Abstract

Asphaltenes, heavy aromatic components of crude oil, are known to adsorb on surfaces and can lead to pipe clogging or hinder oil recovery. Because of their multicomponent structure, the details of their interactions with surfaces are complex. We investigate the effect of the physicochemical properties of the substrate on the extent and mechanism of this adsorption. Using wetting measurements, we relate the initial kinetics of deposition to the interfacial energy of the surface. We then quantify the long-term adsorption dynamics using a quartz crystal microbalance and ellipsometry. Finally, we investigate the mechanism and morphology of adsorption with force spectroscopy measurements as a function of surface chemistry. We determine different adsorption regimes differing in orientation, packing density, and initial kinetics on different substrate functionalizations. Specifically, we find that alkane substrates delay the initial monolayer formation, fluorinated surfaces exhibit fast adsorption but low bonding strength, and hydroxyl substrates lead to a different adsorption orientation and a high packing density of the asphaltene layer.
Introduction
Asphaltenes are complex heavy aromatic molecules contained in crude oil and composed of a polyaromatic core and aliphatic side chains.1−4 This dual morphology is reinforced by the presence of polar substituents in the core such as oxygen, nitrogen, and sulfur (see the Supporting Information for elemental composition)5 and the polarizable nature of the aromatic core.6 These characteristics enable asphaltene molecules to take part in both polar and apolar interactions. As a consequence, they are considered surfactants and have a remarkable affinity for liquid interfaces7−10 and solid substrates.11−14 Their adsorption on the solid walls of pipes can lead to clogging, requiring costly interruption of production and, often, physical cleaning.14−16
In this study, we investigate the adsorption of a sample of asphaltenes on solid surfaces as a function of the type of interactions they can have with the substrate. A series of complementary experimental techniques enables the study of all the phases of adsorption from monolayer formation to long-term kinetics. The combination of these results with force spectroscopy measurements by AFM (Atomic Force Microscopy) gives further insight into the mechanism of asphaltene adsorption as a function of the physicochemical properties of the substrate.
Defined by their solubility in toluene and insolubility in n-alkanes,1 asphaltenes exhibit a micellar behavior as their concentration increases.18 At low concentration, asphaltene molecules of size around 1 nm are isolated and independent. As the concentration increases, their aromatic cores interact, leading to π–π stacking and the formation of nano-aggregates of about 2 nm in size. As the concentration further increases, the aliphatic side chains start interacting and clusters of these nano-aggregates of typical size 5–10 nm appear.18,19 Here, we focus on the single-molecule regime using a model solution of asphaltenes in pure toluene at a concentration of 50 mg/L, below the critical nano-aggregate concentration of 60 mg/L.18 The adsorption of single molecules on solids has been extensively studied in other systems, such as proteins, to avoid biological contamination,20 control fouling on cooking devices,21 or even develop biocoatings in immunology.22 Proteins, also known as surfactants in alimentary foams23 and multiple biological situations,24 could provide an analogy with asphaltenes as their molecular structure is usually described as a combination of hydrophilic polar parts and hydrophobic chains.24,25 The adsorption of proteins on solids is partially controlled by the wetting properties of the surface,26,27 an assertion which remains to be discussed in the case of asphaltenes.
Experimental Methods
Asphaltenes
The asphaltenes used in this study were extracted from Cold Lake vacuum residue by dissolution in toluene and precipitation with n-heptane (C7-asphaltenes). The elemental composition of the sample studied here is available in the Supporting Information.
Silane Functionalization of Silicon Substrates
To prepare the alkane-functionalized substrates, the silicon samples were coated with octadecyltrichlorosilane (Sigma-Aldrich) in the presence of a stoichiometric quantity of water emulsified in the toluene solvent. For the fluoroalkane substrate, the silicon samples were functionalized with 1H,1H,2H,2H perfluoro-octylsilane (Sigma-Aldrich) in the vapor phase for 4 h after plasma cleaning to activate the surface. The surfaces were then rinsed with acetone, isopropyl alcohol, and water before use.
Thiol Functionalization of Gold Surfaces
Gold surfaces were functionalized for quartz crystal microbalance (QCM) measurements and AFM measurements (probe functionalization). The thiols used were mercaptoethanol, decanethiol, and 1H,1H,2H,2H perfluorodecylthiol for the hydroxyl, alkane, and fluoroalkane functionalization, respectively. In each case, the gold surfaces were immersed in a solution of the thiol in ethanol at a concentration of 50 mM for 24–48 h. The surfaces were then rinsed with pure ethanol and water and allowed to dry before use.
Contact Angle Measurements
Contact angles were measured using a Ramé-Hart (model 500-F1) contact angle goniometer. Each pair of advancing and receding contact angle is obtained by sequentially infusing and removing liquid from a droplet on the surface and measuring the contact angle of the quasi-statically moving contact line. When contact angles are reported in this article, they are the average of a minimum of three measurements at different positions on each of the two separate samples to ensure reproducibility of the results.
Atomic Force Microscopy
Atomic force microscopy images were obtained using a Dimension 3100 atomic force microscope with the Nanoscope IV scanned probe controller in the tapping mode. The probes used were tapping mode pyramidal probes with a stiffness of ca. 40 N/m. The images reported here are representative of series of AFM images taken in different spots and on separate samples.
