Abstract
Background
Hospitalized acutely ill medical patients are at risk for fatal and major thromboembolic events. Whether use of extended-duration primary thromboprophylaxis can prevent such events is unknown.
Objectives
The purpose of this study was to evaluate whether extended-duration rivaroxaban reduces the risk of venous and arterial fatal and major thromboembolic events without significantly increasing major bleeding in acutely ill medical patients after discharge.
Methods
MARINER (A Study of Rivaroxaban [JNJ-39039039] on the Venous Thromboembolic Risk in Post-Hospital Discharge Patients) studied acutely ill medical patients with additional risk factors for venous thromboembolism (VTE). Medically ill patients with a baseline creatinine clearance ≥50 ml/min were randomized in a double-blind fashion to rivaroxaban 10 mg or placebo daily at hospital discharge for 45 days. Exploratory efficacy analyses were performed with the intent-to-treat population including all data through day 45. Time-to-event curves were calculated using the Kaplan-Meier method. A blinded independent committee adjudicated all clinical events.
Results
In total, 4,909 patients were assigned to rivaroxaban and 4,913 patients to placebo. The mean age was 67.8 years, 55.5% were men, mean baseline creatinine clearance was 87.8 ml/min, and mean duration of hospitalization was 6.7 days. The pre-specified composite efficacy endpoint (symptomatic VTE, myocardial infarction, nonhemorrhagic stroke, and cardiovascular death) occurred in 1.28% and 1.77% of patients in the rivaroxaban and placebo groups, respectively (hazard ratio: 0.72; 95% confidence interval: 0.52 to 1.00; p = 0.049), whereas major bleeding occurred in 0.27% and 0.18% of patients in the rivaroxaban and placebo groups, respectively (hazard ratio: 1.44; 95% confidence interval: 0.62 to 3.37; p = 0.398).
Conclusions
Extended-duration rivaroxaban in hospitalized medically ill patients resulted in a 28% reduction in fatal and major thromboembolic events without a significant increase in major bleeding. (A Study of Rivaroxaban [JNJ-39039039] on the Venous Thromboembolic Risk in Post-Hospital Discharge Patients [MARINER]; NCT02111564)
Key Words: hospitalized, major bleeding, medically ill, rivaroxaban, thromboembolic events
Abbreviations and Acronyms: CI, confidence interval; CrCl, creatinine clearance; HR, hazard ratio; MI, myocardial infarction; VTE, venous thromboembolism
Central Illustration
A large proportion of the approximately 8 million acute medically ill patients each year in the United States are at risk for venous thromboembolism (VTE) (1). Hospitalization is considered the single most important risk factor for developing these events (2), and the risk of VTE continues beyond hospitalization, especially within the first 6 weeks after discharge (3). Although the relationship between VTE and atherothrombosis/arterial thromboembolism has been known for some time due to the shared mechanisms of inflammation, hypercoagulability, and endothelial injury inherent to both disease processes and due to common patient-level risk factors, such as obesity, dyslipidemia, and tobacco use (4,5), it has only recently been appreciated that medically ill patients are also at increased risk of arterial thromboembolic events in the post-hospital discharge period (6,7). Retrospective data also reveal that extended duration of a prophylactic dose of a direct oral anticoagulant in medically ill patients may reduce the risk of fatal and major arterial thromboembolism by 30% to 50% (6,7).
The previously reported MARINER trial (A Study of Rivaroxaban [JNJ-39039039] on the Venous Thromboembolic Risk in Post-Hospital Discharge Patients) (NCT02111564) (8), randomized medically ill patients with additional risk factors for VTE to extended-duration rivaroxaban (10 mg once daily for 45 days or 7.5 mg once daily for those with a creatinine clearance between 30 and 50 ml/min) or placebo after excluding those at high risk of bleeding. Although the trial did not demonstrate a reduction in the primary endpoint of symptomatic VTE and VTE-related death, key secondary efficacy endpoints revealed a 56% reduction in symptomatic VTE and a 27% reduction in symptomatic VTE and all-cause mortality. The lower 7.5 mg dose of rivaroxaban used in patients with moderate renal insufficiency was found to be ineffective (8), unlike the dose of 10 mg, which had previously been demonstrated to be effective in the MAGELLAN (Venous Thromboembolic Event [VTE] Prophylaxis in Medically Ill Patients; NCT00571649) study of extended thromboprophylaxis in medically ill patients (9). A pre-specified secondary endpoint of the trial was fatal and major venous and arterial thromboembolic events (8). Therefore, this exploratory analysis focused on whether rivaroxaban could reduce the incidence of fatal and major thromboembolic events in patients treated with 10 mg daily of rivaroxaban compared with placebo when given to acutely ill medical patients at the time of discharge for 45 days.
