Abstract
Accumulating evidence has demonstrated that endometrial stromal cells (ESCs) are responsible for the pathogenesis of endometriosis (Ems), which is characterized by the presence of functional endometrial-like tissues outside the uterine cavity. Abnormal expression of microRNAs (miRNAs) in ESCs may be implicated in the etiology of Ems; however, the exact mechanisms have yet to be fully elucidated. The aim of the present study was to investigate the effects of miRNAs on ESCs and the underlying mechanisms. Using a microarray assay, microRNA-16 (miR-16) was found to be significantly downregulated in the ectopic endometrial tissues in patients with Ems, compared with that in eutopic endometrial tissues. Overexpression of miR-16 significantly suppressed the migration and invasion of ESCs, whereas miR-16 inhibition exerted the opposite effects. Furthermore, dual luciferase reporter assay demonstrated that miR-16 directly targeted the inhibitor of nuclear factor (NF)-κB kinase subunit β (IKKβ) and suppressed its translation. It was observed that the expression of IKKβ was upregulated and inversely correlated with miR-16 levels in the ectopic endometrial tissues in patients with Ems. Additionally, knockdown of IKKβ by si-IKKβ mimicked the effects of miR-16 overexpression on ESCs, while the promoting effects of IKKβ overexpression on the migration and invasion of ESCs were attenuated by miR-16 overexpression. Finally, miR-16 inhibited the activation of the NF-κB pathway by targeting IKKβ. Collectively, these results demonstrated that miR-16 may suppress Ems by inhibiting the IKKβ/NF-κB pathway, suggesting that miR-16 may be a useful target in the treatment of Ems.
Keywords: endometriosis, microRNA-16, endometrial stromal cell, inhibitor of nuclear factor κB kinase subunit β/nuclear factor-κB pathway
Introduction
Endometriosis (Ems) is a common gynecological disease that manifests in ~5-20% of women experiencing pelvic pain, 20-50% of infertile women and 6-10% of women of reproductive age (1). Despite major developments in the treatment of Ems over the past decades (2), such as laparoscopic surgery, the recurrence rate remains high. The presence of functional endometrial-like tissues outside the uterine cavity is the main pathological characteristic of this disease, and the major reason for its formation is the excessive invasiveness of endometrial stromal cells (ESCs) (3). Therefore, therapeutic strategies that inhibit the invasion of ESCs may be beneficial in Ems.
MicroRNAs (miRNAs) are single-stranded non-coding RNAs that bind to the target mRNAs and interfere with the translation process (4). Aberrantly expressed miRNAs have been found in endometriotic tissues from patients with Ems, suggesting that miRNAs may play important roles in the development of Ems (5,6). For example, Liang et al demonstrated that miR-200c was downregulated in ectopic endometrial tissues, and overexpression of miR-200c suppressed the growth of endometriotic lesions in a rat model of Ems (7). Notably, several studies have demonstrated that the proliferative and invasive abilities of ESCs are regulated by miRNAs. Meng et al observed that inhibition of miR-126-5p enhanced the migration and invasion of ESCs by promoting the expression of breast cancer anti-estrogen resistance protein 3 (8). Zhang et al reported that miR-141-3p overexpression suppressed the proliferation and migration, and promoted the apoptosis of ESCs via targeting Krüppel-like factor 12 (9); however, the effects of miRNAs on the invasiveness of ESCs remain elusive.
A large number of studies have demonstrated the involvement of the nuclear factor (NF)-κB signaling pathway in the modulation of the invasion of ESCs in Ems (10,11). Inhibitor of NF-κB kinase subunit β (IKKβ) is an important regulator of the NF-κB pathway, in which IKK-mediated phosphorylation of IκB may facilitate NF-κB entering the nucleus and activating the expression of specific genes (12). It has been previously reported that miRNAs are key regulators of ESC function through the NF-κB signaling pathway. For example, Zhang et al demonstrated that miR-138 expression affected the growth of ESCs through the NF-κB signaling pathway (13). However, whether miRNAs affect the invasive ability of ESCs through the IKKβ/NF-κB pathway remains largely unknown.
In the present study, the miRNA expression profile was examined in endometriotic tissues from patients with Ems. The functions of miR-16 in suppressing the invasiveness of ESCs were determined, and the potential underlying mechanism was investigated. Taken together, these findings may provide theoretical evidence for the development of new treatment methods targeting miRNAs for Ems treatment.
Materials and methods
Patients and samples
A total of 20 eutopic and ectopic endometrial tissues were collected from patients who underwent laparoscopic surgery or hysterectomy at the Department of Gynecology of the Maternal and Child Care Service Center of Dongguan City Guangdong Province between October 2016 and October 2017. Control endometrium was obtained from 20 patients without Ems. All participants were legally considered adults, and no minors were included in the present study. Written informed consent was obtained from each patient and the protocol of the present study was approved by the Ethics Committee of the Maternal and Child Care Service Center of Dongguan City Guangdong Province.
Cell culture and transfection
Primary endometrial stromal cells were isolated using three eutopic endometrial tissues, as previously described (14). The cells were maintained in DMEM/F12 supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) in a 5% CO2 incubator at 37°C.
When cells in 6-well plates had grown to ~80% confluence, miR-16 mimics (20 nmol/l), miR-16 inhibitor (20 nmol/l), si-IKKβ (30 nM) or 2 µg pcDNA-IKKβ were transfected into cells at 37°C for 48 h, using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). The miR-16 mimics, mimics negative control (NC), miR-16 inhibitor and inhibitor NC were obtained from RiBoBio Co., Ltd. IKKβ overexpressing vector pcDNA-IKKβ and pcDNA vector were constructed by Qiagen, Inc. In addition, IKKβ siRNA-1, -2, -3 and corresponding negative control siRNA (si-Scramble) were purchased from RiboBio Co., Ltd.
miRNA microarray
Total RNA was extracted from three eutopic and three ectopic endometrium tissues by miRNeasy isolation kit (Qiagen, Inc.) according to the manufacturer's protocol. Total RNA (200 ng) was labeled with fluorescence dye Hy3 or Hy5 using the miRCURY Hy3/Hy5 Power Labeling kit (Exiqon). After hybridization on the miRCURY™ LNA Array (v.18.0, Exiqon), data obtained from Axon GenePix 4000B microarray scanner (Axon Instruments; Molecular Devices, LLC) were imported into the GenePix Pro 6.0 program (Axon Instruments; Molecular Devices, LLC) for analysis (15). The bioinformatics analysis was performed by RiboBio Co., Ltd. Finally, the heatmap of miRNAs with the most marked differences was created using hierarchical clustering in GeneSpring GX software, version 7.3 (Agilent Technologies, Inc.).