AFM Force Spectroscopy
Atomic force spectroscopy measurements were obtained with an Asylum Research MFP-3D scanning probe microscope. The probes used were silicon nitride cantilevers with a gold-plated silicon dioxide spherical particle (5 μm) mounted at the tip and exhibited a stiffness of ca. 0.5 N/m. Before each measurement, the actual stiffness of the probe was measured by measuring its deflection on a glass substrate. Each adhesion force experiment is the result of 210 measurements on a 14 × 15 grid with each measurement separated by 4 μm. The resulting probability distribution function reported here shows the variability of the measurement.
Quartz Crystal Microbalance
QCM experiments were performed on a commercially available instrument from Biolin Scientific (QSense QCM-D, E4 flow cells). Hydrocarbon-resistant Kalrez o-rings were used in the flow cells. The injection flow rate was maintained at 401 μL/min using a peristaltic pump (Cole-Parmer). Solvent-resistant polyvinylchloride solva tubing (Cole-Parmer) was chosen for the peristaltic pump. Teflon inlet tubing and fittings were used as far as possible. The quartz sensors (Biolin Scientific) were physically vapor-deposited with gold. The root-mean-square roughness measured by AFM as reported by the manufacturer is 0.9 nm. The surface of the gold sensors was functionalized using thiol chemistry (see the Supporting Information-2). Prior to experiments, the sensors were rinsed in heptane and dried with nitrogen. During measurements, the pure solvent was first injected for 18–20 h to determine the solvent contribution to the total frequency shift. Subsequently, the asphaltene solution was injected for 48 h and the total frequency shift was measured. The experiments were performed using a flow-through setup. This measurement was replicated on independent samples to verify reproducibility.
Ellipsometry
Film thickness measurements were conducted by ellipsometry using a Woollam spectroscopic ellipsometer (model XLS-100). The wavelength λ of the incident beam was varied from 400 to 1200 nm. The incident angle was kept constant at a value of 57°. The thickness of the coating of a functionalized substrate was obtained by assuming a Cauchy model and a refractive index of 1.45 and was found to be of the order of 3 nm (Supporting Information-2). The thickness of the adsorbed asphaltene layer was estimated by assuming a refractive index of 1.9, as recently estimated by Turgman-Cohen et al.39 Each data point is obtained by averaging a minimum of three measurements on each of the two separate samples.
Results and Discussion
Solid Adsorption
To study the influence of solid surface energy, we prepared smooth functionalized solids, specifically, silicon wafers coated with silanes28 and gold coupons functionalized using thiol chemistry29 (see the Experimental Methods). The AFM images of the functionalized silicon substrate shown in Figure 1a–c demonstrate that the nanometric coating is extremely smooth. Indeed, the root-mean-square roughness r was measured to be ∼0.2 nm, on the order of the sensitivity of the AFM apparatus, for all functionalizations. We consider three types of functionalizations. First, as a polar, hydrophilic substrate, we study the native oxide layer on silicon wafers. In addition, two apolar hydrophobic functionalizations are investigated: an octylsilane, consisting of a straight hydrocarbon chain, and a fluorinated substrate with a perfluoroalkane of the same length.
Figure 1.
Characterizations of three functionalized substrates before and after asphaltene adsorption. AFM pictures of different functionalized substrates before and after asphaltene adsorption. Three functionalized substrates are studied before adsorption (τ = 0 h). A clean bare silicon wafer with hydroxyl groups (a), a silicon wafer grafted with an alkane chain of eight carbons (b), and a fluorinated surface (c) obtained after deposition of trichloro(1H,1H,2H,2H-perfluoroctyl)silane on a silicon wafer were used as smooth functionalized substrates (r ≈ 0.2 nm). The color bar scales from 0 to 12 nm. The size of every AFM picture represents a 1 μm by 1 μm square as better evidenced by the scale bar on the top right corner. After being immersed for a time τ = 48 h in a solution of asphaltenes in toluene, the hydroxyl (d), alkane (e), and fluorinated (f) substrates show a greater root-mean-square roughness (r ≈ 1.2 nm) reinforced by noticeable patchiness in the AFM pictures. The advancing θa (g) and receding θr (h) contact angles of water are compared on these three substrates before (τ = 0 h, blue) and after (τ = 48 h, yellow) adsorption. The solid line represents these contact angles for water on an asphaltene layer obtained by spin coating (100°).
Functionalized silicon substrates were immersed in an asphaltene solution for 48 h. After exposure to asphaltenes, the substrates were rinsed with toluene and dried with nitrogen before being imaged with AFM. The resulting surface topographies, shown in Figure 1d–f, exhibit a significantly increased roughness (r > 1 nm). On all three surfaces, noticeable patchiness is observed with grains around 10 nm in size. This change in morphology indicates that asphaltenes have adsorbed on all three functionalized solids. We investigated the resulting change in physicochemical properties by measuring the contact angles of water on each surface. In Figure 1g, the advancing contact angle θa of water is reported before adsorption (τ = 0 h, in blue) and after 48 h in solution (in yellow). Initially, the functionalized substrates cover a large range of contact angles varying from 40° on the hydroxyl solid to 120° on the fluorinated substrate. After 48 h in the asphaltene solution, the advancing contact angle θa converges toward the same value on all substrates. This value, around 100°, is highlighted in Figure 1g with a solid black line and is identical to the value obtained on a spin-coated layer of asphaltenes. Similarly, the receding contact angle θr of water, reported in Figure 1h, while initially disparate, converges to an intermediate value of around 65°. Note, however, that the receding contact angle on the hydroxyl substrate is significantly smaller than that on the other substrates. Indeed, receding contact angles are known to be highly dependent on surface topographies,30 and the layer of asphaltenes adsorbed on the hydroxyl substrate exhibited slightly different roughnesses compared to the other two (see Figure 1d–f). Crucially, both AFM and contact angles measurements demonstrate that asphaltenes are adsorbed on all solids, regardless of functionalization. After 48 h, the wetting homogeneity of the three substrates suggests complete coverage with asphaltenes.
