Abstract
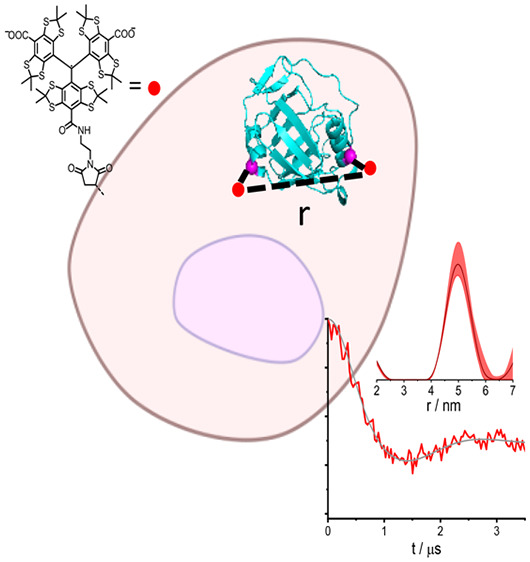
Double-electron electron resonance (DEER) can be used to track the structural dynamics of proteins in their native environment, the cell. This method provides the distance distribution between two spin labels attached at specific, well-defined positions in a protein. For the method to be viable under in-cell conditions, the spin label and its attachment to the protein should exhibit high chemical stability in the cell. Here we present low-temperature, trityl–trityl DEER distance measurements on two model proteins, PpiB (prolyl cis–trans isomerase from E. coli) and GB1 (immunoglobulin G-binding protein), doubly labeled with the trityl spin label, CT02MA. Both proteins gave in-cell distance distributions similar to those observed in vitro, with maxima at 4.5–5 nm, and the data were further compared with in-cell Gd(III)–Gd(III) DEER obtained for PpiB labeled with BrPSPy-DO3A-Gd(III) at the same positions. These results highlight the challenges of designing trityl tags suitable for in-cell distance determination at ambient temperatures on live cells.
The cellular environment is complex and differs considerably in terms of viscosity, confinement, and composition from solution conditions under which protein structure and dynamics are commonly studied. The different conditions may affect conformational equilibria and the stability of proteins, and therefore, tracking proteins conformations in their native environment, the cell, though challenging, is of current high interest. This has been addressed by a number of biophysical methods, among them double-electron electron resonance (DEER, also called PELDOR).1−4 DEER measures the dipolar interaction between two paramagnetic centers, which is proportional to 1/r3, r being the distance between the two paramagnetic centers.5 Since most proteins are diamagnetic, DEER relies on introducing spin labels at well-defined positions in the protein and it provides the distance distribution between the two labels. The insensitivity of DEER to the molecular size and the lack of an environmental background signal make it an attractive method for in-cell structural studies. The transition from solution to in-cell measurements is however challenging because it requires the conjugation between the label and the protein residue, as well as the spin label itself, to be stable in the reducing environment of the cell. Moreover, the measurement sensitivity should be high enough to allow access to protein concentrations close to the physiological range.
The first pioneering in-cell DEER measurements were carried out on a protein1 and on nucleic acids2,3,6 labeled with standard nitroxide spin labels injected into Xenopus oocytes. These works reported the reduction of the nitroxide radical to the corresponding diamagnetic hydroxyl analogue in the cell and this presents a serious obstacle for the development of in-cell DEER. This triggered efforts to synthesize reduction-resistant nitroxides,7 and recently in-cell DEER measurements were performed with such a new spin label.8 Another approach uses spin labels based on redox stable Gd(III) chelates,4,9−15 which feature a high sensitivity at high magnetic fields. In most of these works, the labeled protein was delivered into eukaryotic cells using hypotonic swelling or electroporation.
Trityl-based spin labels comprise yet another new family of spin labels. They possess the exceptional property of having a very narrow EPR spectrum, which ensures high sensitivity and high modulation depth in DEER and other EPR methods for distance measurements.16 Trityl is also unique in terms of its long phase memory time at room temperature, and this enables trityl–trityl DEER measurements at ambient temperatures.17−21 To date, most of the distance measurements involving trityl spin labels were applied to nucleic acids,18,20−27 but a few were also reported for proteins.17,28−31 Trityl-nitroxide28,32 and trityl-Gd(III)30 distance measurements were introduced as well. In-cell distance measurements involving a trityl spin label have so far been reported only for Fe(III)-trityl in cytochrome P450 CYP101 employing the RIDME (relaxation-induced dipolar modulation enhancement) experiment.29 In this system, Fe(III) is intrinsic, and the trityl label was conjugated via an engineered cysteine residue, forming a stable thioether bond to the protein. The protein was injected into oocytes (the final in-cell concentration was 250 μM) and a RIDME trace with a modulation depth of ∼4%, and an evolution time of 1.5 μs was collected. Although this is a significant advance, the reported signal-to-noise ratio (SNR) and the strong background decay pose challenges. In-cell trityl–trityl distance measurements on biomolecules have not been demonstrated so far.