Reverse transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from endometrial tissues and cells using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Reverse transcription of miR-16 and IKKβ was performed using the PrimeScript RT reagent kit (Takara Bio, Inc.) and a reverse transcription kit (Invitrogen; Thermo Fisher Scientific, Inc.), respectively, at 42°C for 1 h. miR-16 and IKKβ expressions were measured using the Exiqon SYBR Green Master Mix (Exiqon) on a LightCycler 480 instrument (Roche Diagnostics). The primers used were as follows: miR-16 F: 5′-TAGCAGCACGTAAATATTGGCG-3′ and R: 5′-TGCGTGTCGTGGAGTC-3′; U6 F: 5′-TGCGGGTGCTCGCTTCGCAGC-3′ and R: 5′-CCAGTGCAGGGTCCGAGGT-3′; IKKβ F: 5′-TGTCAGTGGAAGCCCGGATAG-3′ and R: 5′-AGGTTATGTGCTTCAGCCACCAG-3′; and GAPDH F: 5′-AGGTCGGTGTGAACGGATTTG-3′ and R: 5′-TGTAGACCATGTAGTTGAGGTCA-3′. The reaction mixtures were denatured at 95°C for 3 min, followed by 40 two-step cycles of 95°C for 10 sec and 60°C for 30 sec. The expression of miR-16 and IKKβ in the tissues was normalized to the expression of U6 and GAPDH, respectively. The RT-qPCR assays were performed in triplicate and the relative expression levels were calculated based on the 2−ΔΔCq method (16).
Cell invasion
Transwell chambers (8-µm pore; BD Biosciences) coated with Matrigel (BD Biosciences) were used for the invasion assay. Briefly, primary ESC suspension containing 8×104 cells was added in the top chamber with DMEM/F12, while DMEM/F12 containing 20% FBS was added to the lower chamber. After 24 h of incubation, the cells were stained with 0.1% crystal violet solution for 10 min at room temperature and photographed with an inverted micro-scope (IX71, Olympus Corporation) at a magnification of ×200.
Wound healing assay
After achieving 90% confluence, the transfected primary ESC layer was scratched using a 10-µl pipette tip and the cells were cultured for another 24 h in DMEM/F12. Subsequently, the wound area was captured at 0 and 24 h, and the distance between the edges of the scratch was calculated using ImageJ software, version 1.46 (Rawak Software, Inc.).
Bioinformatics analysis and dual luciferase reporter assay
miRNA target prediction tools, including TargetScan 7.0 (http://targetscan.org/) and miRanda (http://miranda.org) were used to search for the putative targets of miR-16. A sequence containing miR-16 predicted target within the IKKβ 3′-untranslated region (UTR) CUCUUUUUAUUUCACUGCUGCUA or a mutant sequence lacking any complementarity with the miR-16 seed sequence CUCUUUUUAUUUCACACAGCAGA were inserted into pGL3 control vector (Promega Corporation) to generate the reporter vector pGL3-IKKβ 3′-UTR wild-type (wt) and pGL3-IKKβ 3′-UTR mutant (mut). When ESCs reached 60-70% conf1uence in a 24-well plate, 100 ng of Luciferase plasmid was co-transfected with 50 ng of Renilla plasmid (Ambion) and 650 ng of miR-16 mimics or mimics NC using Lipofectamine 2000. At 48 h post-transfection, the luciferase activities were analyzed using the Dual Luciferase Reporter Assay system (Promega Corporation).
Western blot analysis
Western blotting was performed as previously described (17). Briefly, 40 µg extracted protein samples were separated by 12% SDS-PAGE (w/v) and transferred onto a PVDF membrane (EMD Millipore). Subsequently, the membranes were blocked with 5% skimmed milk for 2 h at room temperature, followed by incubation with primary anti-bodies against IKKβ (cat. no. ab178870; 1:500, Abcam), IκB-α (cat. no. sc-373893; 1:1,000, Santa Cruz Biotechnology, Inc.), p-IκB-α (cat. no. 2859; 1:1,000, Cell Signaling Technologies, Inc.), anti-nuclear p-p65 (cat. no. 3033; 1:1,000, Cell Signaling Technologies, Inc.), anti-histone H3 (cat. no. sc-8654; 1:1,000, Santa Cruz Biotechnology, Inc.) and β-actin antibody (cat. no. 4970; 1:1,000, Cell Signaling Technologies, Inc.) at 4°C overnight, followed by HRP-conjugated goat anti-rabbit IgG (cat. no. 205718; 1:10,000, Abcam). The protein bands were developed using an ECL kit (Cytiva) and blot bands were quantified with ImageJ software, version 1.46 (Rawak Software, Inc.).
Statistical analysis
Statistical analysis was performed using GraphPad Prism, version 5.0 (GraphPad Software, Inc.). Data are presented as means ± standard deviation. When only two groups were compared, Wilcoxon test was conducted. Differences between multiple groups were analyzed using one-way ANOVA followed by Tukey's post hoc test. To determine the correlation between the expression of miR-16 and IKKβ, the data were analyzed using Spearman's analysis. P<0.05 was considered to indicate statistically significant differences.
Results
miR-16 is downregulated in ectopic endometrium
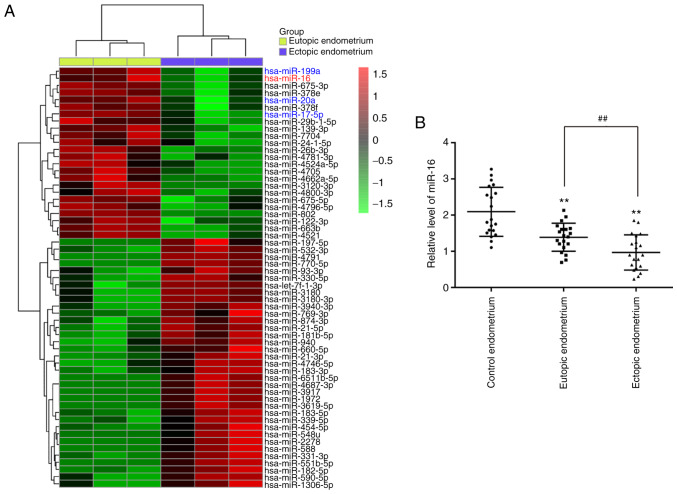
A number of studies have confirmed the aberrant expression patterns of miRNAs in ectopic as well as eutopic endometrial tissues from patients with Ems, which are different compared with those of healthy individuals (18,19). Therefore, using a micro-array assay, we analyzed the differentially expressed miRNAs between eutopic and ectopic endometrial tissues from patients with Ems. The results demonstrated that 24 miRNAs were downregulated and 35 miRNAs were upregulated in ectopic endometrial tissues compared with eutopic endometrial tissues (Fig. 1A). Interestingly, some miRNAs, including miR-16, miR-199a, miR-20a and miR-17-5p, have also been found to be downregulated in previous studies, consistently with our results (20-22). In the present study, miR-16 was selected for further investigation as its expression level was the lowest in the ectopic endometrium. Moreover, previous studies indicated that miR-16 participates in the regulation of cancer cell invasiveness (23-25); however, the effect of miR-16 on the invasiveness of ESCs remains unknown.