Monolayer Formation
We use contact angle measurements to track the formation of the first monolayer on the functionalized solids. Figure 2a shows the wetting transition for water from the original functionalized solids to asphaltene-covered substrates. The initial and final contact angles are similar to the ones shown in Figure 1g. The samples were placed in an asphaltene solution at a concentration of 50 mg/L in toluene and withdrawn regularly to measure the evolution of the contact angles of water (Figure 2a), toluene (Figure 2b), and heptane (Figure 2c). After being withdrawn, they were rinsed with toluene and dried with a nitrogen gun. Then, the contact angles were measured with a goniometer. In Figure 2a–c, each bar represents the advancing (top) and receding (bottom) contact angles, as sketched in Figure 2d. Each reported value is the average of a minimum of five measurements made in at least two different positions on the substrate. The error bar represents the standard deviation of the measurements. In Figure 2a, we observe that the advancing contact angle of water θa reaches a value of around 100° on all solids as expected. Similarly, the contact angles of heptane and toluene also converge to a common value on all substrates. Some deviations are observed on the receding contact angle θr, which are attributed to the patchiness observed by AFM in Figure 1. After 10,000 s (about 3 h), the contact angles reach a steady state, except on the fluorinated substrate which experiences an increase in wetting with toluene and heptane, a possible consequence of long-term modifications in the monolayer, which will be discussed later. However, we interpret both the uniformity of the wetting on all three substrates and the steady state observed on contact angles after 10,000 s as the presence of a complete monolayer of asphaltenes. Any further adsorption of asphaltenes does not further modify the wetting property of the substrates.
Figure 2.
Monolayer formation of asphaltenes during adsorption on three functionalized substrates. (a) Advancing and receding contact angles of water on hydroxyl (top blue), alkane (red), and fluorinated (bottom green) coatings as a function of adsorption time t. (b) Advancing and receding contact angles of toluene on hydroxyl (top blue), alkane (red), and fluorinated (bottom green) coatings as a function of adsorption time t. (c) Advancing and receding contact angles of heptane on hydroxyl (top blue), alkane (red), and fluorinated (bottom green) coatings as a function of adsorption time t. (d) Sketch of the advancing and receding contact angles. (e) Estimation of solid energy of hydroxyl (blue circles), alkane (red squares), and fluorinated (green triangles) coatings during asphaltene adsorption for a time t.
The evolution of the advancing and receding contact angles enables us to visualize the kinetics of the first monolayer formation. In Figure 2a, the contact angles of water on the hydroxyl substrate (in blue) are strongly affected by asphaltene deposition as soon as 1 s after immersion. The advancing contact angle experiences a rapid increase to reach its final value. Deposition is also observed to be fast on fluorinated substrates (in green) where the receding contact angle changes significantly in the first few seconds. Conversely, the water contact angles remained stable on the alkane coating (in red) for about 1000 s. Figure 2b shows that the toluene contact angles also exhibit a difference between the initial kinetics of adsorption on fluorinated and alkane surfaces. We observe a transition after t = 1000 s from the initial physicochemistry of the alkane coating to a modified wetting state induced by asphaltene fouling. On the other hand, the receding contact angle of toluene of fluorinated substrates immediately becomes extremely low. These complementary results demonstrate that the kinetics of the monolayer formation is highly sensitive to the underlying chemical functionalization: fouling is fast on hydroxyl and fluorinated substrates, while it is delayed on an alkane substrate.
In addition, measuring the contact angles
of water, toluene, and
heptane allows us to derive the solid energy of the surfaces. Indeed,
as described by Fowkes,31 the solid–liquid
interfacial energy γls can be expressed as the sum
of a polar component γAB including hydrogen-bonding
and Lewis acid–basis32 effects and
an apolar part γLW denoted after Lifshitz–van
der Waals.32 The polar component can be
further broken down into electron-acceptor γ+ and
electron-donor γ– parts,33 such that the solid energy γs can be written
as γs = γLW + 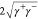 .34 Writing the
solid–liquid interfacial energy in this form leads to a modified
Young equation γl(1 + cos θls) =
2(
.34 Writing the
solid–liquid interfacial energy in this form leads to a modified
Young equation γl(1 + cos θls) =
2( +
+  +
+ 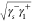 ), where γl is the surface
tension of the liquid and θls is the contact angle
of the liquid on the solid. For a given substrate, we can measure
the contact angle of three different liquids of known surface tension
components to obtain three equations from which we can derive all
three components of the solid surface energy γs.
The advancing contact angle was used in these derivations as it is
considered a signature of the chemistry of a solid and leads to values
of γs in good agreement with the literature for our
native functionalizations.35Figure 2e shows the solid energy as
a function of adsorption time. The solid line represents the solid
energy of an asphaltene layer spin-coated on a wafer (θls ≈ 100° and γs ≈ 28.5
mN/m). The solid energy of the hydroxyl substrate (in blue) decreases
rapidly (1 s) from a high value (50 mN/m) to an intermediate value
(around 28 mN/m) where it remains stable over the rest of the experiment.
