Abstract
Nav1.7 is closely associated with neuropathic pain. Hydrogen sulfide (H2S) has recently been reported to be involved in numerous biological functions, and it has been shown that H2S can enhance the sodium current density, and inhibiting the endogenous production of H2S mediated by cystathionine β-synthetase (CBS) using O-(carboxymethyl) hydroxylamine hemihydrochloride (AOAA) can significantly reduce the expression of Nav1.7 and thus the sodium current density in rat dorsal root ganglion (DRG) neurons. In the present study, it was shown that the fluorescence intensity of H2S was increased in a spared nerve injury (SNI) model and AOAA inhibited this increase. Nav1.7 is expressed in DRG neurons, and the expression of CBS and Nav1.7 were increased in DRG neurons 7, 14 and 21 days post-operation. AOAA inhibited the increase in the expression of CBS, phosphorylated (p)-MEK1/2, p-ERK1/2 and Nav1.7 induced by SNI, and U0126 (a MEK blocker) was able to inhibit the increase in p-MEK1/2, p-ERK1/2 and Nav1.7 expression. However, PF-04856264 did not inhibit the increase in CBS, p-MEK1/2, p-ERK1/2 or Nav1.7 expression induced by SNI surgery. The current density of Nav1.7 was significantly increased in the SNI model and administration of AOAA and U0126 both significantly decreased the density. In addition, AOAA, U0126 and PF-04856264 inhibited the decrease in rheobase, and the increase in action potential induced by SNI in DRG neurons. There was no significant difference in thermal withdrawal latency among each group. However, the time the animals spent with their paw lifted increased significantly following SNI, and the time the animals spent with their paw lifted decreased significantly following the administration of AOAA, U0126 and PF-04856264. In conclusion, these data show that Nav1.7 expression in DRG neurons is upregulated by CBS-derived endogenous H2S in an SNI model, contributing to the maintenance of neuropathic pain.
Keywords: dorsal root ganglion, Nav1.7, cystathionine β-synthetase, hydrogen sulfide, neuropathic pain
Introduction
Neuropathic pain is defined by the International Association for the Study of Pain in 2011 as pain caused by a lesion or disease of the somatosensory nervous system (1). Neuropathic pain is usually caused by peripheral nerve injury and can lead to allodynia or hyperalgesia, that is, the patient feels pain with weak or no stimulation (2).
Studies have shown that dorsal root ganglion (DRG) neurons are closely associated with neuropathic pain. Changes in neurotrophic factors, inflammatory factors and expression of various ion channels in DRG neurons result in increased excitability of DRG neurons, which underlie the development of neuropathic pain (3-7). Voltage-gated sodium channels (VGSCs or Navs) have been classified into nine different subtypes (Nav1.1-1.9), seven of which are expressed on human DRG neurons (Nav1.1-1.3 and Nav1.6-1.9). PCR analysis showed that Nav1.7 is primarily expressed in healthy human DRG neurons (8). Nav1.7 is closely associated with neuropathic pain. Mutations in SCN9A, the gene encoding Nav1.7, are associated with inherited primary erythromelalgia, paroxysmal extreme pain disorder and idiopathic small fiber neuropathies (9-11).
Hydrogen sulfide (H2S), has been reported to be involved in numerous biological functions. Studies have shown that H2S can enhance the sodium current density (12,13), and inhibiting the endogenous production of H2S mediated by the enzyme cystathionine β-synthetase (CBS) with O-(carboxymethyl) hydroxylamine hemihydrochloride (AOAA) can significantly reduce the sodium current density carried by Nav1.7 and Nav1.8 in DRG neurons of rats (14). In addition, mitogen-activated protein kinases (MAPKs) are transducers of cell signaling that have been implicated in neuronal responses to pathologies such as pain. Previous studies have shown that expression of Nav1.7, Nav1.8 and phosphorylated (p)-MAPKs, including p-extracellular signal-regulated kinase (ERK) and p-p38, are significantly increased in the blind-ending axon of the neuroma (15). Furthermore, p-ERK modulates the gating kinetics of Nav1.7 through phosphorylation of Nav1.7 at multiple sites (16).
The aim of the present study was to assess whether endogenous H2S regulated Nav1.7 through the MEK/ERK pathway in DRG neurons, and whether this may underlie the pathogenesis of neuropathic pain.
However, there are few reports regarding the relationship and role of endogenous H2S with Nav1.7 (14). In the present study, it was shown that endogenous H2S activated the MEK/ERK pathway and upregulated the expression and function of Nav1.7 in SNI rats, suggesting that inhibiting endogenous H2S may provide a potentially novel therapeutic method for treatment of neuropathic pain.
Materials and methods
Animals and groups
Male Sprague-Dawley rats, aged 8-12 weeks, and weighing 180-200 g were used in the present study. Two or three rats were housed in each cage and under a 12-h light/dark cycle, and food and water were provided ad libitum. The present study was approved by the Xinjiang Medical University Animal Center (approval no. SCXK Xin2003-0001). Animal experiments were performed in accordance with the Institutional Ethics Review Board at the First Affiliated Hospital of the Shihezi University School of Medicine. A total of 150 rats were randomly divided into five groups: i) Sham group (n=30); ii) SNI+Vehicle group (n=30); iii) SNI+AOAA group (n=30); iv) SNI+U0126 group (n=30); and iv) SNI+PF-04856264 group (n=30). Rats were used for immunofluorescence experiments (n=6 per group), western blot analysis (n=9 per group), patch clamp experiments (n=3 per group) and nociceptive behavioral studies (n=6 per group for thermal nociceptive tests and n=6 per group for acetone experiments).
Reagents and instruments
The primary reagents used in the present study were anti-Nav1.7 mouse monoclonal antibody (cat. no. ab85015; Abcam), anti-Nav1.7 rabbit monoclonal antibody (cat. no. orb447636; Biorbyt), anti-CBS rabbit polyclonal antibody (cat. no. ab96252; Abcam), mouse anti-NF-200 (a marker for myelinated A-fibers; cat. no. ab82259; Abcam), mouse anti-calcitonin gene related peptide (CGRP; a marker of peptidergic C-type neurons; ab81887; Abcam), rabbit anti-p-MEK (cat. no. 2338S; Cell Signaling Technology, Inc.), rabbit anti-MEK (cat. no. 4694S; Cell Signaling Technology, Inc.), rabbit anti-p-ERK (cat. no. 4370S; Cell Signaling Technology, Inc.), rabbit anti-ERK (cat. no. 4695S; Cell Signaling Technology, Inc.), mouse anti-GAPDH (cat. no. ab8245; Abcam) and mouse anti-β-actin (cat. no. ab8226; Abcam). In addition, the following secondary antibodies were used: Goat anti-mouse antibody IgG H&L (HRP) (cat. no. 12-349; Sigma-Aldrich; Merck KGaA), goat anti-rabbit antibody IgG H&L (HRP) (cat. no. 12-348; Sigma-Aldrich; Merck KGaA), goat anti-rabbit IgG H&L (TRITC) (cat. no. ab6718; Abcam), goat anti-mouse IgG H&L (FITC) (cat. no. ab6785; Abcam), goat anti-rabbit IgG H&L (FITC) (cat. no. ab6717; Abcam) and IB4 (FITC-conjugated; a marker for non-peptidergic C-type neurons; cat. no. L2895; Sigma-Aldrich; Merck KGaA). During acute digestion of DRG neurons, collagenase I (cat. no. C0130; Sigma-Aldrich; Merck KGaA), trypsin (cat. no. T1426; cat. no. Sigma-Aldrich; Merck KGaA) and trypsin inhibitor (cat. no. T6522; Sigma-Aldrich; Merck KGaA) were used. PF-04856264 (Sigma-Aldrich; Merck KGaA), AOAA (Sigma-Aldrich; Merck KGaA), dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA), radio-immunoprecipitation assay buffer (RIPA; Thermo Fisher Scientific, Inc.) and phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich; Merck KGaA) and DMEM (Gibco; Thermo Fisher Scientific, Inc.) were also used in the present study.
The equipment used included an automatic thermal radiation stimulator (37370 Plantar Test Apparatus; Ugo Basile SRL), a BX51 fluorescence microscope (Carl Zeiss AG), a patch clamp amplifier (MultiClamp 700B; Axon Instruments; Molecular Devices, LLC), a microelectrode control instrument (P-2000; Sutter Instrument Company), a biological data acquisition system (Digidata 1550A; Axon Instruments; Molecular Devices, LLC), a micromanipulator (MP-225; Sutter Instrument Company) and an inverted microscope (Olympus Corporation).
Establishment of neuropathic pain in rats
Rats were anesthetized with 1% pentobarbital sodium (50 mg/kg) by intraperitoneal (i.p.) injection, as described previously (17). Following anesthesia, three terminal branches of the sciatic nerve were gently separated proximal to the popliteal fossa, and the tibial and the common peroneal nerves were tightly ligated using a 4-0 silk and sectioned distal to the ligation, removing 3 mm of the distal stumps at this level. Subsequently, the wound was closed in layers. The rats were kept in a warm environment until complete recovery from anesthesia. Following the operation, the rats were subjected to single-cage feeding, enhanced animal nutrition, infection prevention and a strict 12 h light/dark cycle. In the Sham group, the sciatic nerve was isolated and exposed but not injured.