Figure 1.
The expression of miR-16 was downregulated in ectopic endometrial tissues from patients with Ems. (A) Heatmap of normalized expression levels of miRNAs in 3 paired eutopic and ectopic endometrial tissues from patients with endometriosis. (B) Reverse transcription-quantitative PCR was performed to determine the expression levels of miR-16 in 20 paired eutopic endometrial and ectopic endometrial from patients with Ems and in 20 endometrial tissues from women without Ems (control endometrium). Data represent the mean ± standard deviation of three independent experiments. One-way ANOVA (Tukey's post hoc test); **P<0.01 vs. control endometrium group; ##P<0.01 vs. eutopic endometrial tissues. Ems, endometriosis.
Next, RT-qPCR was performed to verify the results of the miRNA microarray assay in 20 paired eutopic and ectopic endometrial tissues from patients with Ems. As shown in Fig. 1B, miR-16 was also found to be downregulated in eutopic and ectopic endometrial tissues from patients with Ems compared with the control endometrium group. In addition, miR-16 was found to be significantly lower in ectopic endometrial tissues compared with eutopic endometrial tissues. All data suggest that miR-16 may play a key role in the pathogenesis of Ems.
miR-16 inhibits the invasion and migration of ESCs
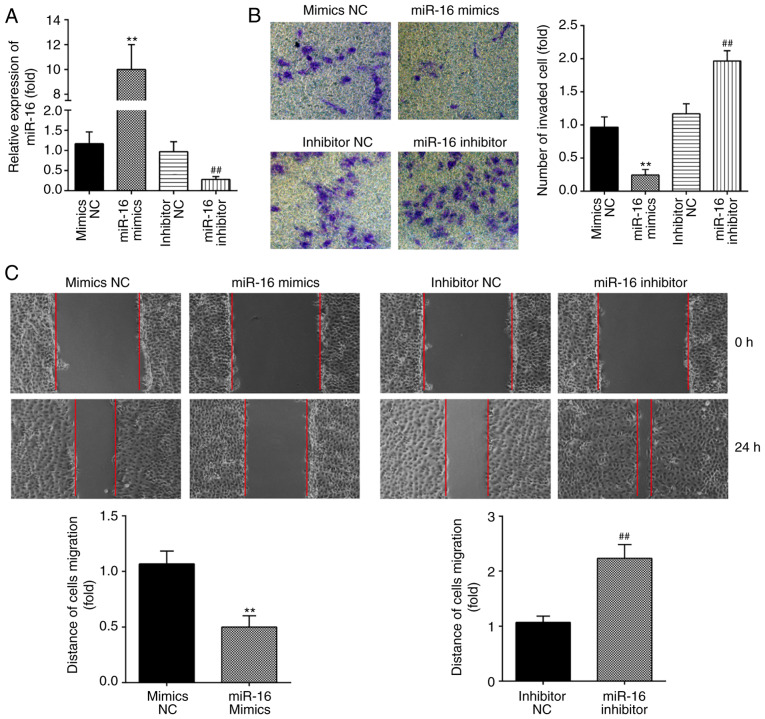
Previous studies have reported that the invasion of ESCs is a major factor leading to the formation of endometriotic lesions (26,27). To investigate the role of miR-16 in the invasiveness of ESCs, miR-16 mimics and miR-16 inhibitor were added to primary ESCs, and RT-qPCR was performed to evaluate the transfection efficiency. As shown in Fig. 2A, the levels of miR-16 were markedly increased following miR-16 mimics transfection, and decreased following miR-16 inhibitor transfection. Subsequently, Transwell and wound healing assays were performed to assess the changes in the invasive and migratory abilities of ESCs. It was observed that miR-16 overexpression markedly suppressed the invasive and migratory abilities of ESCs. By contrast, inhibition of miR-16 enhanced the migration and invasion of ESCs (Fig. 2B and C). Collectively, these findings indicate that miR-16 upregulation suppresses the invasiveness of ESCs.
Figure 2.
miR-16 inhibited the invasion and migration of ESCs. ESCs were transfected with miR-16 mimics or miR-16 inhibitor for 24 h, and were then harvested for subsequent experiments. (A) The transfection efficiency of miR-16 was detected by reverse transcription-quantitative PCR analysis in ESCs at 24 h post-transfection. (B and C) Cell invasion and wound healing assay were used to determine the invasiveness of ESCs at 48 h post-transfection. Data represent the mean ± standard deviation of three independent experiments. One-way ANOVA (Tukey's post hoc test); **P<0.01 vs. mimics NC group, ##P<0.01 vs. inhibitor NC group. ESCs, endometrial stromal cells; NC, negative control.
IKKβ is a direct target of miR-16
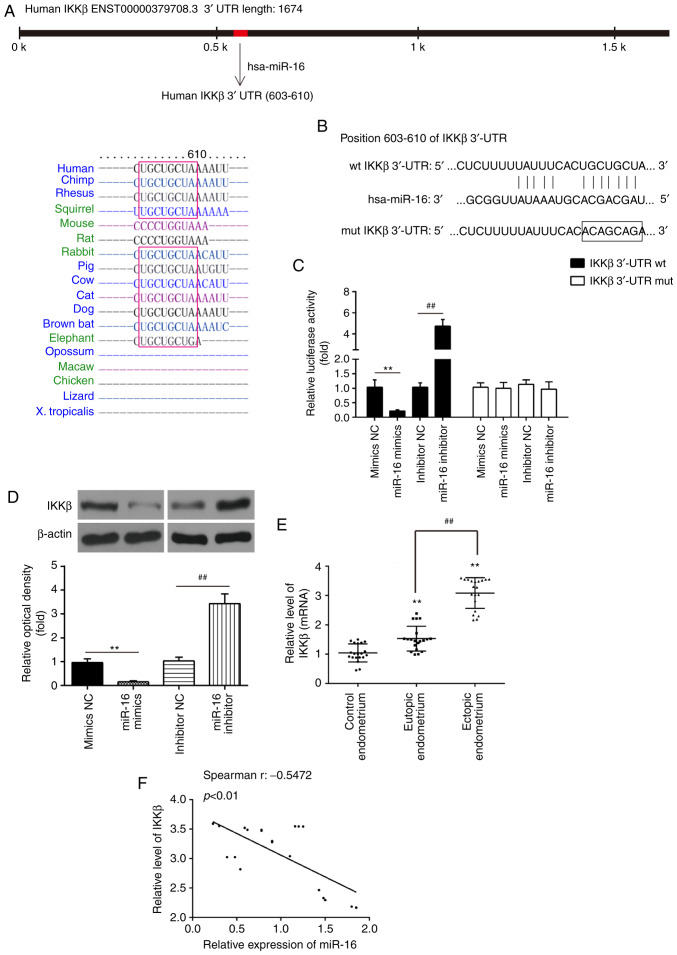
Through bioinformatics prediction using TargetScan 7.0 (http://targetscan.org/) and miRanda (http://miranda.org), a putative target site of miR-16 was found in the 3′-UTR of IKKβ mRNA (Fig. 3A and B). To determine whether miR-16 targets IKKβ, a luciferase reporter assay was performed. The results revealed that miR-16 mimics markedly inhibited the luciferase activity in the IKKβ-3′UTR-wt reporter, and miR-16 inhibitor caused increased luciferase activity; however, no change was observed in cells with co-transfection of IKKβ 3′-UTR-mut with miR-16 (Fig. 3C). To determine whether IKKβ is regulated by miR-16, the protein level of IKKβ was measured by western blotting. As shown in Fig. 3D, IKKβ was markedly decreased following miR-16 mimics transfection, while its expression was increased by miR-16 inhibitor in primary ESCs. In addition, compared with control endometrial tissues, the mRNA level of IKKβ was significantly increased in eutopic and ectopic endometrial tissues (Fig. 3E). In addition, IKKβ was found to be significantly higher in ectopic endometrial tissues compared with its expression in eutopic endometrial tissues. Spearman's analyses were used for correlation analysis and the results indicated that miR-16 expression was inversely correlated with IKKβ expression in ectopic endometrial tissues (r=-0.5472, P<0.01; Fig. 3F). These data indicate that miR-16 directly targets IKKβ and suppresses its translation in primary ESCs.
