Abstract
Background.
Population-based studies of women with epithelial ovarian cancer suggest that black women have worse survival compared to white women. The primary objective of this study was to determine if, at a National Cancer Institute (NCI)-Designated Comprehensive Cancer Center (CCC) serving a diverse racial and socioeconomic population, race is independently associated with differences in survival.
Methods.
A retrospective review of women with EOC diagnosed between 2004–2009 undergoing treatment with follow-up at our institution was performed.
Records were reviewed for demographics, comorbidities (as defined by the Charlson Comorbidity Index (CCI)), tumor characteristics, treatment, progression-free (PFS), and overall survival (OS). Survival was calculated using the Kaplan-Meier method and compared with the log-rank test. Multivariate survival analysis was performed with Cox (proportional hazards) model.
Results.
367 patients met inclusion criteria. 54 (15%) were black and 308 (84%) were white. Compared to white women, black women had higher BMI, lower rates of optimal surgical cytoreduction, lower rates of intraperitoneal chemotherapy, and higher CCI scores. The median PFS for black and white women were 9.7 and 14.6 months, respectively (p = 0.033). The median overall survival was 21.7 months for black women and 42.6 months for white women (p < 0.001). On multivariate analysis, black race independently correlated with a worse overall survival (HR 1.61, 95% CI 1.06–2.43).
Conclusion.
In this cohort, racial disparities may be due to higher medical comorbidities and lower rates of optimal surgical cytoreduction. After accounting for these differences, race remained an independent predictor of worse overall survival.
Keywords: Epithelial ovarian cancer, Racial disparities, Comorbidities
1. Background
Epithelial ovarian cancer (EOC) is the most deadly gynecologic malignancy due to its frequent presentation at an advanced stage and high risk of recurrence following front-line therapy [1]. While white women have a higher incidence of ovarian cancer compared to black women (12.9 per 100,000 versus 9.5 per 100,000) [2], black women have worse survival outcomes. This survival difference appears to be independent of tumor stage, grade, and histology [3–5]. Data is lacking on patient-specific factors that may strongly affect survival outcomes.
We sought to further understand the nature of these racial disparities in women with EOC by closely examining patient, tumor, and treatment characteristics not typically captured in population-based studies. The primary objective of this study is to determine if, at a National Cancer Institute (NCI)-Designated Comprehensive Cancer Center (CCC) that serves as a state-wide referral center for a diverse racial and socioeconomic population, race is independently associated with differences in survival.
2. Methods
This was a retrospective cohort study designed to determine if there was a difference in progression-free survival (PFS) and overall survival (OS) between black and white women at our institution. Eligible subjects included women diagnosed with any stage of EOC between 2004 and 2009. Subjects were identified through the UAB hospital tumor registry, which captures all new cancer diagnoses seen within the UAB health system. To be included in this analysis, patients had to receive primary treatment with follow-up at our institution. All records were reviewed for demographics, medical comorbidities, tumor characteristics, treatment, cancer progression and death. Race was determined by self-identification from the patient’s intake medical forms. Comorbidities were quantified using the Charlson Comorbidity Index (CCI), which is a validated predictor of hospital mortality [6]. The CCI includes 19 medical conditions that are weighted based on their association with hospital mortality. Because all patients had an invasive malignancy, we did not include “metastatic solid tumor” as part of each patient’s score. For our analysis, we separated patients into 3 categories based on CCI: 0, 1, or 2+. To avoid bias, an independent investigator who was not aware of the study’s endpoints extracted each patient’s CCI from their medical record. PFS was calculated from the time of initiation of chemotherapy until disease recurrence or progression according to clinical assessment, rising CA-125, or radiographic evidence of recurrence. Overall survival was calculated from initiation of chemotherapy until last known follow-up or death from any cause. The study was carried out in accordance with the standards of the Institutional Human Subjects Protection Review Board at the University of Alabama at Birmingham (UAB).
Chi-square (χ2) test or Fisher’s exact test for categorical variables and the t-test or Wilcoxon rank sum test for continuous variables were used to compare the difference between white and black patients. Continuous variables were expressed as median ± standard deviation unless stated otherwise. Kaplan-Meier (KM) estimates were performed to detect the difference of the survival curves for white and black patients; the results reported are based on log-rank test. Multivariate survival analysis was performed with the Cox (proportional hazards) model adjusting for age, race, stage, histology, grade, cytoreduction, CCI and BMI. A value of p < 0.05 was considered statistically significant. All analyses were performed using SAS statistical software, version 9.3.55.