Fluorescence probe for measuring H2S
Rats were anesthetized with 1% pentobarbital sodium (50 mg/kg, i.p.) and subsequently sacrificed by cervical dislocation. The L4-L6 DRGs from the SNI surgery side were then removed immediately. WSP-5 (cat. no. C3378; APExBIO), a fluorescent probe for rapid detection of H2S, was dissolved in D-Hanks balanced salt solution for use. The samples were cut into pieces using eye scissors and digestive enzymes (DMEM containing 0.24 mg/ml trypsin and 0.6 mg/ml collagenase I) were used to acutely isolate DRG neurons at 37°C for 2-4 min, followed by centrifugation at 153 × g for 5 min at 4°C. After removing the supernatant, the specimens were placed on a 96-well plate. Samples were incubated with WSP-5 (50 µm/l) at 37°C for 30 min, and the specimens were cleaned with D-Hanks. A fluorescence microscope (magnification, ×100) was used to record the results. When the concentration of H2S in the cell increased, the fluorescence intensity of WSP-5 increased proportionally. Fluorescence intensity was analyzed using CellSens Standard 2.1 Image Acquisition (Olympus Corporation) and Image Pro Plus version 6.0 (Media Cybernetics).
Immunofluorescence experiments
As described previously (18), rats were anesthetized with 1% pentobarbital sodium (50 mg/kg, i.p.) and perfused through the aorta with normal saline followed by fresh 4% PFA in PBS for 10 min. L4, L5 and L6 DRGs were removed rapidly and post-fixed in 4% PFA in PBS for 24 h at room temperature, followed by dehydration in 20 or 30% sucrose in phosphate buffer at 4°C. DRGs were embedded in paraffin and sectioned (5-µm) using a freezing microtome. Sections were washed and incubated with blocking buffer (4% BSA in PBS with 0.2% Tween-20) at room temperature for 30 min. Subsequently, sections were rinsed with 0.01 M PBS three times and incubated with mouse anti-Nav1.7 antibody (1:100) or rabbit anti-CBS antibody (1:100) in a wet box overnight at 4°C. Next, FITC-conjugated goat anti-mouse secondary antibodies (1:200) and FITC-conjugated goat anti-rabbit secondary antibodies (1:200) were incubated with the sections at room temperature for 120 min.
In the co-expression experiments, sections were incubated with a mixture of primary rabbit anti-Nav1.7 antibody (1:100) and mouse anti-NF-200 (1:100)/mouse anti-CGRP (1:100) in a wet box overnight at 4°C. Next, the sections were rinsed with 0.01 M PBS three times, and then incubated with a mixture of the TRITC-conjugated goat anti-rabbit secondary antibody (1:200) and FITC-conjugated goat anti-mouse secondary antibody (1:200) in the dark for 120 min at room temperature. However, in order to detect the co-expression of Nav1.7 and IB4 on DRG neurons, the tissue sections were incubated only with the primary antibody rabbit anti-Nav1.7 antibody (1:100) overnight at 4°C, followed by rinsing three times, and incubation with IB4 (1:500) mixed with TRITC-conjugated goat anti-rabbit secondary antibody (1:200) at room temperature for 120 min in the dark. Immunofluorescence quantification for all target proteins was performed by measuring the mean absorbance following laser confocal microscopy (magnification, ×200; Carl Zeiss AG) and analyzed with ZEN 2009 Light Edition (Carl Zeiss AG).
Western blot analysis
The L4-L6 DRGs from each group were homogenized in RIPA buffer (1 µl PMSF to 100 µl RIPA buffer) with freshly added protease inhibitor (10 mg tissue to 100 µl RIPA buffer), and centrifuged at 15,294 x g for 15 min at 4°C. The protein concentration was determined using the BCA method. Protein aliquots (40 µg/lane) were separated using a Tris-glycine denaturing gradient gel electrophoresis on an 8% SDS-gel using SDS-PAGE. The proteins were then transferred to a PVDF membrane (EMD Millipore), blocked with 5% non-fat milk in TBST buffer (pH 8.0, 10 mmol/l Tris-HCl, 150 mmol/l NaCl and 0.2% Tween-20) for 2 h at room temperature, and then incubated with the following primary antibodies in a box overnight at 4°C: Rabbit anti-Nav1.7 monoclonal antibody (1:1,000), rabbit anti-CBS polyclonal antibody (1:1,000), rabbit anti-p-MEK (1:1,000), rabbit anti-MEK (1:1,000), rabbit anti-p-ERK (1:1,000), rabbit anti-ERK (1:1,000), mouse anti-GAPDH (1:1,000) or mouse anti-β-actin (1:1,000). Following incubation with primary antibodies, the PVDF membrane was washed three times with TBST for 5 min and incubated with horseradish peroxidase-conjugated goat anti-mouse antibody IgG H&L (HRP) (1:10,000) or goat anti-rabbit antibody IgG H&L (HRP) (1:10,000) for 2 h at room temperature. Protein levels were normalized to β-actin or GAPDH using Image J 1.43 (National Institutes of Health).
Acute separation of DRG neurons
Rats were anesthetized with 1% sodium pentobarbital (50 mg/kg, i.p.) and then sacrificed by cervical dislocation. Subsequently, the segmental L4-L6 DRG organization were removed immediately. The redundant nerve fibers and connective tissues were amputated. Next, the ganglia were cut using eye scissors, and gently dissociated using a fire-polished Pasteur pipette and treatment with digestive enzymes (DMEM containing 0.24 mg/ml trypsin and 0.6 mg/ml collagenase I) at 37°C for 2-4 min. Incubation with trypsin inhibitors was then performed at room temperature for 15 min to terminate digestion. Extracellular fluid contained: 150 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2·H2O, 10 mM HEPES and 10 mM D-glucose, and was adjusted to pH 7.4 with NaOH. Extracellular fluid was added to the neuron suspension. The solution osmotic pressure of the extracellular fluid was 300-330 mOsm. The neurons suspension was placed in a 35 mm cell culture dish and used for electrophysiological recording once the neurons had adhered to the wall.
Patch clamp recording
A borosilicate glass blank with core produced by Sutter Instrument Company was transformed into a microelectrode using a P-2000 electrode drawing instrument. Microelectrodes with resistance values between 2-8 MΩ were used for current-clamp recording. The pipette solution contained the following: 130 mM K-gluconate, 10 mM NaCl, 1.2 mM MgCl2·H2O, 2 mM CaCl2, 5 mM EGTA, 10 mM HEPES and 7.5 mM D-glucose, adjusted to pH 7.35 with KOH, and 300-330 mOsm with sucrose. The extracellular solution contained: 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2·H2O, 10 mM HEPES and 11 mM glucose, and was adjusted to pH 7.4 with NaOH and 315 mOsm with sucrose. In the voltage clamp mode, the pipette solution contained the following: 140 mM CsF, 10 mM NaCl, 1 mM EGTA and 10 mM HEPES, adjusted to pH 7.3 with CsOH and 315 mOsm with sucrose. The extracellular bath solution contained the following: 70 mM NaCl, 70 mM choline chloride, 3 mM KCl, 1 mM MgCl2·H2O, 1 mM CaCl2, 10 mM HEPES, 5 mM CsCl, 20 mM tetraethylammonium chloride and 0.1 mM, CdCl2, was adjusted to pH 7.32 with NaOH and 327 mOsm with sucrose. The inverted microscope was used to observe the state of DRG neurons, and the micromanipulator was used to slowly bring the microelectrode into contact with DRG neurons under the microscope. A GΩ seal was formed between the cells and the electrode. After the membrane was broken, cells with a resistance above GΩ were used for experiments. In the voltage clamp mode, the holding potential used was -60 mV; a step protocol (from -100 to 20 mV in 10 mV increments with a pulse duration of 100 msec) was applied to determine the Nav1.7 current. In the current clamp mode, a step protocol (from 0 to 600 pA in 50 pA increments with a pulse duration of 150 msec) was applied to determine the rheobase (the minimum current intensity required to excite the first action potential). Subsequently, another step protocol (duration, 500 msec; amplitude, double-strength of rheobase) was performed to record the number of action potentials. The recorded signal was amplified by a MultiClamp 700B amplifier, filtered at 2.5 kHz, and converted by an Axon DigiData 1550A digital-to-analog/analog-to-digital converter with a sampling frequency of 10-20 kHz. And the 'net' current is obtained by subtracting the 'administration' current from the 'no administration' current, which represents the current sensitive to this drug.