Figure 3.
IKKβ is a direct target of miR-16. (A and B) Predicted miR-16 binding sites on IKKβ. (C) Luciferase assay of ESCs co-transfected with firefly luciferase constructs containing the IKKβ wild-type or mutated 3′-UTRs and miR-16 mimics, mimic NC, miR-16 inhibitor or inhibitor NC, as indicated (n=3). (D) The protein levels of IKKβ were detected by western blotting after miR-16 mimics and miR-16 inhibitor transfection. β-actin was used as loading control. Data represent the mean ± standard deviation of three independent experiments. One-way ANOVA (Tukey's post hoc test); **P<0.01 vs. mimics NC group; ##P<0.01 vs. inhibitor NC group. (E) Reverse transcription-quantitative PCR was performed to determine the expression levels of IKKβ in 20 paired eutopic and ectopic endometrial tissues from patients with Ems and in 20 endometrial tissues from women without Ems (control endometrium). Data represent the mean ± standard deviation of three independent experiments. One-way ANOVA (Tukey's post hoc test); **P<0.01 vs. control endometrium group; ##P<0.01 vs. eutopic endometrial tissues. (F) Spearman's analyses were used for correlation analysis between miR-16 and IKKβ expression levels in ectopic endometrial tissues (r: -0.5472, P<0.01). Ems, endometriosis; ESCs, endometrial stromal cells; IKKβ, inhibitor of nuclear factor-κB kinase subunit β; UTR, untranslated region; NC, negative control.
Knockdown of IKKβ suppresses ESC migration and invasion
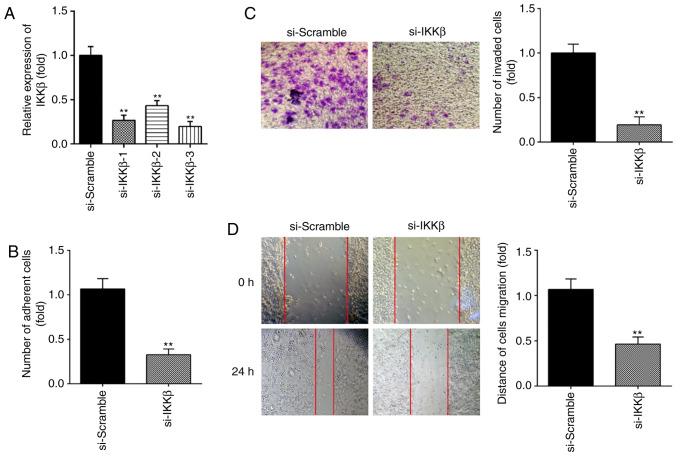
Next, to determine the effect of IKKβ on ESC migration and invasion, si-IKKβ was transfected into primary ESCs to downregulate the expression of IKKβ. As shown in Fig. 4A, IKKβ protein expression was notably decreased following si-IKKβ transfection. si-IKKβ-3 was selected in subsequent assays for its more effective inhibition. It was observed that inhibition of IKKβ markedly suppressed the invasive and migratory abilities of ESCs, similar to the effects of miR-16 mimics (Fig. 4B-D). Collectively, these findings indicate that knockdown of IKKβ inhibits ESC migration and invasion.
Figure 4.
Knockdown of IKKβ suppressed ESC migration and invasion. ESCs were transfected with si-IKKβ or si-Scramble for 48 h, and were then harvested for subsequent experiments. (A) The transfection efficiency of si-IKKβ-1, si-IKKβ-2 and si-IKKβ-3 was detected by reverse transcription-quantitative PCR analysis in ESCs at 24 h post-transfection. (B-D) Cell invasion and wound healing assay were used to determine the invasiveness of ESCs at 24 h post-transfection. Data represent the mean ± standard deviation of three independent experiments. One-way ANOVA (Tukey's post hoc test); **P<0.01 vs. si-Scramble group. ESCs, endometrial stromal cells; IKKβ, inhibitor of nuclear factor-κB kinase subunit β.
miR-16 inhibits the invasion and migration of ESCs by targeting IKKβ
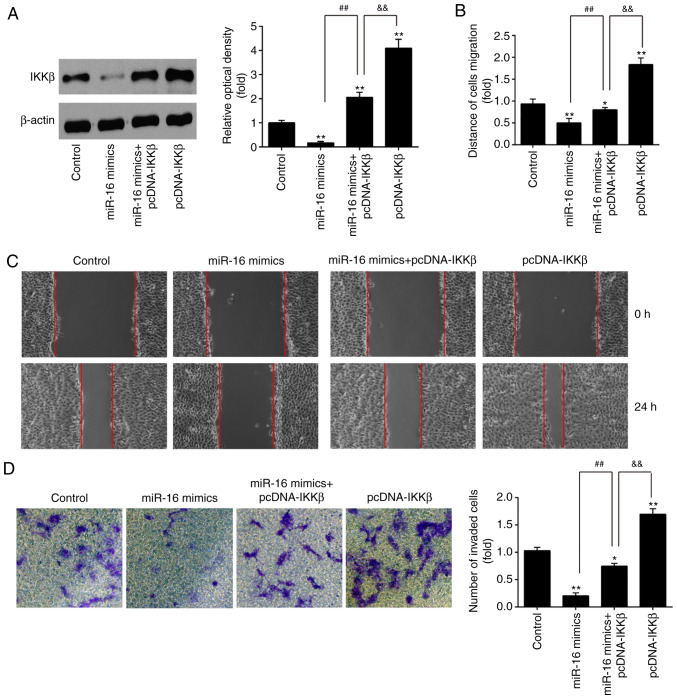
As mentioned above, IKKβ knockdown inhibited ESC migration and invasion; however, whether miR-16 inhibited ESC invasion and migration via inhibiting IKKβ remained unclear. To this end, the IKKβ overexpression vector pcDNA-IKKβ and miR-16 mimics were co-transfected into primary ESCs and incubated for 48 h. The results of western blotting demonstrated that IKKβ was significantly upregulated in the miR-16 mimics + pcDNA-IKKβ group compared with the miR-16 mimics group, whereas the expression level of IKKβ in the pcDNA-IKKβ group was markedly higher compared with that in the miR-16 mimics + pcDNA-IKKβ group (Fig. 5A). These results demonstrated that IKKβ over-expression reversed the inhibitory effects of miR-16 mimics on cell migration and invasion (Fig. 5B-D).
