Abstract

Superamphiphobic surfaces are commonly associated with superior anticontamination and antifouling properties. Visually, this is justified by their ability to easily shed off drops and contaminants. However, on micropillar arrays, tiny droplets are known to remain on pillars’ top faces while the drop advances. This raises the question of whether remnants remain even on nanostructured superamphiphobic surfaces. Are superamphiphobic surfaces really self-cleaning? Here we investigate the presence of microdroplet contaminants on three nanostructured superamphiphobic surfaces. After brief contact with liquids having different volatilities and surface tension (water, ethylene glycol, hexadecane, and an ionic liquid), confocal microscopy reveals a “blanket-like” layer of microdroplets remaining on the surface. It appears that the phenomenon is universal. Notably, when placing subsequent drops onto the contaminated surface, they are still able to roll off. However, adhesion forces can gradually increase by up to 3 times after repeated liquid drop contact. Therefore, we conclude that superamphiphobic surfaces do not warrant self-cleaning and anticontamination capabilities at sub-micrometric length scales.
Keywords: wetting, superhydrophobicity, contamination, microdroplet, adhesion, pinning
Superamphiphobic surfaces have been represented as next-generation self-cleaning materials.1,2 However, is this assignment really justified?3,4 Indeed, liquid drops rolling over superamphiphobic surfaces easily remove macroscopic contamination and the surfaces appear to be clean. No remnants of water and other test liquids are visible by eye or optical shadowgraphy. The contact and roll-off angles can remain almost unaltered even if a second drop was placed on the same spot. Such behaviors appear to indicate the lack of macro-, micro-, and nanoscopic contamination. However, drops are also left on the surfaces for short durations, and different locations are typically used for every test. Here, we highlight the discovery of remnant microdroplets remaining on superamphiphobic surfaces in the Cassie–Baxter state, despite nonwetting contact. In this article, we call these microdroplets “contamination”. Notably, the phenomenon is universal and occurs despite characteristically high contact angles and low sliding angles.
Superamphiphobicity is often defined by low sliding angles of <10° with low surface tension liquids (γ < 30 mN/m).2,5−7 This is facilitated by the near-spherical shape of liquid drops2 resulting from the air cushion upon which the drops partially rest.8 Small contact areas are associated with the self-cleaning properties of superamphiphobic surfaces. Today, the concept of self-cleaning is exploited in developing bioengineering,9,10 microfluidics,11 antimicrobial coatings,12−15 and membrane16−21 technologies. The absence of contact contamination is particularly important in biomedical diagnostics. For instance, highly precise superhydrophilic–superhydrophobic micropatterning has been used to selectively screen and capture bioactive molecules, cells, or enzymes within superhydrophilic spots while being automatically excluded from superhydrophobic domains.11,22,23 To the best of our knowledge, it is still unclear if liquid remnants exist on nanostructured superamphiphobic surfaces after macroscopic liquid contact.1,24−26
Nanostructured superamphiphobic surfaces possess very low adhesion and hysteresis.2 The complete detachment of a large parent drop from nanostructured protrusions may be indeed energetically favorable compared to pinching off a microscopic satellite4 microdroplet. However, self-cleaning28,29 has never been demonstrated down to short temporal time scales and micrometric length scales. It is of importance to both fundamental understanding and applied research to clarify whether remnants form and remain.
Here, both superamphiphobic nanoparticles- (soot-templated or wet-sprayed) and nanofilaments-based surfaces were investigated.6,7,27 They represent state-of-the-art superamphiphobic nanostructured surfaces. The volatility of the investigated liquids ranged from lower vapor pressures in hexadecane (P ≈ 0.2 Pa) to higher vapor pressures in water (P ≈ 103 Pa). Using laser scanning confocal microscopy, we detect microdroplet contamination for several liquids having low volatility. For highly volatile liquids such as water, contamination can sometimes be observed with the naked eye, especially under high humidity (see Movie M1). Notably, a “blanket-like” layer of microdroplets covers the surface after contacting and repelling liquid drops. Long-lasting trails and spots are respectively formed when nonvolatile or hygroscopic liquid drops roll over or sit on these surfaces (Figure S1).
Results/Discussion
Classification of Nanotextures and Liquids
Three superamphiphobic surfaces were used: soot-templated,7 wet-sprayed silica,27 and nanofilament6-based surfaces. Soot-templated surfaces (Figure 1a,b) are composed of fractal-like soot-templated nanoparticles (50–70 nm diameter), covered by a silica shell (20–30 nm thick). The silica shell was fluorinated with a trichloro(1H,1H,2H,2H-perfluorooctyl)silane to lower the surface energy.7 Soot-templated surfaces have a highly porous morphology (Figure 1a). The alternative superamphiphobic nanoparticle surfaces were synthesized by spray-deposition of functionalized fumed silica nanoparticles and condensed nanofilaments, respectively (Figure S2). Wet-spraying nanoparticles gave rise to comparatively densely packed coatings, Figure S2a.27 For nanofilaments, glass slides were immersed in toluene with controlled amounts of water and trichloromethylsilane. After a reaction time of 6 h, glass slides were coated with a 1–2 μm thick coating of nanofilaments (20–50 nm diameters, spaced between 50 and 500 nm, Figure S2b).6 To reduce surface energy, coated slides were fluorinated using 1H,1H,2H,2H-perfluorodecyltrichlorosilane (PFDTS). All surfaces show a high static water contact angle of >150° and low roll-off angles of less than 10° for both 6 μL water and hexadecane drops.6,7,27
Figure 1.
Optical and interference microscopy of microdroplet contamination on soot-templated superamphiphobic surfaces. Top-view SEM images at (a) low and (b) high magnification of soot-templated surfaces.7 (c) Sequence of video images showing the pristine surface with a sessile ethylene glycol drop (30 μL). After 2 min, the drop was removed by a tissue. The drop left a circular imprint (hazy spot) that disappeared after a minute. (d) Rolling of a 30 μL ethylene glycol drop off a tilted surface. Tilt angle: 13°. Trails are shallower compared to the spots. (e) Interference scans were performed using confocal microscopy, mapping the mean penetration depth by the liquid drop into the surface. In this case, the drop remained on the surface. From the interference patterns (f, g, insets) the penetration depth was calculated with respect to time with both (f) ethylene glycol and (g) an ionic liquid (trihexyltetradecylphosphonium bis(trifluoromethyl sulfonyl) imide). Dark regions correspond to constructive and bright regions to destructive interference using a wavelength of 633 nm. A dark-to-bright transition represents λ/4, or 158 nm.
As a model liquid for demonstrating contamination of superamphiphobic coatings, ethylene glycol was used. Ethylene glycol’s low ambient vapor pressure of 8 Pa at 20 °C, coupled to hygroscopicity, minimized evaporative losses upon microdroplet formation. Its relatively high surface tension, γ = 0.0477 N/m, also implies a high-energy barrier against Wenzel wetting on superamphiphobic surfaces.1,2,5−7,28 For comparison, we used water, hexadecane, and a completely nonvolatile ionic liquid (trihexyltetradecylphosphonium bis(trifluoromethyl sulfonyl) imide (Iolitec, >98%, abbreviated [P6,6,6,14]+[TFSI]−). Surfaces after brief contact with the ionic liquid were investigated under high vacuum using X-ray photoelectron spectroscopy and scanning electron microscopy.
