Abstract
Acute lung injury (ALI) is a severe inflammatory lung disease with a rapid onset. The anti-inflammatory functions of microRNA-93 (miRNA/miR-93) have been described in various types of tissue injury and disease. However, the biological role of miR-93 and its molecular mechanisms underlying the initiation and progression of ALI have not yet been reported, at least to the best of our knowledge. The present study aimed to investigate the regulatory effects exerted by miR-93 in ALI. Using an in vivo murine model of ALI induced by lipopolysaccharide (LPS), miR-93 expression was found to be downregulated in the lung tissues and bronchoalveolar lavage fluid (BALF) compared with the control group. Following agomiR-93 injection, it was observed that agomiR-93 attenuated lung injury, as evidenced by decreased lung permeability, a reduced lung wet/dry weight ratio and an increased survival rate of the mice. Concomitantly, agomiR-93 significantly reduced LPS-induced the interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α levels in BALF. Of note, Toll-like receptor 4 (TLR4), an upstream regulator of the nuclear factor (NF)-κB signaling pathway, was directly suppressed by miR-93 in RAW 264.7 cells. Importantly, agomiR-93 induced a significant suppression of the TLR4/myeloid differentiation primary response 88 (MyD88)/NF-κB signaling pathway, as demonstrated by the downregulation of MyD88, and the phosphorylation of IκB-α and p65 in the lung tissues of mice with ALI. Taken together, the findings of the present study indicate that miR-93 attenutes LPS-induced lung injury by regulating the TLR4/MyD88/NF-κB signaling pathway, suggesting that miR-93 may prove to be a potential therapeutic target for ALI.
Keywords: acute lung injury, microRNA-93, inflammation, Toll-like receptor 4/myeloid differentiation primary response 88/nuclear factor-κB signaling pathway
Introduction
Acute lung injury (ALI) is a severe type of lung disease that is associated with a high mortality rate in critically ill patients (1,2). However, even though supportive treatments have been developed, there is still an urgent need for the development of novel therapeutic strategies for the treatment of patients with ALI (3). An excessive inflammatory response in the lung tissues is a main risk factor for the initiation and development of ALI (4). Therefore, the effective inhibition of inflammation may be beneficial for improving the current therapeutic approaches for the management of ALI.
Lipopolysaccharide (LPS), the active component of bacterial endotoxin, is an important pathogenic factor in the progression of ALI (5,6). It has been reported that LPS can induce extensive damage and the inflammatory reaction of neutrophils, and has been widely used to mimic ALI in vitro and in vivo (7,8). A number of studies have demonstrated that the LPS-induced inflammatory response is regulated by Toll-like receptor 4 (TLR4) (9). In ALI, TLR4 triggers the activation of the nuclear factor (NF)-κB signaling pathway and promotes the secretion of pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6, finally resulting in damage to the lung tissue (10-13). Extensive research has revealed that the silencing of TLR4 can reduce the expression of the intracellular adaptor protein, myeloid differentiation primary response 88 (MyD88), and can decrease NF-κB activity, leading to the inhibition of pro-inflammatory cytokines in ALI (14). Thus, the proper regulation of the TLR4/NF-κB pathway may prove to be an effective strategy with which to supress the development of ALI.
MicroRNAs (miRNAs or miRs) are a family of short, small, non-coding RNAs (an average size of 22 nucleotides), which negatively regulate target gene expression through either translation repression or RNA degradation (15). The potential regulatory role of miRNAs in lung diseases via the regulation of crucial factors or key pathways, has been widely reported, including in ALI (16). For example, Ling et al demonstrated that the inhibition of miR-494 attenuated the inflammatory response in rats with ALI through the NAD(P)H quinone dehydrogenase 1 (NQO1)-mediated nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway (17). Another study demonstrated that the enforced expression of miR-125b attenuated the development of ALI using the LPS-induced model (18). These studies suggest that targeting miRNAs may be beneficial for the suppression of the development of ALI. Recently, miR-93, a member of the miR-17 family, was reported to be involved in the regulation of the inflammatory response in various types of tissue injury and diseases (19-21), in addition to its anti-tumor effects. However, little attention has been paid to the function of miR-93 in the inflammatory response during ALI.
Thus, the present study investigated the expression of miR-93 in a mouse model of ALI induced by LPS. The role of miR-93 in the regulation of the inflammatory response during ALI was further investigated. The findings presented herein suggest that miR-93 may prove to be a novel therapeutic strategy for ALI.
Materials and methods
Animals
All animal experiments were performed according to the guidelines of the Chinese Council on Animal Care and ethical approval was obtained for the use of animals prior to the commencement of the study from the Ethics Committee of the West China Second University Hospital, Sichuan University. Male BALB/c mice (weighing 18-22 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. The BALB/c mice were housed under standard conditions (12-h light/dark cycle, temperature of 25-27°C and a humidity of ~40%) with free access to food and water throughout the duration of the experiments. All mice were randomly divided into 4 groups (n=6/group) as follows: i) The control group; ii) the LPS group; iii) the LPS + agomir-93 group; and iv) the LPS + agomir-NC group. In the control group, mice were injected intravenously (via the tail vein) with an intra-tracheal instillation of 2 mg/kg normal saline (NS); in the LPS group, mice were injected intravenously (via the tail vein) with an intra-tracheal instillation of 2 mg/kg LPS; in the agomir-93 or agomir negative control (NC) groups, mice were injected intravenously (via the tail vein) with agomir-93 or agomir NC [8 mg/kg (22), RiboBio Co., Ltd.] at 24 h prior to the injection of 2 mg/kg LPS. Pentobarbital sodium (50 mg/kg, intraperitoneal injection) was used for anesthesia prior to each surgical procedure, and all efforts were made to minimize animal suffering. No mice were found dead during the process of anesthesia. If an animal reached the defined humane endpoints [a body weight loss of >15% in 1-2 days or an overall body weight loss of >20%, or displayed obvious signs of suffering (e.g., lethargy, squinted eyes, dehydration or hunched back)], it was humanely euthanized. Sacrifice was performed by an intraperitoneal injection of pentobarbital sodium (50 mg/kg) followed by cervical dislocation, and mortality was confirmed by observation (23) and the lung tissues and bronchoalveolar lavage fluid (BALF) were then collected for subsequent analysis.
In addition, the survival experiments were performed in 4 groups of mice (n=10/group) (LPS, Control, LPS + agomir-93 and LPS + agomir-NC). If an animal reached the defined humane endpoints mentioned above, it was humanely euthanized. The remaining mice were humanely euthanized 96 h later. The survival rate of the mice was observed from 0 to 96 h using the Kaplan Meier method.