Nociceptive behavioral study
According to our previous study (18), the threshold responses to painful thermal stimuli were evaluated on the surgical side hind paw at day 1 pre-operation and at days 1, 3, 7, 14 and 21 post-operation in each group. Each rat was tested three times at intervals of 5 min, and the mean of the three tests was obtained. Data are expressed as thermal withdrawal latency in sec. As described previously (19), acetone experiments were performed on the plantar surface of the surgical side hind paw with 20 µl acetone by measuring the duration of paw withdrawal in the 30 sec immediately following acetone application. These experiments were evaluated 1 day before operation and 1, 3, 7, 14 and 21 days post-operation in each group. Each day, cold allodynia measurements were repeated three times, and the mean was calculated. The next stimulus was administered once the animal was quiet, and data were expressed as the time that the paw was lifted in sec.
Drug administration
AOAA, an inhibitor of CBS, was dissolved in normal saline and immediately injected intrathecally into the animals (10 µg/kg body weight) 30 min before the nociceptive behavioral study. An equivalent volume of normal saline was injected intrathecally in the SNI+Vehicle group. For western blotting and immunofluorescence experiments, AOAA was administered for 21 consecutive days from the day of surgery (14,20). In the patch clamp experiments, AOAA (final concentration of 1 µm) was incubated with acutely dissociated DRG neurons (modeling for 21 days) for 1 h. An equivalent volume of normal saline was added during the incubation in the SNI+Vehicle group.
U0126, an inhibitor of MEK, was freshly dissolved in DMSO. For the nociceptive behavioral study, U0126 (10 µg dissolved in 10 µl 10% DMSO) was administered intrathecally 30 min before the test. For western blotting and immunofluorescence experiments, U0126 (10 µg dissolved in 10 µl 10% DMSO) was administered intrathecally daily for 21 consecutive days from the day of SNI surgery (20). For the patch clamp experiments, U0126 (final concentration of 1 µm) was incubated with acutely dissociated DRG neurons for 20 min (16).
PF-04856264, a specific inhibitor of Nav1.7, was freshly prepared in DMSO according to the manufacturer's protocol. For the nociceptive behavioral study, an i.p. injection of PF-04856264 (30 mg/kg body weight) was performed 10 min before the test (21). For western blot analysis and immunofluorescence experiments, PF-04856264 (30 mg/kg body weight) was administered intrathecally daily for 21 consecutive days from the day of surgery. For the patch clamp experiments, PF-04856264 (1 µm) was incubated with acutely dissociated DRG neurons for 1 h (22).
Statistical analysis
Data are presented as the mean ± standard error of the mean of three independent experiments. SPSS version 17.0 (SPSS, Inc.) was used for statistical analysis. Firstly, the distribution of the data was determined using a Shapiro-Wilk's or One-Sample Kolmogorov-Smirnov test. Variables with normal distribution were analyzed using ANOVA followed by Bonferroni's post hoc test. Whereas variables not normally distributed were analyzed using a Friedman test or Kruskal-Wallis test with a Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Detection of H2S concentration changes in DRG neurons using a chlorine ion fluorescence probe
Firstly, DRG neurons were acutely dissociated and WSP-5 was used to detect the concentration of H2S in DRG neurons. The intensity of green fluorescence is directly proportional to the concentration of H2S in DRG neurons. The experiment evaluated changes in the intracellular H2S concentrations of the L4-L6 DRG neurons on the surgical side of rats in the Sham group, SNI+Vehicle group and SNI+AOAA group. The results demonstrated that the concentration of H2S in L4-L6 DRG neurons in the SNI+Vehicle group was significantly increased (P<0.05) compared with the Sham group. In addition, compared with the SNI+Vehicle group, the concentration of H2S in the SNI+AOAA group was significantly decreased (P<0.05; Fig. 1). This indicates that the change of H2S in DRG is closely associated with pain. However, acute isolation may affect the activity of DRG neurons and the concentration of H2S in neurons, thus affecting the intensity of the H2S fluorescence.
Figure 1.
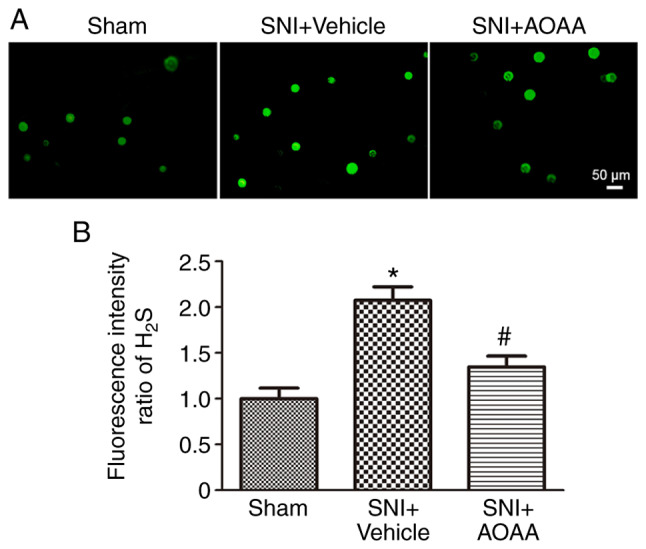
Fluorescence intensity of H2S in different groups. (A) Intensity of H2S fluorescence probes in different groups. Scale bar, 50 µm. (B) Statistical analysis of the H2S fluorescence intensity in different groups. *P<0.05 vs. Sham group; #P<0.05 vs. SNI+Vehicle group. n=9 per group. SNI, spared nerve injury; AOAA, O-(carboxymethyl) hydroxylamine hemihydro-chloride; H2S, hydrogen sulfide.
Expression of Nav1.7 and CBS in DRG neurons of SNI rats
Immunofluorescence was performed to observe the expression of Nav1.7 in the DRG neurons. The results revealed that Nav1.7 was expressed in different types of DRG neurons, including NF-200-marked DRG neurons, CGRP-marked DRG neurons and IB4-marked DRG neurons (Fig. 2).
Figure 2.
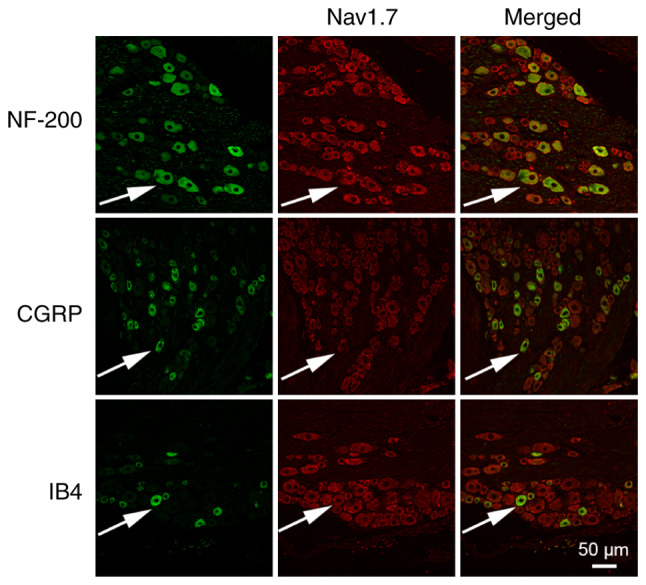
Co-expression of Nav1.7 with NF-200 (a marker of A-type neurons), CGRP (a marker of peptidergic C-type neurons) and IB4 (a marker of non-peptidergic C-type neurons) on dorsal root ganglion neurons. Arrows indicate co-labeled neurons. Scale bar, 50 µm. Nav1.7, voltage-gated sodium channel 1.7; CGRP, calcitonin gene related peptide.
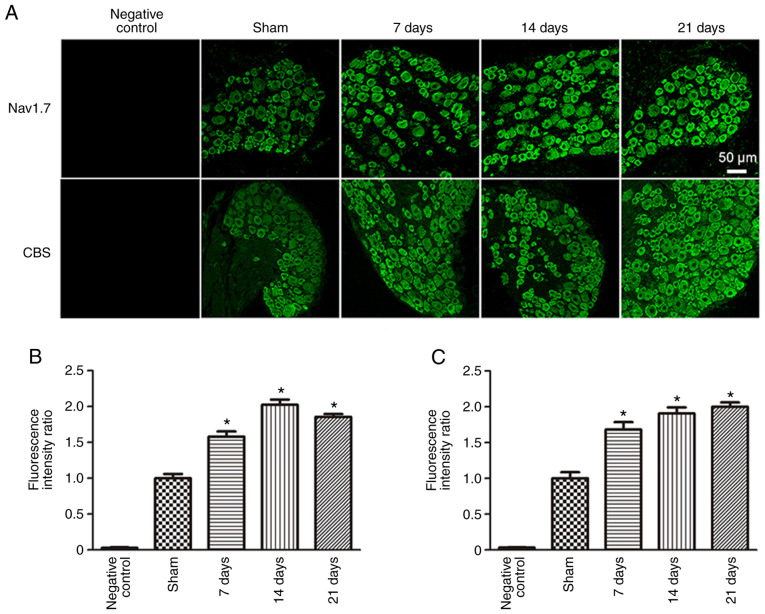
To observe changes in both Nav1.7 and CBS protein expression levels in the DRG neurons of rats following SNI surgery, immunofluorescence and western blotting were used. Both Nav1.7 and CBS were expressed in DRG neurons. Compared with the Sham group (1.00±0.06), the fluorescence intensity ratio of Nav1.7 was significantly increased 7 (1.58±0.07; P<0.05), 14 (2.03±0.07; P<0.05) and 21 days (1.85±0.04; P<0.05) post-SNI surgery (Fig. 3). In addition, compared with the Sham group (1.0±0.09), the fluorescence intensity ratio of CBS was also significantly increased 7 (1.68±0.10; P<0.05), 14 (1.91±0.08; P<0.05) and 21 days (2.00±0.06; P<0.05) post-SNI surgery.