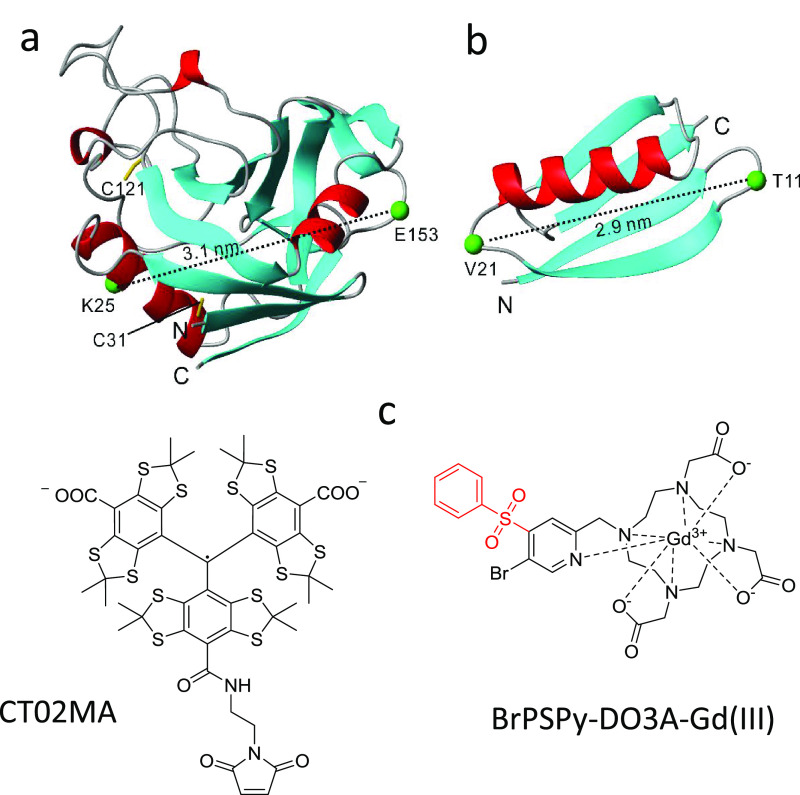
In this work, we report on W-band (94.9 GHz) in-cell trityl–trityl DEER measurements on two proteins, the K25C/E153C mutant of prolyl cis–trans isomerase B from E. coli (PpiB) and the T11C/V21C mutant of immunoglobulin G-binding protein (GB1) labeled with the CT02MA tag30 (see Figure 1). The structures of these proteins were determined by X-ray crystallography33 and NMR, both in solution and in the solid state.34−36 The structure of GB1 was also determined in cells.34,35 In addition, we compared the PbiB K25C/E153C results to those obtained with the same protein labeled with BrPSPy-DO3A-Gd(III) both in vitro and in cell.15
Figure 1.
Structural representation of (a) PpiB and (b) GB1, of which the cysteine mutation at K25 and E153 in PpiB (PDB code: 2NUL(33)) and T11 and V21 in GB1 (PDB code: 2QMT(36)) are shown; the two corresponding Cα atoms, connected by a dashed line, are denoted. The native cysteine residues C31 and C121 in PpiB are relatively buried and were not modified with trityl tags when labeling takes place at 277 K. (c) Molecular structure of the CT02MA and BrPSPy-DO3A-Gd(III) spin labels used in this work.
PpiB contains two native cysteines, C31 and C121, which, according to the X-ray crystal structure,33 are relatively buried and therefore are not expected to be accessible to spin labeling.37 Indeed, wild-type PpiB does not react with CT02MA under common reaction conditions at 303 K (Figure S1). However, when the CT02MA ligation reaction was carried out at 303 K for K25C/E153C, labeling at C31 and C121 became evident (see Figure S1).
We optimized the ligation conditions using the K25C mutant, which is close to C31 (Figure S2), and therefore, it can affect C31, and this can further perturb the 3D structure. We prepared 15N labeled PpiB K25C and investigated by NMR whether the structure of the protein was modified and C31 and C121 were labeled. Incubation of the K25C mutant with 1 equiv of CT02MA in 20 mM Tris buffer, pH 7.2 at 293 K for 5 h broadened the peaks of only a few residues that are close to K25C (Figure S3a) owing to paramagnetic relaxation enhancement (PRE), but all other peaks remained in the same positions, indicating no change in the 3D structure of the protein. Furthermore, 10 h incubation with 3.5 equiv of CT02MA at 293 K gave a similar 15N-HSQC spectrum with only additional nonspecific PRE broadening observed (Figure S3b). In both cases, there was no evidence for labeling of C31 and C121 (Figure S4). In contrast, increasing the ligation temperature to 308 K resulted in broadening beyond detection of almost all cross-peaks and some new cross-peaks with a narrow dispersion, typically assigned to the unfolded polypeptides, were detected (Figure S3c). The attachment of CT02MA at C25 probably decreases the stability of the secondary structure in the ligation region, which at a 308 K renders C31 more solvent accessible than the wild type protein, thus allowing labeling. The labeling of C31 further disturbs the 3D structure of PpiB, resulting in exposing C121 for labeling, which is consistent with the MALDI-TOF results shown in Figure S1. On the basis of mass spectrometry and NMR, we concluded that the labeling of C31 and C121 with CT02MA led to the denaturation of the protein. Accordingly, to achieve selective labeling of PpiB with CT02MA at K25C and E153C, the ligation reaction should be carried out below 293 K; we performed it at 277 K (Figure S1).