RT-qPCR analysis
Total RNA was isolated from the lung tissues or BALF using the miRNeasy mini kit (Qiagen, Inc.). The reverse transcription of miR-93 was performed using the miScript II RT kit and the reverse transcription kit (Invitrogen; Thermo Fisher Scientific, Inc.), respectively. miR-93 expression was measured using Exiqon SYBR-Green Master Mix (Exiqon) on a Light Cycler instrument (Bio-Rad Laboratories, Inc.). The reaction mixtures were denatured at 95°C for 3 min, followed by 40 two-step cycles of 95°C for 10 sec and 60°C for 30 sec. The primers for RT-qPCR analysis were as follows: miR-93 forward, 5′-AGG CCC AAA GTG CTG TTC GT-3′ and reverse, 5′-GTG CAG GGT CCG AGG T-3′; U6 forward, 5′-TGC GGG TGC TCG CTT CGC AGC-3′ and reverse, 5′-CCA GTG CAG GGT CC GAG GT-3′. miRNA relative expression levels were analyzed using the 2−ΔΔcq method (24) and determined by normalization to U6.
Histopathological evaluation of lung tissues
At 24 h after the LPS challenge, the mice were sacrificed and the left lung tissues of mice were obtained and fixed in in 4% (v/v) paraformaldehyde, embedded in paraffin, sectioned at 4 µm thickness. Then, the fixed lung tissues were stained with hematoxylin and eosin (H&E) for the evaluation of the severity of lung injury. Images were captured using a microscope (Nikon Corp.), and the lung injury score was assessed by two pathologists as previously described (25). In brief, i) the thickness of alveolar walls; ii) infiltration of inflammatory cells; iii) hemorrhage; and iv) alveolar congestion were graded according to the following scale: 0, no damage; 1, mild damage; 2, moderate damage; 3, severe damage; 4, maximal damage.
Evaluation of lung permeability
Pulmonary capillary permeability was assessed using the Evans blue (EB) dye extravasation method, as previously described (26). Briefly, EB dye (20 mg/kg, Sigma-Aldrich; Merck KGaA) in normal saline was injected into the mice in each group (the Control group; the LPS group; the LPS + agomir-93 group; the LPS + agomir-NC group; n=6/group) through via the tail vein prior to the termination of the experiment. The mice were sacrificed after dye injection at 2 h, and EB dye was then extracted using formamide for 18 h at 60°C and the absorbance was measured at 620 nm. The dye concentration was reported as the ratio of absorbance relative to the amount of dry lung tissue (µg/100 mg).
Lung wet/dry (W/D) ratio
The lung W/D ratio was used to calculate pulmonary edema. The right lungs of the mice were obtained and immediately weighted, then placed in an incubator at 80°C for 60 h to obtain the dry weight.
Measurement of IL-6, IL-1β and TNF-α levels
The concentrations of IL-6 (ab100712), IL-1β (ab197742) and TNF-α (ab100747) in BALF were analyzed using ELISA kits (Abcam) according to the kit instructions.
Immunohistochemical staining
Immunohistochemistry (IHC) and IHC scoring systems were performed as described previously (27,28). 4-µm-thick formalin-fixed, paraffin-embedded serial sections of lung tissue were blocked and then incubated with a primary antibody specific for nuclear phosphorylated (p-)p65 (1:4,000 dilution; ab53489, Abcam) in 10% rabbit serum overnight at 4°C. The sections were then treated with a secondary antibody conjugated to horseradish peroxidase (1:10,000 dilution; ab7090, Abcam) overnight at 4°C. After the colorimetric reaction was completed, the sections were observed under a light microscope (Eclipse E100, Nikon; ×40 magnification). IHC scores are calculated as follows: IHC score=1× (number of weakly stained cells in the field) + 2× (number of moderately stained cells in the field) + 3× (number of intensely stained cells in the field) (29).
Cell culture and transfection
RAW 264.7 macrophages are widely used as a model of LPS-induced ALI, and play an essential role in the regulation of the inflammatory response (30,31). Therefore, RAW 264.7 cells were selected for use in an ex vivo experiment as the present study focused on the ALI-induced inflammatory response. The RAW264.7 cell line was obtained from ATCC. The cells were maintained in DMEM supplemented with 10% FBS (Sigma-Aldrich; Merck KGaA), 1% penicillin and streptomycin (Sigma-Aldrich; Merck KGaA) at 37°C and a 5% CO2 incubator.
When the RAW264.7 cells in a 6-well plate grown to approximately 80% confluency, miR-93 mimics (20 nmol/l) and miR-93 inhibitor (20 nmol/l) were transfected into the cells at 37°C for 24 h, using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). miR-93 mimics, miR-93 inhibitor and the corresponding control vectors were purchased from RiboBio Co., Ltd.
Luciferase assay
miRNA target prediction tools, including miRanda (https://miranda.org.uk/) and TargetScan Release 7.0 (http://targetscan.org/) were used to search for the putative targets of miR-93. The dual-luciferase reporter assay was performed as described previously (32). Briefly, partial sequences of TLR4 3'-UTR containing miR-93 binding sites was amplified by PCR and cloned into the dual-luciferase reporter vector (pmirGLO; Promega Corporation) (wild-type pmirGLO-TLR4-3'-UTR, wt). Mutation of the predicted binding site (mutation pmirGLO-TLR4-mut-3'-UTR) was generated using the QuikChange Site-Directed Mutagenesis kit (Stratagene; Agilent Technologies, Inc.) according to the manufacturer's instructions. RAW264.7 cells were co-transfected with miR-93 mimics, miR-93 inhibitor and the luciferase reporter plasmids using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). At 48 h post-transfection, luciferase activities were detected using the dual luciferase reporter kit (Beyotime Institute of Biotechnology) with Renilla luciferase activity as an internal control.