Figure 3.
Immunofluorescence results of Nav1.7 and CBS in dorsal root ganglion neurons of spared nerve injury rats. (A) Expression of Nav1.7 and CBS in each group. Scale bar, 50 µm. Fluorescence intensity ratios of (B) Nav1.7 and (C) CBS in each group. *P<0.05 vs. Sham group. n=6 per group. Nav1.7, voltage-gated sodium channel 1.7; CBS, cystathionine β-synthetase.
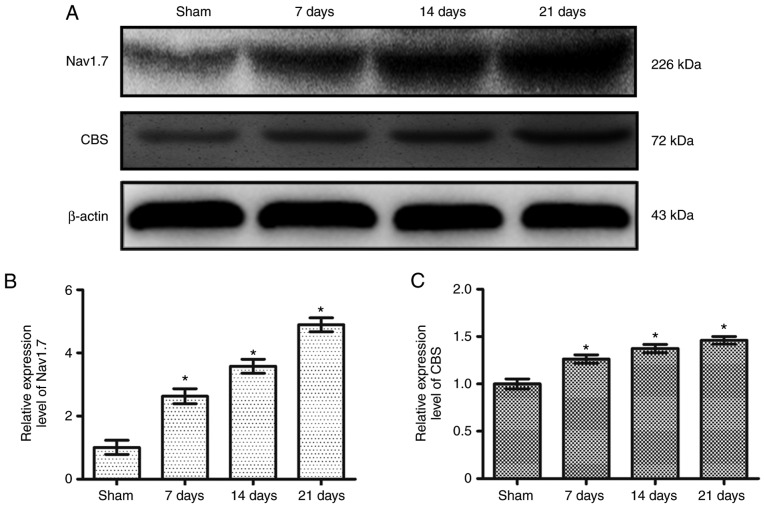
Compared with the Sham group (1.00±0.22), the relative expression level of Nav1.7 was significantly increased at 7 (2.63±0.24; P<0.05), 14 (3.58±0.22; P<0.05) and 21 days (4.88±0.22; P<0.05) post-SNI surgery (Fig. 4A and B). Furthermore, compared with the Sham group (1.0±0.05), the relative expression level of CBS was also significantly increased at 7 (1.26±0.04; P<0.05), 14 (1.37±0.04; P<0.05) and 21 days (1.46±0.04; P<0.05) post-SNI surgery (Fig. 4A and C). The changes observed were most prominent after 21 days; therefore, in all subsequent experiments, measurements were taken 21 days after surgery.
Figure 4.
Protein expression levels of Nav1.7 and CBS in dorsal root ganglion neurons of spared nerve injury rats. (A) Expression levels of Nav1.7 and CBS in each group. Relative expression of (B) NaV1.7 and (C) CBS in each group. * P<0.05 vs. Sham group. n=6 per group. Nav1.7, voltage-gated sodium channel 1.7; CBS, cystathionine β-synthetase.
Endogenous H2S upregulates the expression of Nav1.7 by activating the MEK/ERK pathway
To investigate how H2S regulates the expression of Nav1.7 in DRG neurons of rats following SNI surgery, western blotting was performed following treatment with AOAA, U0126 and PF-04856264 (Fig. 5A).
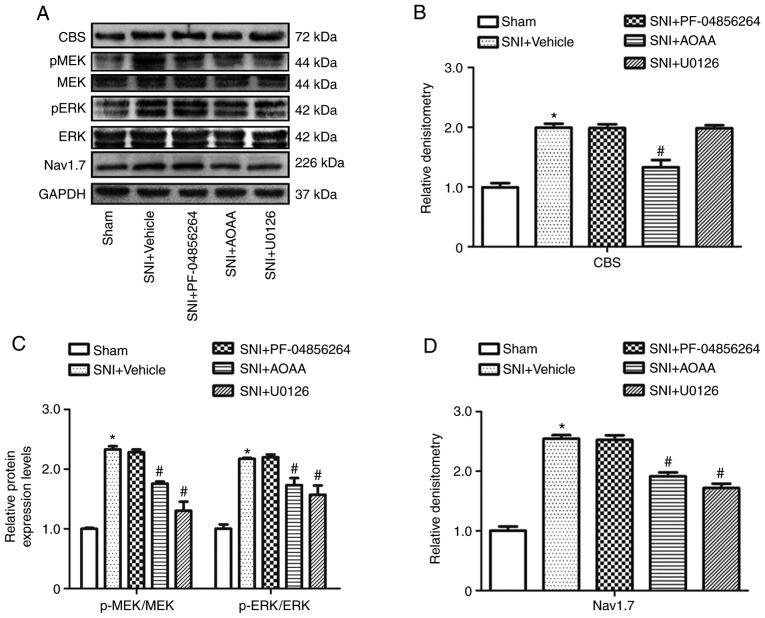
Figure 5.
Changes in protein expression levels following the administration of AOAA, U0126 or PF-04856264 in dorsal root ganglions of SNI rats. (A) Expression levels of CBS, MEK, p-MEK, ERK, p-ERK, Nav1.7 and GAPDH in each group. Relative expression of (B) CBS, (C) p-MEK/MEK, p-ERK/ERK and (D) Nav1.7 in each group. *P<0.05 vs. Sham group. #P<0.05 vs. SNI+Vehicle group. n=6 per group. AOAA, O-(carboxymethyl) hydroxylamine hemihydrochloride; SNI, spared nerve injury; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase; p, phosphorylated; cystathionine β-synthetase; Nav1.7, voltage-gated sodium channel 1.7.
Compared with the Sham group (1.0±0.04), the relative expression level of CBS (1.99±0.04; P<0.05) was increased significantly in the SNI+Vehicle group. Compared with the SNI+Vehicle group (1.99±0.04), the relative expression of CBS was reduced significantly in the SNI+AOAA group (1.33±0.07; P<0.05). However, there was no significant difference in the relative expression of CBS following administration of U0126 (1.98±0.03; P>0.05) and PF-04856264 (1.99±0.04; P>0.05; Fig. 5B).
Compared with the Sham group, the relative ratios of p-MEK/MEK and p-ERK/ERK were significantly increased in DRG neurons after SNI surgery (P<0.05). In addition, compared with the SNI+Vehicle group, the relative ratios of p-MEK/MEK and p-ERK/ERK were reduced significantly in the SNI+AOAA and SNI+U0126 groups (P<0.05). However, there were no significant differences in relative ratios of p-MEK/MEK and p-ERK/ERK following administration of PF-04856264 (P>0.05; Fig. 5C).
Compared with the Sham group (1.0±0.04), the relative expression of Nav1.7 was increased significantly in the SNI+Vehicle group (2.54±0.03; P<0.05). Compared with the SNI+Vehicle group (2.54±0.03), the relative expression level of Nav1.7 was reduced significantly in the SNI+AOAA group (1.91±0.04; P<0.05). In addition, the relative expression of Nav1.7 was also reduced significantly in the SNI+U0126 group (1.72±0.04; P<0.05; Fig. 5D).