Similarly, the solid energy of the fluorinated substrate (in green)
starts from a low value (highly apolar solid, γs ≈
10 mN/m) and eventually reaches a value close to that of an asphaltene
layer. Conversely, the solid energy of the alkane substrate does not
change significantly over time. Indeed, the initial surface energy
of the alkane substrate is close to the value of the solid energy
of an asphaltene layer.
), where γl is the surface
tension of the liquid and θls is the contact angle
of the liquid on the solid. For a given substrate, we can measure
the contact angle of three different liquids of known surface tension
components to obtain three equations from which we can derive all
three components of the solid surface energy γs.
The advancing contact angle was used in these derivations as it is
considered a signature of the chemistry of a solid and leads to values
of γs in good agreement with the literature for our
native functionalizations.35Figure 2e shows the solid energy as
a function of adsorption time. The solid line represents the solid
energy of an asphaltene layer spin-coated on a wafer (θls ≈ 100° and γs ≈ 28.5
mN/m). The solid energy of the hydroxyl substrate (in blue) decreases
rapidly (1 s) from a high value (50 mN/m) to an intermediate value
(around 28 mN/m) where it remains stable over the rest of the experiment.
Similarly, the solid energy of the fluorinated substrate (in green)
starts from a low value (highly apolar solid, γs ≈
10 mN/m) and eventually reaches a value close to that of an asphaltene
layer. Conversely, the solid energy of the alkane substrate does not
change significantly over time. Indeed, the initial surface energy
of the alkane substrate is close to the value of the solid energy
of an asphaltene layer.
Combining these observations with the contact angle measurements on the different substrates, the initial adsorption of asphaltenes appears to be driven thermodynamically by the lowering of the solid–liquid interfacial energy. Indeed, the surfaces with a significant initial solid–liquid interfacial energy (hydroxyl and fluorinated) show the fastest initial kinetics, while the alkane substrate show a delayed adsorption which we attribute to the small interfacial energy between the substrate and the toluene solvent.
Long-Term Adsorption
The long-term adsorption kinetics, beyond the monolayer formation, was also studied quantitatively using two complementary techniques. The QCM provides information about the added mass because of the adsorption of asphaltenes on a functionalized gold-coated quartz crystal (see the Experimental Methods). By tracking the evolution of the resonant frequency f of the crystal as it is exposed to asphaltenes, the adsorbed mass m is estimated via the Sauerbrey equation36,37 as a function of time. To speed up the adsorption and limit the frequency drift of the instrument during the experiment, the asphaltene solution used in this experiment was of a higher concentration (400 mg/L) in a mixed heptane–toluene (30–70 wt %) solvent. The solution was flown over the sensors (Q = 400 μL/min) for 8 h. Complementarily, we measure the thickness h of the asphaltene layer adsorbed on functionalized silicon substrates in static conditions exposed to a 50 mg/L solution in toluene using ellipsometry. On measuring the reflection of incident polarized light on a reflective substrate, a spectroscopic ellipsometer enables the determination of the refractive index of the material and the thickness of transparent layers (see the Experimental Methods). The wavelength λ of the incident beam is varied from 400 to 1200 nm. The incident angle was kept constant at a value of 57°. To obtain baseline measurements and evaluate the effectiveness of the treatments, the functionalization coatings were studied first. Modeling the functionalized solid as a superposition of a 1 mm-thick layer of silicon providing reflectiveness and a layer of alkyl-silanes of thickness h0 and a refractive index of 1.45, we obtained the coating thickness h0 for the various substrates, detailed in the Supporting Information and in good agreement with results previously reported in the literature.28,38 The measured thickness of our coatings is a few nanometers, assuming a homogeneous coating. The visualization of the various coating by AFM (Figure 1a–c) validates the uniformity of the film. The functionalized solids are then immersed in an asphaltene solution (50 mg/L in toluene) and withdrawn regularly. Once withdrawn, the samples are immediately rinsed in toluene and dried with a nitrogen gun. To study the effect of the rinsing process, we verified that immersing the samples in pure toluene for a time up to several hours does not affect the measured thickness of the adsorbed layer. The measurements, therefore, reflect the thickness of the irreversibly adsorbed asphaltenes on the solids. As recently discussed by Turgman-Cohen et al.,39 we assume a refractive index of 1.9 for asphaltenes. Each data point is obtained after scanning three different spots on at least two samples. Thus, the value reported is the average of a minimum of six measurements. The standard deviation is reported as the error bar.
The QCM measurements (Figure 3a) show a monotonic increase of the adsorbed mass m with the adsorption time t on all three functionalized substrates. The adsorbed mass reaches a final value between 100 and 1000 ng/cm2. However, the measurements are significantly different on the three functionalizations. On the hydroxyl substrate (in blue), the mass exhibits a rapid initial increase followed by a level-off to steady-state kinetics after about 3 h (10,000 s). The same fast initial and slow steady-state kinetics are observed on the fluorinated substrate (in green), although the final adsorbed mass is less than half of that on the hydroxyl solid. The alkane substrate (in red) exhibits a markedly slower initial adsorption before reaching the plateau. This is consistent with the slow monolayer formation on the alkane substrate previously discussed.
Figure 3.