Western blot analysis
Western blot analysis was performed as previously described (32,33). Total protein was extracted using RIPA buffer (Beyotime Institute of Biotechnology). The extraction and isolation of nuclear and cytoplasmic proteins were performed using the Nuclear and Cytoplasmic Protein Extraction kit (Beyotime Institute of Biotechnology). The concentrations of total cellular protein were determined using a BCA assay kit (Pierce; Thermo Fisher Scientific, Inc.). Briefly, 40 µg extracted protein samples were transferred onto polyvinylidene difluoride (PVDF, Millipore) membranes and then blocked with 5% skim milk at 4°C overnight. Each membrane was then probed with primary antibodies against TLR4 (1:2,000, ab9870), nuclear p-p65 (1:1,500, ab53489), p65 (1:2,000, ab86299), p-IκBα (1:2,000, ab133462), IκBα (1:2,000, ab32518), MyD88 (1:1,000, ab199247), Histone 3 (1:1,000, ab1791) and β-actin (1:1,000, ab8226) (all from Abcam) at 4°C overnight, followed by HRP-conjugated goat anti-rabbit IgG (1:10,000; cat. no. ab205718; Abcam). The protein bands were developed using an ECL kit (GE Healthcare) and blot bands were quantified using ImageJ software version 1.46 (Rawak Software, Inc.).
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc.) was used to perform the statistical analyses. Each experiment was performed at least 3 times. Numerical data are presented as the means ± SD. Statistical significance between 2 groups was determined using a Student's t-test. Comparisons among multiple groups were performed using one-way analysis of variance (ANOVA) with Tukey's post hoc test. The Kaplan-Meier method was performed to calculate the survival rates, and multiple comparisons were made using the Bonferroni method. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-93 is downregulated in mice with ALI
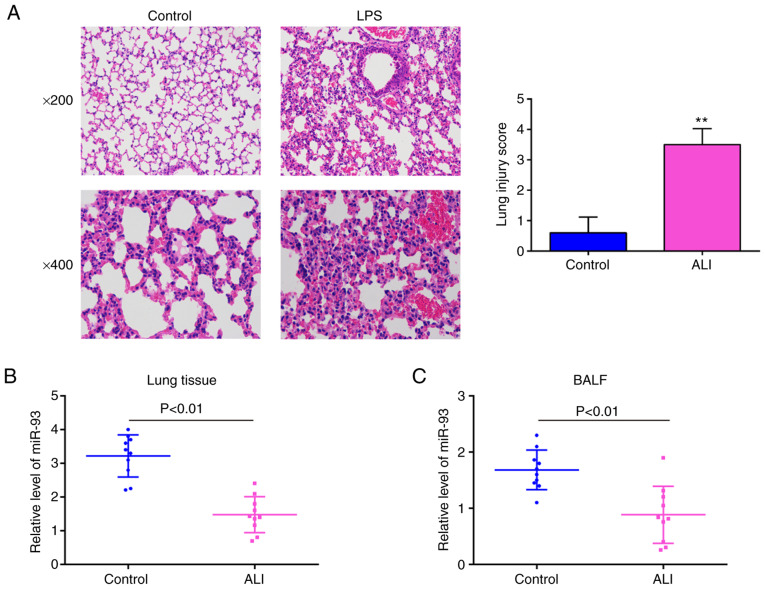
As is known, the mouse model of LPS-induced ALI is widely used to simulate the pathological conditions of lung injury in vivo (34). Following the injection of mice with LPS (2 mg/kg) for 24 h, lung histopathological changes were evaluated. As shown in Fig. 1A, the lungs of the mice in the ALI group displayed significant pathological changes, including obvious inflammatory cell infiltration and widespread alveolar wall thickness, compared with that in the control group. Moreover, lung injury was scored using a semi-quantitative histopathological score system, and the lung injury score was significantly increased in the ALI group, compared with that in the control group. The above-mentioned data indicated that the mouse model of ALI was successfully established. Subsequently, the levels of miR-93 in the lung tissues and in BALF from the mice with ALI were examined by RT-qPCR. It was observed that the expression levels of miR-93 were significantly downregulated in the lung tissues and in BALF, compared with those in the control group (Fig. 1B and C). These data suggest that miR-93 is involved in the pathogenesis of ALI.
Figure 1.
miR-93 is downregulated in mice with LPS-induced ALI. Mice (n=6 in each group) were treated with a single dose of LPS (2 mg/kg) or normal saline and total RNA was isolated from their lungs 24 h following treatment. (A) Staining images of lung tissues following hematoxylin and eosin staining and the pathological changes in lung tissues were evaluated semi-quantitatively based on a histological examination. Original magnification, ×200 and ×400. Data are the means ± SD (n=3) of one representative experiment, **P<0.01 vs. the control group. (B and C) miR-93 expression was determined by RT-qPCR in lung tissues and BALF of mice challenged with LPS (n=10). P<0.01 vs. the control group. LPS, lipopolysaccharide; ALI, acute lung injury.
miR-93 attenuates LPS-induced ALI in mice
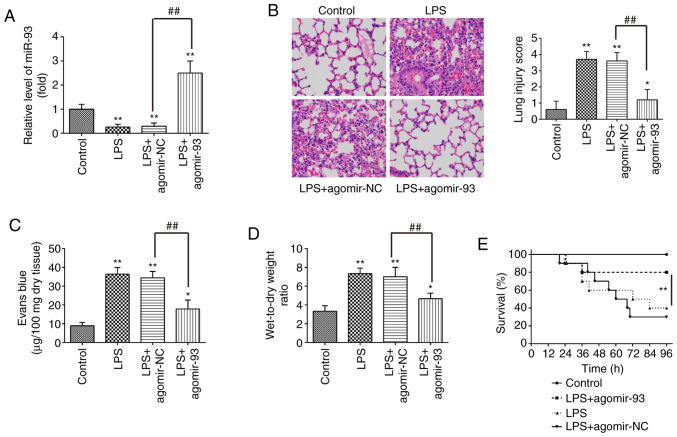
To further investigate the roles of miR-93 in LPS-induced ALI, the mice were intravenously injected with agomir-93 or agomir NC prior to LPS administration. As shown in Fig. 2A, miR-93 expression in the lung tissues from mice with ALI was notably increased compared with that in the agomir NC group. Subsequently, H&E staining was carried out to detect the inflammatory status in the lungs. The results revealed that the agomir-93 injection markedly attenuated the histopathological lesions, compared with those in the ALI group (Fig. 2B). Lung micro-vascular permeability was assessed by EB extravasation. As shown in Fig. 2C, pulmonary permeability was significantly increased in the ALI group, compared with that in the control group, whereas the increased pulmonary permeability was markedly alleviated after the agomir-93 injection. The lung W/D ratio was evaluated to indicate pulmonary edema. It was found that the lung W/D ratio was significantly increased in the ALI group, compared with that in the control group, whereas the increased lung W/D ratio was markedly decreased after the agomir-93 injection (Fig. 2D). In addition, only 40% of the mice administered LPS survived up to 96 h, whereas 80% of the mice that were injected with agomiR-93 survived following exposure to LPS (Fig. 2E). Collectively, these results indicate that agomir-93 injection was able to improve LPS induced lung injury.