Endogenous H2S upregulates Nav1.7 expression by activating the MEK/ERK pathway and increasing the Nav1.7-carried current density
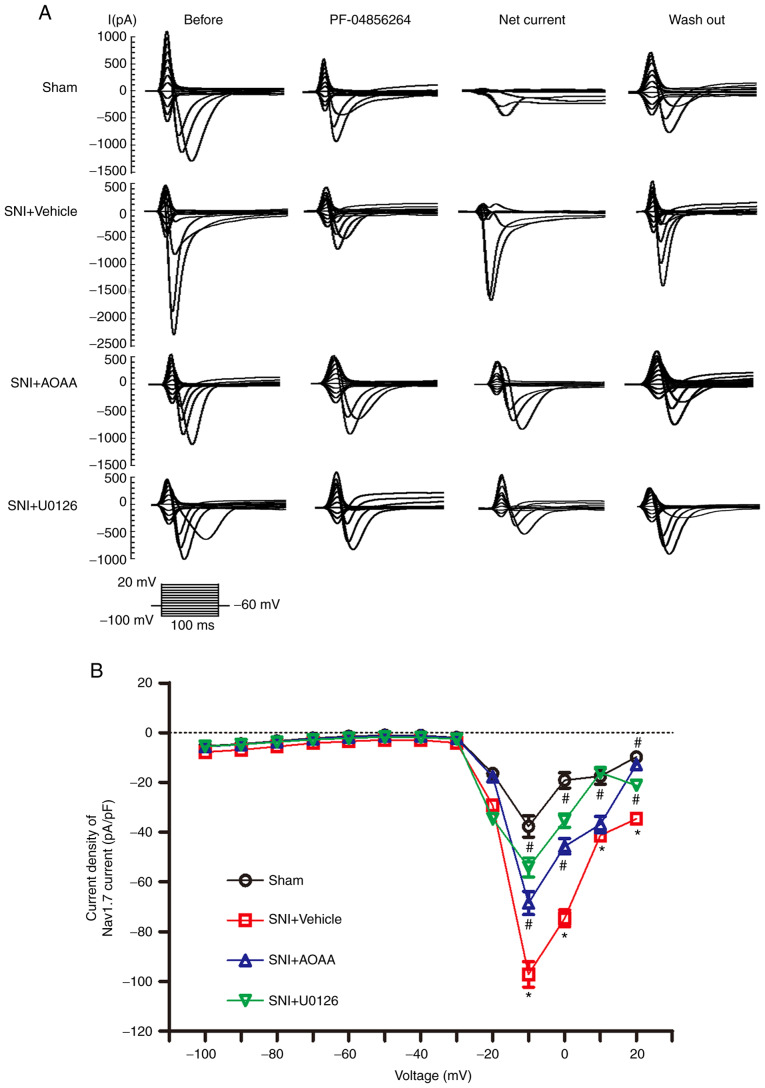
To investigate whether endogenous H2S upregulates Nav1.7 expression via the MEK/ERK signaling pathway and increases the sodium current carried by Nav1.7 in DRG neurons, patch-clamp experiments were performed. The 'Net' current was obtained by subtracting the PF-04856264 from the 'Before' current, which represents the current sensitive to PF-04856264, and the Net current was taken to represent the current carried by Nav1.7 (Fig. 6A). For a more accurate measure of the current 'felt' by the cells, the current density was calculated.
Figure 6.
Changes in the current density carried by Nav1.7 following administration of AOAA or U0126 in DRG neurons of SNI rats. (A) Representative traces showing the sodium current in DRG neurons stimulated using a step protocol from -100 to 20 mV in 10 mV increments with a pulse duration of 100 msec. Before: Traces before cells were perfused with PF-04856264. PF-04856264: Traces after incubation with PF-04856264 for 60 sec. Net current: Traces obtained by subtracting the traces of PF-04856264 from the traces of Before. Wash out: Traces after the extracellular fluid was washed for 4 min. (B) Current density-voltage curves of DRG neurons from each group. *P<0.05 vs. Sham group. #P<0.05 vs. SNI+Vehicle group. n=7 per group. DRG, dorsal root ganglion; SNI, spared nerve injury; AOAA, O-(carboxymethyl) hydroxylamine hemihydrochloride; Nav1.7, voltage-gated sodium channel 1.7.
At the voltage of -10 mV, compared with the Sham group (-37.69±4.31), the current density of Nav1.7 was significantly increased in the SNI+Vehicle group (-97.19±5.14; P<0.05). Compared with the SNI+Vehicle group, administration of AOAA (-68.53±4.66; P<0.05) and U0126 (-54.18±3.78; P<0.05) significantly decreased the current density of Nav1.7 (Fig. 6B). At the voltage of 0 mV, the current density of Nav1.7 was significantly increased in the SNI+Vehicle group (-74.71±3.38) compared with Sham group (-19.18±3.06; P<0.05). Compared with the SNI+Vehicle group, administration of AOAA (-45.69±3.07; P<0.05) and U0126 (-35.40±2.69; P<0.05) significantly decreased the current density of Nav1.7. Furthermore, at the voltage of 20 mV, the current density of Nav1.7 was significantly increased in the SNI+Vehicle group (-34.71±1.45) compared with the Sham group (-9.79±1.58; P<0.05). Compared with the SNI+Vehicle group, administration of AOAA (-12.84±1.32; P<0.05) and U0126 (-21.25±1.39; P<0.05) significantly decreased the current density of Nav1.7. At the voltage of 10 mV, the current density of Nav1.7 was significantly increased in the SNI+Vehicle group (-41.35±1.6) compared with the Sham group (-17.47±3.16; P<0.05). Compared with the SNI+Vehicle group, administration of U0126 (-16.10±1.74; P<0.05) decreased the current density of Nav1.7. However, the current density of Nav1.7 was not changed after administration of AAOA (-36.93±3.22; P>0.05).
Endogenous H2S upregulates Nav1.7 by activating the MEK/ERK signaling pathway, resulting in increased excitability of DRG neurons
To investigate whether endogenous H2S results in upregulated expression of Nav1.7 by the MEK/ERK pathway and enhances the excitability of DRG neurons, patch-clamp experiments were performed (Fig. 7). The rheobase (the minimum current intensity required to excite the first action potential) and the number of the action potentials were used as indicators of the excitability of DRG neurons.
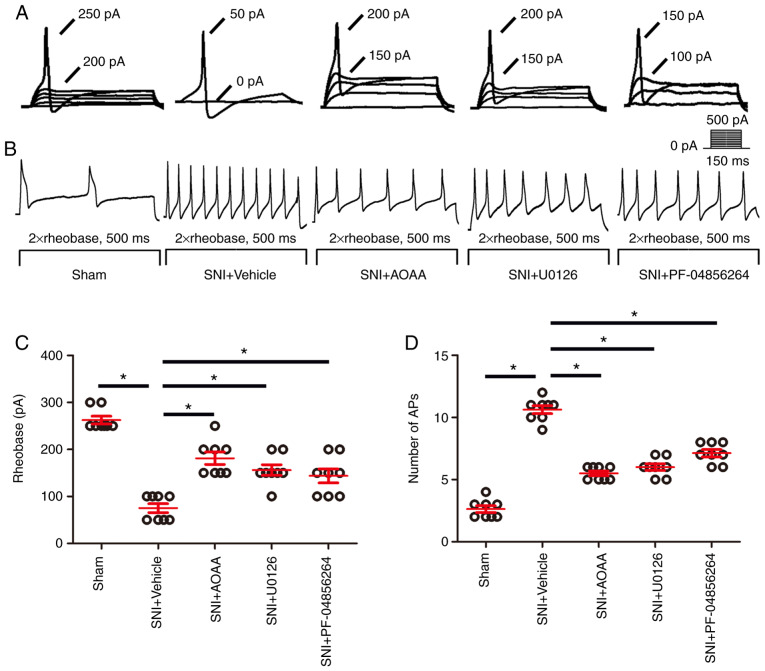
Figure 7.
AOAA, U0126 and PF-04856264 reduce the increase in excitability of DRGs in SNI rats. (A) Representative traces showing the rheobases of action potentials evoked by current injections to DRG neurons of rats in each group. (B) Typical traces showing the action potentials elicited by twice the strength of the rheobase for 500 msec in DRG neurons of rats in each group. (C) Scatter plot showing the statistical comparison of the rheobase of action potentials in each group. (D) Scatter plot showing the statistical comparison of the number of action potentials elicited by twice the strength of the rheobase for 500 msec in each group. *P<0.05. n=8 for each group. DRG, dorsal root ganglion; SNI, spared nerve injury; AOAA, O-(carboxymethyl)hydroxylamine hemihydrochloride. DRG, dorsal root ganglion; SNI, spared nerve injury; AOAA, O-(carboxymethyl) hydroxylamine hemihydrochloride.
Compared with the Sham group (262.5±8.18), the rheo-base of action potentials was significantly decreased in the SNI+Vehicle group (75.00±9.45; P<0.05). Compared with the SNI+Vehicle group, administration of AOAA (181.13±16.33), U0126 (156.25±11.33) and PF-04856264 (143.75±14.75) significantly increased the rheobase of action potentials (P<0.05; Fig. 7C).
Compared with the Sham group (2.63±0.26), the number of action potentials was significantly increased in the SNI+Vehicle group (10.63±0.32; P<0.05). Compared with the SNI+Vehicle group, administration of AOAA (5.50±0.19), U0126 (6.0±0.27) and PF-04856264 (7.13±0.30) significantly decreased the number of action potentials (P<0.05; Fig. 7D).
Endogenous H2S alters the behavior of rats by upregulating expression of Nav1.7
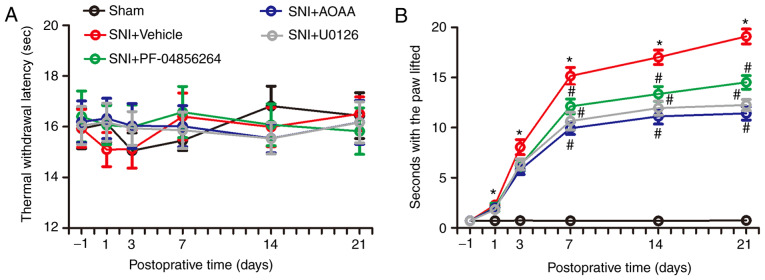
In order to investigate the changes in nociceptive behavior of rats following SNI surgery, and to detect changes in nociceptive behavior in SNI rats after administration of AOAA, U0126 or PF-04856264, a Hargreaves test and acetone experiments were performed. There were no significant differences between each group in terms of thermal withdrawal latency (TWL) values (F=0.130; P=0.9416; Fig. 8A). However, the acetone experiments demonstrated that, compared with the Sham group, the time animals spent with the paw lifted in the SNI+Vehicle group increased significantly between days 1 and 21 (P<0.05). Compared with the SNI+Vehicle group, administration of AOAA or U0126 significantly decreased the time with the paw lifted between days 7 and 21 (P<0.05). In addition, compared with the SNI+Vehicle group, administration of PF-04856264 significantly decreased the time with the paw lifted between days 14 and 21 (P<0.05; Fig. 8).