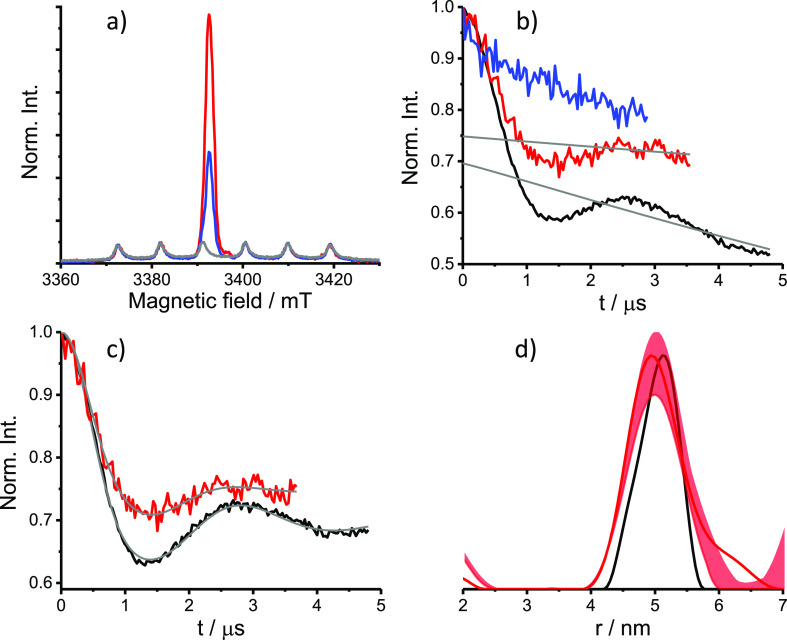
The DEER time domain traces of solution PpiB K25C/E153C-CT02MA, measured at 50 K, and the derived distance distribution are presented in Figure 2. Experimental details are given in the SI, along with echo-detected EPR (ED-EPR) and echo-decay measurements (Figures S5–S7). The latter gave a phase memory time, TM, of 2.1 μs, limiting the DEER evolution time to 4.5 μs at most. Intrigued by this result, we compared the TM values of the free CT02MA in D2O/glycerol-d8 and H2O/glycerol with those of PpiB K25C/E153C-CT02MA in D2O/glycerol-d8 and H2O/glycerol (see Figure S7, Table S2). Interestingly, for the latter, the solvent deuteration had a rather minor effect on TM. This is in strong contrast to PpiB K25C/E153C labeled with BrPSPy-DO3A-Gd(III) and maleimide-proxy (M-Proxy) (see Figure S7, Table S2), where solvent deuteration increases TM considerably.
Figure 2.
DEER results for PpiB K25C/E153C-CT02MA. (a) W-band ED-EPR spectra of HeLa cells incubated 4 h (red) and 12 h (blue) after electroporation and of the control sample with 4 h incubation but without the electroporation step (gray). The ED-EPR traces were normalized to the intensity of endogenous Mn(II). (b) Primary DEER traces and background function (gray) of the 4 and 12 h in-cell sample (red and blue, respectively), compared with the solution sample (75 μM in D2O/glycerol-d8, black). (c) DEER traces shown in panel b after background removal and the fitting traces obtained with Tikhonov regularization using DeerAnalysis (gray).40 (d) Distance distributions. The uncertainty range obtained using a background decay validation of the in-cell sample is shown in light red.
The distance distribution has a peak at 5 nm, a width of 1 nm at half height, and is in good agreement with the distance distribution predicted from the crystal structure, as derived from mtsslWizard, a PyMOL plugin38 (see Figure S8). We have used DEER with chirp pulses to avoid potential orientation selection.30 The modulation depth, λ, of 30 ± 5%, depending on the measurement conditions and the background function used (see SI and Figure S10), is higher than what we observed earlier at W-band for ubiquitin and for GB1 measured under the same conditions.30 We attribute this to the higher labeling efficiency of PpiB K25C/E153C, the absence of free tag and of oxidized tag.30 DEER was also measured with rectangular pulses and the results were similar (Figure S10). The calculated λ value for DEER with observe and rectangular pump pulses of 25/50 and 40 ns, respectively, having a frequency difference of 20 MHz is 42% and for 30 MHz difference is 28%. The calculations of λ were done as described earlier.30 These values are close to the experimental values of 35% and 32% (Figure S10) and are consistent with high labeling efficiency. For reference, we also prepared PpiB K25C/E153C labeled with M-Proxyl and BrPSPy-DO3A-Gd(III); the distance distribution obtained showed a maximum at 4.5 nm, which is somewhat shorter than that given by CT02MA, again in agreement with the predicted distance distribution from the crystal structure (see Figure S8 and S9).
PpiB K25C/E153C-CT02MA was delivered into HeLa cells by electroporation,11 and cells were collected and frozen after 4 and 12 h incubation at 37 °C as described earlier.12,13,15 The resulting in-cell ED-EPR spectra (Figure 2a) show, in addition to the narrow trityl spectrum, signals of endogenous Mn(II). A control experiment was carried out under the same conditions, but without the electroporation step, and the resulting ED-EPR spectrum consisted of only the Mn(II) signals (Figure 2a). The echo decay of the in-cell samples was faster than that of the solution sample (Figure S6). We did not wash the cells with D2O before freezing them because the use of deuterated solvent did not extend the phase memory time significantly as described above. The DEER data of cells frozen 4 h after electroporation (Figure 2b) reveal a clear oscillation with a reduction in λ from 32% to 25%, and the derived distance distribution was very similar to that observed in solution (Figure 2c,d). The sample, incubated for 12 h at 37 °C after delivery, exhibited a considerable reduction in the ED-EPR signal (55% using the Mn(II) signal as an internal standard), and no DEER modulation could be detected (Figure 2a,b).