Figure 2.
miR-93 attenuates lung injury in mice with LPS-induced ALI. Mice were injected intravenously with agomir-93 and control agomir (8 mg/kg) 24 h prior to the LPS (2 mg/kg) challenge. Following the LPS administration for 24 h, the mice were sacrificed and the lung tissues were collected for analysis. (A) miR-93 expression was assessed in lung tissues in the model of ALI induced by LPS (n=6 in each group). (B) Staining images of lung tissues following hematoxylin and eosin staining and the pathological changes in lung tissues were evaluated semi-quantitatively based on a histological examination (n=6 in each group). Original magnification, ×100. (C) Lung permeability was assessed using the Evans blue dye extravasation method (n=6 in each group). (D) The lung W/D ratio was determined to evaluate pulmonary edema at 24 h after the LPS challenge (n=6 in each group). Data are the means ± SD (n=3) of one representative experiment, *P<0.05, **P<0.01 vs. the control group. ##P<0.01 vs. the LPS + agomir-NC group. (E) Survival rates were determined using the Kaplan-Meier method. **P<0.01 vs. the LPS alone group. LPS, lipopolysaccharide; ALI, acute lung injury; W/D, wet/dry ratio.
miR-93 suppresses the LPS-induced inflammatory response in mice
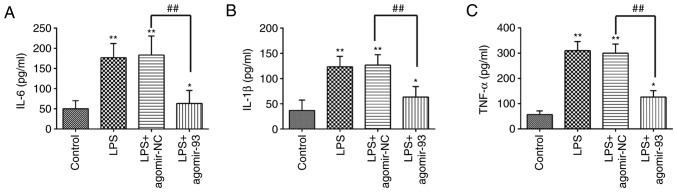
Currently, ALI is recognized as an unbalanced inflammatory response, and the inflammatory process is regulated by multiple miRNAs (35-37). In the present study, to further investigate whether miR-93 affects the inflammatory response induced by LPS in mice, ELISA was performed to examine the levels of pro-inflammatory cytokines, including IL-6, IL-1β, and TNF-α in BALF from mice with ALI. As shown in Fig. 3, exposure to LPS resulted in a significant increase in the production of IL-6, IL-1β and TNF-α. By contrast, these increased levels of pro-inflammatory cytokines were markedly attenuated by the agomir-93 injection. These data thus suggest that the agomir-93 injection alleviated lung injury by suppressing the inflammatory response in mice with ALI.
Figure 3.
miR-93 suppresses the inflammatory response in mice with LPS-induced ALI. Mice were injected intravenously with agomir-93 and control agomir (8 mg/kg) 24 h prior to the LPS (2 mg/kg) challenge. After the LPS administration for 24 h, the mice were sacrificed and BALF was collected for analysis. (A) IL-6, (B) IL-1β, and (C) TNF-α levels in BALF were measured using commercial ELISA kits. Data are the means ± SD (n=3) of one representative experiment, *P<0.05, **P<0.01 vs. the control group. ##P<0.01 vs. the LPS + agomir-NC group. LPS alone group. LPS, lipopolysaccharide; ALI, acute lung injury; BALF, bronchoalveolar lavage fluid.
TLR4 is a target of miR-93 in RAW264.7 cells
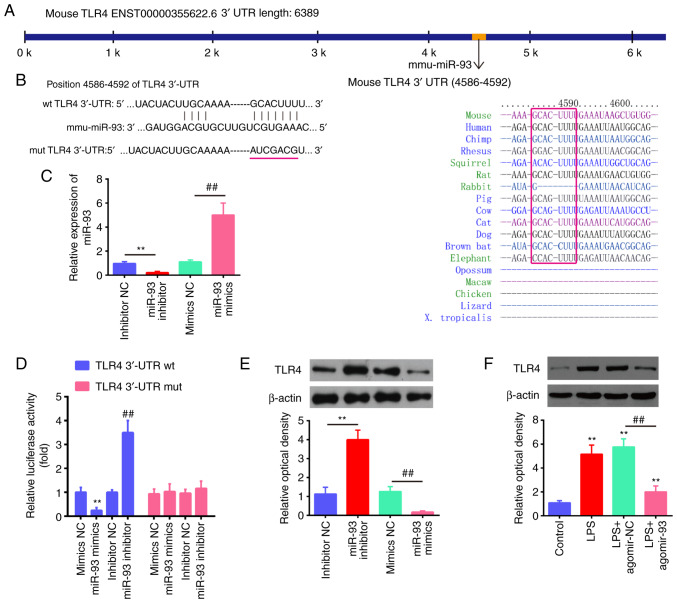
According to previous research, TLR4 is a common receptor of LPS, and plays important roles in the inflammatory response in ALI (38-42). Through bioinformatics prediction using TargetScan 7.0 and miRanda, the present study found that miR-93 directly targeted the TLR4 gene and that the target sequences were highly conserved among species (Fig. 4A and B). Notably, a previous study demonstrated that TLR4 was a direct target of miR-93 in chondrocytes (20). However, the association between miR-93 and TLR4 in ALI has not yet been clarified. To validate the possibility that TLR4 is directly targeted by miR-93, a luciferase reporter assay was performed. As shown in Fig. 4C, the expression levels of miR-93 were significantly increased following transfection with miR-93 mimics, while they were decreased by transfection with miR-93 inhibitor in the RAW264.7 cells, demonstrating the sufficient transfection efficacy. It was observed that transfection with miR-93 mimics markedly inhibited the luciferase activity in the TLR4-3'UTR wt reporter, and that the miR-93 inhibitor induced an increased luciferase activity; however, no changes were observed in the cells transfected with the TLR4 3'-UTR-mut plasmid (Fig. 4D), indicating a targeting interaction between miR-93 and TLR4. In addition, western blot analysis demonstrated that TLR4 protein expression was decreased by transfection with miR-93 mimics, whereas it was increased by transfection with miR-93 inhibitor in the RAW264.7 cells (Fig. 4E). The effect of agomir-93 on the protein expression of TLR4 protein in vivo was also assessed by western blot analysis. It was found that exposure to LPS significantly increased the protein expression of TLR4 compared with the control group, whereas this promoting effect of LPS on TLR4 expression was attenuated by agomir-93 (Fig. 4F). These results suggest that miR-93 targets TLR4 and suppresses its expression induced by LPS in vitro and in vivo.
Figure 4.