Figure 8.
Changes in nociceptive behavior in each group. (A) Thermal withdrawal latency of rats in each group. (B) Time with the paw lifted for rats in each group. *P<0.05 vs. Sham group. #P<0.05 vs. SNI+Vehicle group. n=6 per group. SNI, spared nerve injury; AOAA, O-(carboxymethyl) hydroxylamine hemihydrochloride.
Discussion
The primary findings of the present study were: i) The concentration of H2S in DRG neurons was increased after SNI modeling; ii) the expression levels of Nav1.7 and CBS were increased in DRG neurons of SNI rats; iii) endogenous H2S upregulates the expression of Nav1.7 by activating the MEK/ERK signaling pathway; iv) endogenous H2S upregulates Nav1.7 by activating the MEK/ERK pathway, resulting in increased Nav1.7 carried current, and thus an increase in the excitability of DRG neurons; and v) endogenous H2S alters the behavior of rats by upregulating Nav1.7. Together, these results suggest that endogenous H2S and Nav1.7 serve an important role in neuropathic pain.
Previous studies have shown that Nav1.7, one of the nine subtypes of VGSCs, serves an important role in human pain disorders, including idiopathic small fiber neuropathies, paroxysmal extreme pain disorder, inherited erythromelalgia and congenital indifference to pain (23-26). Upon plantar incision surgery, the expression of Nav1.7 in L4-L6 DRG neurons of rats is increased, as well as the cumulative pain score. Those changes are inhibited when rats are pretreated with SCN9A-RNA interference lentivirus delivered via an intrathecal tube (4). He et al (25) confirmed that R1488*, a variant of SCN9A, results in a complete loss-of-function of Nav1.7, which is consistent with variants in this gene in subjects with congenital insensitivity to pain. Geha et al (27) demonstrated that pharmacotherapy guided by genomic analysis, molecular modeling and functional profiling attenuated neuropathic pain in patients carrying an S241T Nav1.7 mutant channel. In the present study, it was shown that Nav1.7 is expressed in different types of DRG neurons (NF-200, CGRP and IB4) and the expression of Nav1.7 was increased in L4-L6 DRG neurons of SNI rats. Previous studies have reported that there is a relationship between the diameter of DRG neurons in rats and their excitatory typing, and the excitability of small and medium-sized cells was higher compared with that of small and medium-sized cells, indicating that small and medium-sized cells play a more important role in the generation of neuropathic pain (28,29). In addition, our previous study (30) demonstrated that the changes in excitatory typing of DRG neurons with different sizes potentially explains the mechanisms of neuropathic pain, and after SNI surgery the excitatory type of DRG neurons in rats changed, with the proportion of type 1 and type 2 cells increased, but the proportion of type 3 cells decreased. Therefore, neurons with excitatory changes were selected to be recorded in the patch clamp experiment. This result is consistent with the findings of a previous study (30). In addition, in the present study, the excitability of rat DRG neurons increased, and rats developed cold allodynia following SNI surgery, which was inhibited by the Nav1.7 specific blocker PF-04856264.
An increasing number of studies have shown that endogenous H2S has a variety of physiological functions, including considerable support for a role of H2S as a neuromodulator (31-33) or an endogenous gaseous transmitter (34). Under physiological conditions, H2S has been shown to regulate key neuronal functions, including modulation of inward or outward currents on dorsal raphe serotonergic neurons in vitro (35), or regulating the release of corticotrophin-releasing hormone from the hypothalamus (36). H2S is an important endogenous vasoactive factor and is a gaseous opener of K+-ATP channels in vascular smooth muscle cells (34). CBS and systathionine γ-lyase (CSE) are two important enzymes involved in the generation of endogenous H2S (37-41), which are expressed in the spinal cord and colon, and detectable quantities of H2S are produced by these tissues in the presence of L-cysteine, a CSE/CBS substrate (42). CBS and CSE expression have been observed in several mammalian tissues, including liver, kidney, brain, ileum and blood lymphocytes (34). In the cardiovascular system, H2S is predominantly derived from CSE, and modulates endothelium-dependent and endothelium-independent vasodilatation (43), whereas CBS-derived H2S is a physiologically relevant neuromodulator in the central nervous system (CNS) (44). Consistent with this view, it has been shown that H2S is present at relatively high levels in the mammalian brain, and that in the CNS, the activity of CBS is >30-fold greater than that of CSE (45). Xu et al (46) reported that CBS, but not CSE, is expressed by colon-specific sensory neurons. Similarly, the expression of CBS in L4-L6 DRG neurons was also shown in the present immunofluorescence experiments. These results suggest that the CBS-H2S pathway may serve an important role in the nervous system. Previous studies have shown that sodium channels, T-type calcium channels, transient receptor potential cation channel subfamily V member 1 receptors, transient receptor potential cation channel subfamily A member 1 receptors and N-methyl-D-aspartic acid receptors are targets of H2S-induced pain hypersensitivity (47-53). Yan et al (14) demonstrated that the expression of CBS increased in DRG neurons in a lumbar disc herniation pain model in rats, and that the administration of AOAA partially reversed this change. In the present study, the expression of CBS increased in DRG neurons of SNI rats. Furthermore, it has previously been shown that the activity of sodium channels is enhanced by upregulation of CBS expression in DRG neurons (54), and that treatment with AOAA significantly suppresses the expression of Nav1.7 and Nav1.8 in DRG neurons (14). Wang et al (55) reported that AOAA treatment significantly hyperpolarized the resting potential of colon-specific DRG neurons and enhanced the rheobase. The results of the present study demonstrated that the concentration of H2S in DRG neurons increased following SNI modeling and the administration of AOAA partially reversed this change. Furthermore, the increased expression of Nav1.7 and enhanced excitability of DRG neurons induced by SNI surgery was partially reversed by inhibiting CBS with AOAA. The reduced number of action potentials and the increased rheobase may explain the reversal in neuronal excitability following application of AOAA.
H2S can regulate various physiological and pathophysiological processes by regulating MAPK. Lan et al (56) demonstrated that H2S protects PC12 cells against chemical hypoxia-induced injuries by inhibition of reactive oxygen species-mediated activation of the ERK1/2 and p38-MAPK signaling pathways. Gobbi et al (57) reported that H2S impairs the growth and adhesion of keratinocyte cells by inhibiting the Raf-MAPK-ERK signaling pathway. Additionally, it has been shown that ERK serves an active role in mediating H2S-induced apoptosis in human aorta smooth muscle cells by activating caspase-3 (58). Stamboulian et al (16) reported that p-ERK phosphorylates Nav1.7 and induces hyperpolarized activation and fast inactivation of Nav1.7. U0126 inhibits the expression of p-ERK (16,59). The ERK signaling cascade regulates a variety of important cellular processes including growth, proliferation and pain (60,61). Activated cAMP response element-binding protein (CREB) is known to exhibit neuroprotective and growth promoting effects (62). Sabbir and Fernyhough (63) reported that 1 h of treatment with muscarinic toxin 7 and pirenzepine activates ERK through muscarinic acetylcholine type 1 receptor and induces a significant increase in levels of p-CREB in cultured sensory neurons. However, in the present study, it was demonstrated that p-MEK and p-ERK levels were increased in DRG neurons of SNI rats, and these changes were reversed by administration of AOAA, but not by administration of PF-04856264. In addition, administration of U0126 inhibited the increase in Nav1.7 expression, suggesting that p-MEK/p-ERK regulated the expression of Nav1.7 in DRG neurons of SNI rats (Fig. 9). The process of p-ERK regulating p-CREB needs to be further evaluated in future experiments. Furthermore, there may be a certain relationship between p-CREB and Nav1.7 (Fig. 9), which needs to be further researched in subsequent experiments.
Figure 9.
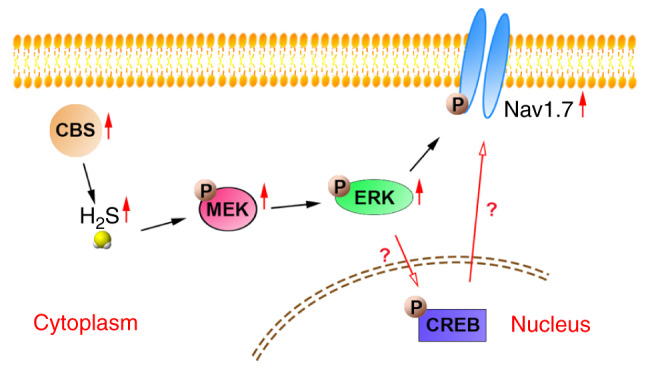
Schematic diagram of the CBS/H2S-MEK/ERK signaling pathway-mediated regulation of Nav1.7 expression. p-ERK may regulate the expression of Nav1.7 via CREB in the nucleus. Nav1.7, voltage-gated sodium channel 1.7; ERK, extracellular signal-regulated kinase; MEK, mitogen-activated protein kinase kinase; p, phosphorylated; CBS, cysta-thionine β-synthetase; CREB, cAMP-response element binding protein; H2S, hydrogen sulfide.