We tested the stability of the labeled protein toward reduction in solution by adding glutathione (Figure S11). Incubation of 30 μM PpiB K25C/E153C-CT02MA with 0.1 mM glutathione for 4 h at 37 °C decreased the modulation depth to 28%, and after 12 h incubation, the modulation depth was further reduced to ∼17%. Exposure to 0.5 mM glutathione for 4 h reduced the depth to 17% and after 12 h, the trityl ED-EPR spectrum vanished. The stability of a trityl radical was also tested in 5 mM ascorbic acid, and a 25% decrease in the spin concentration was reported, whereas incubation for 2 h in a cell extract of Xenopus laevis oocytes led to a 40% loss of spin concentration.7 The decrease in the modulation depth is primarily due to reduction of trityl, as evident by the reduction in the ED-EPR signal intensity. In addition, the increased relative intensity of the overlapping Mn(II) signal adds to the reduced modulation depth. It is also likely that potential thiol exchange processes between intracellular glutathione and the succinimidyl thioether moiety in the protein-CT02MA conjugate,41 contribute to the loss of modulation depth. These results provide additional evidence that CT02MA labeled PpiB indeed delivered into the cell as spin labels proteins facing the external side of the membrane are not sensitive to reduction39
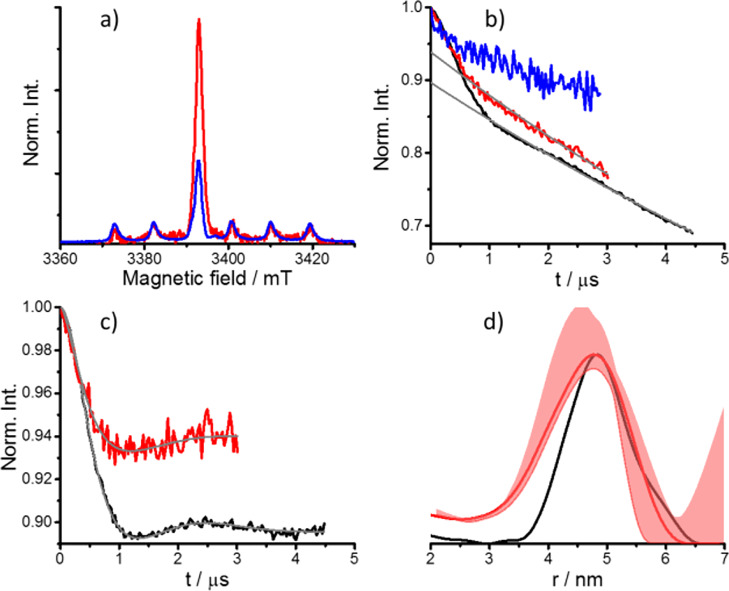
The DEER results obtained for GB1 T11C/V21C-CT02MA are presented in Figure 3. The DEER trace exhibits a 10% modulation depth as reported earlier.30 Considering that 100% labeling generates λ ≈ 30%, we estimated the labeling efficiency for GB1 to be about 57% per site. We have also estimated the labeling efficiency from the intensity of the ED-EPR spectrum using the free tag as reference and found for 93% PpiB and 82% for GB1. In this calculation, the protein concentration was determined using UV–vis prior to labeling and we assumed that there was 10% protein loss owing to the labeling process for both proteins. It was not possible to determine the protein concentration after labeling with this method because of the overlapping signals of the CT02MA.30 The higher labeling efficiency obtained from the signal intensity can arise from the presence of some free CT02MA in the GB1 sample. Solution and in-cell echo decays are shown in Figure S3b. In spite of the relatively low modulation depth and the short echo decay times, we were able to record DEER traces in cells frozen after incubation for 4 h postdelivery, with a reduction in λ down to 6%. The distance distribution is somewhat broader than in solution; this can be attributed to the shorter evolution time of the DEER trace. Freezing after 12 h incubation post delivery led to a significant reduction (60%) in the signal intensity (Figure 3a), and no DEER effect could be observed, similar to the results obtained for PpiB.
Figure 3.
DEER results for GB1 T11C/V21C-CT02MA. (a) W-band ED-EPR spectra of HeLa cells frozen after incubation for 4 h (red) and 12 h (blue) postdelivery. The ED-EPR traces were normalized to the intensity of endogenous Mn(II). (b) Primary DEER traces and background function (gray) of the 4 h (red) and 12 h (blue) in-cell samples compared with the solution sample (50 μM in D2O/glycerol-d8, black). (c) DEER traces shown in panel b after background removal and the fitting traces obtained with Tikhonov regularization using DeerAnalysis (gray). (d) Distance distributions. The uncertainty range obtained using background decay validation for the in-cell sample is shown in light red.