TLR4 is a direct target of miR-93. (A and B) The putative binding site of miR-93 and TLR4 is shown. (C) The expression levels of miR-93 were measured by RT-qPCR following transfection with miR-93 mimics or miR-93 inhibitor in RAW264.7 cells. (D) Luciferase activity of RAW264.7 cells was detected by a dual luciferase assay. Data are the means ± SD (n=3) of one representative experiment, **P<0.01 vs. mimics NC, ##P<0.01 vs. inhibitor NC. (E) Protein expression levels of TLR4 following transfection with miR-93 mimics or miR-93 inhibitor measured by western blot analysis. Data are the means ± SD (n=3) of one representative experiment, **P<0.01 vs mimics NC, ##P<0.01 vs inhibitor NC. (F) Mice were injected intravenously with agomir-93, and agomir NC (8 mg/kg) 24 h prior to the LPS (2 mg/kg) challenge. After the LPS administration for 24 h, the mice were sacrificed and lung tissues were then collected for the detection of the protein expression levels of TLR4 by western blot analysis. Data are the means ± SD (n=3) of one representative experiment, **P<0.01 vs. control group. ##P<0.01 vs. the LPS + agomiR-NC group. LPS, lipopolysaccharide; ALI, acute lung injury; TLR4, Toll-like receptor 4.
miR-93 inhibits LPS-induced inflammatory responses through the TLR4/MyD88/NF-κB pathway
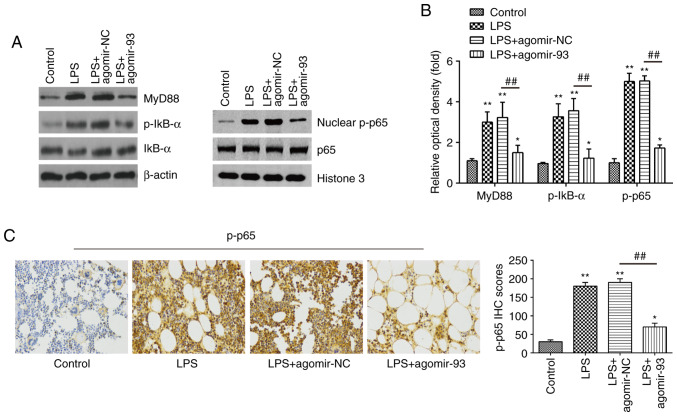
Previous studies have reported that the NF-κB pathway, acting as the downstream signaling effector of TLR4, plays a crucial role in LPS-induced ALI (43,44). Thus, the present study sought to determine whether miR-93 influences the TLR4/MyD88/NF-κB pathway in a mouse model of ALI. The expression levels of key proteins of this pathway, including MyD88, IκB-α, p-IκB-α, p65 and nuclear p-p65 were measured by western blot analysis. As shown in Fig. 5A and B, exposure to LPS significantly increased the expression levels of MyD88, p-IκB-α and nuclear p-p65, compared with those in the control group. However, the promoting effects of LPS on the expression levels of these proteins were markedly attenuated by agomir-93 injection. Similar results were observed for the expression of p-p65, as determined by IHC staining (Fig. 5C). Collectively, these results suggest that miR-93 inhibited the TLR4-mediated activation of the NF-κB pathway, and thus suppressed the inflammatory response in LPS treated mice (Fig. 6).
Figure 5.
miR-93 inhibits the LPS-induced inflammatory response through the TLR4/MyD88/NF-κB pathway. Mice were injected intravenously with agomir-93, and agomir NC (8 mg/kg) 24 h prior to the LPS (2 mg/kg) challenge. After the LPS administration for 24 h, the mice were sacrificed and lung tissues were then collected for analysis. (A) The levels of MyD88, IκB-α, p-IκB-α, p65 and nuclear p-p65 were measured by western blot analysis. (B) The bands were semi-quantitatively analyzed using Image J software, and normalized to β-actin density. (C) The expression of nuclear p-p65 was determined by IHC (×400 magnification) and IHC scores are calculated. Data are the means ± SD (n=3) of one representative experiment. *P<0.05, **P<0.01 vs. control group. ##P<0.01 vs. LPS + agomiR-NC group. LPS, lipopolysaccharide; ALI, acute lung injury; TLR4, Toll-like receptor 4; MyD88, myeloid differentiation primary response 88.
Figure 6.
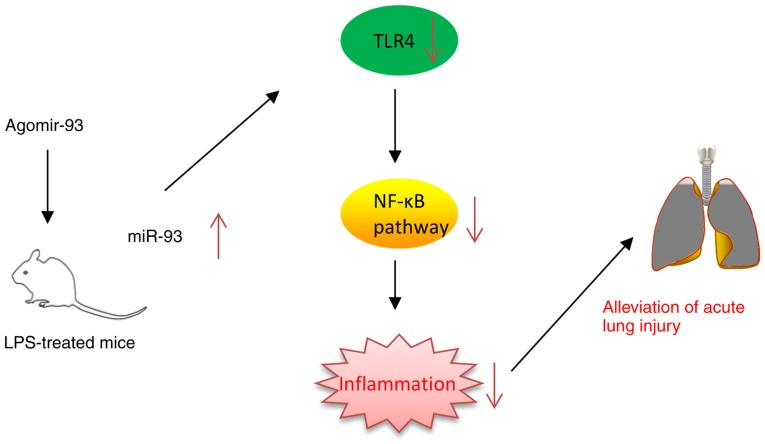
Schematic diagram of the signaling pathway in which miR-93 attenuates LPS-induced acute lung injury. AgomiR-93 injection attenuated LPS-induced inflammation in a model of ALI by blocking the activation of the TLR4/MyD88/NF-κB signaling pathway. LPS, lipopolysaccharide; ALI, acute lung injury; TLR4, Toll-like receptor 4; MyD88, myeloid differentiation primary response 88.
Discussion
In the present study, it was found that miR-93 expression was downregulated in lung tissues and BALF from mice with LPS-induced ALI. Agomir-93 injection attenuated LPS-induced lung injury through the suppression of the pulmonary inflammatory response by blocking the TLR4/MyD88/NF-κB pathway in mice. On the basis of this, miR-93 may thus serve as a potential therapeutic target for the treatment of ALI.
