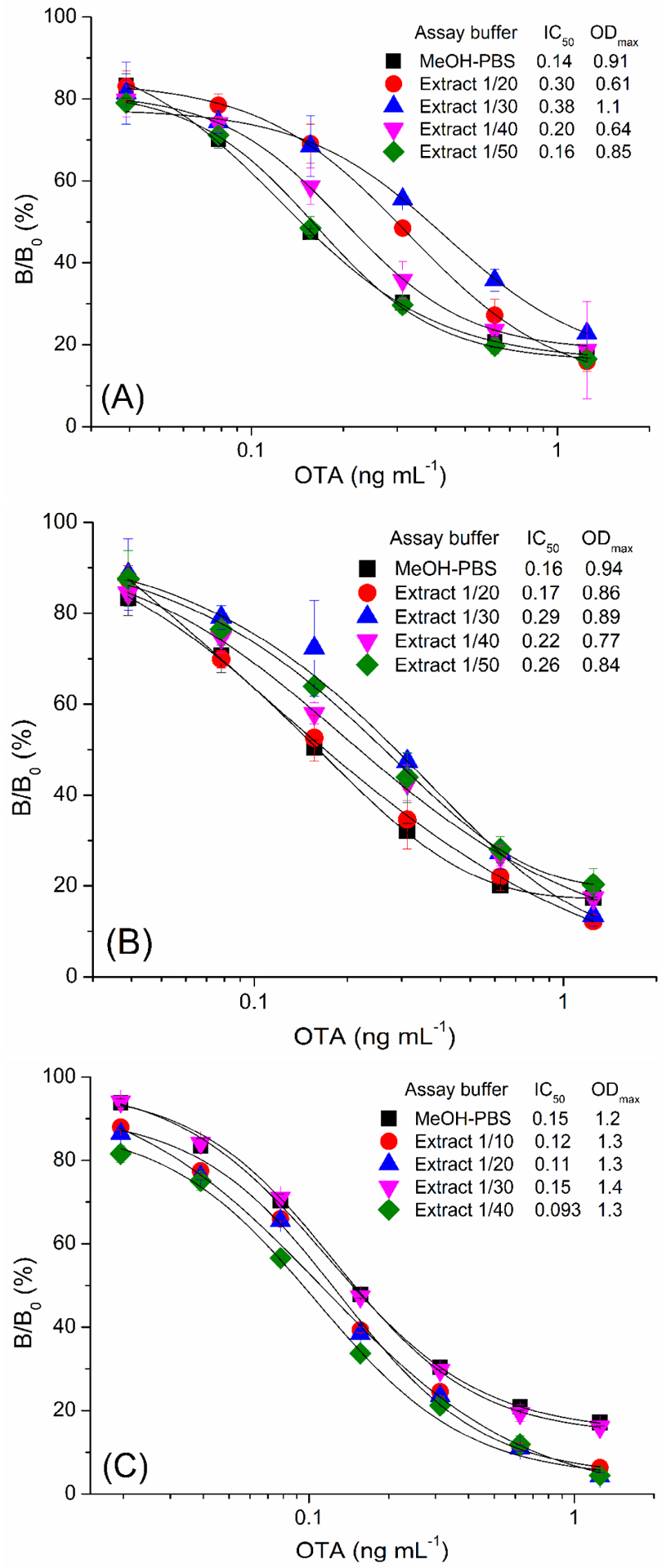
The standard inhibition curves of Nb28-AviTag-based BA-ELISA in 5% methanol-0.5×PBS (MeOH-PBS), barley extracts (A), oats extracts (B), and rice extracts (C). The error bars represent the standard deviation of triplicate tests.
3.6. Assay validation
The spiking and recovery study was performed to evaluate the accuracy and precision of the Nb-AviTag-based BA-ELISA for cereal sample analysis. The cereal samples (barley, oats, and rice) spiked with various concentrations of OTA standard (3, 5, 10, and 20 μg kg−1) were pretreated by extraction. The content of recovered OTA in extracting solution was determined by the Nb-AviTag-based BA-ELISA (Table 2).The average recovery and the relative standard deviation (RSD) of intra-assay ranged from 84% to 137% and from 0.64% to 4.6%, respectively. In respect of inter-assay, the average recovery and RSD were in the range of 86%−133% and 0.88%−7.8%, respectively. To further assess the effectiveness of the developed method, six OTA contaminated samples were detected by the Nb-AviTag-based BA-ELISA, and the results were validated by LC-MS/MS (Table 3). The OTA content of six cereal samples tested by the developed method ranged was in a range of 1.1–7.2 μg kg−1, and correlated well with that tested by LC-MS/MS35 (Figure S-4 in SI). The OTA content of oats sample No. 2 tested by both methods exceeded the maximum level of OTA in cereal (5 μg kg−1) regulated by the European Union. Thus, the above experiments showed an accurate and precise BA-ELISA using the Nb-AviTag for detecting OTA in cereal.