3. Results
367 patients met inclusion criteria. 54 (15%) were black, 308 (84%) were white, and 5 (1%) were other races and were excluded from further analysis. The majority of women were treated with primary surgical resection and post-operative chemotherapy, while only 16 patients (4%) received neoadjuvant chemotherapy. Compared to white women, black women had higher BMI (29.8 vs. 27.8, p = 0.02), lower rates of optimal surgical cytoreduction (54% vs. 76%, p = 0.002), and lower rates of intraperitoneal (IP) chemotherapy (0% vs. 9%, p = 0.013). Tumor histology also varied between the two groups with greater percentages of clear cell, endometrioid and mucinous tumors being present in black women. Age, stage, and grade did not vary significantly between races. Although the most common CCI in both groups was 0, black women were more likely to have a CCI of 1 or 2+ (p = 0.002). Full clinical and demographic factors are displayed in Table 1.
Table 1.
Comparison of clinical and demographic factors between black and white women with epithelial ovarian cancer.
| Black (N = 54) | White (N = 308) | p-Value | |
|---|---|---|---|
| Age (Std dev) | 62.7 (11.8) | 63.7 (11.9) | NSa |
| BMI (Std dev) | 29.8 (5.8) | 27.7 (6.3) | 0.02 |
| Grade (N, %) | NS | ||
| 1 | 2 (4%) | 8 (3%) | |
| 2 | 14(26%) | 54(18%) | |
| 3 | 34 (63%) | 226 (73%) | |
| Unknown | 4 (7%) | 20 (6%) | |
| Stage (N, %) | NS | ||
| 1 | 4 (7%) | 19 (6%) | |
| 2 | 8 (15%) | 53 (17%) | |
| 3 | 35 (65%) | 209 (68%) | |
| 4 | 7 (13%) | 27 (9%) | |
| Histology (N, %) | 0.001 | ||
| Papillary serous | 33 (61%) | 206 (67%) | |
| Clear cell | 2 (4%) | 10 (3%) | |
| Endometrioid | 7 (13%) | 33 (11%) | |
| Mucinous adenocarcinoma | 7 (13%) | 5 (2%) | |
| NOS | 0 (0%) | 10 (3%) | |
| Other (mixed, signet ring) | 5 (9%) | 44(14%) | |
| Charlson Comorbidity Index | 0.002 | ||
| 0 | 22 (41%) | 199(65%) | |
| 1 | 20 (37%) | 79 (26%) | |
| 2+ | 12(22%) | 30 (10%) | |
| Cytoreduction status | 0.002 | ||
| <1 cm (optimal) | 29 (54%) | 233 (76%) | |
| >1cm (suboptimal) | 25 (46%) | 73 (23%) | |
| Unknown | 0 (0%) | 2 (1%) | |
| IP chemotherapy | 0.002 | ||
| Yes | 0 (0%) | 28 (9%) | |
| No | 54 (100%) | 280 (91%) | |
| Neoadjuvant chemotherapy | NS | ||
| Yes | 1 (2%) | 15 (5%) | |
| No | 53 (98%) | 293 (95%) |
NS = not significant.
PFS and OS varied significantly between races. The median PFS for black and white women were 9.7 and 14.6 months, respectively (p = 0.036) (Fig. 1A), while the median overall survival was 21.7 months for black women and 42.6 months for white women (p b 0.005) (Fig. 1B). On multivariate analysis, race did not independently influence the PFS, although it did significantly increase the hazard ratio for OS (HR 1.61, 95% CI 1.06–2.43). Factors that independently affected PFS and OS in this model included age, stage, CCI and cytoreduction status (Tables 2, 3). A CCI of 2 or greater showed a 30% increased risk of suboptimal surgical cytoreduction, although this did not reach statistical significance (RR 1.3, 95% CI 0.82–2.1). When stratified by cytoreduction status, white women again had higher overall survival compared to black women after both optimal (54.6 vs. 32.7 months, p = 0.004) and suboptimal (20.7 vs. 12.4 months, p = 0.004) cytoreduction.
Fig. 1.