A number of studies have demonstrated the involvement of miRNAs in the regulation of the inflammatory response in ALI. Several miRNAs, such as miR-24 and miR-27b, have been shown to attenuate lung injury in vitro and in vivo (45,46). miR-93 has previously been reported to play an important role in inflammatory diseases. For example, in a mouse model of cerebral ischemia reperfusion (CIR), miR-93 overexpression was shown to inhibit the inflammatory response by the regulation of IRAK4 expression (21). Xu et al found that miR-93 suppressed pro-inflammatory cytokine production in a rat model of acute ocular inflammation (47). However, to date, the role of miR-93 in the pulmonary inflammatory responses of ALI has not yet been extensively studied. In the present study, it was found that miR-93 expression was significantly downregulated in lung tissues and BALF from mice with LPS-induced ALI. It was also observed that agomiR-93 injection markedly attenuated LPS-induced lung damage by suppressing the inflammatory response in mice, which indicates the anti-inflammatory roles of miR-93 in ALI. However, the molecular mechanisms underlying the attenuating effects of miR-93 on ALI remain unclear.
TLR4 is a well-known pathogen recognition receptor that has been proposed to contribute to the inflammatory response in LPS-induced ALI, and its inhibition may provide further therapeutic value (48). For example, Guo et al demonstrated that the inhibition of TLR4 lessened the severity of ALI in a rat model (49). Xia et al reported that YiQiFuMai (YQFM), re-developed based on the well-known traditional Chinese medicinal formula, Sheng-Mai-San, improved the recovery of lung tissue damage induced by ALI in mice through the inhibition of the TLR4 pathway (50). Notably, a previous study demonstrated that miR-93 inhibited chondrocyte inflammation in osteoarthritis (OA) by targeting TLR4 (20). Another study by Tang et al demonstrated that miR-93 protected cardiomyocytes against the LPS-induced inflammatory response by inhibiting TLR4 expression (51). In the present study, TLR4 was identified as a target of miR-93 in RAW264.7 cells and its expression was negatively regulated by miR-93 in mice with ALI. These results suggest that miR-93 may exert its anti-inflammatory effects through the suppression of TLR4.
It is a growing consensus that the TLR4/NF-κB signaling pathway has been implicated in the inflammatory responses of different diseases, including ALI (41,52,53). For example, Meng et al reported that umbelliferone (Umb) protected against LPS-induced lung injury by alleviating the inflammatory response through the inhibition of the TLR4/NF-κB pathway in mice (54). Previous studies have reported that miRNAs play a regulatory role in the inflammatory response induced by ALI through the TLR4/NF-κB pathway. For example, Yang et al demonstrated that the upregulation of miR-140-5p inhibited inflammatory cyto-kine production in ALI through the TLR4/MyD88/NF-κB pathway (36). Ju et al found that miR-27a alleviated LPS-induced lung injury and the inflammatory response in mice by modulating this pathway (37). Given the important role of the TLR4/MyD88/NF-κB signaling pathway in ALI, the present study aimed to investigate whether miR-93 attenuates LPS-induced lung injury by regulating the TLR4/MyD88/NF-κB signaling pathway. The findings of the present study revealed that agomir-93 injection significantly inhibited the LPS-induced activation of the TLR4/MyD88/NF-κB signaling pathway in vivo. These results suggest that the protective effect of miR-93 on LPS induced lung injury may be mediated via the TLR4/MyD88/NF-κB signaling pathway.
In conclusion, the findings of the present study demonstrate that miR-93 may exert protective effects against LPS-induced ALI both in vitro and in vivo by targeting the TLR4/MyD88/NF-κB signaling pathway. These results suggest that miR-93 might hold promise as a potential therapeutic target for use in the treatment of ALI.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Key R&D projects of science and Technology Department of Sichuan Province (grant no. 2020YFS0104).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
HG and DX performed the experiments, contributed to data analysis and wrote the manuscript. LG and XL conceptualized the study design, contributed to data analysis and experimental materials. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All animal studies were performed according to the guidelines of the Chinese Council on Animal Care and ethical approval was obtained for the use of animals prior to the start of the study from the ethics committee of the West China Second University Hospital, Sichuan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. Functional disability 5 years after acute respi-ratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 2.Mendez JL, Hubmayr RD. New insights into the pathology of acute respiratory failure. Curr Opin Crit Care. 2005;11:29–36. doi: 10.1097/00075198-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Cao J, Feng J, Wu Q, Chen BY. A study of noninvasive positive-pressure mechanical ventilation in the treatment of acute lung injury with a complex critical care ventilator. J Int Med Res. 2014;42:788–798. doi: 10.1177/0300060514522205. [DOI] [PubMed] [Google Scholar]
- 4.Tsushima K, King LS, Aggarwal NR, De Gorordo A, D'Alessio FR, Kubo K. Acute lung injury review. Intern Med. 2009;48:621–630. doi: 10.2169/internalmedicine.48.1741. [DOI] [PubMed] [Google Scholar]
- 5.Deng J, Wang DX, Liang AL, Tang J, Xiang DK. Effects of baicalin on alveolar fluid clearance and α-ENaC expression in rats with LPS-induced acute lung injury. Can J Physiol Pharmacol. 2017;95:122–128. doi: 10.1139/cjpp-2016-0212. [DOI] [PubMed] [Google Scholar]