Methods
Study design
The MARINER protocol and study results have been reported previously (8,10). The protocol was approved by local ethics committees, and all subjects provided written informed consent. Briefly, MARINER was a multicenter, prospective, randomized, double-blind, placebo-controlled, event-driven study that evaluated rivaroxaban (10 mg daily in patients with creatinine clearance [CrCl] ≥50 ml/min or 7.5 mg daily with CrCl 30 to <50 ml/min at baseline) versus placebo for 45 days beyond hospital discharge to prevent symptomatic VTE and VTE-related death in acutely ill medical patients. Eligible patients were 40 years of age or older, were hospitalized for at least 3 and no more than 10 consecutive days prior to randomization for a specific acute medical illness, and had other risk factors for VTE. Other VTE risk factors were demonstrated by a total modified IMPROVE (International Medical Prevention Registry on Venous Thromboembolism) VTE risk score of ≥4 or VTE risk score of 2 or 3 with D-dimer >2× the upper limit of normal. Patients received thromboprophylaxis during the index hospitalization with low-molecular-weight heparin or unfractionated heparin. Patients with an increased risk of bleeding were excluded from the study. The primary hypothesis was that rivaroxaban was superior to placebo for the prevention of the composite of symptomatic VTE (lower extremity deep vein thrombosis and nonfatal pulmonary embolism [PE]) and VTE-related death (death due to PE or death in which PE could not be ruled out). The primary hypothesis of this exploratory analysis was that rivaroxaban was superior to placebo for the prevention of the pre-specified composite of symptomatic VTE, myocardial infarction (MI), nonhemorrhagic stroke, and cardiovascular (CV) death in the stratum of patients with a baseline CrCl ≥50 ml/min receiving the 10 mg dose of rivaroxaban.
Statistical methods
The point estimate for the hazard ratio (HR) and corresponding 95% confidence interval (CI) for the 10 mg rivaroxaban dose versus placebo were provided based on the Cox proportional hazards model. Patients were analyzed according to the treatment group they were randomized to, regardless of the actual treatment received. All statistical tests were interpreted at a nominal (without adjustment for multiplicity) 2-sided significance level of 0.05, and all CIs at a nominal 2-sided level of 95%. The Kaplan-Meier method was used to summarize the time-to-event analyses.
Efficacy and safety outcomes
The efficacy outcome of the composite of symptomatic VTE, MI, nonhemorrhagic stroke, and CV death was a pre-specified secondary endpoint, and individual outcomes were verified by objective testing and autopsy reports, where available using standardized definitions (8). The principal safety outcome of major bleeding was based on the International Society on Thrombosis and Haemostasis bleeding criteria and included fatal bleeding, bleeding into a critical organ, or bleeding that led to a drop of ≥2 g/dl of hemoglobin or a transfusion of 2 U or more of blood. Bleeding events were analyzed based on time from randomization to the first occurrence. All endpoints were adjudicated by a blinded clinical events committee.