In conclusion, endogenous H2S serves an important role in the development and maintenance of neuropathic pain by activating the MEK/ERK pathway to upregulate the Nav1.7 channel. These results may assist in the identification of novel drug targets for the treatment of neuropathic pain. One limitation of the present study was that the mechanisms underlying the increase in Nav1.7 were not determined, and thus require further study.
Acknowledgments
This study was performed at the Department of Physiology and the Key Laboratory of Xinjiang Endemic and Ethnic Diseases of Xinjiang Provincial, Shihezi University School of Medicine (Shihezi, China).
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 81560081).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JQS, LL and KTM conceived and designed the study, implementation of the study and writing of the manuscript: JJT, CYT and QYC performed the experiments and wrote the manuscript. WYS, YZ, ZWQ and MZ analyzed and described the data. WYS, LL and KTM were involved in critically revising the manuscript for important intellectual content. All authors read and approved the final manuscript and agree to be responsible for all aspects of the study.
Ethics approval and consent to participate
Animal experiments were approved by the Institutional Ethics Review Board at the First Affiliated Hospital of the Shihezi University School of Medicine (approval no. A2017-169-01) and were performed in accordance with the Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals of the International Association for the Study of Pain.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice AS, Treede RD. A new definition of neuropathic pain. Pain. 2011;152:2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Wang K, Lin M, Li Q, Hong Y. Inhibition of morphine tolerance by MrgC receptor via modulation of interleukin-1β and matrix metalloproteinase 9 in dorsal root ganglia in rats. Eur J Pharmacol. 2017;815:10–17. doi: 10.1016/j.ejphar.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Sun J, Li N, Duan G, Liu Y, Guo S, Wang C, Zhu C, Zhang X. Increased Na1.7 expression in the dorsal root ganglion contributes to pain hypersensitivity after plantar incision in rats. Mol Pain. 2018;14:1744806918782323. doi: 10.1177/1744806918782323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai H, Asaoka N, Miyake T, Nagayasu K, Nakagawa T, Shirakawa H, Kaneko S. Neurotropin inhibits neuronal activity through potentiation of sustained K currents in primary cultured DRG neurons. J Pharmacol Sci. 2018;137:313–316. doi: 10.1016/j.jphs.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Pachuau J, Martin-Caraballo M. Expression pattern of T-type Ca (2+) channels in embryonic chick nodose ganglion neurons. Dev Neurobiol. 2007;67:1901–1914. doi: 10.1002/dneu.20563. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura N, Nagami E, Matsushita Y, Kayano T, Shibuya I. Constitutive activity of transient receptor potential vanilloid type 1 triggers spontaneous firing in nerve growth factor-treated dorsal root ganglion neurons of rats. IBRO Rep. 2018;5:33–42. doi: 10.1016/j.ibror.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang W, Berta T, Kim YH, Lee S, Lee SY, Ji RR. Expression and role of voltage-gated sodium channels in human dorsal root ganglion neurons with special focus on Nav1.7, species differences, and regulation by paclitaxel. Neurosci Bulletin. 2018;34:4–12. doi: 10.1007/s12264-017-0132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koenig J, Werdehausen R, Linley JE, Habib AM, Vernon J, Lolignier S, Eijkelkamp N, Zhao J, Okorokov AL, Woods CG, et al. Regulation of Nav1.7: A conserved SCN9A natural anti-sense transcript expressed in dorsal root ganglia. PLoS One. 2015;10:e0128830. doi: 10.1371/journal.pone.0128830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DT, Rossignol E, Najem K, Ospina LH. Bilateral congenital corneal anesthesia in a patient with SCN9A mutation, confirmed primary erythromelalgia, and paroxysmal extreme pain disorder. J AAPOS. 2015;19:478–479. doi: 10.1016/j.jaapos.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS, Estacion M, Lauria G, Vanhoutte EK, Gerrits MM, et al. Gain of function Naν1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71:26–39. doi: 10.1002/ana.22485. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Qu R, Hu S, Xiao Y, Jiang X, Xu GY. Upregulation of cystathionine β-synthetase expression contributes to visceral hyperalgesia induced by heterotypic intermittent stress in rats. PLoS One. 2012;7:e53165. doi: 10.1371/journal.pone.0053165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu S, Xiao Y, Zhu L, Li L, Hu CY, Jiang X, Xu GY. Neonatal maternal deprivation sensitizes voltage-gated sodium channel currents in colon-specific dorsal root ganglion neurons in rats. Am J Physiol Gastrointest Liver Physiol. 2013;304:G311–G321. doi: 10.1152/ajpgi.00338.2012. [DOI] [PubMed] [Google Scholar]
- 14.Yan J, Hu S, Zou K, Xu M, Wang Q, Miao X, Yu SP, Xu GY. Inhibition of cystathionine β-synthetase suppresses sodium channel activities of dorsal root ganglion neurons of rats with lumbar disc herniation. Sci Rep. 2016;6:38188. doi: 10.1038/srep38188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004;2004:reE14. doi: 10.1126/stke.2522004re14. [DOI] [PubMed] [Google Scholar]
- 16.Stamboulian S, Choi JS, Ahn HS, Chang YW, Tyrrell L, Black JA, Waxman SG, Dib-Hajj SD. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J Neurosci. 2010;30:1637–1647. doi: 10.1523/JNEUROSCI.4872-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casals-Díaz L, Casas C, Navarro X. Changes of voltage-gated sodium channels in sensory nerve regeneration and neuropathic pain models. Restor Neurol Neurosci. 2015;33:321–334. doi: 10.3233/RNN-140444. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Gao CX, Wang YP, Ma KT, Li L, Yin JW, Dai ZG, Wang S, Si JQ. The association between the expression of PAR2 and TMEM16A and neuropathic pain. Mol Med Rep. 2018;17:3744–3750. doi: 10.3892/mmr.2017.8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norcini M, Sideris A, Martin Hernandez LA, Zhang J, Blanck TJ, Recio-Pinto E. An approach to identify microRNAs involved in neuropathic pain following a peripheral nerve injury. Front Neurosci. 2014;8:266. doi: 10.3389/fnins.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu D, Wu X, Grabauskas G, Owyang C. Butyrate-induced colonic hypersensitivity is mediated by mitogen-activated protein kinase activation in rat dorsal root ganglia. Gut. 2013;62:1466–1474. doi: 10.1136/gutjnl-2012-302260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deuis JR, Wingerd JS, Winter Z, Durek T, Dekan Z, Sousa SR, Zimmermann K, Hoffmann T, Weidner C, Nassar MA, et al. Analgesic effects of GpTx-1, PF-04856264 and CNV1014802 in a mouse model of Nav17-mediated pain. Toxins (Basel) 2016;8:pii: E78. doi: 10.3390/toxins8030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Reichert E, Cohen AE. Optical electrophysiology for probing function and pharmacology of voltage-gated ion channels. ELife. 2016;5:pii: e15202. doi: 10.7554/eLife.15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi JS, Boralevi F, Brissaud O, Sánchez-Martín J, Te Morsche RH, Dib-Hajj SD, Drenth JP, Waxman SG. Paroxysmal extreme pain disorder: A molecular lesion of peripheral neurons. Nat Rev Neurol. 2011;7:51–55. doi: 10.1038/nrneurol.2010.162. [DOI] [PubMed] [Google Scholar]
- 24.Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W, Young GT, Zhang B, Cox PJ, Cho LT, John S, Paciga SA, Wood LS, Danziger N, Scollen S, Vangjeli C. Functional confirmation that the R1488* variant in SCN9A results in complete loss-of-function of Na1.7. BMC Med Genet. 2018;19:124. doi: 10.1186/s12881-018-0643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummins TR, Dib-Hajj SD, Waxman SG. Electrophysiological properties of mutant Nav1.7 sodium channels in a painful inherited neuropathy. J Neurosci. 2004;24:8232–8236. doi: 10.1523/JNEUROSCI.2695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geha P, Yang Y, Estacion M, Schulman BR, Tokuno H, Apkarian AV, Dib-Hajj DS, Waxman SG. Pharmacotherapy for pain in a family with inherited erythromelalgia guided by genomic analysis and functional profiling. JAMA Neurol. 2016;73:659–667. doi: 10.1001/jamaneurol.2016.0389. [DOI] [PubMed] [Google Scholar]