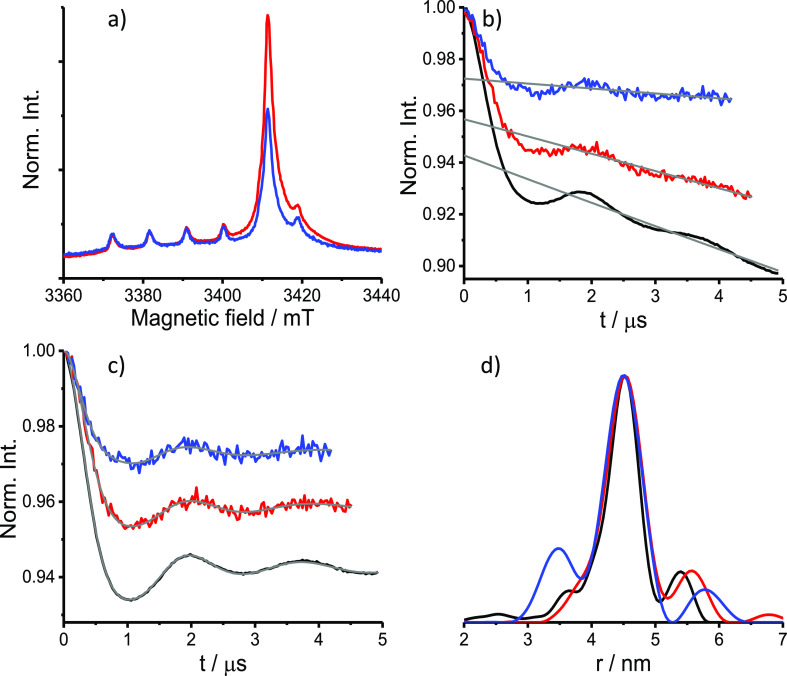
We also carried out in-cell measurements, under the same conditions, with PpiB K25C/E153C labeled with BrPSPy-DO3A-Gd(III), to better understand the origin of the reduced ED-EPR signal and the absence of a DEER effect for PpiB K25C/E153C-CT02MA incubated for 12 h after cell delivery. The question is whether the decrease arises from protein degradation and expulsion from the cell or it is due to spin label reduction? BrPSPy-DO3A-Gd(III) has a higher selectivity to K25C and E153C, compared with CT02MA, and the native cysteine were not accessible even at 303 K (Figure S1). The DEER results are shown in Figure 4. For this sample, a longer evolution time could be used because of the longer echo decay time for both solution and in-cell samples (see Figures S6c and S7). We also observed a reduction in the ED-EPR signal intensity after 12 h, as reported earlier,12,13 due to reduced protein concentration in the cell, but in contrast to the CT02MA-labeled protein, here also after 12 h of incubation a clear DEER trace with a good SNR could be obtained. A reduction in the modulation depth was detected and attributed, at least in part, to the increase in the relative contributions of the endogenous Mn(II) signals, which overlap with the Gd(III) signal (see Figure 4a).12,13,15 Comparison of these results with those of PpiB labeled with CT02MA indicates a significant reduction of CT02MA and maybe a thiol exchange of protein-CT02MA with the reactive thiol groups in cells that cause the disappearance of the DEER effect.41
Figure 4.
DEER results for PpiB K25C/E153C-BrPy-DO3A-Gd(III). (a) W-band ED-EPR spectra in HeLa cells incubated 4 h (red) and 12 h (blue) after electroporation. The ED-EPR traces were normalized to the intensity of endogenous Mn(II). (b) Primary DEER traces and background function (gray) of the 4 h (red) and 12 h (blue) in-cell samples compared with the solution sample (50 μM, black). (c) DEER traces shown in panel b after background removal and the fitting traces obtained with Tikhonov regularization using DeerAnalysis (gray). (d) Distance distributions.
We estimated the in-cell spin concentration from the EPR signal intensity using the endogenous Mn(II) signals as an internal standard (see Table 1 and SI for details). The results show somewhat higher in-cell spin concentrations for the Gd(III)-labeled sample at 4 h incubation time and, more importantly, a milder reduction over time compared with the CT02MA-labeled proteins, reflecting the higher intracellular stability of Gd(III).
Table 1. Estimated in-Cell Spin Concentration for Samples Studied in This Work.
| in-cell
EPR spin concentration (μM)a |
||
|---|---|---|
| 4 h | 12 h | |
| PpiB-CT02MA | 31.8 ± 6.7 | 11.7 ± 3.1 |
| PpiB-BrPy-DO3A-Gd(III) | 46.1 ± 12.5 | 29.0 ± 8.3 |
| GB1-CT02MA | 35.4 ± 7.4 | 12.5 ± 3.2 |
Protein concentration is about half that of the spin concentration if we consider 100% labeling efficiency.
To conclude, we have demonstrated the feasibility of low temperature trityl–trityl DEER distance measurements in human HeLa cells on two doubly labeled proteins featuring means distances of 4.5–5 nm. Labeling with CT02MA withstands an in-cell incubation of 4 h, which is sufficient for most applications. We also noted the shorter phase memory time, compared with BrPSPy-DO3A-Gd(III), which we suspect is due to the methyl groups.42 This shortcoming, which compromises sensitivity, can be amended by replacing the methyl groups with another group, like −CH2CH2OH. The CT02MA label may also be more limited in terms of labeling efficiency and perturbation of the protein structure, compared to BrPSPy-DO3A-Gd(III). Nonetheless, the main advantage of in-cell trityl–trityl distance measurements over Gd(III)–Gd(III) is their potential for room temperature distance measurements on biomolecules in live cells, as opposed to frozen cells. While this is still a far reaching goal, it provides an important incentive for further development of trityl tags optimized for in-cell measurements.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC)-Israel Science Foundation (ISF) grant (Grant Nos. 21761142004 and 2484/17) to X.C.S. and D.G., a Major National Scientific Research Project in China (2016YFA0501202), and the Natural Science Foundation of China (21673122 and 21473095) to X.C.S. This research was made possible in part by the historic generosity of the Harold Perlman Family (D.G.). D.G. holds the Erich Klieger Professorial Chair in Chemical Physics.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpclett.9b03208.