- 6.Fragoso IT, Ribeiro EL, Gomes FO, Donato MA, Silva AK, Oliveira AC, Araújo SM, Barbosa KP, Santos LA, Peixoto CA. Diethylcarbamazine attenuates LPS-induced acute lung injury in mice by apoptosis of inflammatory cells. Pharmacol Rep. 2017;69:81–89. doi: 10.1016/j.pharep.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Lou J, Mao YY, Lai TW, Liu LY, Zhu C, Zhang C, Liu J, Li YY, Zhang F, et al. Activation of MTOR in pulmonary epithelium promotes LPS-induced acute lung injury. Autophagy. 2016;12:2286–2299. doi: 10.1080/15548627.2016.1230584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsia TC, Yin MC. Post-Intake of S-Ethyl cysteine and S-Methyl cysteine improved LPS-Induced acute lung injury in mice. Nutrients. 2016;8:E507. doi: 10.3390/nu8080507. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Porcherie A, Cunha P, Trotereau A, Roussel P, Gilbert FB, Rainard P, Germon P. Repertoire of Escherichia coli agonists sensed by innate immunity receptors of the bovine udder and mammary epithelial cells. Vet Res. 2012;43:14. doi: 10.1186/1297-9716-43-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wieland CW, Florquin S, Maris NA, Hoebe K, Beutler B, Takeda K, Akira S, van der Poll T. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable haemophilus influenzae from the mouse lung. J Immunol. 2005;175:6042–6049. doi: 10.4049/jimmunol.175.9.6042. [DOI] [PubMed] [Google Scholar]
- 11.Vilahur G, Badimon L. Ischemia/reperfusion activates myocardial innate immune response: The key role of the toll-like receptor. Front Physiol. 2014;5:496. doi: 10.3389/fphys.2014.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuffa LG, Fioruci-Fontanelli BA, Mendes LO, Ferreira Seiva FR, Martinez M, Fávaro WJ, Domeniconi RF, Pinheiro PF, Delazari Dos Santos L, Martinez FE. Melatonin attenuates the TLR4-mediated inflammatory response through MyD88- and TRIF-dependent signaling pathways in an in vivo model of ovarian cancer. BMC Cancer. 2015;15:34. doi: 10.1186/s12885-015-1032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng J, Zou Y, Chen J, Qin F, Chen X, Chen X, Dai S. sTLR4/sMD-2 complex alleviates LPS-induced acute lung injury by inhibiting pro-inflammatory cytokines and chemokine CXCL1 expression. Exp Ther Med. 2018;16:4632–4638. doi: 10.3892/etm.2018.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 16.Suo T, Chen GZ, Huang Y, Zhao KC, Wang T, Hu K. MiRNA-1246 suppresses acute lung injury-induced inflammation and apoptosis via the NF-κB and Wnt/β-catenin signal pathways. Biomed Pharmacother. 2018;108:783–791. doi: 10.1016/j.biopha.2018.09.046. [DOI] [PubMed] [Google Scholar]
- 17.Ling Y, Li ZZ, Zhang JF, Zheng XW, Lei ZQ, Chen RY, Feng JH. MicroRNA-494 inhibition alleviates acute lung injury through Nrf2 signaling pathway via NQO1 in sepsis-associated acute respiratory distress syndrome. Life Sci. 2018;210:1–8. doi: 10.1016/j.lfs.2018.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Guo Z, Gu Y, Wang C, Zhang J, Shan S, Gu X, Wang K, Han Y, Ren T. Enforced expression of miR-125b attenuates LPS-induced acute lung injury. Immunol Lett. 2014;162:18–26. doi: 10.1016/j.imlet.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Hua Q, Chen Y, Liu Y, Li M, Diao Q, Xue H, Zeng H, Huang L, Jiang Y. Circular RNA 0039411 is involved in neodymium oxide-induced inflammation and antiproliferation in a human bronchial epithelial cell line via sponging miR-93-5p. Toxicol Sci. 2019;170:69–81. doi: 10.1093/toxsci/kfz074. [DOI] [PubMed] [Google Scholar]
- 20.Ding Y, Wang L, Zhao Q, Wu Z, Kong L. MicroRNA93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NFKB signaling pathway. Int J Mol Med. 2019;43:779–790. doi: 10.3892/ijmm.2018.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian F, Yuan C, Hu L, Shan S. MicroRNA-93 inhibits inflammatory responses and cell apoptosis after cerebral ischemia reperfusion by targeting interleukin-1 receptor-associated kinase 4. Exp Ther Med. 2017;14:2903–2910. doi: 10.3892/etm.2017.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Z, Zhang C, Cheng L, Hu M, Tao H, Song L. The microRNA miR-17 regulates lung FoxA1 expression during lipopolysaccharide-induced acute lung injury. Biochem Biophys Res Commun. 2014;445:48–53. doi: 10.1016/j.bbrc.2014.01.108. [DOI] [PubMed] [Google Scholar]
- 23.Carbone L, Carbone ET, Yi EM, Bauer DB, Lindstrom KA, Parker JM, Austin JA, Seo Y, Gandhi AD, Wilkerson JD. Assessing cervical dislocation as a humane euthanasia method in mice. J Am Assoc Lab Anim Sci. 2012;51:352–356. [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Barreto TR, Costola-de-Souza C, Margatho RO, Queiroz- Hazarbassanov N, Rodrigues SC, Felício LF, Palermo-Neto J, Zager A. Repeated Domperidone treatment modulates pulmonary cytokines in LPS-induced acute lung injury in mice. Int Immunopharmacol. 2018;56:43–50. doi: 10.1016/j.intimp.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Duan Y, Learoyd J, Meliton AY, Leff AR, Zhu X. Inhibition of Pyk2 blocks lung inflammation and injury in a mouse model of acute lung injury. Respir Res. 2012;13:4. doi: 10.1186/1465-9921-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu T, Kasamatsu A, Yamamoto A, Koike K, Ishige S, Takatori H, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H, Uzawa K. Annexin A10 in human oral cancer: Biomarker for tumoral growth via G1/S transition by targeting MAPK signaling pathways. PLoS One. 2012;7:e45510. doi: 10.1371/journal.pone.0045510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyoda M, Kasamatsu A, Ishigami T, Nakashima D, Endo-Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H, Uzawa K. Epithelial cell transforming sequence 2 in human oral cancer. PLoS One. 2010;5:e14082. doi: 10.1371/journal.pone.0014082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyama T, Ogawara K, Kasamatsu A, Okamoto A, Kasama H, Minakawa Y, Shimada K, Yokoe H, Shiiba M, Tanzawa H, Uzawa K. ANGPTL3 is a novel biomarker as it activates ERK/MAPK pathway in oral cancer. Cancer Med. 2015;4:759–769. doi: 10.1002/cam4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Yang D, Cao X, Wang F, Jiang H, Guo H, Du L, Guo Q, Yin X. LFG-500, a newly synthesized flavonoid, attenuates lipopolysaccharide-induced acute lung injury and inflammation in mice. Biochem Pharmacol. 2016;113:57–69. doi: 10.1016/j.bcp.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Lei C, Jiao Y, He B, Wang G, Wang Q, Wang J. RIP140 down-regulation alleviates acute lung injury via the inhibition of LPS-induced PPARγ promoter methylation. Pulm Pharmacol Ther. 2016;37:57–64. doi: 10.1016/j.pupt.