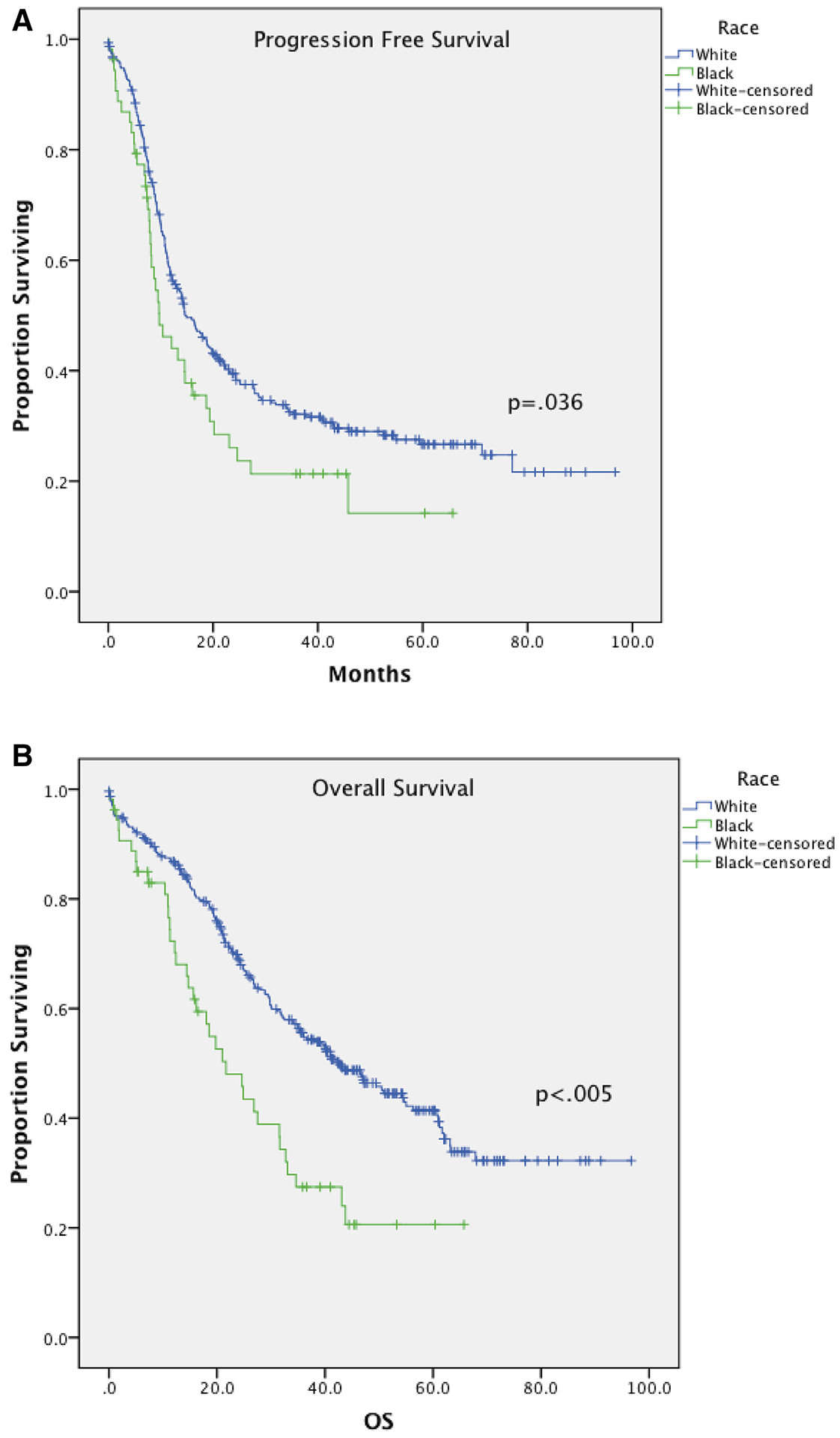
A: Progression free survival stratified by race. B: Overall survival stratified by race
Table 2.
Adjusted progression free survival analysis (selected variables only).
| Variable | Hazard ratio | 95% CI | p value |
|---|---|---|---|
| Black race | 1.21 | 0.82–1.77 | 0.339 |
| Age | 1.02 | 1.00–1.03 | 0.019 |
| Stage | 1.83 | 1.44–2.33 | <0.001 |
| CCIa | 1.16 | 1.04–1.28 | 0.007 |
| Suboptimal cytoreduction | 1.58 | 1.17–2.14 | 0.003 |
CCI - Charlson Comorbidity Index.
Table 3.
Adjusted overall survival analysis (selected variables only).
| Variable | Hazard ratio | 95% CI | p value |
|---|---|---|---|
| Black race | 1.61 | 1.06–2.43 | 0.024 |
| Age | 1.02 | 1.01–1.04 | 0.005 |
| Stage | 1.74 | 1.32–2.29 | <0.001 |
| CCIa | 1.19 | 1.07–1.34 | 0.002 |
| Suboptimal cytoreduction | 2.07 | 1.48–2.89 | <0.001 |
CCI - Charlson Comorbidity Index.