Results
Baseline characteristics
A total of 4,909 patients were assigned to the rivaroxaban 10 mg group (baseline CrCl ≥50 ml/min) and 4,913 patients to matching placebo and are included in the intent-to-treat (ITT) population. The ITT analysis set included all randomized patients who had valid informed consent. Baseline characteristics were well balanced between treatment groups (Table 1 ). The mean age of this population was 67.8 years, 55.5% were men, and 96.5% were white. The most frequently reported admitting diagnosis for patients overall was heart failure with reduced ejection fraction ≤45% (37.2%) and the mean duration of hospitalization was 6.7 ± 2.4 days. Overall, 52.0% of patients had a baseline CrCl level ≥80 ml/min and 47.9% had a baseline CrCl 50 to <80 ml/min with a mean baseline creatine clearance of 87.8 ml/min. At baseline, aspirin was used in 51.9% of patients; a statin was used by 41.7%. A history of reduced or preserved ejection fraction heart failure (47.7%), coronary artery disease (31.6%), diabetes (28.7%), and hyperlipidemia (20.0%) were also common in the population. A history of cancer was reported by 8.3% of patients, while patients with active cancer undergoing chemotherapy were excluded. Total modified IMPROVE VTE risk factor scores of 2, 3, and ≥4 were reported for 35.8%, 28.4%, and 35.8% of patients, respectively.
Table 1.
Characteristics of Patients at Baseline (ITT)
| Rivaroxaban 10 mg (n = 4,909) | Placebo (n = 4,913) | |
|---|---|---|
| Male | 55.3 | 55.7 |
| Mean age, yrs | 67.8 | 67.7 |
| White race | 96.3 | 96.7 |
| Mean BMI, kg/m2 | 29.5 | 29.3 |
| Reason for hospitalization | ||
| Heart failure | 37.4 | 37.0 |
| Respiratory insufficiency or exacerbation of chronic obstructive pulmonary disease | 27.6 | 28.0 |
| Ischemic stroke | 15.5 | 15.7 |
| Infectious disease | 17.9 | 17.7 |
| Inflammatory disease | 1.5 | 1.7 |
| Mean duration of index hospitalization, days | 6.7 | 6.7 |
| D-dimer >2× upper limit of normal during the index hospitalization | 68.7 | 68.7 |
| Mean baseline CrCl | 87.9 | 87.8 |
| Baseline aspirin | 51.9 | 49.1 |
| Current or former smoker | 48.2 | 48.3 |
| Baseline statin | 41.9 | 41.4 |
| Baseline U.S.-approved thromboprophylaxis | 71.2 | 71.5 |
| History of hypertension | 78.2 | 78.8 |
| History of diabetes | 29.5 | 27.9 |
| History of heart failure | 47.9 | 47.5 |
| History of coronary artery disease | 32.1 | 31.1 |
| History of hyperlipidemia | 20.5 | 19.6 |
| History of cancer | 8.0 | 8.7 |
| IMPROVE VTE risk factor score | ||
| 2 | 35.5 | 36.0 |
| 3 | 29.3 | 27.4 |
| ≥4 | 35.1 | 36.4 |
Values are % or mean (where indicated). Note: intention-to-treat (ITT): all randomized patients who had valid signed informed consent. U.S.-approved thromboprophylaxis includes enoxaparin, dalteparin, and heparin. Patients who have both U.S.-approved and other baseline use of thromboprophylaxis are included in the U.S.-approved thromboprophylaxis category.
BMI = body mass index; CrCl = creatine clearance; IMPROVE VTE = International Medical Prevention Registry on Venous Thromboembolism venous thromboembolism model.