Optical Visualization of Macro-scale Drop Contamination: Trails and Spots
We performed two different protocols. First, we deposited a drop of ethylene glycol (30 μL) onto a soot-templated surface. After 2 min, the ethylene glycol drop was removed by absorbing it into a piece of tissue paper (Figure 1c, Movie M2). Upon removal, an imprint of the drop’s original footprint remained (Figure 1c). Second, we let ethylene glycol drops (approximately 30 μL) roll over a soot-templated surface (Figure 1d, Movie M3). This resulted in the gradual and temporal generation of an imprinted trail (Figure 1d). In both experiments, contamination appears to be a fuzzy white region that disappears after approximately 1 min exposure to the ambient environment, presumably by evaporation. Afterward, the domain appears visually identical to that before contact.
To understand how liquid remnants remain on the surface, an important question is, how deep does the liquid enter the superamphiphobic layer while being in contact with the surface? We quantified the size and kinetics of surface impalement using a confocal microscope in reflection mode (Leica TCS SP8, low numerical aperture objective Leica HC PL APO 10×/0.4) and a HeNe laser at 633 nm. Upon drop deposition, interference patterns appear (Figure 1f,g, inset, Figure S3). Interference fringes gradually change with time. A switch of the intensity from bright to dark corresponds to an increase in depth equal to λ/4 (λ = 633 nm). We measured the kinetics of the average penetration depth over an area of 200 × 200 μm2 (10 000 points) and a time span of 600 s for a drop of ethylene glycol (Figure 1f, Movie M4) and ionic liquid (Figure 1g, Movie M5). For both liquids, the penetration depth is below the size of one nanoparticle in the first few seconds. Impalement progressed slowly.
Despite negligible impalement within the first few seconds, microdroplet remnants are formed upon drop contact (Movie M6, <1 s). We confirm that, despite changes in surface penetration depth, microdroplet density reaches a maximum (up to 75% of final) within the first 30 s (Figure S4), whereas ethylene glycol gradually penetrates into the structure. There is no significant influence of time-dependent penetration on the onset of microdroplet formation. At a time scale of 10 min, the liquid impaled the superamphiphobic surface between 0.2 and 2 μm (Figure 1f,g). This indicates that any nano- and microdroplet contamination remained on the topmost nanoparticle structures. Despite surface impalement and contamination, superamphiphobicity persists and is verified by very low sliding angles: For instance: ethylene glycol roll-off angles were found to be 1.8 ± 0.2°, while hexadecane drops rolled off at 2.3 ± 1.5° on soot-templated surfaces (10 μL drops).
Confocal Microscopy Visualization of Microscale Drop Contamination: Microdroplets
To monitor the depth profile of microdroplet remnants, confocal microscopy was focused on the XZ-plane. A dyed drop of ethylene glycol was left to sit on a soot-templated surface for approximately 30 s. Thereafter, the drop was rolled off, leaving behind microscopic spots (Figure 2a). It is important to reiterate that microdroplet formation occurs immediately upon contact (Movie M6, <1 s). Dynamic observation of the XZ-plane during drop roll-off revealed how the microdroplets are formed. They appear to be peeled off from the large drop by the surface, giving rise to multiple fluorescent microspots (Figure 2b–e). The microdroplets are localized to the topmost layers of the superamphiphobic soot-templated surface, with no signs of Wenzel wetting. The Cassie–Baxter state persists. Such an observation supports the depth impalement data provided by interference microscopy (Figure 2e).
Figure 2.
Confocal microscopy imaging of an ethylene glycol drop rolling off a soot-templated superamphiphobic surface. (a) Sketch of a drop (blue) rolling over a nanoparticle-based soot-templated surface. Microdroplets (blue) remain. The particles (gray) are hydrophobized (green) to lower the surface energy. (b–e) A fluorescence-dyed ethylene glycol drop (blue) was rolled off a superamphiphobic surface while dynamically observing surface fluorescence. XZ-plane showing the vertical, Z-axial contact line of a drop. The images were taken using an inverted laser scanning confocal microscope using a 40× air objective. Ethylene glycol was dyed with ATTO 647-ester at a concentration of 10 μg/mL (blue). The bulk drop and thus microdroplets appear blue. Reflection from the interface between the glass and the superamphiphobic coating appears red. All particle spheres represented in the schematics should be considered as agglomerates instead of individual nanoparticles.
To investigate the influence of morphology, we investigated alternative superamphiphobic surfaces composed of nanofilaments (layer thickness of approximately 2 μm)6 and wet-sprayed nanoparticles (approximately 3–5 μm thick).27 After 150 s of drop deposition, the drop was rolled off. Microdroplets were present on both surfaces. XY- and XZ-planes showing microdroplet profiles are included in Figure S5 for reference. Microdroplet sizes and densities were determined using ImageJ’s ParticleAnalyzer package. The package analyzes 8-bit threshold images of microdroplets without the background reflection under no spheroidal constraints (0–1). Confocal images show that the contamination patterns are similar on both superamphiphobic nanoparticles and nanofilaments (Figure 3a,b). Microdroplet size distributions were nonsymmetric and non-normal. Ethylene glycol microdroplets were measured at an average of 0.5 μm, ranging from 0.2 to 1.0 μm (soot-templated nanoparticles), 2.1 μm, ranging from 0.5 to 3.8 μm (wet-sprayed nanoparticles), and 0.9 μm, ranging from 0.3 to 1.9 μm (nanofilaments). Microdroplets on nanofilaments appear smaller compared to the soot-templated surfaces (Figure 3a,b). However, they are actually partially hidden within the reflection plane (black-red) as the substrate/air interface is much closer as compared to the latter.
Figure 3.
Imaging of the “blanket-like” coverage of ethylene glycol microdroplets on soot-templated and nanofilament surfaces. Confocal images of the XY-plane taken close to the surface–air interface of (a) soot-templated nanoparticles and (b) nanofilaments after drop removal. Surface coverages of ethylene glycol microdroplets on soot-templated nanoparticles, wet-sprayed nanoparticles, and nanofilaments are approximately 22 × 103, 2 × 103, and 26 × 103 microdroplets per millimeter square, respectively. (c, d) Microdroplets were found on the accumulated XZ section at the air–surface interface. Evidently, none of the microdroplets impale deep into the nanostructured surface. (e–g) Scanning electron microscopy of the soot-templated surface after a nonvolatile (ionic liquid) drop was removed. The dark spots reflect the previous positions of the microdroplets. These imprints are composed of multiple dispersed subagglomerate zones that are approximately 1–10 μm in diameters.
Microdroplets on wet-sprayed nanoparticles were noticeably larger in volume (Figure S5). The persistent observation of microdroplets on differently structured superamphiphobic surfaces is indicative of a universal phenomenon. To further investigate macroscopic consistencies in the penetration depth of the microdroplets, we analyzed the side profiles (accumulated XZ, 100 μm in plane) (Figure 3c,d and Figure S5). Despite the dense and blanket-like coverage of microdroplets, the contamination is limited only to the topmost layer of all superamphiphobic surfaces. This thus preserves the effective Cassie–Baxter (air-gaps) state.