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Yao Y, Sun F, Lei M. MiR-25 inhibits sepsis-induced cardiomyocyte apoptosis by targetting PTEN. Biosci Rep. 2018;38:BSR20171511. doi: 10.1042/BSR20171511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Q, Xu HX, Li JP, Wang S, Fu Z, Jia J, Wang L, Zhu ZF, Lu R, Yao Z. Growth differentiation factor 15 induces growth and metastasis of human liver cancer stem-like cells via AKT/GSK-3β/β-catenin signaling. Oncotarget. 2017;8:16972–16987. doi: 10.18632/oncotarget.15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Zeng L, Zhang T, Liu J, Wang W. Casticin inhibits lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol. 2016;789:172–178. doi: 10.1016/j.ejphar.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 35.Alghetaa H, Mohammed A, Sultan M, Busbee P, Murphy A, Chatterjee S, Nagarkatti M, Nagarkatti P. Resveratrol protects mice against SEB-induced acute lung injury and mortality by miR-193a modulation that targets TGF-β signalling. J Cell Mol Med. 2018;22:2644–2655. doi: 10.1111/jcmm.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Liu D, Xi Y, Li J, Liu B, Li J. Upregulation of miRNA-140-5pinhibits inflammatory cytokines in acute lung injury through the MyD88/NF-κB signaling pathway by targeting TLR4. Exp Ther Med. 2018;16:3913–3920. doi: 10.3892/etm.2018.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ju M, Liu B, He H, Gu Z, Liu Y, Su Y, Zhu D, Cang J, Luo Z. MicroRNA-27a alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis through modulating TLR4/MyD88/NF-κB pathway. Cell Cycle. 2018;17:2001–2018. doi: 10.1080/15384101.2018.1509635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, Yang F, Yu X, Wang B, Yang Y, Zhou X, Cheng R, Xia S, Zhou X. MiR-16 inhibits NLRP3 inflammasome activation by directly targeting TLR4 in acute lung injury. Biomed Pharmacother. 2019;112:108664. doi: 10.1016/j.biopha.2019.108664. [DOI] [PubMed] [Google Scholar]
- 39.Dong Z, Yuan Y. Accelerated inflammation and oxidative stress induced by LPS in acute lung injury: Lotanhibition by ST1926. Int J Mol Med. 2018;41:3405–3421. doi: 10.3892/ijmm.2018.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu X, Liu S, Zhu J, Ni H. Dachengqi decoction alleviates acute lung injury and inhibits inflammatory cytokines production through TLR4/NF-κB signaling pathway in vivo and in vitro. J Cell Biochem. 2019;120:8956–8964. doi: 10.1002/jcb.27615. [DOI] [PubMed] [Google Scholar]
- 41.Liu JX, Li X, Yan FG, Pan QJ, Yang C, Wu MY, Li G, Liu HF. Protective effect of forsythoside B against lipopolysaccharide-induced acute lung injury by attenuating the TLR4/NF-κB pathway. Int Immunopharmacol. 2019;66:336–346. doi: 10.1016/j.intimp.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 42.Deng G, He H, Chen Z, OuYang L, Xiao X, Ge J, Xiang B, Jiang S, Cheng S. Lianqinjiedu decoction attenuates LPS-induced inflammation and acute lung injury in rats via TLR4/NF-κB pathway. Biomed Pharmacother. 2017;96:148–152. doi: 10.1016/j.biopha.2017.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang F, Zhu M, Jiang N, Zhang M, Feng L, Jia X. Paeonol ameliorates lipopolysaccharides-induced acute lung injury by regulating TLR4/MyD88/NF-κB signaling pathway. Pharmazie. 2019;74:101–106. doi: 10.1691/ph.2019.8860. [DOI] [PubMed] [Google Scholar]
- 44.Wang D, Wang X, Tong W, Cui Y, Li X, Sun H. Umbelliferone alleviates lipopolysaccharide-induced inflammatory responses in acute lung injury by down-regulating TLR4/MyD88/NF-κB signaling. Inflammation. 2019;42:440–448. doi: 10.1007/s10753-018-00953-4. [DOI] [PubMed] [Google Scholar]
- 45.Lin Y, Yang Y. MiR-24 inhibits inflammatory responses in LPS-induced acute lung injury of neonatal rats through targeting NLRP3. Pathol Res Pract. 2019;215:683–688. doi: 10.1016/j.prp.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Huang L, Zhu G, Pei Z, Zhang W. Downregulated microRNA-27b attenuates lipopolysaccharide-induced acute lung injury via activation of NF-E2-related factor 2 and inhibition of nuclear factor κB signaling pathway. J Cell Physiol. 2019;234:6023–6032. doi: 10.1002/jcp.27187. [DOI] [PubMed] [Google Scholar]
- 47.Xu Y, Jin H, Yang X, Wang L, Su L, Liu K, Gu Q, Xu X. MicroRNA-93 inhibits inflammatory cytokine production in LPS-stimulated murine macrophages by targeting IRAK4. FEBS Lett. 2014;588:1692–1698. doi: 10.1016/j.febslet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 48.He X, Qian Y, Li Z, Fan EK, Li Y, Wu L, Billiar TR, Wilson MA, Shi X, Fan J. TLR4-Upregulated IL-1β and IL-1RI promote alveolar macrophage pyroptosis and lung inflammation through an autocrine mechanism. Sci Rep. 2016;6:31663. doi: 10.1038/srep31663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo S, Jiang K, Wu H, Yang C, Yang Y, Yang J, Zhao G, Deng G. Magnoflorine ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-κB and MAPK activation. Front Pharmacol. 2018;9:982. doi: 10.3389/fphar.2018.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia Y, S D, Jiang S, Fan R, Wang Y, Wang Y, Tang J, Zhang Y, He RL, Yu B, Kou J. YiQiFuMai lyophilized injection attenuates particulate matter-induced acute lung injury in mice via TLR4-mTOR-autophagy pathway. Biomed Pharmacother. 2018;108:906–913. doi: 10.1016/j.biopha.2018.09.088. [DOI] [PubMed] [Google Scholar]
- 51.Tang B, Xuan L, Tang M, Wang H, Zhou J, Liu J, Wu S, Li M, Wang X, Zhang H. MiR-93-3p alleviates lipopolysaccharide-induced inflammation and apoptosis in H9c2 cardiomyocytes by inhibiting toll-like receptor 4. Pathol Res Pract. 2018;214:1686–1693. doi: 10.1016/j.prp.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 52.Sun XJ, Li XQ, Wang XL, Tan WF, Wang JK. Sevoflurane inhibits nuclear factor-κB activation in lipopolysaccha-ride-induced acute inflammatory lung injury via toll-like receptor 4 signaling. PLoS One. 2015;10:e0122752. doi: 10.1371/journal.pone.0122752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bai S, Zhou H, Wu L. Bone marrow stromal cells improved functional recovery in spinal cord injury rats partly via the Toll-like receptor-4/nuclear factor-κB signaling pathway. Exp Ther Med. 2019;17:444–448. doi: 10.3892/etm.2018.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meng X, Liu J, Wang H, Chen P, Wang D. MicroRNA-126-5p downregulates BCAR3 expression to promote cell migration and invasion in endometriosis. Mol Cell Endocrinol. 2019;494:110486. doi: 10.1016/j.mce.2019.110486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.