4. Discussion
Racial disparities in ovarian cancer outcomes are complex and multifactorial, and are likely influenced by differences in medical comorbidities, tumor biology, access to and receipt of guideline-adherent care, and sociocultural factors. In this retrospective cohort study, we found that black women with EOC had worse PFS and OS compared to white women. This difference was not subtle: median PFS was almost 5 months shorter for black women and median OS was almost twice as long for white women compared to black women (42.6 vs. 21.7 months). The reasons for these survival differences are multifactorial. Compared to white women, black women were more likely to have more comorbidities and a higher BMI. They were less likely to undergo optimal cytoreductive surgery and less likely to receive IP chemotherapy. When accounting for these variables in multivariate analysis, race was not an independent predictor of PFS although it did still correlate with worse OS.
Although it has been known for over a decade that there are racial disparities in EOC survival, few detailed analyses of differences that impact outcomes between groups exist. Our data support findings by Ross et al., who similarly found that black women in the Deep South have lower rates of optimal cytoreduction and IP chemotherapy, with poorer outcomes even after accounting for socioeconomic status [7]. In an analysis of the National Cancer Data Base, Bristow et al. demonstrated that black race was independently associated with NCCN guideline non-adherence and worse overall survival [8]. Utilizing another large database encompassing patients from nine states, Goff et al. found that comprehensive surgical staging was less likely in black and Hispanic women compared to white women, even when controlling for annual house-hold income [9]. Bristow et al. found similar results in an analysis of a Maryland health services database [10]. Regarding chemotherapy, Wright and colleagues analyzed the SEER database and found that black race was an independent risk factor for delayed chemotherapy [11]. These analyses suggest that treatment rendered may be different between races, although the question regarding why treatment differences occur still remain.
In our study, we found multiple differences between black and white women with EOC, which may play a role in survival disparities. First, we observed a dramatic difference in rates of optimal cytoreduction, which has been well established to affect survival [12,13]. Additionally, we found that both BMI as well as the CCI were higher in black compared with white women with ovarian cancer. Interestingly, CCI score did not directly correlate with cytoreduction status. Women with a comorbidity score of zero were no more likely to be optimally surgically cytoreduced compared to women with a score of 2 or greater. It remains unclear from our data why rates of optimal cytoreduction are so different between races. We hypothesize that it may be due to more extensive tumor burden in black compared to white women or to more patient comorbidities that are not necessarily captured in the CCI that may have affected the level of surgical aggressiveness. However, even when compared to white women with similar cytoreduction status, black women still had significantly shorter OS in both the optimal and suboptimal groups.
A strength of our study is the use of detailed data to evaluate the impact of medical comorbidities, BMI and specific treatment on patient outcomes. Furthermore, the patients in this study all received primary treatment in a CCC with access to fellowship-trained providers as well as clinical trials. As with other retrospective data both bias and confounding are potential weaknesses in our study, as is the small sample size. Specifically, decisions regarding therapy may not be accurately captured in the medical record including the rationale for non-utilization of IP therapy or exact reasons for suboptimal surgical cytoreduction. For example, patients who lived outside of the metro area may have opted to receive traditional IV chemotherapy every three weeks instead of the IP regimen which required more visits to our institution. Administration of neoadjuvant chemotherapy (NAC) was uncommon during the study period, and there was not a difference in the rate of NAC between groups. Additional data is needed to capture differences in administration of NAC since 2009, given new guidance in support of this practice [14]. Due to limitations in data we did not take SES into account in our analysis, however as mentioned above, racial disparities persist despite adjustment for factors related to SES therefore this may not have impacted our findings.
In conclusion, our data demonstrate and corroborate observations from others that black women have worse survival than white women when treated for EOC. These outcomes are consistent with a large body of data that has shown racial disparities in ovarian cancer, not only related to socioeconomic and health-related risk factors, but also to inequity in treatment based on race. Our study highlights both the importance of recognizing disparities in outcomes and the importance of recognizing actionable factors that may contribute to these disparities. As a result of these observations, we performed a quality improvement project at our institution which utilized a checklist for providers to assess perioperative morbidity and to help reinforce decisions regarding planned cytoreduction versus triage to neoadjuvant chemotherapy and clinical trial enrollment [15]. By using a standard preoperative algorithm for all patients, our project increased our rate of optimal surgical cytoreduction. As demonstrated by our work and that of many others, racial differences in the treatment of ovarian cancer is one contributor to poorer outcomes for black women, and is a clear area for improvement. It is no longer enough to describe the disparities in health outcomes for women with gynecologic cancers. Going forward we must emphasize the provider’s role in ensuring health equity for all patients, and incorporate this into every facet of education, research and clinical care.