- 28.Fang X, Djouhri L, McMullan S, Berry C, Waxman SG, Okuse K, Lawson SN. Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. J Neurosci. 2006;26:7281–7292. doi: 10.1523/JNEUROSCI.1072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Cavanaugh DJ, Nemenov MI, Basbaum AI. The modality-specific contribution of peptidergic and non-peptidergic nociceptors is manifest at the level of dorsal horn nociresponsive neurons. J Physiol. 2013;591:1097–1110. doi: 10.1113/jphysiol.2012.242115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan CY, Ma KT, Si JQ, Zhou Y, Qu ZW, Zhang M, Chen QY, Tian JJ, Xu ZZ, Deng SY. Type of excitability of DRG neurons in rats with neuropathic pain. Chin J Mod Med. 2019;29:1–7. [Google Scholar]
- 31.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol. 2002;26:13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- 33.Moore PK, Bhatia M, Moochhala S. Hydrogen sulfide: From the smell of the past to the mediator of the future? Trends Pharmacol Sci. 2003;24:609–611. doi: 10.1016/j.tips.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Wang R. Two's company, three's a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 35.Kombian SB, Reiffenstein RJ, Colmers WF. The actions of hydrogen sulfide on dorsal raphe serotonergic neurons in vitro. J Neurophysiol. 1993;70:81–96. doi: 10.1152/jn.1993.70.1.81. [DOI] [PubMed] [Google Scholar]
- 36.Dello Russo C, Tringali G, Ragazzoni E, Maggiano N, Menini E, Vairano M, Preziosi P, Navarra P. Evidence that hydrogen sulphide can modulate hypothalamopituitary-adrenal axis function: In vitro and in vivo studies in the rat. J Neuroendocrinol. 2000;12:225–233. doi: 10.1046/j.1365-2826.2000.00441.x. [DOI] [PubMed] [Google Scholar]
- 37.Eto K, Kimura H. A novel enhancing mechanism for hydrogen sulfide-producing activity of cystathionine beta-synthase. J Biol Chem. 2002;277:42680–42685. doi: 10.1074/jbc.M205835200. [DOI] [PubMed] [Google Scholar]
- 38.Julian D, Statile JL, Wohlgemuth SE, Arp AJ. Enzymatic hydrogen sulfide production in marine invertebrate tissues. Comp Biochem Physiol A Part, Mol Integr Physiol. 2002;133:105–115. doi: 10.1016/S1095-6433(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Jhee KH, Kruger WD. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J Biol Chem. 2004;279:52082–52086. doi: 10.1074/jbc.C400481200. [DOI] [PubMed] [Google Scholar]
- 40.Yusuf M, Kwong Huat BT, Hsu A, Whiteman M, Bhatia M, Moore PK. Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulfide biosynthesis. Biochem Biophys Res Commun. 2005;333:1146–1152. doi: 10.1016/j.bbrc.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Levonen AL, Lapatto R, Saksela M, Raivio KO. Human cystathionine gamma-lyase: Developmental and in vitro expression of two isoforms. Biochem J. 2000;347:291–295. doi: 10.1042/bj3470291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith HS. Hydrogen sulfide's involvement in modulating nociception. Pain Physician. 2009;12:901–910. [PubMed] [Google Scholar]
- 43.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boehning D, Snyder SH. Novel neural modulators. Ann Rev Neurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- 45.Awata S, Nakayama K, Suzuki I, Sugahara K, Kodama H. Changes in cystathionine gamma-lyase in various regions of rat brain during development. Biochem Mol Biol Int. 1995;35:1331–1338. [PubMed] [Google Scholar]
- 46.Xu GY, Winston JH, Shenoy M, Zhou S, Chen JD, Pasricha PJ. The endogenous hydrogen sulfide producing enzyme cystathionine-beta synthase contributes to visceral hypersensitivity in a rat model of irritable bowel syndrome. Mol Pain. 2009;5:44. doi: 10.1186/1744-8069-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatakeyama Y, Takahashi K, Tominaga M, Kimura H, Ohta T. Polysulfide evokes acute pain through the activation of nociceptive TRPA1 in mouse sensory neurons. Mol Pain. 2015;11:24. doi: 10.1186/s12990-015-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maeda Y, Aoki Y, Sekiguchi F, Matsunami M, Takahashi T, Nishikawa H, Kawabata A. Hyperalgesia induced by spinal and peripheral hydrogen sulfide: Evidence for involvement of Cav3.2 T-type calcium channels. Pain. 2009;142:127–132. doi: 10.1016/j.pain.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 49.Kawabata AT, Ishiki K, Nagasawa K, Yoshida S, Maeda Y, Takahashi T, Sekiguchi F, Wada T, Ichida S, Nishikawa H. Hydrogen sulfide as a novel nociceptive messenger. Pain. 2007;132:74–81. doi: 10.1016/j.pain.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 50.Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H, Ishii I, De Giorgio R, Campi B, Schemann M. Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology. 2006;131:1542–1552. doi: 10.1053/j.gastro.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 51.Krueger D, Foerster M, Mueller K, Zeller F, Slotta-Huspenina J, Donovan J, Grundy D, Schemann M. Signaling mechanisms involved in the intestinal pro-secretory actions of hydrogen sulfide. Neurogastroenterol Motil. 2010;22:1224–1231. e319–320. doi: 10.1111/j.1365-2982.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhao S, Liu FF, Wu YM, Jiang YQ, Guo YX, Wang XL. Upregulation of spinal NMDA receptors mediates hydrogen sulfide-induced hyperalgesia. J Neurol Sci. 2016;363:176–181. doi: 10.1016/j.jns.2016.02.058. [DOI] [PubMed] [Google Scholar]
- 53.Hu S, Xu W, Miao X, Gao Y, Zhu L, Zhou Y, Xiao Y, Xu GY. Sensitization of sodium channels by cystathionine β-synthetase activation in colon sensory neurons in adult rats with neonatal maternal deprivation. Exp Neurol. 2013;248:275–285. doi: 10.1016/j.expneurol.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 54.Qu R, Tao J, Wang Y, Zhou Y, Wu G, Xiao Y, Hu CY, Jiang X, Xu GY. Neonatal colonic inflammation sensitizes voltage-gated Na (+) channels via upregulation of cystathionine β-synthetase expression in rat primary sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2013;304:G763–G772. doi: 10.1152/ajpgi.00466.2012. [DOI] [PubMed] [Google Scholar]
- 55.Wang HJ, Xu X, Xie RH, Rui YY, Zhang PA, Zhu XJ, Xu GY. Prenatal maternal stress induces visceral hypersensitivity of adult rat offspring through activation of cystathionine- β-synthase signaling in primary sensory neurons. Mol Pain. 2018;14:1744806918777406. doi: 10.1177/1744806918777406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lan A, Liao X, Mo L, Yang C, Yang Z, Wang X, Hu F, Chen P, Feng J, Zheng D, Xiao L. Hydrogen sulfide protects against chemical hypoxia-induced injury by inhibiting ROS-activated ERK1/2 and p38MAPK signaling pathways in PC12 cells. PLoS One. 2011;6:e25921. doi: 10.1371/journal.pone.0025921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gobbi G, Ricci F, Malinverno C, Carubbi C, Pambianco M, Panfilis GD, Vitale M, Mirandola P. Hydrogen sulfide impairs keratinocyte cell growth and adhesion inhibiting mitogen-activated protein kinase signaling. Lab Invest. 2009;89:994–1006. doi: 10.1038/labinvest.2009.61. [DOI] [PubMed] [Google Scholar]
- 58.Yang G, Sun X, Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004;18:1782–1784. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- 59.Yang F, Sun W, Yang Y, Wang Y, Li CL, Fu H, Wang XL, Yang F, He T, Chen J. SDF1-CXCR4 signaling contributes to persistent pain and hypersensitivity via regulating excitability of primary nociceptive neurons: Involvement of ERK-dependent Nav1.8 up-regulation. J Neuroinflammation. 2015;12:219. doi: 10.1186/s12974-015-0441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, Zhang Y, Huo Y, Wang D, Hong Y. Adrenomedullin mediates tumor necrosis factor-α-induced responses in dorsal root ganglia in rats. Brain Res. 2016;1644:183–191. doi: 10.1016/j.brainres.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 61.Ma W, Zheng WH, Powell K, Jhamandas K, Quirion R. Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: An in vitro and in vivo study. Eur J Neurosci. 2001;14:1091–1104. doi: 10.1046/j.0953-816x.2001.01731.x. [DOI] [PubMed] [Google Scholar]
- 62.Wang WX, Wu Q, Liang SS, Zhang XK, Hu Q, Chen QH, Huang HJ, Xu L, Lou FQ. Dexmedetomidine promotes the recovery of neurogenesis in aged mouse with postoperative cognitive dysfunction. Neurosci Lett. 2018;677:110–116. doi: 10.1016/j.neulet.2018.03.043. [DOI] [PubMed] [Google Scholar]
- 63.Sabbir MG, Fernyhough P. Muscarinic receptor antagonists activate ERK-CREB signaling to augment neurite outgrowth of adult sensory neurons. Neuropharmacology. 2018;143:268–81. doi: 10.1016/j.neuropharm.2018.09.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.