Efficacy and safety outcomes
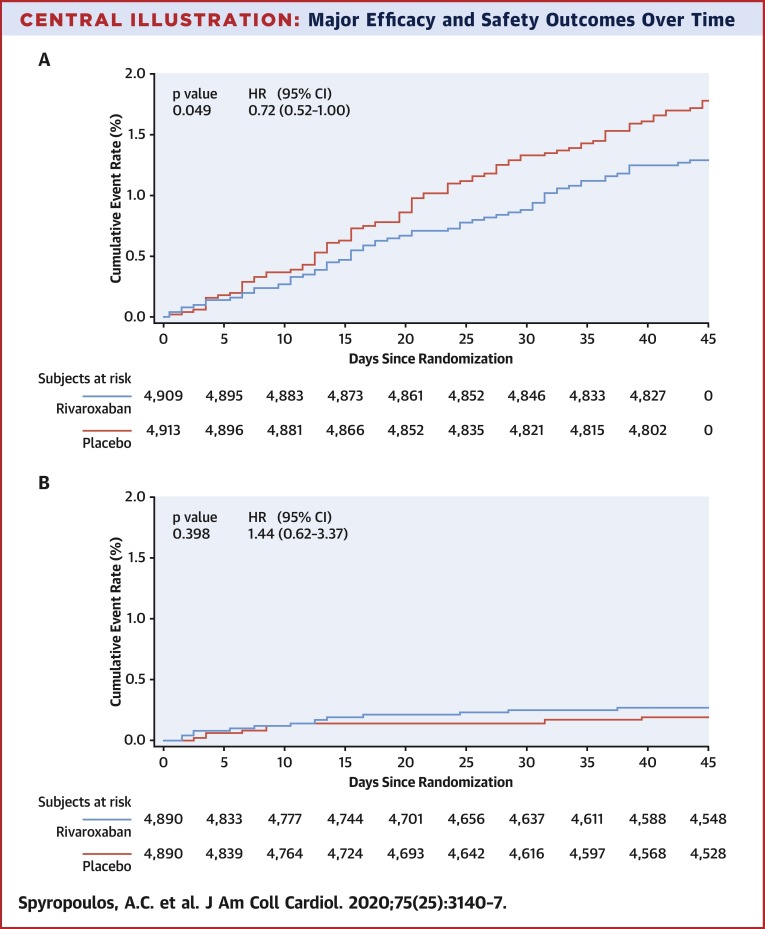
The composite endpoint of symptomatic VTE, MI, nonhemorrhagic stroke, and CV death occurred in 1.28% of patients randomized to rivaroxaban and in 1.77% of those on placebo (HR: 0.72; 95% CI: 0.52 to 1.00; p = 0.049) (Table 2 ). All incidence of the components of the composite endpoint, except MI, numerically tended to favor rivaroxaban. Symptomatic lower-extremity deep vein thrombosis and symptomatic nonfatal PE showed greater risk reduction with rivaroxaban with a relative risk reduction of 80% (HR: 0.20; 95% CI: 0.04 to 0.91) and 62% (HR: 0.36; 95% CI: 0.12 to 1.14), respectively (Table 2). Kaplan-Meier curves for the composite of symptomatic VTE, MI, nonhemorrhagic stroke, and CV death are presented in the Central Illustration .
Table 2.
Time to First Occurrence of Composite Outcome and Components (ITT)
| Endpoint | Rivaroxaban 10 mg | Placebo | Rivaroxaban 10 mg vs. Placebo |
|
|---|---|---|---|---|
| HR (95% CI)∗ | p Value† | |||
| Composite of symptomatic VTE (DVT and nonfatal PE), MI, nonhemorrhagic stroke, CV death | 63/4,909 (1.28) | 87/4,913 (1.77) | 0.72 (0.52–1.00) | 0.049 |
| Symptomatic lower extremity DVT | 2/4,909 (0.04) | 10/4,913 (0.20) | 0.20 (0.04–0.91) | — |
| Symptomatic nonfatal PE | 4/4,909 (0.08) | 11/4,913 (0.22) | 0.36 (0.12–1.14) | — |
| MI | 13/4,909 (0.26) | 8/4,913 (0.16) | 1.62 (0.67–3.92) | — |
| Nonhemorrhagic stroke | 13/4,909 (0.26) | 24/4,913 (0.49) | 0.54 (0.28–1.06) | — |
| CV death | 39/4,909 (0.79) | 42/4,913 (0.85) | 0.93 (0.60–1.44) | — |
Values are n/N (%) unless otherwise indicated. CV death includes VTE-related death. All events were adjudicated by the clinical event committee. Intention-to-treat (ITT): all randomized patients who had valid signed informed consent.
CI = confidence interval; CV = cardiovascular; DVT = deep vein thrombosis; HR = hazard ratio; MI = myocardial infarction; PE = pulmonary embolism; VTE = venous thromboembolism.
HRs (95% CIs) are from Cox proportional hazards model with treatment as the only covariate.
The p value (2-sided) for superiority of rivaroxaban versus placebo from Cox proportional hazards model.
Central Illustration.