Quantification of adsorbed asphaltenes on three functionalized substrates. (a) QCM measurements of asphaltenes adsorbed on hydroxyl (blue circles), alkane (red squares), and fluorinated (green triangles) coatings. The adsorbed mass rapidly increases on hydroxyl and fluorinated solids, whereas the increase is delayed by a few thousands of seconds on the alkane substrate. (b) Ellipsometry measurements of asphaltenes adsorbed on hydroxyl (blue circles), alkane (red squares), and fluorinated (green triangles) coatings. The thickness of hydroxyl and fluorinated substrates seems to be similar, whereas the kinetics is slower on alkane chains. Moreover, the long-term thickness seems to be higher on alkane.
The ellipsometry measurements, reported in Figure 3b on a semi-log scale, show similar but complementary results. On the hydroxyl (blue circles) and fluorinated substrates (green triangles), the thickness h of the asphaltene layer exhibits a very similar behavior. The thickness measured after 1 s is larger than 1 nm, which represents the order of magnitude of the expected size for a monolayer of asphaltenes as expected from the contact angle measurements from Figure 2. This validates the use of ellipsometry for quantification despite the relative patchiness of our asphaltene layers reported in Figure 1. Then, the thickness increases by a factor of 5 between 1 and 104 s. The alkane layer (red squares), like in the QCM results, displays slower initial kinetics. The asphaltene layer thickness is lower than 1 nm until t ≈ 1000 s, indicating slow monolayer formation. Then, after a few thousands of seconds, the kinetics speed up and, after 106 s (about 2 weeks) in solution, the thickness of asphaltenes reaches a value of about 15 nm on the alkane substrate, whereas it remains of the order of 6 nm on the two other substrates.
Despite differences in measurements (mass m and thickness h) and in configurations (in situ measurement under flow conditions with the QCM and a posteriori on dried substrates by ellipsometry), both quantitative techniques are in good agreement with respect to the trends. Indeed, measurements obtained both by the QCM (Figure 3a) and ellipsometry (Figure 3b) show two distinct kinetics depending on the functionalization. On both the hydroxyl and fluorinated substrates, the initial adsorption is rapid: after 1 s, a thickness of 1 nm of asphaltenes is measured by ellipsometry, whereas it remains lower than 1 nm for a few thousands of seconds on the alkane coating. Conversely, at a longer timescale, the steady-state kinetics is faster on alkane chains. However, there are key differences between the QCM and ellipsometry measurements in the ratio between the measured masses and thicknesses. Indeed, from the QCM measurements, the maximum mass is obtained on the hydroxyl substrate, whereas the maximum thickness is reached at a long timescale on the alkane substrate. This suggests a difference in the adsorbed layer density. In fact, from these measurements, we can estimate the relative density of the asphaltene layer on the hydroxyl substrate to be twice that on the other substrates (see Supporting Information-2). Thus, a key factor is the conformation adopted by the asphaltene molecule during adsorption. Indeed, asphaltene molecules exhibit two major interaction pathways: they can form polar interactions with the polar substituents and the polarizable polyaromatic core.6 They can also participate in apolar interactions with the aliphatic side chains. Depending on which of these predominate, the geometrical conformation of the adsorbed molecules may vary.
Adsorption Mechanism
We used atomic force spectroscopy to probe the molecular interactions between the asphaltene molecules and a functionalized surface. This technique uses a functionalized spherical probe at the tip of an AFM cantilever. The deflection of the cantilever is related to the force exerted on the probe by the substrate through the spring constant, k. This enables the measurement of interaction forces on the order of pico- to nano-newtons. Specifically, the process is shown schematically in Figure 4a. The probe is brought close to the substrate until it jumps to contact under the action of local attractive forces. It is pressed on the surface until a user-defined trigger force, Ftrig, is reached and kept there for a tunable dwell time tdw. The probe is then retracted at constant speed until it detaches from the surface. The deflection measured just prior to detachment gives the adhesion force Fadh between the probe and the surface. A typical experiment consists of 210 measurements of the adhesion force on a grid of 14 by 15 points separated by 4 μm. The resulting force histogram is then converted into a probability density function of the force measured. When the force measured varied, we could not identify any topographical structures at the micrometer scale and attribute the variation in force to stochastic effects such as the orientation of the probe and the localized grains observed in Figure 1. The probe size and applied force were kept constant for all experiments unless otherwise stated to obtain comparable force measurements. However, the contact area between the probe and the surface depends on the applied force, bead radius, materials considered, and interfacial tension. To normalize the measurements, the adhesion strength σ is reported as the force measured per unit contact area as described by the DMT model.40 The probes consisted of spherical polystyrene beads 5 μm in diameter coated with a gold layer attached at the end of triangular cantilevers of spring constant 1 < k < 3 nN/nm. They were functionalized with thiol chemistry with the same functional groups as the QCM and ellipsometry study (see Supporting Information-2).
Figure 4.
Force adhesion spectroscopy—probing orientation and adhesion. (a) Principle of the adhesion force microscopy technique used. (b) Left: probability density function of the adhesion strength measured between an alkane-functionalized probe and asphaltenes adsorbed on a surface with hydroxyl, alkane, and fluoroalkane termination. Right: schematic of the measurement performed with the hypothesized orientation of the asphaltene molecules on hydroxyl and alkane substrates. (c) Left: probability density function of the adhesion strength measured between probes functionalized with hydroxyl, alkane, and fluoroalkane termination and a surface covered with asphaltenes adsorbed on a hydroxyl substrate after a dwell time of 5 s. Right: schematic of the measurement performed with functionalized beads on a layer of asphaltenes adsorbed on a hydroxyl substrate.