Table 2.
Recoveries of OTA tested by the Nb-AviTag-based BA-ELISA
| Matrix | OTA spiked (μg/kg) | Mean ± SD (μg/kg) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Intra-assay (n = 3)a | ||||
| barley | 3 | 3.5 ± 0.12 | 117 | 3.4 |
| 5 | 4.2 ± 0.060 | 84 | 1.4 | |
| 10 | 10 ± 0.14 | 100 | 1.4 | |
| 20 | 25 ± 0.72 | 125 | 2.9 | |
| oats | 3 | 3.1 ± 0.020 | 103 | 0.64 |
| 5 | 4.8 ± 0.11 | 96 | 2.3 | |
| 10 | 8.8 ± 0.28 | 88 | 3.2 | |
| 20 | 23 ± 0.45 | 115 | 1.9 | |
| rice | 3 | 4.1 ± 0.060 | 137 | 1.5 |
| 5 | 5.2 ± 0.18 | 104 | 3.5 | |
| 10 | 9.2 ± 0.42 | 92 | 4.6 | |
| 20 | 20 ± 0.50 | 100 | 2.5 | |
| Inter-assay (n = 3)b | ||||
| barley | 3 | 3.4 ± 0.050 | 113 | 1.5 |
| 5 | 4.4 ± 0.050 | 88 | 1.1 | |
| 10 | 10 ± 0.34 | 100 | 3.4 | |
| 20 | 25 ± 0.22 | 125 | 0.88 | |
| oats | 3 | 3.0 ± 0.060 | 100 | 2.0 |
| 5 | 4.6 ± 0.080 | 92 | 1.7 | |
| 10 | 8.6 ± 0.11 | 86 | 1.3 | |
| 20 | 23 ± 0.67 | 115 | 2.9 | |
| rice | 3 | 4.0 ± 0.17 | 133 | 4.2 |
| 5 | 5.2 ± 0.07 | 104 | 1.3 | |
| 10 | 9.9 ± 0.67 | 99 | 6.8 | |
| 20 | 18 ± 1.4 | 90 | 7.8 | |
Each assay was conducted three times on the same day.
The assays were conducted on three different days.
Table 3.
Sample analysis by the Nb-AviTag-based BA-ELISA and LC-MS/MS
| Sample | Nb-AviTag-based BA-ELISA(Mean ± SD, n = 4) | RSD (%) | LC-MS/MS35 (Mean ± SD, n = 3) | RSD (%) |
|---|---|---|---|---|
| barley | ||||
| 1 | 2.3 ± 0.10 | 4.3 | 1.8 ± 0.14 | 7.8 |
| oats | ||||
| 1 | 1.1 ± 0.050 | 4.5 | 0.82 ± 0.10 | 12 |
| 2 | 7.2 ± 0.50 | 6.9 | 7.5 ± 0.11 | 1.5 |
| rice | ||||
| 1 | 1.9 ± 0.050 | 2.6 | 2.0 ± 0.090 | 4.5 |
| 2 | 1.7 ± 0.10 | 5.9 | 1.8 ± 0.17 | 9.4 |
| 3 | 1.3 ± 0.090 | 6.9 | 1.4 ± 0.18 | 13 |
Conclusions
Thus, we reported a rapid, sensitive, and selective BA-ELISA using an Nb-AviTag fusion protein for rapid detection of OTA in cereal. This study exhibits the advantages of Nbs on detecting low molecular weight compounds. Nbs are more economical to develop and produce than conventional monoclonal antibodies, and still retain the advantages of monoclonal antibody technology. Nb is ease of genetic manipulation by recombinant DNA technology as demonstrated here. Moreover, we have also demonstrated the AviTag fusion molecules can work as ideal probes in immunoassays for analysis of low molecular weight compounds. Not only does the AviTag fusion technology yield homogeneous biotinylated binding agents with a defined analyte/reporter molar ratio by using an in vivo biotinylation strategy, but the high specificity and affinity between streptavidin and biotin can increase the sensitivity of the procedure.
Supplementary Material
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31760493) and the Scientific Research Foundation of Hainan University (kyqd1631). Partial support was provided by the National Institute of Environmental Health Sciences Superfund Research Program (P42-ES04699).