Experimental details, MALDI-TOF spectra, NMR spectra, ED-EPR spectra, echo-decays, additional DEER data, stability of CT02MA labeled PpiB (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Igarashi R.; Sakai T.; Hara H.; Tenno T.; Tanaka T.; Tochio H.; Shirakawa M. Distance Determination in Proteins inside Xenopus Laevis Oocytes by Double Electron-Electron Eesonance Experiments. J. Am. Chem. Soc. 2010, 132 (24), 8228–8229. 10.1021/ja906104e. [DOI] [PubMed] [Google Scholar]
- Azarkh M.; Okle O.; Singh V.; Seemann I. T.; Hartig J. S.; Dietrich D. R.; Drescher M. Long-Range Distance Determination in a DNA Model System inside Xenopus Laevis Oocytes by in-Cell Spin-Label EPR. ChemBioChem 2011, 12 (13), 1992–5. 10.1002/cbic.201100281. [DOI] [PubMed] [Google Scholar]
- Krstic I.; Haensel R.; Romainczyk O.; Engels J. W.; Doetsch V.; Prisner T. F. Long-Range Distance Measurements on Nucleic Acids in Cells by Pulsed EPR Spectroscopy. Angew. Chem., Int. Ed. 2011, 50 (22), 5070–5074. 10.1002/anie.201100886. [DOI] [PubMed] [Google Scholar]
- Martorana A.; Bellapadrona G.; Feintuch A.; Di Gregorio E.; Aime S.; Goldfarb D. Probing Protein Conformation in Cells by EPR Distance Measurements Using Gd3+ Spin Labeling. J. Am. Chem. Soc. 2014, 136 (38), 13458–13465. 10.1021/ja5079392. [DOI] [PubMed] [Google Scholar]
- Jeschke G. DEER Distance Measurements on Proteins. Annu. Rev. Phys. Chem. 2012, 63, 419–46. 10.1146/annurev-physchem-032511-143716. [DOI] [PubMed] [Google Scholar]
- Azarkh M.; Singh V.; Okle O.; Dietrich D. R.; Hartig J. S.; Drescher M. Intracellular Conformations of Human Telomeric Quadruplexes Studied by Electron Paramagnetic Resonance Spectroscopy. ChemPhysChem 2012, 13 (6), 1444–1447. 10.1002/cphc.201100980. [DOI] [PubMed] [Google Scholar]
- Jagtap A. P.; Krstic I.; Kunjir N. C.; Hansel R.; Prisner T. F.; Sigurdsson S. T. Sterically Shielded Spin Labels for in-Cell EPR Spectroscopy: Analysis of Stability in Reducing Environment. Free Radical Res. 2015, 49 (1), 78–85. 10.3109/10715762.2014.979409. [DOI] [PubMed] [Google Scholar]
- Karthikeyan G.; Bonucci A.; Casano G.; Gerbaud G.; Abel S.; Thome V.; Kodjabachian L.; Magalon A.; Guigliarelli B.; Belle V.; Ouari O.; Mileo E. A Bioresistant Nitroxide Spin Label for in-Cell EPR Spectroscopy: In Vitro and in Oocytes Protein Structural Dynamics Studies. Angew. Chem., Int. Ed. 2018, 57 (5), 1366–1370. 10.1002/anie.201710184. [DOI] [PubMed] [Google Scholar]
- Qi M.; Gross A.; Jeschke G.; Godt A.; Drescher M. Gd(III)-PyMTA Label Is Suitable for in-Cell EPR. J. Am. Chem. Soc. 2014, 136 (43), 15366–15378. 10.1021/ja508274d. [DOI] [PubMed] [Google Scholar]
- Mascali F. C.; Ching H. Y. V.; Rasia R. M.; Un S.; Tabares L. C. Using Genetically Encodable Self-Assembling Gd(III) Spin Labels to Make in-Cell Nanometric Distance Measurements. Angew. Chem., Int. Ed. 2016, 55, 11041–11043. 10.1002/anie.201603653. [DOI] [PubMed] [Google Scholar]
- Theillet F. X.; Binolfi A.; Bekei B.; Martorana A.; Rose H. M.; Stuiver M.; Verzini S.; Lorenz D.; van Rossum M.; Goldfarb D.; Selenko P. Structural Disorder of Monomeric Alpha-Synuclein Persists in Mammalian Cells. Nature 2016, 530 (7588), 45–50. 10.1038/nature16531. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Yang F.; Gong Y. J.; Chen J. L.; Goldfarb D.; Su X. C. A Reactive, Rigid Gd(III) Labeling Tag for in-Cell EPR Distance Measurements in Proteins. Angew. Chem., Int. Ed. 2017, 56 (11), 2914–2918. 10.1002/anie.201611051. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Yang F.; Gong Y.-J.; Bahrenberg T.; Feintuch A.; Su X.-C.; Goldfarb D. High Sensitivity in-Cell EPR Distance Measurements on Proteins Using an Optimized Gd(III) Spin Label. J. Phys. Chem. Lett. 2018, 9 (20), 6119–6123. 10.1021/acs.jpclett.8b02663. [DOI] [PubMed] [Google Scholar]
- Dalaloyan A.; Martorna A.; Barak Y.; Gataulin D.; Reuveny E.; Howe A.; Elbaum M.; Albeck A.; Unger T.; Frydman V.; Abdelkader E. H.; Otting O.; Goldfarb D. Tracking Conformational Changes in Calmodulin in Vitro, in Cell Extract, and in Cells by EPR Distance Measurements. ChemPhysChem 2019, 20, 1860–1868. 10.1002/cphc.201900341. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Yang F.; Li X. Y.; Su X. C.; Goldfarb D. In-Cell EPR Distance Measurements on Ubiquitin Labeled with a Rigid PyMTA-Gd(III) Tag. J. Phys. Chem. B 2019, 123 (5), 1050–1059. 10.1021/acs.jpcb.8b11442. [DOI] [PubMed] [Google Scholar]