To examine the universal nature of microdroplet remnants, we investigated whether other common liquids, such as hexadecane and undecane, also form microdroplets (Movie M7). The model superamphiphobic soot-templated surface was used. Submicrometric droplets from nonvolatile hexadecane (P0 = 0.2 Pa) could be seen for almost 1 min (Figure S6). Their mean diameter was 0.7 μm, with a minimum detectable size of 0.2 μm. The actual minimum size could be smaller owing to the resolution limit of confocal microscopy.
According to
the Kelvin equation,29 the
vapor pressure of a liquid Pv increases
to 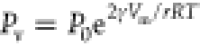 . Here, P0 is
the saturated vapor pressure over a planar surface, Vm is the molar volume of the liquid,30R is the gas constant, and T is temperature. The coefficient 2γVm/RT, also called Kelvin length, is 5.1 nm for ethylene
glycol, 6.5 nm for hexadecane, and 4.2 nm for undecane. The overpressure,
above ambient saturation (P0), can range
from 1% to 7% with microdroplet radii, r, from 0.1
to 1 μm for hexadecane. The slightly volatile undecane microdroplets
(P0 = 55 Pa) were only stable within the
order of a few seconds (Figure S7). Hence,
we were not able to capture full 3D images. Notably, despite the high
vapor pressure of ethylene glycol (P0 =
8 Pa), these microdroplets were at least as persistent as hexadecane
droplets. The high stability of ethylene glycol microdroplets may
be caused by the hygroscopic nature of ethylene glycol, where microdroplets
are sustained by the absorption of water.
. Here, P0 is
the saturated vapor pressure over a planar surface, Vm is the molar volume of the liquid,30R is the gas constant, and T is temperature. The coefficient 2γVm/RT, also called Kelvin length, is 5.1 nm for ethylene
glycol, 6.5 nm for hexadecane, and 4.2 nm for undecane. The overpressure,
above ambient saturation (P0), can range
from 1% to 7% with microdroplet radii, r, from 0.1
to 1 μm for hexadecane. The slightly volatile undecane microdroplets
(P0 = 55 Pa) were only stable within the
order of a few seconds (Figure S7). Hence,
we were not able to capture full 3D images. Notably, despite the high
vapor pressure of ethylene glycol (P0 =
8 Pa), these microdroplets were at least as persistent as hexadecane
droplets. The high stability of ethylene glycol microdroplets may
be caused by the hygroscopic nature of ethylene glycol, where microdroplets
are sustained by the absorption of water.
Electron Microscope Imaging of Microdroplets
The ultralow vapor pressure of ionic liquids, on the order of 10–10 Pa,31 makes the observation of microdroplets possible in high vacuum. To visualize the morphology of these microdroplet contaminants, trails of 30 μL drops were investigated optically and by scanning electron microscopy. Spots and trails do not macroscopically vanish (Movie M8). Under the SEM, it appears that microdroplets damage the superamphiphobic nanostructures. This results in clustered domains of nanoparticle agglomerates. These agglomerates were approximately 1–10 μm in dimension (Figure 3e–g). Agglomerated clusters were coated in a thin layer of ionic liquid, indicating local collapse of nanostructures by capillary forces (Figure 3g, darker and thicker agglomerates). Encapsulation of agglomerated clusters by microdroplets likely happens for all liquids, as the microdroplets could be as large as a few micrometers. Observing the presence of other liquids is difficult due to inherent volatility under high vacuum.
To verify that dark regions visible in SEM were remnants of the ionic liquid, we investigated the chemical composition of the surface by X-ray photoelectron spectroscopy (Figure S8). The transitions expected for the neat ionic liquid, i.e., P 2p, S 2p, P 2s, S 2s, C 1s, N 1s, O 1s, F 1s, F KLL, and O KLL are found in the survey spectra acquired on the trails/spots of the ionic liquid on the soot-templated surfaces. Key atomic signatures for the presence of ionic liquid are indicated by the phosphorus, sulfur, and nitrogen signals. For the pristine superamphiphobic soot-templated surfaces, the transitions P 2p, S 2p, P 2s, S 2s, and N 1s are absent. Thus, the spectra with a clear N 1s transition prove the presence of remnant ionic liquids on the surface. Here, the ionic liquid used represents a model analog system for SEM and XPS: Readers should note that different liquids will result in slightly different wetting behavior.
Microdroplet Formation
The formation of microdroplets relies on the interplay between the actual local contact angle (θlocal) and the inherent receding contact angle (θrec). The inherent receding contact angle (θrec) is also known as the characteristic contact angle that a liquid drop takes with an equivalent flat surface. Capillary bridges are formed between a receding surface of a drop and the top faces of surface protrusions. During the receding motion, these capillary bridges are stretched and at some point will break. For pinned contact lines, the breaking point is defined by the pinning centers (Figure 4a). For freely moving contact lines, the local contact angle decreases to the point where it matches the material’s inherent receding contact angle (Figure 4b). Remnants then depend on how far the contact line has receded when the liquid capillary bridge has thinned to the point of rupture. In both cases, the formation of microdroplet remnants is coupled to agglomerate-induced bridging, forming clusters that make up the micrometric droplets that we observe (Figure 4c). Considering that local agglomerate geometry and interagglomerate proximity significantly influences the formation of the microdroplets, it is difficult to develop a quantitative theoretical model for such stochastic surfaces.
Figure 4.
Geometrical pinning of liquids to a spherical asperity. (a) Case 1, pinning: The contact angle and contact line are pinned at the local contact angle (θlocal) until the capillary bridge (double-sided arrow) ruptures. (b) Case 2, depinning: The contact angle and contact line reach the inherent receding contact angle (θrec) and the latter begins sliding. However, thinning of the capillary bridge continues (double-sided arrow), eventually rupturing and breaks before complete depinning of the contact line. In both instances, a remnant droplet is formed. However, because of experimental resolution, visualization of remnants on a single nanoparticle having a diameter of approximately 80 nm is not possible. Therefore, we are only able to visualize the remnants integrating several nanoparticles. (c) Surrounding remnant droplets merge, forming the micrometric droplets that we observe.
The remnant microdroplets gradually penetrate into sublayers on a longer time scale. This is supported by observations from contacted ionic liquids and high-vacuum scanning electron microscopy. Liquid micrometric microdroplets are integrated into both the top (“contact points”) and sublayers (“inside”) of the nanoparticle aggregations. A series of images depicting mild to severe surface penetration is included in Figure S9.
Hydrophilic defects could also reduce the inherent receding contact angle (θrec) and pin the contact line. To investigate the possible influence of defects in a control experiment, a glass slide was fluoro-silanized under the same conditions as the superamphiphobic soot surface (60 min, 50 mbar), Figure S10. We then moved (immobilized and dragged by a metal plate) a drop of fluorescence-dyed (ATTO 647-ester at 10 μg/mL) ethylene glycol over its surface with velocities of 10 and 300 μm/s. Afterward we imaged the glass surface with a confocal microscope. No remaining microdroplets were detected. This indicates that the surface was homogeneously silanized or that defects were so small that they cannot be responsible for microdroplet remnants. We conclude that the primary effect behind the formation of microdroplets is by pinning to the top faces of surface protrusions rather than to hydrophilic defects.