Figure 5.
miR-16 inhibited the invasion and migration of ESCs by targeting IKKβ. ESCs were co-transfected with miR-16 mimics and pcDNA-IKKβ for 48 h, and were then harvested for subsequent experiments. (A) The transfection efficiency of pcDNA-IKKβ was detected by western blotting in ESCs at 24 h post-transfection. β-actin was used as the loading control. (B) The number of attached cells was counted at 24 h post-transfection under a microscope at ×200 magnification. (C and D) Cell invasion and wound healing assay were used to determine the invasiveness of ESCs at 24 h post-transfection. Data represent the mean ± standard deviation of three independent experiments. One-way ANOVA (Tukey's post hoc test); *P<0.05, **P<0.01 vs. control group, ##P<0.01 vs. miR-16 mimics group; &&P<0.01 vs. pcDNA-IKKβ. ESCs, endometrial stromal cells; IKKβ, inhibitor of nuclear factor-κB kinase subunit β.
Furthermore, the effect of IKKβ overexpression on the migration and invasion of ESCs was evaluated. It was observed that the invasion and migration of ESCs was markedly enhanced in the pcDNA-IKKβ alone group (Fig. 5B-D). However, following miR-16 mimics co-transfection, the promoting effects of pcDNA-IKKβ on cell migration and invasion were notably reversed. These data suggest that miR-16 may inhibit ESC invasion and migration by suppressing IKKβ expression.
miR-16 blocks the activation of the NF-κB pathway via suppression of IKKβ
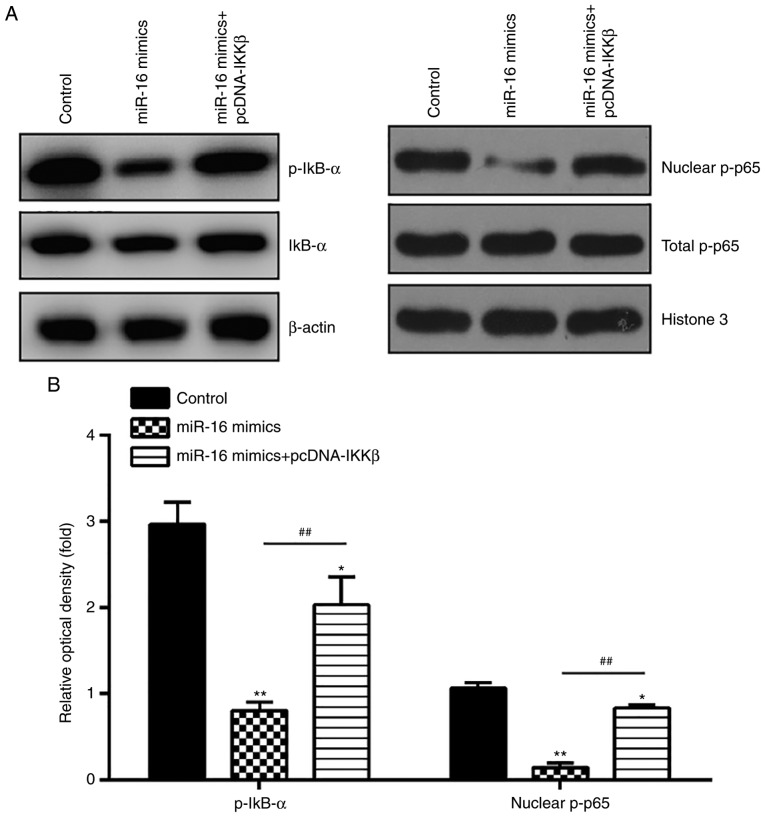
It is well known that IKKβ is an important regulator of the NF-κB pathway, and activation of NF-κB signaling is implicated in ESC function, including cell invasion and proliferation (28-30). To determine whether miR-16 affects the activation of the NF-κB pathway in primary ESCs, the expressions of key proteins in this pathway, namely nuclear p-p65, p-IκB-α and IκB-α, were detected by western blotting. The results demonstrated that miR-16 mimics significantly reduced the expression levels of p-IκB-α and nuclear p-p65, whereas IKKβ overexpression reversed the suppressive effects of miR-16 on this pathway (Fig. 6A and B). These data indicate that miR-16 inhibits NF-κB pathway activation by suppressing IKKβ.
Figure 6.
miR-16 blocked the activation of NF-κB pathway via suppression of IKKβ. ESCs were co-transfected with miR-16 mimics and pcDNA-IKKβ for 48 h, and were then harvested for subsequent experiments. (A) The protein expressions of p-IκB-α, IκB-α and nuclear p-p65 were determined by western blot analysis. β-actin was used as the loading control. (B) The bands were semi-quantitatively analyzed using Image J software, and normalized to total IκB-α or Histone 3 density. Data represent the mean ± standard deviation of three independent experiments. One-way ANOVA (Tukey's post hoc test); *P<0.05, **P<0.01 vs. control group, ##P<0.01 vs. miR-16 mimics group. ESCs, endometrial stromal cells; IKKβ, inhibitor of nuclear factor-κB kinase subunit β; NF-κB, nuclear factor-κB.
Discussion
The present study demonstrated that miR-16 is downregulated in eutopic and ectopic endometrial tissues from patients with Ems compared with the endometrium of healthy individuals. Moreover, miR-16 overexpression attenuated ESC adhesion, migration and invasion in vitro. Notably, our data demon-strated that the upregulation of miR-16 exerted anti-invasive effects by suppressing the IKKβ/NF-κB pathway in primary ESCs (Fig. 7). These findings suggest that miR-16 may serve as a potential therapeutic target for the management of Ems.
Figure 7.