- Meyer A.; Jassoy J. J.; Spicher S.; Berndhauser A.; Schiemann O. Performance of PELDOR, RIDME, SIFTER, and DQC in Measuring Distances in Trityl Based Bi- and Triradicals: Exchange Coupling, Pseudosecular Coupling and Multi-Spin Effects. Phys. Chem. Chem. Phys. 2018, 20 (20), 13858–13869. 10.1039/C8CP01276H. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Liu Y.; Borbat P.; Zweier J. L.; Freed J. H.; Hubbell W. L. Pulsed ESR Dipolar Spectroscopy for Distance Measurements in Immobilized Spin Labeled Proteins in Liquid Solution. J. Am. Chem. Soc. 2012, 134 (24), 9950–2. 10.1021/ja303791p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevelev G. Y.; Krumkacheva O. A.; Lomzov A. A.; Kuzhelev A. A.; Rogozhnikova O. Y.; Trukhin D. V.; Troitskaya T. I.; Tormyshev V. M.; Fedin M. V.; Pyshnyi D. V.; Bagryanskaya E. G. Physiological-Temperature Distance Measurement in Nucleic Acid Using Triarylmethyl-Based Spin Labels and Pulsed Dipolar EPR Spectroscopy. J. Am. Chem. Soc. 2014, 136 (28), 9874–9877. 10.1021/ja505122n. [DOI] [PubMed] [Google Scholar]
- Kuzhelev A. A.; Strizhakov R. K.; Krumkacheva O. A.; Polienko Y. F.; Morozov D. A.; Shevelev G. Y.; Pyshnyi D. V.; Kirilyuk I. A.; Fedin M. V.; Bagryanskaya E. G. Room-Temperature Electron Spin Relaxation of Nitroxides Immobilized in Trehalose: Effect of Substituents Adjacent to NO-Group. J. Magn. Reson. 2016, 266, 1–7. 10.1016/j.jmr.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Krumkacheva O.; Bagryanskaya E.. Trityl Radicals as Spin Labels. In Electron Paramagnetic Resonance; Chechik V., Murphy D. M., Eds.; RSC Publishing, 2017; Vol. 25, pp 35–60. [Google Scholar]
- Shevelev G. Y.; Gulyak E. L.; Lomzov A. A.; Kuzhelev A. A.; Krumkacheva O. A.; Kupryushkin M. S.; Tormyshev V. M.; Fedin M. V.; Bagryanskaya E. G.; Pyshnyi D. V. A Versatile Approach to Attachment of Triarylmethyl Labels to DNA for Nanoscale Structural EPR Studies at Physiological Temperatures. J. Phys. Chem. B 2018, 122 (1), 137–143. 10.1021/acs.jpcb.7b10689. [DOI] [PubMed] [Google Scholar]
- Shevelev G. Y.; Krumkacheva O. A.; Lomzov A. A.; Kuzhelev A. A.; Trukhin D. V.; Rogozhnikova O. Y.; Tormyshev V. M.; Pyshnyi D. V.; Fedin M. V.; Bagryanskaya E. G. Triarylmethyl Labels: Toward Improving the Accuracy of EPR Nanoscale Distance Measurements in Dnas. J. Phys. Chem. B 2015, 119 (43), 13641–13648. 10.1021/acs.jpcb.5b03026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzhelev A. A.; Shevelev G. Y.; Krumkacheva O. A.; Tormyshev V. M.; Pyshnyi D. V.; Fedin M. V.; Bagryanskaya E. G. Saccharides as Prospective Immobilizers of Nucleic Acids for Room-Temperature Structural EPR Studies. J. Phys. Chem. Lett. 2016, 7 (13), 2544–2548. 10.1021/acs.jpclett.6b01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomzov A. A.; Sviridov E. A.; Shernuykov A. V.; Shevelev G. Y.; Pyshnyi D. V.; Bagryanskaya E. G. Study of a DNA Duplex by Nuclear Magnetic Resonance and Molecular Dynamics Simulations. Validation of Pulsed Dipolar Electron Paramagnetic Resonance Distance Measurements Using Triarylmethyl-Based Spin Labels. J. Phys. Chem. B 2016, 120 (23), 5125–5133. 10.1021/acs.jpcb.6b03193. [DOI] [PubMed] [Google Scholar]
- Krumkacheva O.; Bagryanskaya E. EPR-Based Distance Measurements at Ambient Temperature. J. Magn. Reson. 2017, 280, 117–126. 10.1016/j.jmr.2017.02.015. [DOI] [PubMed] [Google Scholar]
- Kuzhelev A.; Akhmetzyanov D.; Denysenkov V.; Shevelev G.; Krumkacheva O.; Bagryanskaya E.; Prisner T. High-Frequency Pulsed Electron-Electron Double Resonance Spectroscopy on DNA Duplexes Using Trityl Tags and Shaped Microwave Pulses. Phys. Chem. Chem. Phys. 2018, 20 (41), 26140–26144. 10.1039/C8CP03951H. [DOI] [PubMed] [Google Scholar]
- Kuzhelev A. A.; Krumkacheva O. A.; Ivanov M. Y.; Prikhod’ko S. A.; Adonin N. Y.; Tormyshev V. M.; Bowman M. K.; Fedin M. V.; Bagryanskaya E. G. Pulse EPR of Triarylmethyl Probes: A New Approach for the Investigation of Molecular Motions in Soft Matter. J. Phys. Chem. B 2018, 122 (36), 8624–8630. 10.1021/acs.jpcb.8b07714. [DOI] [PubMed] [Google Scholar]