Lowering the liquid surface tension (γ) decreases the material’s receding contact angle θrec. This reduction facilitates droplet pinning and makes the formation of microdroplet remnants easier during drop detachment. In contrast, the viscosity of the liquids did not appear to influence the size of capillary bridges during necking and rupture, at least not in the experimentally accessible range of velocities of 10–300 μm/s (capillary number from 10–6 to 10–4). This range also indicates that hydrodynamic effects are negligible.
In summary, geometrical pinning of liquid droplets occurs when a liquid is withdrawn from surface protrusions. For water, remnants go unnoticed because the microdroplets immediately evaporate. However, non- or poorly volatile solvents may not or will take longer time scales to vaporize, thus leaving visible microdroplets.
Influence on the Definitions of Superamphiphobicity and Self-Cleaning Properties
An important question is, do microdroplet remnants of previous drops influence the roll-off angles of subsequent drops? To answer this question, 30 μL drops were deposited on a defined spot for periods between 10 and 60 min. Then, they were removed with a tissue. Within 10 s, a 10 μL test drop of the same liquid was placed onto the footprint of the larger drop and the surface was gradually tilted to determine the roll-off angle within the next 10 s. For water, the roll-off angle, α, remained low and constant at α = 1° (Figure 5a,b, blue). The roll-off angle for ethylene glycol only slightly increased over time, rising from approximately 2° to 4° at 60 min. After 60 min, a dense white spot was formed, showing macroscopic surface contamination (Figure 5b, inset).
Figure 5.
Microdroplet clusters on soot-templated surfaces: Influence on wettability. Time-resolved dynamic analysis of increasing roll-off angles with respect to three different liquids: water, ethylene glycol, and the ionic liquid [P6,6,6,14]+[TFSI]−. (a) The roll-off angle of water and ethylene glycol remained below 5°. The roll-off angle of the ionic liquid gradually rose to 47° after 60 min. (b) The roll-off angle for ethylene glycol rose from 2° to 4°. This is accompanied by the formation of a dense white fuzzy spot on the surface (inset, at 60 min).
The roll-off angle of the ionic liquid gradually increased with increasing drop sitting time (Figure 5a, purple). After 60 min of sitting time, the roll-off angle increased to 47°. The drop gradually impaled into the superamphiphobic layer,32 hinting toward a partial Cassie-to-Wenzel transition. Notably, the extent of surface contamination by ionic liquids can vary. Individually prepared drop trails and spots can show greatly differing roll-off angles, up to between 25° and 45°. Due to the low surface tension of an ionic liquid, γ = 0.028 N/m, the impalement pressure is low; that is, the drop easily impales the surface (Figures S9, S11–S13). Here, ethylene glycol also impales the surface, but it evaporates, at least partially.
Microdroplet Formation and Impact on Adhesion and Wetting
An increasing roll-off angle indicates that the normal adhesion force to a drop should increase. In order to quantify the temporal dynamics of adhesion forces, we attached a 30 μm diameter droplet of ionic liquid to a hydrophobized tipless AFM cantilever and performed force curves on a pristine soot-templated surface (Figure 6a). The spring constant of the cantilever, k = 7.7 N/m, was determined using the built-in thermal tune method of the AFM control software prior to picking up the droplet. After attaching the ionic-liquid droplet, around 200 force curves were recorded at a rate of 0.25 Hz and a maximum applied force of 10 ± 5 nN. From the measured shift in the thermal noise resonance33 of the cantilever with an attached droplet we could calculate the mass loss for every contact. The frequency increased by 112 Hz after 200 contacts. This indicates a mass loss of 20 fg from the droplet-probe after contact (Figure 6b). Taking into account the density of this ionic liquid (1070 kg/m3), a mass loss of 20 fg would correspond to a spherical droplet of 0.3–0.4 μm in diameter, representing the liquid remnants left behind on the soot-templated surface.
Figure 6.
Adhesion properties of microdroplets. (a) A pristine soot-templated surface was tested using droplet-probe force microscopy. A total of 200 force curves were taken under repeated contacts of a nonvolatile ionic liquid drop with the surface on two locations. (b) The shift in the thermal noise spectra of the droplet-probe cantilever, before (black) and after 200 cycles (cyan), corresponds to a mass loss of 20 fg. (c) The maximum tip-to-sample distance decreases around 25 nm between the first and last measurement. (d) Time-dependent adhesion on an initially pristine spot. Adhesion on the pristine surface starts at approximately 200–400 nN and increases over time up to 500–550 nN after gradual contamination.
While the drop is periodically approached and retracted at a rate of 0.25 Hz and at constant maximal load, the maximum tip–sample distance decreases around 25 nm over 6 min of force curve measurements (Figure 6c). The dominant contribution is likely due to localized pulling up of the soot-templated nanoparticles by capillary forces (SEM, Figure 3g and Figure S14). Finally, the adhesion force gradually increases from around 300 nN to 550 nN between the first and last force curve (Figure 6d, red line). The effective contact area per microdroplet increases the net contribution of adhesion. It should be noted that the adhesion force varied by up to 200 nN depending on the specific position. This variation is likely induced by the microrough nature of the soot-templated surface.
To estimate the maximum adhesion force, Fadh, we assume that the bottom side of the drop experiences partial contact with protrusions of the soot-templated surface. From confocal microscope images, we counted a uniform distribution of wetted protrusions (Methods), at approximately 16 000 per mm2. In addition, prior SEM analysis reveals that protrusions are typically not single nanospheres but agglomerates of nanospheres that form the relevant contact points. Here, the protrusions represent where microdroplets are left behind: comprising agglomerates coated with a thin layer of liquid. We further deduce, for simplicity, that these protrusions have an effective diameter of between 0.5 and 1 μm. The capillary force required to pull the drop of one such agglomerate is estimated to be the Fadh.
| 1 |
For the ionic liquid, we assume Fadh ≈ πDγ, for 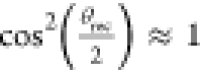 . Thus, a protrusion with a diameter of D between
0.5 and 1 μm, γ = 0.028 N/m, experiences
an adhesion force of between 44 and 88 nN. The estimated contact area
between the surface and the ionic liquid drop on the AFM cantilever
was 165 μm2 (see Methods).
Therefore, we estimate an average of approximately 2.6 contact points,
leading to an adhesion force Fadh = 2.6
× (44 to 88) nN ≈ 115–230 nN. This agrees within
an order of accuracy to the experimentally determined increase in
adhesion force of 250 nN, due to microdroplet contamination (Figure 6d).
. Thus, a protrusion with a diameter of D between
0.5 and 1 μm, γ = 0.028 N/m, experiences
an adhesion force of between 44 and 88 nN. The estimated contact area
between the surface and the ionic liquid drop on the AFM cantilever
was 165 μm2 (see Methods).
Therefore, we estimate an average of approximately 2.6 contact points,
leading to an adhesion force Fadh = 2.6
× (44 to 88) nN ≈ 115–230 nN. This agrees within
an order of accuracy to the experimentally determined increase in
adhesion force of 250 nN, due to microdroplet contamination (Figure 6d).