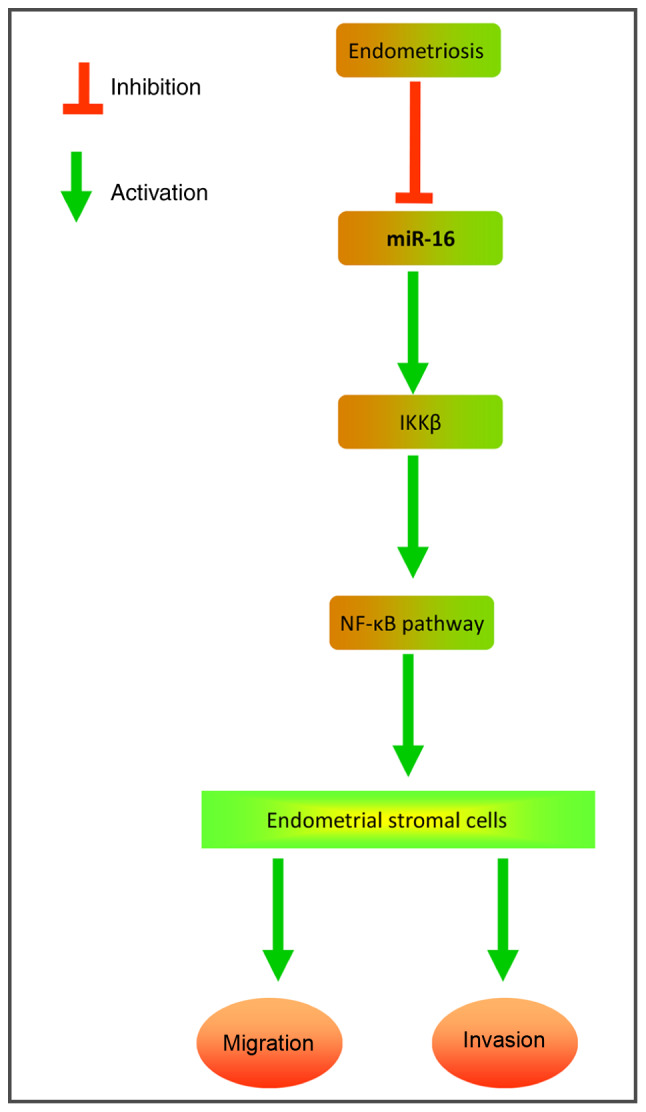
Schematic diagrams showing that miR-16 is downregulated in ectopic endometrium from patients with Ems, and that miR-16 suppressed the expression of IKKβ, subsequently blocked the activation of NF-κB pathway, thereby inhibiting the invasion and migration of ESCs. Ems, endometriosis; ESCs, endometrial stromal cells; IKKβ, inhibitor of nuclear factor-κB kinase subunit β; NF-κB, nuclear factor-κB.
Several miRNAs have been previously identified to contribute to the development of Ems (31-33). For example, Jia et al reported that miR-17-5p, miR-20a and miR-22 are downregulated in plasma samples from patients with Ems, and investigated their prognostic value (20). Ma et al observed that injection of miR-142-3p significantly attenuated ectopic endometriotic lesions in vivo (34). Li et al also found that miR-451a inhibition reduced established Ems lesions in a murine model (35). Zhu et al demonstrated that miR-488 plays a protective role in mice with artificial Ems by targeting Frizzled-7 (36). All these findings outline a potential molecular target for Ems treatment. In the present study, using a miRNA microarray, a number of miRNAs were found to be markedly deregulated. In particular, we focused on miR-16, the expression of which was the most notably decreased in endometriotic tissues, which was in agreement with a previous study (22), indicating that miR-16 may be involved in the initiation and progression of Ems.
It has been demonstrated that increased adhesion and invasion of ESCs are the major causes of the formation of endometriotic lesions (37,38). Accumulating evidence has demonstrated that miR-16 expression is downregulated in various types of human cancer, and the invasion and migration of cancer cells were suppressed by the upregulation of miR-16. For example, Li et al observed that miR-16 overexpression exerts an anti-invasive effect on ovarian cancer via the regulation of the Wnt/β-catenin pathway (23). Jiao et al demonstrated that miR-16 overexpression in osteosarcoma cells suppressed their migration and invasion by targeting RAB23 (25). However, the effects of miR-16 on the migration and invasion of ESCs were not extensively investigated. The present study demonstrated that miR-16 upregulation markedly suppressed the migration and invasion of primary ESCs. However, the precise mechanism remains unclear.
IKKβ, one of the catalytic subunits of the IKK complex, has been reported to be a critical regulator of the activation of NF-κB signaling pathway (39). It has previously been demonstrated that downregulating IKKβ activity may inhibit the activation of NF-κB signaling and attenuate ESC migration and invasion (21). Although IKKβ was shown to be a target of miR-16 in nasal epithelial cells (40), it has not been determined whether IKKβ is also a direct target of miR-16 in ESCs. In the present study, IKKβ was proven to be a target of miR-16, and its translation was suppressed by miR-16 in ESCs. Additionally, it was observed that the levels of IKKβ were upregulated and inversely correlated with miR-16 levels in ectopic endometrial tissues. Furthermore, the knockdown of IKKβ mimicked the effects of miR-16 overexpression on the migration and invasion of ESCs, whereas overexpression of IKKβ markedly reversed these effects, indicating that miR-16 may exert its anti-invasive effects by suppressing IKKβ expression in ESCs. Numerous in vitro and in vivo studies have suggested that NF-κB plays an important role in regulating key cell processes in Ems, such as cell migration, invasion and angiogenesis (41,42). Constitutive activation of the NF-κB pathway has been found in peritoneal endometriotic lesions and inhibition of NF-κB may reduce Ems development and persistence (11,43). Given the association between IKKβ and the NF-κB pathway, it is possible that miR-16 attenuates ESC invasiveness partly through IKKβ/NF-κB pathway inhibition. Indeed, it was observed that miR-16 overexpression decreased IκB-α phosphorylation and lowered NF-κB nuclear translocation, which suggests that miR-16 may block NF-κB pathway activation through downregulating IKKβ protein expression; this may be the underlying basis of miR-16-mediated suppression of ESC invasiveness.
It is generally accepted that the establishment and development of Ems require retrograde menstruation or endometrial fragments, which contain aberrantly activated of eutopic ESCs. This is followed by the migration, invasion, implantation and proliferation of these ESCs on the surface or inner part of pelvic tissues or organs, which occurs simultaneously with a controlled local inflammatory response and angiogenesis (44). Multiple studies have suggested that ESCs in patients with Ems possess different properties compared with the ESCs of healthy controls (45,46). Ectopic ESCs exhibit increased invasive capacity compared with that of their eutopic counterparts from patients with Ems and those from non-endometriotic controls (47,48). Therefore, identification of the underlying mechanisms that alter the invasiveness of ESCs may be a major step toward better understanding the pathogenesis of Ems. In the present study, it should be noted that miR-16 overexpression inhibited the migration and invasion of ESCs, which are dependent on the downregulation of the downstream molecule IKKβ. The adhesion ability of ESCs in the pathological process of Ems does not appear to be as important as their invasive capacity (8). Therefore, the ESC adhesion ability was not evaluated in the present study.