To investigate the orientation of the adsorbed asphaltene molecules on a functionalized substrate, we measure the adhesion strength, σ, between an alkane-functionalized (C8) probe and a surface covered with asphaltenes adsorbed on the three functionalizations studied: hydroxyl, alkane, and fluoroalkane. The substrates were immersed for 48 h in a solution of 50 mg/L of asphaltenes in toluene and then rinsed with toluene to remove the loosely bound asphaltenes and dried with a nitrogen gun. The presence of asphaltenes on the surfaces was verified by measuring the advancing water contact angle which was 100 ± 4° on all substrates. Figure 4b shows the resulting probability density of the adhesion force measured with Ftrig = 10 nN and no dwell time. Two regimes were identified: the asphaltenes adsorbed on hydroxyl substrates exhibited a large spread of measured forces and two distinct peaks suggesting a bimodal adhesion mechanism. Conversely, asphaltenes adsorbed on alkane and fluoroalkane substrates exhibited a smaller mean adhesion strength with the alkane probe and a single-mode distribution. This indicates that the interactions between the asphaltenes and the alkane chains on the probe were of a different nature in these cases, suggesting a different orientation of the asphaltene molecules adsorbed on the substrate. Asphaltenes are expected to expose their aromatic core to polar interfaces, such as the hydroxyl wafer, as shown by Andrews et al.41 at the water–air interface. Similarly, Abraham et al.42 have shown that asphaltenes can re-orient themselves after adsorbing on a solid surface when exposed to polar media (e.g., water). The difference in behavior of the adhesion strength measured in Figure 4b is a hallmark of different orientations for asphaltenes adsorbed on alkane and fluorinated substrates. These results are consistent with the hypothesis that asphaltenes adsorb face-on on hydroxyl substrates to maximize the polar–polar interactions between the substituents in the aromatic center and the hydroxyl groups, as illustrated in Figure 4b (right schematic). Conversely, on very apolar substrates such as alkane and fluoroalkane coatings, the side chains of the asphaltenes can intercalate in the brush formed by alkane (or fluoroalkane) functionalization leading to a side-on adsorption mode.
The different modes of adsorption of the asphaltenes on the substrate also suggest a difference in the packing density because of the planar shape of asphaltene molecules. The molecules adsorbed on a hydroxyl substrate should indeed lead to a higher packing density than the orthogonal geometry suggested for the other substrates. This explains the discrepancy observed between the QCM and ellipsometry measurements where the mass of asphaltenes adsorbed on the hydroxyl substrate appeared disproportionately large compared to the thickness measured.
However, despite apparently similar orientations, differences persist between the fluorinated and alkane substrates in the long-term adsorption trends. In Figure 3a,b, asphaltenes seem to adsorb less on fluorinated substrates. To explain the remaining difference between alkane and fluoroalkane substrates in the steady-state adsorption kinetics observed on the QCM and ellipsometry, the adhesion strength, σ, between a functionalized probe and a layer of asphaltenes adsorbed on a hydroxyl substrate was measured. The probes were functionalized with a hydroxyl-terminated mercaptoethanol, an octanethiol, and a perfluorodecylthiol to mimic the functionalizations used for the QCM and ellipsometry studies. Figure 4c (left panel) shows the probability density function of the adhesion strength, σ, measured at equilibrium, after a dwell time of 5 s on the surface. The adhesion strength of the fluoroalkane-functionalized probe with the asphaltene-coated surface is significantly lower than that for the other two functionalizations. This suggests a different adsorption mechanism and, possibly, a reversible adsorption mode on the fluoroalkane substrate consistent with the fast mass and thickness buildup despite the low adhesion. Moreover, this possible desorption could explain the partial wetting experienced by toluene and heptane on the fluorinated substrate after a long time of adsorption (t > 10,000 s in Figure 2). On fluoroalkane, the low adhesion with asphaltenes could induce a permanent transient state where asphaltene molecules are released and spots on the substrate remain uncovered. Conversely, the adhesion force between the alkane- and hydroxyl-functionalized probes and the asphaltene layer was significantly larger, indicating high adhesion energy and suggesting an irreversible adsorption mechanism.
Conclusions
These insights show that although asphaltene adsorption is prevalent on all types of surfaces, the mechanism and kinetics strongly depend on the substrate and the type of interactions it can have with asphaltenes. This suggests the possibility of tailoring the surface functionalization to the application. In particular, we showed that the polar interactions of the substituents and polyaromatic core with hydroxyl-terminated surfaces lead to a higher packing density than the other substrates. Separately, the alkane-functionalized surface displays a significant delay in adsorption kinetics, on the order of 1000 s, which would make it suitable for protecting analytical devices, such as temperature or pressure probes, to prevent asphaltene contamination during testing of the oil. Finally, while fluorinated surfaces exhibit fast monolayer formation, they also show low binding energy which could enable easier removal using shear induced by liquid flow.
Acknowledgments
The authors would like to acknowledge Mohsen Yeganeh, Robert Colby, Ma Ning, Li Quizi, and Srinivasan Rajagopalan from ExxonMobil for their input and discussions. The study was supported by ExxonMobil as part of the MIT Energy Initiative.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.langmuir.0c00029.