Conclusions
The observation that liquid drops in temporary contact with superliquid-repellent surfaces leave nano- or microdroplets behind changes the concept of self-cleaning. In fact, the brief contact with a macroscopic rolling or sitting drop will result in a “blanket-like” coverage of microdroplets, even for nanostructured superamphiphobic surfaces. Contamination at this level appears to be a universal phenomenon that occurs with multiple liquids and surfaces. Perfect self-cleaning surfaces are only possible with pure and volatile liquids. For nonvolatile liquids, the formation of microdroplet contaminants is sufficient in gradually influencing superwetting performance and adhesion properties. Thus, caution should be exercised when utilizing these surfaces for (1) nonvolatile liquids,25 (2) anticontamination and antifouling in sub-micrometer cell biology13,14,34−36 (bacteria ≈ 1 μm, virus ≈ 100 nm)37 or dissolved compounds (inorganic salts), while (3) accepting contamination posed by long-term usage.
Methods/Experimental
Test Liquids
The liquids used, with their surface tensions, were water (72.8 mN/m), ethylene glycol (47.7 mN/m, 99.8%, Aldrich), an ionic liquid, trihexyltetradecylphosphonium bis(trifluoromethyl sulfonyl) imide (Iolitec, >98%, abbreviated [P6,6,6,14]+[TFSI]−, 28.8 mN/m, Wilhelmy plate measurements), n-hexadecane (27.5 mN/m, >99%, Aldrich), and n-undecane (24.7 mN/m, >99%, Aldrich). Unless otherwise indicated, surface tensions were provided by the manufacturers. All experiments were performed at between 30% and 60% relative humidity, at a temperature of 20–25 °C.
Synthesis of Surfaces
Soot-Templated Superamphiphobic Surface
Superamphiphobic soot surfaces were synthesized based on our previous work,7 which entails four distinctive steps: (1) soot deposition, (2) silica templating, (3) calcination, and (4) fluoro-functionalization. The particle surface is composed of fractal-like soot nanoparticles (50–70 nm diameter), covered by a silica shell (20–30 nm thick). The silica shell was then fluorinated with 1H,1H,2H,2H-perfluorodecyltrichlorosilane using chemical vapor deposition to lower the surface energy (Figure 1).5 A pristine superamphiphobic nanoparticle surface tends to have a highly porous and opened morphology, characterized by overhanging nanoparticle agglomerates.
Dense Nanoparticulate Superamphiphobic Surface
The alternative superamphiphobic nanoparticle surface was synthesized by the functionalization of fumed silica nanoparticles using 1H,1H,2H,2H-perfluorodecyltrichlorosilane.27 The functionalized particles reached a functionalization density of approximately 54 w/w%. Wet-spraying solvated nanoparticles gave rise to comparatively densely packed coatings (Figure S2).27
Nanofilament-based Superamphiphobic Surface
Glass slides (170 μm, 20 × 60 mm, Marienfeld) were immersed in 100 mL of toluene with controlled amounts of water (180–250 ppm) and trichloromethylsilane (0.4 mL). Trichloromethylsilane hydrolyzes and reacts with the hydroxyl groups on glass, forming silicone nanofilaments. After a reaction time of 6 h, glass slides were coated with a 1–2 μm thick layer of nanofilaments (20–50 nm diameters, spaced between 50 and 500 nm).6,15 To achieve superamphiphobicity, the nanofilaments were activated in an oxygen plasma and modified with 1H,1H,2H,2H-perfluorodecyltrichlorosilane using chemical vapor deposition.
Soot-templated nanoparticles are between 20 and 35 μm thick, spray nanoparticle coatings are approximately 5 μm thick, while nanofilaments were approximately 1–2 μm thick.
Contact Angles
Dynamic and static contact angles were recorded using an OCA 35 contact angle goniometer (Dataphysics, Germany, zoom factor 0.7). All surfaces show a high static water contact angle of >150° and low roll-off angles of less than 10° for both 6 μL water and hexadecane drops.6,7,27 Roll-off angles were assessed by tilting the surfaces at 1°/s until the drop starts rolling off (20 ms per frame). The contact angle, roll-off angle, and contact angle hysteresis were computed by a commercially available program (SCA20). Results are presented as mean ± standard deviations.
Dynamic Visual Capture of Drop Spots and Trails
Water, hexadecane, undecane, ethylene glycol, and the ionic liquid ([P6,6,6,14]+[TFSI]−) were used in testing the presence of macroscopically visible drop spots and trails (Figure S15) at 30–60% relative humidity and 20–25 °C. Spots: To study possible microdroplet remnants, we deposited 30 μL drops of various liquids for approximately 6–10 min before its removal (soaked up/slide off). Drops were rolled off the sample by tilting or soaked up by a tissue. Then we imaged the surface from the top by a dynamic single-lens reflex camera (Nikon D3300, 1×, ambient or LED illumination, 3–5 min). Drop spots disappear in a timespan of a few seconds to a few minutes for liquids having finite volatility. Trails: A drop deposition system is based on a syringe pump that deposits 1 to 3 mL/min of liquid, via drops that detach from a 25G needle nozzle. The surface is tilted at around 13°. The drops roll down the surface with varying periodicity. Drop trails were captured on video (Nikon D3300, 5 min, 1080 p, 50 fps), forming under continuous flow, and disappearing when the flow is halted.
Ethylene Glycol
Visual observation of spots and trails formed from ethylene glycol are persistent for approximately 1-2 minutes. Thereafter, the macroscopic observation is lost after gradual vaporization of the spots or trails. The dried surfaces appear pristine.
Ionic Liquid ([P6,6,6,14]+[TFSI]−)
Drop deposition was replicated under similar conditions to the experiments based on ethylene glycol, but with lasting spots and trails. This is likely caused by its inability to evaporate after forming microdroplets.
X-ray Photoelectron Spectroscopy
XPS analyses were carried out with a Kratos Axis Ultra DLD (Kratos Ltd., Manchester, UK) using a monochromatic Al Kα X-ray source (1486.6 eV, emission current: 10 mA, anode voltage: 15 kV). The instrument base pressure remained below 8.0 × 10–10 Pa. The instrument work function was calibrated to a binding energy of 84.0 eV for metallic gold (Au 4f7/2). The charge neutralizer was used for all analyses (filament current: 2.1 A, charge balance: 3.45 V, and filament bias: 1.5 V). The charge neutralization was monitored with the help of the C 1s peak for adventitious carbon. Survey spectra were acquired at a pass energy of 80 eV with 20 sweeps and an energy step of 1 eV. The high-resolution spectra were acquired at a pass energy of 20 eV with 10 sweeps and an energy step of 0.1 eV. The analysis area was 300 μm × 700 μm. Data were processed using the commercial software CasaXPS (version 2.3.16, Casa Software Ltd., Chichester, UK). All spectra were recorded in the spectroscopy mode utilizing the hybrid lens mode. For each sample, at least three independent measurements were performed. The binding energies were calibrated using the C 1s peak for adventitious carbon at a binding energy of 284.8 eV, with an associated error of ∼0.1–0.2 eV.38 No argon ion sputter cleaning has been performed prior to analysis.
Surface Analysis: Confocal Microscopy
An inverted laser scanning confocal microscope (Leica TCS SP8) was used to dynamically observe the progression of the wetting line down the Z-axis of superamphiphobic surfaces (nanoparticles and nanofilaments). The ionic liquid, [P6,6,6,14]+[TFSI]−, hexadecane, and undecane were dyed with Lumogen Red 300 (λexcitation = 553 nm, λemission = 610 nm), at a concentration of 10 μg/mL. Ethylene glycol was dyed with ATTO 647-ester (λexcitation = 620 nm, λemission = 647 nm) at a concentration of 10 μg/mL. These dye concentrations did not change the surface tension of the drops. The drops were placed onto the substrate after commencing the confocal measurement.