There were certain limitations to the present study: i) The sample size was relatively small; ii) whether other signaling pathways are also regulated by miR-16 remains to be determined in future studies; and iii) the exact mechanism underlying the effect of miR-16 in ESC invasion remains to be fully elucidated. Thus, further research is required to address these issues.
In conclusion, we herein demonstrated that miR-16 is down-regulated in endometriotic tissues, and miR-16 overexpression inhibited primary ESC migration and invasion through regulation of the IKKβ/NF-κB pathway. These findings may help with the development of novel strategies targeting the miR-16/IKKβ/NF-κB signaling pathway in the prophylaxis of Ems. In the future, we aim to investigate the role of miR-16 in the prevention and treatment of Ems in vivo.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during the present study are included in this published article.
Authors' contributions
XW, RR and MS performed the experiments, contributed to data analysis and wrote the manuscript. XW, RR and MS analysed the data. JL conceptualized the study design, contributed to data analysis and experimental materials. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All individuals provided informed consent for the use of their tissue specimens for clinical research. The present study was approved by the Maternal and Child Care Service Center of Dongguan city Guangdong Province Ethics Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ponandai-Srinivasan S, Andersson KL, Nister M, Saare M, Hassan HA, Varghese SJ, Peters M, Salumets A, Gemzell-Danielsson K, Lalitkumar PGL. Aberrant expression of genes associated with stemness and cancer in endometria and endometrioma in a subset of women with endometriosis. Hum Reprod. 2018;33:1924–1938. doi: 10.1093/humrep/dey241. [DOI] [PubMed] [Google Scholar]
- 2.Duffy JM, Arambage K, Correa FJ, Olive D, Farquhar C, Garry R, Barlow DH, Jacobson TZ. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;3:CD011031. doi: 10.1002/14651858.CD011031.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: Pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 4.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 5.Toloubeydokhti T, Pan Q, Luo X, Bukulmez O, Chegini N. The expression and ovarian steroid regulation of endometrial micro-RNAs. Reprod Sci. 2008;15:993–1001. doi: 10.1177/1933719108324132. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, Lessey BA, Giudice LC. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod. 2009;15:625–631. doi: 10.1093/molehr/gap068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang Z, Chen Y, Zhao Y, Xu C, Zhang A, Zhang Q, Wang D, He J, Hua W, Duan P. miR-200c suppresses endometriosis by targeting MALAT1 in vitro and in vivo. Stem Cell Res Ther. 2017;8:251. doi: 10.1186/s13287-017-0706-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng X, Liu J, Wang H, Chen P, Wang D. MicroRNA-126-5p downregulates BCAR3 expression to promote cell migration and invasion in endometriosis. Mol Cell Endocrinol. 2019;494:110486. doi: 10.1016/j.mce.2019.110486. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Yan J, Pan X. miR-141-3p affects apoptosis and migration of endometrial stromal cells by targeting KLF-12. Pflugers Arch. 2019;471:1055–1063. doi: 10.1007/s00424-019-02283-2. [DOI] [PubMed] [Google Scholar]
- 10.Takenaka Y, Taniguchi F, Miyakoda H, Takai E, Terakawa N, Harada T. Lipopolysaccharide promoted proliferation and invasion of endometriotic stromal cells via induction of cyclo-oxygenase-2 expression. Fertil Steril. 2010;93:325–327. doi: 10.1016/j.fertnstert.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Ramos R, Donnez J, Defrere S, Leclercq I, Squifflet J, Lousse JC, Van Langendonckt A. Nuclear factor-kappa B is constitutively activated in peritoneal endometriosis. Mol Hum Reprod. 2007;13:503–509. doi: 10.1093/molehr/gam033. [DOI] [PubMed] [Google Scholar]
- 12.Pan MH, Lin-Shiau SY, Lin JK. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem Pharmacol. 2000;60:1665–1676. doi: 10.1016/S0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhang A, Wang G, Jia L, Su T, Zhang L. Exosome-mediated microRNA-138 and vascular endothelial growth factor in endo-metriosis through inflammation and apoptosis via the nuclear factor-kB signaling pathway. Int J Mol Med. 2019;43:358–370. doi: 10.3892/ijmm.2018.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140:4809–4820. doi: 10.1210/endo.140.10.7070. [DOI] [PubMed] [Google Scholar]
- 15.Wei D, Shen B, Wang W, Zhou Y, Yang X, Lu G, Yang J, Shao Y. MicroRNA199a-5p functions as a tumor suppressor in oral squamous cell carcinoma via targeting the IKKβ/NFkB signaling pathway. Int J Mol Med. 2019;43:1585–1596. doi: 10.3892/ijmm.2019.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Dai L, Gu L, Ding C, Qiu L, Di W. TWEAK promotes ovarian cancer cell metastasis via NF-kappaB pathway activation and VEGF expression. Cancer Lett. 2009;283:159–167. doi: 10.1016/j.canlet.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Gu L, Ni J, Hu P, Hu K, Shi YL. MiR-183 regulates ITGB1P expression and promotes invasion of endometrial stromal cells. Biomed Res Int. 2015;2015:340218. doi: 10.1155/2015/340218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan ML, Chen LR, Tsao HM, Chen KH. Risk of gestational hypertension-preeclampsia in women with preceding endometriosis: A nationwide population-based study. Plos One. 2017;12:e0181261. doi: 10.1371/journal.pone.0181261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia SZ, Yang Y, Lang J, Sun P, Leng J. Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum Reprod. 2013;28:322–330. doi: 10.1093/humrep/des413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai L, Gu L, Di W. MiR-199a attenuates endometrial stromal cell invasiveness through suppression of the IKKβ/NF-kB pathway and reduced interleukin-8 expression. Mol Hum Reprod. 2012;18:136–145. doi: 10.1093/molehr/gar066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braza-Boils A, Salloum-Asfar S, Mari-Alexandre J, Arroyo AB, González-Conejero R, Barceló-Molina M, García-Oms J, Vicente V, Estellés A, Gilabert-Estellés J, Martínez C. Peritoneal fluid modifies the microRNA expression profile in endometrial and endometriotic cells from women with endometriosis. Hum Reprod. 2015;30:2292–2302. doi: 10.1093/humrep/dev204. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Yang L, Sun Y, Wu X. MicroRNA-16 inhibits migration and invasion via regulation of the Wnt/β-catenin signaling pathway in ovarian cancer. Oncol Lett. 2019;17:2631–2638. doi: 10.3892/ol.2019.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin W, Chen F, Wang K, Song Y, Fei X, Wu B. miR-15a/miR-16 cluster inhibits invasion of prostate cancer cells by suppressing TGF-β signaling pathway. Biomed Pharmacother. 2018;104:637–644. doi: 10.1016/j.biopha.2018.05.041. [DOI] [PubMed] [Google Scholar]