Footnotes
Conflict of interest disclosure
The authors declare no competing financial interest.
References
- (1).Amézqueta S; Schorr-Galindo S; Murillo-Arbizu M; González-Peñas E; López de Cerain A; Guiraud JP OTA-producing fungi in foodstuffs: A review. Food Control 2012, 26, 259–268. [Google Scholar]
- (2).Lee HJ; Ryu D Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J. Agric. Food Chem 2017, 65, 7034–7051. [DOI] [PubMed] [Google Scholar]
- (3).Huertas-Pérez JF; Arroyo-Manzanares N; García-Campaña AM; Gámiz-Gracia L Solid phase extraction as sample treatment for the determination of ochratoxin A in foods: A review. Crit. Rev. Food Sci 2017, 57, 3405–3420. [DOI] [PubMed] [Google Scholar]
- (4).Tao Y; Xie S; Xu F; Liu A; Wang Y; Chen D; Pan Y; Huang L; Peng D; Wang X; Yuan Z Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol 2018, 112, 320–331. [DOI] [PubMed] [Google Scholar]
- (5).Duarte SC; Lino CM; Pena A Ochratoxin A in feed of food-producing animals: An undesirable mycotoxin with health and performance effects. Vet. Microbio 2011, 154, 1–13. [DOI] [PubMed] [Google Scholar]
- (6).International Agency for Research on Cancer (IARC). In Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins; World Health Organization: IARC Lyon France, 1993; Vol. 56, pp. 489–521. [Google Scholar]
- (7).Commissions of the Euopean Communities. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- (8).Giovannoli C; Passini C; Di Nardo F; Anfossi L; Baggiani C Determination of ochratoxin A in Italian red wines by molecularly imprinted solid phase extraction and HPLC analysis. J. Agric. Food Chem 2014, 62, 5220–5225. [DOI] [PubMed] [Google Scholar]
- (9).Qi D; Fei T; Liu H; Yao H; Wu D; Liu B Development of multiple heart-cutting two-dimensional liquid chromatography coupled to quadrupole-orbitrap high resolution mass spectrometry for simultaneous determination of aflatoxin B1, B2, G1, G2, and ochratoxin A in snus, a smokeless tobacco product. J. Agric. Food Chem 2017, 65, 9923–9929. [DOI] [PubMed] [Google Scholar]
- (10).Campone L; Piccinelli AL; Celano R; Pagano I; Russo M; Rastrelli L Rapid and automated on-line solid phase extraction HPLC–MS/MS with peak focusing for the determination of ochratoxin A in wine samples. Food Chem. 2018, 244, 128–135. [DOI] [PubMed] [Google Scholar]
- (11).Soleas GJ; Yan J; Goldberg DM Assay of ochratoxin A in wine and beer by high-pressure liquid chromatography photodiode array and gas chromatography mass selective detection. J. Agric. Food Chem 2001, 49, 2733–2740. [DOI] [PubMed] [Google Scholar]
- (12).Liu X; Xu Y; He Q; He Z; Xiong Z Application of mimotope peptides of fumonisin B1 in peptide ELISA. J. Agric. Food Chem 2013, 61, 4765–4770. [DOI] [PubMed] [Google Scholar]
- (13).Zhang Y; Wang L; Shen X; Wei X; Huang X; Liu Y; Sun X; Wang Z; Sun Y; Xu Z; Eremin SA; Lei H Broad-specificity immunoassay for simultaneous detection of ochratoxins A, B, and C in millet and maize. J. Agric. Food Chem 2017, 65, 4830–4838. [DOI] [PubMed] [Google Scholar]
- (14).Jiang W; Beier RC; Luo P; Zhai P; Wu N; Lin G; Wang X; Xu G Analysis of pirlimycin residues in beef muscle, milk, and honey by a biotin–streptavidin-amplified enzyme-linked immunosorbent assay. J. Agric. Food Chem 2016, 64, 364–370. [DOI] [PubMed] [Google Scholar]
- (15).Zou X; Chen C; Huang X; Chen X; Wang L; Xiong Y Phage-free peptide ELISA for ochratoxin A detection based on biotinylated mimotope as a competing antigen. Talanta 2016, 146, 394–400. [DOI] [PubMed] [Google Scholar]
- (16).Qiu Y; Li P; Dong S; Zhang X; Yang Q; Wang Y; Ge J; Hammock BD; Zhang C; Liu X Phage-mediated competitive chemiluminescent immunoassay for detecting Cry1Ab toxin by using an anti-idiotypic camel nanobody. J. Agric. Food Chem 2018, 66, 950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Li C; Mi T; Oliveri Conti G; Yu Q; Wen K; Shen J; Ferrante M; Wang Z Development of a screening fluorescence polarization immunoassay for the simultaneous detection of fumonisins B1 and B2 in Maize. J. Agric. Food Chem 2015, 63, 4940–4946. [DOI] [PubMed] [Google Scholar]