Chemical characterization of the asphaltenes used; characterization of the surface functionalizations; and comparison between the QCM and ellipsometry (PDF)
Author Contributions
∥ H.-L.G. and P.B. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Mullins O. C. The Asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 393–418. 10.1146/annurev-anchem-061010-113849. [DOI] [PubMed] [Google Scholar]
- Schuler B.; Meyer G.; Peña D.; Mullins O. C.; Gross L. Unraveling the Molecular Structures of Asphaltenes by Atomic Force Microscopy. J. Am. Chem. Soc. 2015, 137, 9870–9876. 10.1021/jacs.5b04056. [DOI] [PubMed] [Google Scholar]
- Schuler B.; Fatayer S.; Meyer G.; Rogel E.; Moir M.; Zhang Y.; Harper M. R.; Pomerantz A. E.; Bake K. D.; Witt M.; et al. Heavy Oil Based Mixtures of Different Origins and Treatments Studied by Atomic Force Microscopy. Energy Fuels 2017, 31, 6856–6861. 10.1021/acs.energyfuels.7b00805. [DOI] [Google Scholar]
- Schuler B.; Zhang Y.; Collazos S.; Fatayer S.; Meyer G.; Pérez D.; Guitián E.; Harper M. R.; Kushnerick J. D.; Peña D.; et al. Characterizing Aliphatic Moieties in Hydrocarbons with Atomic Force Microscopy. Chem. Sci. 2017, 8, 2315–2320. 10.1039/c6sc04698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.-L.; Fogler H. S. Stabilization of Asphaltenes in Aliphatic Solvents Using Alkylbenzene-Derived Amphiphiles. 1. Effect of the Chemical Structure of Amphiphiles on Asphaltene Stabilization. Langmuir 1994, 10, 1749–1757. 10.1021/la00018a022. [DOI] [Google Scholar]
- Hansen Solubility Parameters: A User’s Handbook; American Library Association, 2000; Vol. 37. [Google Scholar]
- Taylor K. C.; Hawkins B. F.. Emulsions in Enhanced Oil Recovery. Emulsions: Fundamentals and Applications in the Petroleum Industry; ACS Advances in Chemistry; American Chemical Society, 1992; pp 263–293. [Google Scholar]
- McLean J. D.; Kilpatrick P. K. Effects of Asphaltene Solvency on Stability of Water-in-Crude-Oil Emulsions. J. Colloid Interface Sci. 1997, 189, 242–253. 10.1006/jcis.1997.4807. [DOI] [PubMed] [Google Scholar]
- Kilpatrick P. K. Water-in-Crude Oil Emulsion Stabilization: Review and Unanswered Questions. Energy Fuels 2012, 26, 4017–4026. 10.1021/ef3003262. [DOI] [Google Scholar]
- Rane J. P.; Harbottle D.; Pauchard V.; Couzis A.; Banerjee S. Adsorption Kinetics of Asphaltenes at the Oil-Water Interface and Nanoaggregation in the Bulk. Langmuir 2012, 28, 9986–9995. 10.1021/la301423c. [DOI] [PubMed] [Google Scholar]
- Adams J. J. Asphaltene Adsorption, a Literature Review. Energy Fuels 2014, 28, 2831–2856. 10.1021/ef500282p. [DOI] [Google Scholar]
- Hoepfner M. P.; Limsakoune V.; Chuenmeechao V.; Maqbool T.; Fogler H. S. A Fundamental Study of Asphaltene Deposition. Energy Fuels 2013, 27, 725–735. 10.1021/ef3017392. [DOI] [Google Scholar]
- Zahabi A.; Gray M. R.; Dabros T. Kinetics and Properties of Asphaltene Adsorption on Surfaces. Energy Fuels 2012, 26, 1009–1018. 10.1021/ef2014698. [DOI] [Google Scholar]
- Bennett C. A. Theory Describing Asphaltene Adhesion Fouling Inside Heat Exchanger Tubes. Heat Transfer Eng. 2012, 33, 1246–1250. 10.1080/01457632.2012.692295. [DOI] [Google Scholar]
- Watkinson A. P. Deposition from Crude Oils in Heat Exchangers. Heat Transfer Eng. 2007, 28, 177–184. 10.1080/01457630601064413. [DOI] [Google Scholar]
- Asomaning S.; Watkinson A. P. Petroleum Stability and Heteroatom Species Effects in Fouling of Heat Exchangers by Asphaltenes. Heat Transfer Eng. 2000, 21, 10–16. 10.1080/014576300270852. [DOI] [Google Scholar]
- Mullins O. C. The Modified Yen Model. Energy Fuels 2010, 24, 2179–2207. 10.1021/ef900975e. [DOI] [Google Scholar]
- Hoepfner M. P.; Vilas Bôas Fávero C.; Haji-Akbari N.; Fogler H. S. The Fractal Aggregation of Asphaltenes. Langmuir 2013, 29, 8799–8808. 10.1021/la401406k. [DOI] [PubMed] [Google Scholar]
- Banerjee I.; Pangule R. C.; Kane R. S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- Lalande M.; Tissier J.-P. Fouling of Heat Transfer Surfaces Related to Β-Lactoglobulin Denaturation During Heat Processing of Milk. Biotechnol. Prog. 1985, 1, 131–139. 10.1002/btpr.5420010210. [DOI] [PubMed] [Google Scholar]
- Engvall E.; Perlmann P. ELISA III. Quantitation of Specific Antibodies by Enzyme-Labeled Anti-Immunoglobulin in Antigencoated Tubes. J. Immunol. 1972, 109, 129. 10.1038/npg.els.0004021. [DOI] [PubMed] [Google Scholar]