HIGHLIGHTS.
Black women with ovarian cancer have shortened PFS and OS compared to white women.
Black women have more comorbidities as measured by the Charlson Comorbidity Index.
Rates of optimal surgical cytoreduction are higher in white women.
These differences do not account for all differences in survival between races.
Footnotes
Conflict of interest statement
Funding support was provided in part by NIH 3P30CA013148–43S3 and 5K12HD0012580–15 to Dr. Leath. All other authors declare that they have no conflicts of interest.
References
- [1].Siegel R, Naishadham D, Jemal A, Cancer statistics, 2013, CA Cancer J. Clin 63 (1) (2013) 11–30. [DOI] [PubMed] [Google Scholar]
- [2].American Cancer Society, Cancer Facts and Figures 2012, American Cancer Society, Atlanta, GA, 2013. [Google Scholar]
- [3].Chan JK, Zhang M, Hu JM, Shin JY, Osann K, Kapp DS, Racial disparities in surgical treatment and survival of epithelial ovarian cancer in United States, J. Surg. Oncol 97 (2) (2008) 103–107. [DOI] [PubMed] [Google Scholar]
- [4].Barnholtz-Sloan JS, Tainsky MA, Abrams J, Severson RK, Qureshi F, Jacques SM, et al. , Ethnic differences in survival among women with ovarian carcinoma, Cancer 94 (6) (2002. March 15) 1886–1893. [DOI] [PubMed] [Google Scholar]
- [5].Terplan M, Schluterman N, McNamara EJ, Tracy JK, Temkin SM, Have racial disparities in ovarian cancer increased over time? An analysis of SEER data, Gynecol. Oncol 125 (1) (2012) 19–24. [DOI] [PubMed] [Google Scholar]
- [6].Charlson ME, Pompei P, Ales KL, MacKenzie CR, A new method of classifying prognostic comorbidity in longitudinal studies: development and validation, J. Chronic Dis 40 (5) (1987) 373–383. [DOI] [PubMed] [Google Scholar]
- [7].Ross J, Braswell KV, da Silva LM, et al. , Unraveling the etiology of ovarian cancer racial disparity in the deep south: is it nature or nurture? Gynecol. Oncol 145 (2) (2017) 329–333. [DOI] [PubMed] [Google Scholar]
- [8].Bristow RE, Powell MA, Al-Hammadi N, et al. , Disparities in ovarian cancer care quality and survival according to race and socioeconomic status, J. Natl. Cancer Inst 105 (11) (2013) 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Goff BA, Matthews BJ, Larson EH, Andrilla CH, Wynn M, Lishner DM, et al. , Predictors of comprehensive surgical treatment in patients with ovarian cancer, Cancer 109 (10) (2007) 2031–2042. [DOI] [PubMed] [Google Scholar]
- [10].Bristow RE, Zahurak ML, Ibeanu OA, Racial disparities in ovarian cancer surgical care: a population-based analysis, Gynecol. Oncol 121 (2) (2011) 364–368. [DOI] [PubMed] [Google Scholar]
- [11].Wright J, Doan T, McBride R, Jacobson J, Hershman D, Variability in chemotherapy delivery for elderly women with advanced stage ovarian cancer and its impact on survival, Br. J. Cancer 98 (7) (2008) 1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bristow RE, Chi DS, Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis, Gynecol. Oncol 103 (3) (2006) 1070–1076. [DOI] [PubMed] [Google Scholar]
- [13].Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ, Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis, J. Clin. Oncol 20 (5) (2002) 1248–1259. [DOI] [PubMed] [Google Scholar]
- [14].Wright AA, Bohlke K, Armstrong DK, et al. , Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of clinical oncology clinical practice guideline, J. Clin. Oncol 34 (28) (2016) 3460–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shah MM, Leath CA, Daily LR, McGwin G, Estes JM, Alvarez RD, Straughn JM, Does a standardized preoperative algorithm of clinical data improve outcomes in patients with ovarian cancer? A quality improvement project, Int. J. Gynecol. Cancer 25 (5) (2015) 798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]