Ethylene Glycol and Ionic Liquid
Time-varied XYT planes were first recorded under the reflection mode, with a zoom factor of 1.0 in the XZ plane and a line average of 2 (bidirectional). A dry objective, Leica HC PL APO 10×, NA 0.4, was used. Lasers of 458, 561, and 633 nm were used at ca. 15.4% power. All three lasers were only used during interference analysis, providing improved resolution for surface penetration measurements. This was performed for up to 10 min at a frame rate of 1.18 frames per second (fps). This scan type was performed only for the soot-templated superamphiphobic surface owing to the continued variation in dynamics up until the 10th minute. Thereafter, the drops were removed, and the XYZ plane (3D) was recorded with a line average of 16, with bidirectional scanning. A zoom factor of 1.0 was used with a dry objective, Leica HC PL APO 40×, NA 0.85, in order to match the location.
Ethylene Glycol
Lasers of 458 and 633 nm were used at ca. 15.4% power under reflection and fluorescence mode, respectively. The XYZ stack was recorded over 200 steps as a 3D image. XZT planes were also recorded at 1.58 fps within various time frames.
Ionic Liquid
Lasers of 458 and 561 nm were used at ca. 15.4% power under reflection and fluorescence mode, respectively. The XZT plane was recorded at 0.095 fps over 30 min to map macroscopic penetration after 10 min. The XYZ stack was recorded over 150 steps as a 3D image. For ionic liquid on soot-templated surfaces, a long-term penetration experiment was performed; the depth of penetration by the wetting line is presented as a percentage of the total height of the coating. Several quantitative tests were performed, where a percentage of the penetration was measured in real time before removing the drop, enabling a quantification of this effect.
Other liquids (dyed ionic liquid, [P6,6,6,14]+[TFSI]−, hexadecane, undecane, and ethylene glycol) and surfaces (nanoparticles and nanofilaments) were also analyzed using the XZT mode: Various XZT scans were performed to analyze the effect of liquid on morphology. The ethylene glycol variant was used on soot-templated superamphiphobic nanoparticles, wet-spray-deposited superamphiphobic nanoparticles, and superamphiphobic nanofilaments. These tests were aimed at demonstrating the universal nature of the phenomenon.
Interference Analysis
Interference forms due to reflections from the nearly planar glass surface and the drop interface and allows the determination of changes in the position of the drop with nanometer precision. We employed a confocal microscope in reflection mode (Leica TCS SP8) with a low numerical aperture objective (Leica HC PL APO 10×/0.4) and a HeNe laser at 633 nm to have a nearly collimated beam. Upon drop deposition, interference patterns appear, which dynamically change with time. This measurement was performed for up to 10 min. For ethylene glycol, we present interference depth measurements alongside droplet count measurements. As we cannot run interference microscopy in tandem with droplet count, a separate experiment was performed, where we analyzed the presence of microdroplet remnants every 30 s using a sitting drop.
Surface Analysis: Scanning Electron Microscopy
Preformed drop trails and spots were first made by depositing a 30 μL drop of [P6,6,6,14]+[TFSI]− for approximately 10–15 min before its timely removal (soaked up/slide off). These trails and spots are then coated with a thin layer of platinum (3 nm) using sputtering, before analysis with scanning electron microscopy (Zeiss, LEO 1530 Gemini). A working voltage of 3 kV, coupled to an aperture of 10 μm and a working distance of 2 mm, is used.
Wetting Analysis: Roll-off Angles
Superhydro(oleo)phobicity was assessed through the measurement of static and sliding angles (CAs and SAs), by placing and averaging 3 drops of water and respective low surface tension liquids (10 μL, dispensed at 1 μL/s) on sample surfaces using the sessile drop method. Roll-off angles (α) were assessed by tilting these surfaces at 1°/s until the drop starts sliding off (20 ms per frame). Surfaces are then allowed to be partially infiltrated (or not) by sitting a liquid drop (30 μL, dispensed at 1 μL/s) of varying surface tension at the same spot for sequential timings (10, 20, 30, and 60 min) before it was rolled off. Roll-off angles were then measured on the same spots as per above. Separate measurements of the roll-off angles were also made on preformed trails and spots, by depositing a 30 μL drop of [P6,6,6,14]+[TFSI]− for approximately 10–15 min before its timely removal (soaked up/slide off). Thereafter, 3 drops of [P6,6,6,14]+[TFSI]− (10 μL, dispensed at 1 μL/s) were placed on sample surfaces using the sessile drop method. A fluorinated-glass surface was also used to test static contact angles for the ionic liquid and ethylene glycol.
Atomic Force Microscopy
AFM force spectroscopy measurements were carried out using a NanoWizard 4 system from JPK Instruments. Tipless AFM cantilevers from Mikromash were used (3-lever series). These cantilevers were hydrophobized by placing them within a desiccator in a vacuum for 30 min while being exposed to a trichloro(1H,1H,2H,2H-perfluorooctyl)silane chemical vapor. After hydrophobizing the cantilever, the force constant was calibrated within the commercial AFM setup using the Sader method.39 The cantilever used for the experiment had a force constant of 7.7 N/m. Single drops of the ionic liquid, trihexyltetradecylphosphonium, were attached to the end of a tipless cantilever using the micropositioning system of a JPK Nanowizard IV AFM. In order to do so, a macroscopic drop of this liquid is first placed on a microscope glass slide. The hydrophobized cantilever is then set to gently touch the surface of the macroscopic ionic liquid drop. When retracted from the macroscopic drop, a microscopic droplet is attached to the cantilever. This liquid probe is then used to press against the test surface. All measurements were performed while an ion fan was set to gently blow air on the surface, thus avoiding electrical charging of the interface with successive force curves.
The force calibration was performed by increasing the pressing set point force (20–25 nN) up to the point until where adhesion increases (during contact). This pressing force stabilizes at 10 ± 5 nN, which was used to apply a quasi-identical force on the gradually contaminated nanoparticulate surface. This lowering of the actual applied force with respect to the set point (20–25 nN) occurs because of a slight tilt remaining in the zero force line. To gain information on the temporal dynamics of the adhesion force, we recorded around 200 force curves at the same position, i.e., ∼13 min of measurements. For comparison, the maximum applied load was kept constant using the trigger function of the AFM. It appears that the decrease in penetration depth is a combination of drop deformation, penetration, and even cantilever deflection.
Computation of Adhesion Forces
According to the SEM and confocal analysis, the microstructural dimension is approximately 1 μm. Based on n = 0.016 microdroplet per micrometer square, a specific contact adhesion is experienced by the cantilever drop. This implies a specific contact adhesion of 1.4 nN/μm2, respectively. However, eq 1 does not provide the net adhesion induced by a contacting cantilever drop because the area of contact by the cantilever drop to the surface remains unknown. This can be calculated from the load, which corresponds to the capillary force, using eq 2,40,41 where
| 2 |
where ΔP is Laplace pressure within the drop, θ0A is the top plate (AFM cantilever) advancing contact angle, θA is the bottom plate (surface) advancing contact angle, r is the radius of the cantilever drop that is in contact with the cantilever, while F represents the capillary force (10 nN). The Laplace pressure is given by
| 3 |
where h is the drop height considering a deformation δ of 100 nm (13 μm to 100 nm). Equations 2 and 3 are thus combined, to calculate θA, required to calculate the contact area.
| 4 |
The contact angle
of the ionic liquid
on the AFM cantilever, θ0A, was measured to be approximately 45°.