- Cooper A.; Kennedy M. W. Biofoams and Natural Protein Surfactants. Biophys. Chem. 2010, 151, 96–104. 10.1016/j.bpc.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe M.; Verdes D.; Seeger S. Understanding Protein Adsorption Phenomena at Solid Surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. 10.1016/j.cis.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Ghosh S.; Bull H. B. Adsorbed Films of Bovine Serum Albumin: Tensions at Air-Water Surfaces and Paraffin-Water Interfaces. Biochim. Biophys. Acta 1963, 66, 150–157. 10.1016/0006-3002(63)91178-8. [DOI] [PubMed] [Google Scholar]
- Lee S. H.; Ruckenstein E. Adsorption of Proteins onto Polymeric Surfaces of Different Hydrophilicities - a Case Study with Bovine Serum Albumin. J. Colloid Interface Sci. 1988, 125, 365–379. 10.1016/0021-9797(88)90001-X. [DOI] [Google Scholar]
- Sigal G. B.; Mrksich M.; Whitesides G. M. Effect of Surface Wettability on the Adsorption of Proteins and Detergents. J. Am. Chem. Soc. 1998, 120, 3464–3473. 10.1021/ja970819l. [DOI] [Google Scholar]
- Jönsson U.; Olofsson G.; Malmqvist M.; Rönnberg I. Chemical Vapour Deposition of Silanes. Thin Solid Films 1985, 124, 117–123. 10.1016/0040-6090(85)90253-6. [DOI] [Google Scholar]
- Bain C. D.; Troughton E. B.; Tao Y. T.; Evall J.; Whitesides G. M.; Nuzzo R. G. Formation of Monolayer Films by the Spontaneous Assembly of Organic Thiols from Solution onto Gold. J. Am. Chem. Soc. 1989, 111, 321–335. 10.1021/ja00183a049. [DOI] [Google Scholar]
- Joanny J. F.; De Gennes P. G. A Model for Contact Angle Hysteresis. J. Chem. Phys. 1984, 81, 552–562. 10.1063/1.447337. [DOI] [Google Scholar]
- Fowkes F. M. Attractive Forces At Interfaces. Ind. Eng. Chem. 1964, 56, 40–52. 10.1021/ie50660a008. [DOI] [Google Scholar]
- Van Oss C. J.; Good R. J.; Chaudhury M. K.; Oss C. Additive and Nonadditive Surface Tension Components and the Interpretation of Contact Angles. Langmuir 1988, 4, 884–891. 10.1021/la00082a018. [DOI] [Google Scholar]
- Kollman P.; Rothenberg S. Theoretical Studies of Basicity. Proton Affinities, Li+Affinities, and H-Bond Affinities of Some Simple Bases. J. Am. Chem. Soc. 1977, 99, 1333–1342. 10.1021/ja00447a008. [DOI] [Google Scholar]
- Good R. J. Contact Angle, Wetting, and Adhesion: A Critical Review. J. Adhes. Sci. Technol. 1992, 6, 1269–1302. 10.1163/156856192X00629. [DOI] [Google Scholar]
- Azimi G.; Cui Y.; Sabanska A.; Varanasi K. K. Scale-Resistant Surfaces: Fundamental Studies of the Effect of Surface Energy on Reducing Scale Formation. Appl. Surf. Sci. 2014, 313, 591–599. 10.1016/j.apsusc.2014.06.028. [DOI] [Google Scholar]
- Sauerbrey G. Verwendung von Schwingquarzen Zur Wägung Dünner Schichten Und Zur Mikrowägung. Z. Phys. 1959, 155, 206–222. 10.1007/BF01337937. [DOI] [Google Scholar]
- Kankare J. Sauerbrey Equation of Quartz Crystal Microbalance in Liquid Medium. Langmuir 2002, 18, 7092–7094. 10.1021/la025911w. [DOI] [Google Scholar]
- Kallury K. M. R.; Thompson M.; Tripp C. P.; Hair M. L. Interaction of Silicon Surfaces Silanized with Octadecylchlorosilanes with Octadecanoic Acid and Octadecanamine Studied by Ellipsometry, X-Ray Photoelectron Spectroscopy, and Reflectance Fourier Transform Infrared Spectroscopy. Langmuir 1992, 8, 947–954. 10.1021/la00039a034. [DOI] [Google Scholar]
- Turgman-Cohen S.; Fischer D. A.; Kilpatrick P. K.; Genzer J. Asphaltene Adsorption onto Self-Assembled Monolayers of Alkyltrichlorosilanes of Varying Chain Length. ACS Appl. Mater. Interfaces 2009, 1, 1347–1357. 10.1021/am900203u. [DOI] [PubMed] [Google Scholar]
- Derjaguin B. V.; Muller V. M.; Toporov Y. P. Effect of Contact Deformations on the Adhesion of Particles. J. Colloid Interface Sci. 1975, 53, 314–326. 10.1016/0021-9797(75)90018-1. [DOI] [Google Scholar]
- Andrews A. B.; McClelland A.; Korkeila O.; Demidov A.; Krummel A.; Mullins O. C.; Chen Z. Molecular Orientation of Asphaltenes and PAH Model Compounds in Langmuir-Blodgett Films Using Sum Frequency Generation Spectroscopy. Langmuir 2011, 27, 6049–6058. 10.1021/la200466b. [DOI] [PubMed] [Google Scholar]
- Abraham T.; Christendat D.; Karan K.; Xu Z.; Masliyah J. Asphaltene-Silica Interactions in Aqueous Solutions: Direct Force Measurements Combined with Electrokinetic Studies. Ind. Eng. Chem. Res. 2002, 41, 2170–2177. 10.1021/ie0107690. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