- Joseph B.; Tormyshev V. M.; Rogozhnikova O. Y.; Akhmetzyanov D.; Bagryanskaya E. G.; Prisner T. F. Selective High-Resolution Detection of Membrane Protein-Ligand Interaction in Native Membranes Using Trityl-Nitroxide Peldor. Angew. Chem., Int. Ed. 2016, 55 (38), 11538–42. 10.1002/anie.201606335. [DOI] [PubMed] [Google Scholar]
- Jassoy J. J.; Berndhauser A.; Duthie F.; Kuhn S. P.; Hagelueken G.; Schiemann O. Versatile Trityl Spin Labels for Nanometer Distance Measurements on Biomolecules in Vitro and within Cells. Angew. Chem., Int. Ed. 2017, 56 (1), 177–181. 10.1002/anie.201609085. [DOI] [PubMed] [Google Scholar]
- Giannoulis A.; Yang Y.; Gong Y.-J.; Tan X.; Feintuch A.; Carmieli R.; Bahrenberg T.; Liu Y.; Su X.-C.; Goldfarb D. DEER Distance Measurements on Trityl/Trityl and Gd(III)/Trityl Labelled Proteins. Phys. Chem. Chem. Phys. 2019, 21, 10217–10227. 10.1039/C8CP07249C. [DOI] [PubMed] [Google Scholar]
- Jassoy J. J.; Heubach C. A.; Hett T.; Bernhard F.; Haege F. R.; Hagelueken G.; Schiemann O. Site Selective and Afficient Spin Labeling of Proteins with a Maleimide-Functionalized Trityl Radical for Pulsed Dipolar EPR Spectroscopy. Molecules 2019, 24 (15), 2735. 10.3390/molecules24152735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzhelev A. A.; Krumkacheva O. A.; Shevelev G. Y.; Yulikov M.; Fedin M. V.; Bagryanskaya E. G. Room-Temperature Distance Measurements Using RIDME and the Orthogonal Spin Labels Trityl/Nitroxide. Phys. Chem. Chem. Phys. 2018, 20 (15), 10224–10230. 10.1039/C8CP01093E. [DOI] [PubMed] [Google Scholar]
- Edwards K. J.; Ollis D. L.; Dixon N. E. Crystal Structure of Cytoplasmic Escherichia Coli Peptidyl-Prolyl Isomerase: Evidence for Decreased Mobility of Loops Upon Complexation. J. Mol. Biol. 1997, 271 (2), 258–65. 10.1006/jmbi.1997.1151. [DOI] [PubMed] [Google Scholar]
- Müntener T.; Häussinger D.; Selenko P.; Theillet F.-X. In-Cell Protein Structures from 2D NMR Experiments. J. Phys. Chem. Lett. 2016, 7 (14), 2821–2825. 10.1021/acs.jpclett.6b01074. [DOI] [PubMed] [Google Scholar]
- Pan B. B.; Yang F.; Ye Y.; Wu Q.; Li C.; Huber T.; Su X. C. 3d Structure Determination of a Protein in Living Cells Using Paramagnetic NMR Spectroscopy. Chem. Commun. 2016, 52 (67), 10237–40. 10.1039/C6CC05490K. [DOI] [PubMed] [Google Scholar]
- Frericks Schmidt H. L.; Sperling L. J.; Gao Y. G.; Wylie B. J.; Boettcher J. M.; Wilson S. R.; Rienstra C. M. Crystal Polymorphism of Protein GB1 Examined by Solid-State NMR Spectroscopy and X-Ray Diffraction. J. Phys. Chem. B 2007, 111 (51), 14362–14369. 10.1021/jp075531p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C.; Chen J. L.; Yang Y.; Huang F.; Otting G.; Su X. C. Selective 15N-Labeling of the Side-Chain Amide Groups of Asparagine and Glutamine for Applications in Paramagnetic NMR Spectroscopy. J. Biomol. NMR 2014, 59 (4), 251–61. 10.1007/s10858-014-9844-0. [DOI] [PubMed] [Google Scholar]
- Hagelueken G.; Ward R.; Naismith J. H.; Schiemann O. Mtsslwizard: In Silico Spin-Labeling and Generation of Distance Distributions in Pymol. Appl. Magn. Reson. 2012, 42 (3), 377–391. 10.1007/s00723-012-0314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B.; Sikora A.; Bordignon E.; Jeschke G.; Cafiso D. S.; Prisner T. F. Distance Measurement on an Endogenous Membrane Transporter in E. Coli Cells and Native Membranes Using EPR Spectroscopy. Angew. Chem., Int. Ed. 2015, 54 (21), 6196–6199. 10.1002/anie.201501086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke G.; Chechik V.; Ionita P.; Godt A.; Zimmermann H.; Banham J.; Timmel C. R.; Hilger D.; Jung H. Deeranalysis2006 - A Comprehensive Software Package for Analyzing Pulsed ELDOR Data. Appl. Magn. Reson. 2006, 30 (3–4), 473–498. 10.1007/BF03166213. [DOI] [Google Scholar]
- Fontaine S. D.; Reid R.; Robinson L.; Ashley G. W.; Santi D. V. Long-Term Stabilization of Maleimide-Thiol Conjugates. Bioconjugate Chem. 2015, 26 (1), 145–52. 10.1021/bc5005262. [DOI] [PubMed] [Google Scholar]
- Zecevic A. N. A.; Eaton G. R.; Eaton S. S.; Lindgren M. Dephasing of Electron Spin Echoes for Nitroxyl Radicals in Glassy Solvents by Non-Methyl and Methyl Protons. Mol. Phys. 1998, 95 (6), 1255–1263. 10.1080/00268979809483256. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.