Solving for θA gives the geometrical drop-to-surface
contact angle. This angle can then be fitted to a spherical cap, assuming
small deformations, for a radius of contact,  (Figure S16).
The corresponding contact area is ∼165 μm2.
(Figure S16).
The corresponding contact area is ∼165 μm2.
Acknowledgments
This work was supported by the European Union’s Horizon 2020 research and innovation program LubISS No. 722497 (W.W., A.N., P.B., D.V.), the ERC Advanced Grant No. 340391 “SUPRO” (H.-J.B., D.V.), the German Research Foundation (DFG) with the Collaborative Research Center 1194 (H.-J.B.), and the Priority Programme SPP 2171 (D.V. and H.-J.B.). T.P.C. acknowledges funding support from Conicyt by Fondecyt Initiation 11160664. We thank A. Sharifi for technical support and F. Geyer, K. Hegner, L. Hauer, and M. D’Acunzi for stimulating discussions. We also thank J. Thiel and M. D’Acunzi for their help in fabricating the superamphiphobic soot-templated and nanofilament surfaces.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.9b08211.
Figures containing supplementary SEMs, interference and confocal microscopy data, XPS data, control experiments, and optical photographs/images of the phenomenon/experiments (PDF)
Supporting movie (AVI)
Supporting movie (AVI)
Supporting movie (AVI)
Supporting movie (AVI)
Supporting movie (AVI)
Supporting movie (AVI)
Supporting movie (AVI)
Supporting movie (AVI)
Author Contributions
W.S.Y.W. carried out the experiments and characterization unless otherwise stated below and wrote the manuscript. P.B. performed the XPS experiments. P.P. analyzed drop penetration using interference. A.K. supported the confocal analysis. T.C. and M.K. performed and analyzed the adhesion force experiments. A.N. helped with data interpretation. W.S.Y.W., D.V., and H.-J. contributed to experimental planning, data analysis, and manuscript preparation. All authors reviewed and approved the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Pan S.; Guo R.; Björnmalm M.; Richardson J. J.; Li L.; Peng C.; Bertleff-Zieschang N.; Xu W.; Jiang J.; Caruso F. Coatings Super-Repellent to Ultralow Surface Tension Liquids. Nat. Mater. 2018, 17, 1040–1047. 10.1038/s41563-018-0178-2. [DOI] [PubMed] [Google Scholar]
- Yong J.; Chen F.; Yang Q.; Huo J.; Hou X. Superoleophobic Surfaces. Chem. Soc. Rev. 2017, 46, 4168–4217. 10.1039/C6CS00751A. [DOI] [PubMed] [Google Scholar]
- Papadopoulos P.; Mammen L.; Deng X.; doris v.; Butt H.-J. Pinning-Induced Variations of the Contact Angle of Drops on Microstructured Surfaces. Chem. Lett. 2012, 41, 1343–1345. 10.1246/cl.2012.1343. [DOI] [Google Scholar]
- Butt H.-J.; Vollmer D.; Papadopoulos P. Super Liquid-Repellent Layers: The Smaller the Better. Adv. Colloid Interface Sci. 2015, 222, 104–109. 10.1016/j.cis.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Deng X.; Mammen L.; Butt H.-J.; Vollmer D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science 2012, 335, 67–70. 10.1126/science.1207115. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Seeger S. Superoleophobic Coatings with Ultralow Sliding Angles Based on Silicone Nanofilaments. Angew. Chem., Int. Ed. 2011, 50, 6652–6656. 10.1002/anie.201101008. [DOI] [PubMed] [Google Scholar]
- Deng X.; Mammen L.; Butt H.-J.; Vollmer D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science 2012, 335, 67. 10.1126/science.1207115. [DOI] [PubMed] [Google Scholar]
- Cassie A.; Baxter S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546–551. 10.1039/tf9444000546. [DOI] [Google Scholar]
- Fan L.; Li B.; Zhang J. Antibioadhesive Superhydrophobic Syringe Needles Inspired by Mussels and Lotus Leafs. Adv. Mater. Interfaces 2015, 2, 1500019. 10.1002/admi.201500019. [DOI] [Google Scholar]
- Li H.; Yang Q.; Li G.; Li M.; Wang S.; Song Y. Splitting a Droplet for Femtoliter Liquid Patterns and Single Cell Isolation. ACS Appl. Mater. Interfaces 2015, 7, 9060–9065. 10.1021/am509177s. [DOI] [PubMed] [Google Scholar]
- Ueda E.; Levkin P. A. Emerging Applications of Superhydrophilic-Superhydrophobic Micropatterns. Adv. Mater. 2013, 25, 1234–1247. 10.1002/adma.201204120. [DOI] [PubMed] [Google Scholar]
- Epstein A. K.; Hong D.; Kim P.; Aizenberg J. Biofilm Attachment Reduction on Bioinspired, Dynamic, Micro-Wrinkling Surfaces. New J. Phys. 2013, 15, 095018. 10.1088/1367-2630/15/9/095018. [DOI] [Google Scholar]
- Freschauf L. R.; McLane J.; Sharma H.; Khine M. Shrink-Induced Superhydrophobic and Antibacterial Surfaces in Consumer Plastics. PLoS One 2012, 7, e40987 10.1371/journal.pone.0040987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlet K.; Movafaghi S.; Dasi L. P.; Kota A. K.; Popat K. C. Antibacterial Activity on Superhydrophobic Titania Nanotube Arrays. Colloids Surf., B 2018, 166, 179–186. 10.1016/j.colsurfb.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer F.; D’Acunzi M.; Yang C.-Y.; Müller M.; Baumli P.; Kaltbeitzel A.; Mailänder V.; Encinas N.; Vollmer D.; Butt H.-J. How to Coat the Inside of Narrow and Long Tubes with a Super-Liquid-Repellent Layer—A Promising Candidate for Antibacterial Catheters. Adv. Mater. 2019, 31, 1801324. 10.1002/adma.201801324. [DOI] [PubMed] [Google Scholar]
- Liao Y.; Wang R.; Fane A. G. Engineering Superhydrophobic Surface on Poly(Vinylidene Fluoride) Nanofiber Membranes for Direct Contact Membrane Distillation. J. Membr. Sci. 2013, 440, 77–87. 10.1016/j.memsci.2013.04.006. [DOI] [Google Scholar]
- Park K.-C.; Chhatre S. S.; Srinivasan S.; Cohen R. E.; McKinley G. H. Optimal Design of Permeable Fiber Network Structures for Fog Harvesting. Langmuir 2013, 29, 13269–13277. 10.1021/la402409f. [DOI] [PubMed] [Google Scholar]