- 25.Jiao ZH, Wang JD, Wang XJ. MicroRNA-16 suppressed the invasion and migration of osteosarcoma by directly inhibiting RAB23. Eur Rev Med Pharmacol Sci. 2018;22:2598–2605. doi: 10.26355/eurrev_201805_14953. [DOI] [PubMed] [Google Scholar]
- 26.Lee MY, Kim SH, Oh YS, Heo SH, Kim KH, Chae HD, Kim CH, Kang BM. Role of interleukin-32 in the pathogenesis of endometriosis: In vitro, human and transgenic mouse data. Hum Reprod. 2018;33:807–816. doi: 10.1093/humrep/dey055. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee K, Jana S, DasMahapatra P, Swarnakar S. EGFR-mediated matrix metalloproteinase-7 up-regulation promotes epithelial-mesenchymal transition via ERK1-AP1 axis during ovarian endometriosis progression. FASEB J. 2018;32:4560–4572. doi: 10.1096/fj.201701382RR. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal BB, Sung B. NF-kB in cancer: A matter of life and death. Cancer Discov. 2011;1:469–471. doi: 10.1158/2159-8290.CD-11-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya Y, Osaki K, Kanamoto M, Nakao Y, Takahashi E, Higuchi T, Kamata H. Distinct B subunits of PP2A regulate the NF-kB signalling pathway through dephosphorylation of IKKβ, IkBα and RelA. FEBS Lett. 2017;591:4083–4094. doi: 10.1002/1873-3468.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H, Zhong Q, Xia Y, Li E, Wang S, Ren R. MicroRNA-2861 targets STAT3 and MMP2 to regulate the proliferation and apoptosis of ectopic endometrial cells in endometriosis. Pharmazie. 2019;74:243–249. doi: 10.1691/ph.2019.8881. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Li G, Sheng X, Zhang S. Upregulation of miR33b promotes endometriosis via inhibition of Wnt/β-catenin signaling and ZEB1 expression. Mol Med Rep. 2019;19:2144–2152. doi: 10.3892/mmr.2019.9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update. 2010;16:142–165. doi: 10.1093/humupd/dmp034. [DOI] [PubMed] [Google Scholar]
- 34.Ma L, Li Z, Li W, Ai J, Chen X. MicroRNA-142-3p suppresses endometriosis by regulating KLF9-mediated autophagy in vitro and in vivo. RNA Biol. 2019;16:1733–1748. doi: 10.1080/15476286.2019.1657352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li M, Zhou Y, Taylor HS. miR-451a inhibition reduces established endometriosis lesions in mice. Reprod Sci. 2019;26:1506–1511. doi: 10.1177/1933719119862050. [DOI] [PubMed] [Google Scholar]
- 36.Zhu H, Cao XX, Liu J, Hua H. MicroRNA-488 inhibits endometrial glandular epithelial cell proliferation, migration, and invasion in endometriosis mice via Wnt by inhibiting FZD7. J Cell Mol Med. 2019;23:2419–2430. doi: 10.1111/jcmm.14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 38.Mei J, Jin LP, Ding D, Li MQ, Li DJ, Zhu XY. Inhibition of IDO1 suppresses cyclooxygenase-2 and matrix metallopro-teinase-9 expression and decreases proliferation, adhesion and invasion of endometrial stromal cells. Mol Hum Reprod. 2012;18:467–476. doi: 10.1093/molehr/gas021. [DOI] [PubMed] [Google Scholar]
- 39.Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 40.Gao Y, Yu Z. MicroRNA16 inhibits interleukin13induced inflammatory cytokine secretion and mucus production in nasal epithelial cells by suppressing the IkB kinase β/nuclear factor-kB pathway. Mol Med Rep. 2018;18:4042–4050. doi: 10.3892/mmr.2018.9394. [DOI] [PubMed] [Google Scholar]
- 41.Zhang JJ, Xu ZM, Zhang CM, Dai HY, Ji XQ, Wang XF, Li C. Pyrrolidine dithiocarbamate inhibits nuclear factor-kB pathway activation, and regulates adhesion, migration, invasion and apoptosis of endometriotic stromal cells. Mol Hum Reprod. 2011;17:175–181. doi: 10.1093/molehr/gaq090. [DOI] [PubMed] [Google Scholar]
- 42.Zhang JJ, Xu ZM, Dai HY, Ji XQ, Duan YY, Zhang CM, Qin DY. Application of the nuclear factor-kB inhibitor pyrrolidine dithiocarbamate for the treatment of endometriosis: An in vitro study. Fertil Steril. 2010;94:2942–2944. doi: 10.1016/j.fertnstert.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Huber AV, Huber JC, Kolbus A, Imhof M, Nagele F, Loizou D, Kaufmann U, Singer CF. Systemic HCG treatment in patients with endometriosis: A new perspective for a painful disease. Wien Klin Wochenschr. 2004;116:839–843. doi: 10.1007/s00508-004-0296-5. [DOI] [PubMed] [Google Scholar]
- 44.Iwase A, Kotani T, Goto M, Kobayashi H, Takikawa S, Nakahara T, Nakamura T, Kondo M, Bayasula, Nagatomo Y, Kikkawa F. Possible involvement of CD10 in the development of endometriosis due to its inhibitory effects on CD44-dependent cell adhesion. Reprod Sci. 2014;21:82–88. doi: 10.1177/1933719113488449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lessey BA, Young SL. Integrins and other cell adhesion molecules in endometrium and endometriosis. Semin Reprod Endocrinol. 1997;15:291–299. doi: 10.1055/s-2008-1068759. [DOI] [PubMed] [Google Scholar]
- 46.Vercellini P, Aimi G, Panazza S, Vicentini S, Pisacreta A, Crosignani PG. Deep endometriosis conundrum: Evidence in favor of a peritoneal origin. Fertil Steril. 2000;73:1043–1046. doi: 10.1016/S0015-0282(00)00420-9. [DOI] [PubMed] [Google Scholar]
- 47.Agic A, von Wussow U, Starzinski-Powitz A, Diedrich K, Altevogt P, Hornung D. Inhibition of cell proliferation, adhesion, and invasion with an anti-L1-cell adhesion molecule monoclonal antibody in an in vitro endometriosis model. Fertil Steril. 2010;94:1102–1104. doi: 10.1016/j.fertnstert.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Zhang Z, Xiong W, Zhang L, Xiong Y, Li N, He H, Du Y, Liu Y. Hypoxia-inducible factor-1α promotes endometrial stromal cells migration and invasion by upregulating autophagy in endometriosis. Reproduction. 2017;153:809–820. doi: 10.1530/REP-16-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during the present study are included in this published article.