- (18).Zhang X; Eremin SA; Wen K; Yu X; Li C; Ke Y; Jiang H; Shen J; Wang Z Fluorescence polarization immunoassay based on a new monoclonal antibody for the detection of the zearalenone class of mycotoxins in maize. J. Agric. Food Chem 2017, 65, 2240–2247. [DOI] [PubMed] [Google Scholar]
- (19).Wu Y; Zeng L; Xiong Y; Leng Y; Wang H; Xiong Y Fluorescence ELISA based on glucose oxidase-mediated fluorescence quenching of quantum dots for highly sensitive detection of Hepatitis B. Talanta 2018, 181, 258–264. [DOI] [PubMed] [Google Scholar]
- (20).Ma M; Wen K; Beier RC; Eremin SA; Li C; Zhang S; Shen J; Wang Z Chemiluminescence resonance energy transfer competitive immunoassay employing hapten-functionalized quantum dots for the detection of sulfamethazine. ACS Appl. Mater. Inter 2016, 8, 17745–17750. [DOI] [PubMed] [Google Scholar]
- (21).Adler-Storthz K; Kendall C; Kennedy RC; Henkel RD; Dreesman GR Biotin-avidin-amplified enzyme immunoassay for detection of herpes simplex virus antigen in clinical specimens. J. Clin. Microbiol 1983, 18, 1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wang L; Zhang Y; Gao X; Duan Z; Wang S Determination of chloramphenicol residues in milk by enzyme-linked immunosorbent assay: Improvement by biotin−streptavidin-amplified system. J. Agric. Food Chem 2010, 58, 3265–3270. [DOI] [PubMed] [Google Scholar]
- (23).Bu D; Zhuang H; Zhou X; Yang G Biotin–streptavidin enzyme-linked immunosorbent assay for detecting tetrabromobisphenol A in electronic waste. Talanta 2014, 120, 40–46. [DOI] [PubMed] [Google Scholar]
- (24).Guesdon JL Immunoenzymatic techniques applied to the specific detection of nucleic acids: A review. J. Immunol. Methods 1992, 150, 33–49. [DOI] [PubMed] [Google Scholar]
- (25).Oyama H; Tanaka E; Kawanaka T; Morita I; Niwa T; Kobayashi N Anti-idiotype scFv–enzyme fusion proteins: A clonable analyte-mimicking probe for standardized immunoassays targeting small biomarkers. Anal. Chem 2013, 85, 11553–11559. [DOI] [PubMed] [Google Scholar]
- (26).Cui X; He Q; Shen D; Jiang Z; Chen Y; Zhao S; Hammock BD Production and characterization of a single-chain variable fragment-alkaline phosphatase fusion protein for glycocholic acid detection in a one-step enzyme-linked immunosorbent assay. Anal. Methods 2018, 10, 2629–2635. [Google Scholar]
- (27).Yu X; Tao X; Shen J; Zhang S; Cao X; Chen M; Wang W; Wang Z; Wen K A one-step chemiluminescence immunoassay for 20 fluoroquinolone residues in fish and shrimp based on a single chain Fv-alkaline phosphatase fusion protein. Anal. Methods 2015, 7, 9032–9039. [Google Scholar]
- (28).Chen M; Ding S; Wen K; Xie S; Wang Q; Pei X; Xie J; Wang Z; Jiang H Development of a fluorescence-linked immunosorbent assay for detection of avermectins using a fluorescent single-domain antibody. Anal. Methods 2015, 7, 3728–3734. [Google Scholar]
- (29).Wang X; Searle AK; Hohmann JD; Liu AL; Abraham M-K; Palasubramaniam J; Lim B; Yao Y; Wallert M; Yu E; Chen Y-C; Peter K Dual-targeted theranostic delivery of miRs arrests abdominal aortic aneurysm development. Mol. Ther 2018, 26, 1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Austerberry JI; Dajani R; Panova S; Roberts D; Golovanov AP; Pluen A; van der Walle CF; Uddin S; Warwicker J; Derrick JP; Curtis R The effect of charge mutations on the stability and aggregation of a human single chain Fv fragment. Eur. J. Pharm. Biopharm 2017, 115, 18–30. [DOI] [PubMed] [Google Scholar]
- (31).Hamers-Casterman C; Atarhouch T; Muyldermans S; Robinson G; Hammers C; Songa EB; Bendahman N; Hammers R Nature 1993, 363, 446–448. [DOI] [PubMed] [Google Scholar]