- Garrod R. P.; Harris L. G.; Schofield W. C. E.; McGettrick J.; Ward L. J.; Teare D. O. H.; Badyal J. P. S. Mimicking a Stenocara Beetle’s Back for Microcondensation Using Plasmachemical Patterned Superhydrophobic-Superhydrophilic Surfaces. Langmuir 2007, 23, 689–693. 10.1021/la0610856. [DOI] [PubMed] [Google Scholar]
- Paven M.; Papadopoulos P.; Schöttler S.; Deng X.; Mailänder V.; Vollmer D.; Butt H.-J. Super Liquid-Repellent Gas Membranes for Carbon Dioxide Capture and Heart-Lung Machines. Nat. Commun. 2013, 4, 2512. 10.1038/ncomms3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L.; Zhang Z.; Mai Z.; Ma Y.; Liu B.; Jiang L.; Zhu D. A Super-Hydrophobic and Super-Oleophilic Coating Mesh Film for the Separation of Oil and Water. Angew. Chem., Int. Ed. 2004, 43, 2012–2014. 10.1002/anie.200353381. [DOI] [PubMed] [Google Scholar]
- Razmjou A.; Arifin E.; Dong G.; Mansouri J.; Chen V. Superhydrophobic Modification of TiO2 Nanocomposite Pvdf Membranes for Applications in Membrane Distillation. J. Membr. Sci. 2012, 415–416, 850–863. 10.1016/j.memsci.2012.06.004. [DOI] [Google Scholar]
- Feng W.; Li L.; Du X.; Welle A.; Levkin P. A. Single-Step Fabrication of High-Density Microdroplet Arrays of Low-Surface-Tension Liquids. Adv. Mater. 2016, 28, 3202–3208. 10.1002/adma.201505972. [DOI] [PubMed] [Google Scholar]
- Feng W.; Li L.; Ueda E.; Li J.; Heißler S.; Welle A.; Trapp O.; Levkin P. A. Surface Patterningvia Thiol-Yne Click Chemistry: An Extremely Fast and Versatile Approach to Superhydrophilic-Superhydrophobic Micropatterns. Adv. Mater. Interfaces 2014, 1, 1400269. 10.1002/admi.201400269. [DOI] [Google Scholar]
- Vahabi H.; Wang W.; Movafaghi S.; Kota A. K. Free-Standing, Flexible, Superomniphobic Films. ACS Appl. Mater. Interfaces 2016, 8, 21962–21967. 10.1021/acsami.6b06333. [DOI] [PubMed] [Google Scholar]
- Pan S.; Kota A. K.; Mabry J. M.; Tuteja A. Superomniphobic Surfaces for Effective Chemical Shielding. J. Am. Chem. Soc. 2013, 135, 578–581. 10.1021/ja310517s. [DOI] [PubMed] [Google Scholar]
- Golovin K.; Lee D. H.; Mabry J. M.; Tuteja A. Transparent, Flexible, Superomniphobic Surfaces with Ultra-Low Contact Angle Hysteresis. Angew. Chem., Int. Ed. 2013, 52, 13007–13011. 10.1002/anie.201307222. [DOI] [PubMed] [Google Scholar]
- Wong W. S. Y. Surface Chemistry Enhancements for the Tunable Super-Liquid Repellency of Low-Surface-Tension Liquids. Nano Lett. 2019, 19, 1892–1901. 10.1021/acs.nanolett.8b04972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W. S. Y.; Liu G.; Nasiri N.; Hao C.; Wang Z.; Tricoli A. Omnidirectional Self-Assembly of Transparent Superoleophobic Nanotextures. ACS Nano 2017, 11, 587–596. 10.1021/acsnano.6b06715. [DOI] [PubMed] [Google Scholar]
- Butt H.-J.; Golovko D. S.; Bonaccurso E. On the Derivation of Young’s Equation for Sessile Drops: Nonequilibrium Effects Due to Evaporation. J. Phys. Chem. B 2007, 111, 5277–5283. 10.1021/jp065348g. [DOI] [PubMed] [Google Scholar]
- Rowley R. L.; Widling W. V.; Daubert T. E.; Zundel N. A.; Marshall T. L.; Danner R. P.; Adams M. E.. Physical and Thermodynamic Properties of Pure Chemicals: Dippr 801 Evaluated Process Design Data Supplement 11/12; Taylor & Francis: Oxfordshire, UK, 2002. [Google Scholar]
- Kabo G. J.; Blokhin A. V.; Paulechka Y. U.; Kabo A. G.; Shymanovich M. P.; Magee J. W. Thermodynamic Properties of 1-Butyl-3-Methylimidazolium Hexafluorophosphate in the Condensed State. J. Chem. Eng. Data 2004, 49, 453–461. 10.1021/je034102r. [DOI] [Google Scholar]
- Papadopoulos P.; Vollmer D.; Butt H.-J. Long-Term Repellency of Liquids by Superoleophobic Surfaces. Phys. Rev. Lett. 2016, 117, 046102. 10.1103/PhysRevLett.117.046102. [DOI] [PubMed] [Google Scholar]
- Natalio F.; Corrales T. P.; Wanka S.; Zaslansky P.; Kappl M.; Lima H. P.; Butt H.-J.; Tremel W. Siliceous Spicules Enhance Fracture-Resistance and Stiffness of Pre-Colonial Amazonian Ceramics. Sci. Rep. 2015, 5, 13303. 10.1038/srep13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Wang L.; Levänen E. Superhydrophobic Surfaces for the Reduction of Bacterial Adhesion. RSC Adv. 2013, 3, 12003–12020. 10.1039/c3ra40497h. [DOI] [Google Scholar]
- Privett B. J.; Youn J.; Hong S. A.; Lee J.; Han J.; Shin J. H.; Schoenfisch M. H. Antibacterial Fluorinated Silica Colloid Superhydrophobic Surfaces. Langmuir 2011, 27, 9597–9601. 10.1021/la201801e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryaprabha T.; Sethuraman M. G. Fabrication of Copper-Based Superhydrophobic Self-Cleaning Antibacterial Coating over Cotton Fabric. Cellulose 2017, 24, 395–407. 10.1007/s10570-016-1110-z. [DOI] [Google Scholar]
- Alberts B.; Johnson A.; Lewis J.; Raff M.; Roberts K.; Walter P.. Molecular Biology of the Cell; Garland Science: New York, 2002. [Google Scholar]
- Miller D. J.; Biesinger M. C.; McIntyre N. S. Interactions of CO2 and CO at Fractional Atmosphere Pressures with Iron and Iron Oxide Surfaces: One Possible Mechanism for Surface Contamination?. Surf. Interface Anal. 2002, 33, 299–305. 10.1002/sia.1188. [DOI] [Google Scholar]
- Sader J. E.; Chon J. W. M.; Mulvaney P. Calibration of Rectangular Atomic Force Microscope Cantilevers. Rev. Sci. Instrum. 1999, 70, 3967–3969. 10.1063/1.1150021. [DOI] [Google Scholar]
- Butt H.-J.; Roisman I. V.; Brinkmann M.; Papadopoulos P.; Vollmer D.; Semprebon C. Characterization of Super Liquid-Repellent Surfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 343–354. 10.1016/j.cocis.2014.04.009. [DOI] [Google Scholar]
- Fang W.; Guo H.-Y.; Li B.; Li Q.; Feng X.-Q. Revisiting the Critical Condition for the Cassie-Wenzel Transition on Micropillar-Structured Surfaces. Langmuir 2018, 34, 3838–3844. 10.1021/acs.langmuir.8b00121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