- (32).Greenberg AS; Avila D; Hughes M; Hughes A; McKinney EC; Flajnik MF A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature 1995, 374, 168–173. [DOI] [PubMed] [Google Scholar]
- (33).Muyldermans S Nanobodies: natural single-domain antibodies. Annu. Rev. Biochem 2013, 82, 775–797. [DOI] [PubMed] [Google Scholar]
- (34).Liu X; Xu Y; Xiong Y; Tu Z; Li Y; He Z; Qiu Y; Fu J; Gee SJ; Hammock BD VHH phage-based competitive real-time immuno-polymerase chain reaction for ultrasensitive detection of ochratoxin A in cereal. Anal. Chem 2014, 86, 7471–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Liu X; Xu Y; Wan D; Xiong Y; He Z; Wang X; Gee SJ; Ryu D; Hammock BD Development of a nanobody-alkaline phosphatase fusion protein and its application in a highly sensitive direct competitive fluorescence enzyme immunoassay for detection of ochratoxin A in cereal. Anal. Chem 2015, 87, 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Liu X; Tang Z; Duan Z; He Z; Shu M; Wang X; Gee SJ; Hammock BD; Xu Y Nanobody-based enzyme immunoassay for ochratoxin A in cereal with high resistance to matrix interference. Talanta 2017, 164, 154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Sun Z; Duan Z; Liu X; Deng X; Tang Z Development of a nanobody-based competitive dot ELISA for visual screening of ochratoxin A in cereals. Food Anal. Methods 2017, 10, 3558–3564. [Google Scholar]
- (38).Tang Z; Wang X; Lv J; Hu X; Liu X One-step detection of ochratoxin A in cereal by dot immunoassay using a nanobody-alkaline phosphatase fusion protein. Food Control 2018, 92, 430–436. [Google Scholar]
- (39).Wang J; Majkova Z; Bever CRS; Yang J; Gee SJ; Li J; Xu T; Hammock BD One-step immunoassay for tetrabromobisphenol A using a camelid single domain antibody–alkaline phosphatase fusion protein. Anal. Chem 2015, 87, 4741–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Wang J; Bever CRS; Majkova Z; Dechant JE; Yang J; Gee SJ; Xu T; Hammock BD Heterologous antigen selection of camelid heavy chain single domain antibodies against tetrabromobisphenol A. Anal. Chem 2014, 86, 8296–8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Tang X; Li P; Zhang Q; Zhang Z; Zhang W; Jiang J Time-resolved fluorescence immunochromatographic assay developed using two idiotypic nanobodies for rapid, quantitative, and simultaneous detection of aflatoxin and zearalenone in maize and its products. Anal. Chem 2017, 89, 11520–11528. [DOI] [PubMed] [Google Scholar]
- (42).Shu M; Xu Y; Liu X; Li Y; He Q; Tu Z; Fu J; Gee SJ; Hammock BD Anti-idiotypic nanobody-alkaline phosphatase fusion proteins: Development of a one-step competitive enzyme immunoassay for fumonisin B1 detection in cereal. Anal. Chim. Acta 2016, 924, 53–59. [DOI] [PubMed] [Google Scholar]
- (43).Bever CRS; Majkova Z; Radhakrishnan R; Suni I; McCoy M; Wang Y; Dechant J; Gee S; Hammock BD Development and utilization of camelid VHH antibodies from alpaca for 2,2′,4,4′-tetrabrominated diphenyl ether detection. Anal. Chem 2014, 86, 7875–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Liu Y; Jiang D; Lu X; Wang W; Xu Y; He Q Phage-mediated immuno-PCR for ultrasensitive detection of Cry1Ac protein based on nanobody. J. Agric. Food Chem 2016, 64, 7882–7889. [DOI] [PubMed] [Google Scholar]
- (45).He T; Wang Y; Li P; Zhang Q; Lei J; Zhang Z; Ding X; Zhou H; Zhang W Nanobody-based enzyme immunoassay for aflatoxin in agro-products with high tolerance to cosolvent methanol. Anal. Chem 2014, 86, 8873–8880. [DOI] [PubMed] [Google Scholar]
- (46).Wang Y; Wang H; Li P; Zhang Q; Kim HJ; Gee SJ; Hammock BD Phage-displayed peptide that mimics aflatoxins and its application in immunoassay. J. Agric. Food Chem 2013, 61, 2426–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