Major Efficacy and Safety Outcomes Over Time
(A) Time to first occurrence of composite: venous thromboembolism, myocardial infarction, nonhemorrhagic stroke, and cardiovascular death up to day 45 (rivaroxaban 10 mg daily vs. placebo; intention to treat). Includes all data from randomization to day 45 (inclusive). Patients who do not have events are censored on the minimum of last visit before or on death or day 45. (B) Time to first occurrence of major bleeding on-treatment (rivaroxaban 10 mg daily vs. placebo; safety population). On-treatment includes all data from randomization to 2 days after the last dose of the study drug (inclusive). Subjects who do not have events are censored on the minimum of last visit before or on death, or last dose +2 days. CI = confidence interval; HR = hazard ratio.
The bleeding analysis used the safety population, which for the 10 mg dose included 4,890 in both the rivaroxaban and placebo groups for a total of 9,780 patients. Major bleeding occurred in 13 (0.27%) and 9 (0.18%) patients in the rivaroxaban and placebo groups, respectively (HR: 1.44; 95% CI: 0.62 to 3.37; p = 0.398) (Table 3 ). When evaluated by type of major bleeding, a greater proportion of patients receiving 10 mg rivaroxaban than placebo had major bleeding events with a fall in hemoglobin of ≥2 g/dl (0.22% and 0.12%, respectively) and transfusion of >2 U of packed red blood cells (or whole blood) than patients in the placebo group (0.16% and 0.06%, respectively). There were 2 critical site bleeds in each group, and 2 fatal bleeds in the rivaroxaban group. A Kaplan-Meier analysis of the timing of major bleeding occurrence is shown in the Central Illustration.
Table 3.
Time to First Occurrence of Major Bleeding Event, On-Treatment (Safety Analysis Set)
| Endpoint | Rivaroxaban 10 mg | Placebo | Rivaroxaban 10 mg vs. Placebo |
|
|---|---|---|---|---|
| HR (95% CI)∗ | p Value† | |||
| ISTH major bleeding | 13/4,890 (0.27) | 9/4,890 (0.18) | 1.44 (0.62–3.37) | 0.398 |
| Fall in hemoglobin of ≥2 g/dl | 11/4,890 (0.22) | 6/4,890 (0.12) | 1.83 (0.68–4.95) | — |
| Transfusion of ≥2 U of packed red blood cells or whole blood | 8/4,890 (0.16) | 3/4,890 (0.06) | 2.66 (0.71–10.04) | — |
| Critical site | 2/4,890 (0.04) | 2/4,890 (0.04) | 1.00 (0.14–7.10) | — |
| Fatal outcome | 2/4,890 (0.04) | 0/4,890 | NA | NA |
Values are n/N (%) unless otherwise indicated. All adjudicated by the clinical event committee. Safety analysis set: all intention-to-treat patients who take at least 1 dose of study drug.
ISTH = International Society on Thrombosis and Haemostasis; NA = not applicable; other abbreviations as in Table 2.
HRs (95% CIs) are from Cox proportional hazards model with treatment as the only covariate.
p value (2-sided) for superiority of rivaroxaban vs. placebo from Cox proportional hazards model.
Discussion
This analysis demonstrates that compared with placebo, extended-duration thromboprophylaxis with low-dose rivaroxaban (10 mg daily) started after hospital discharge leads to a significant 28% reduction in the combined risk of fatal and major thromboembolic events without a significant increase in major bleeding in hospitalized medically ill patients. This benefit appears to begin after the first week of treatment and continues until the end of study at 45 days. There was a 0.49% absolute risk reduction of efficacy events and a 0.09% absolute increase in the risk of major bleeding, suggesting a net clinical benefit.
A meta-analysis of arterial thrombosis (including MI and ischemic stroke) of older studies involving ∼11,000 medically ill inpatients receiving heparin-based prophylaxis did not find a reduction of these events compared with control subjects (odds ratio: 1.95; 95% confidence interval: 0.89 to 4.27) (11). The authors concluded that arterial thrombotic events appeared to be under-reported in trials of venous thromboprophylaxis, and they recommended systematic monitoring of such events in future trials. In the MARINER study, data on arterial events were captured using a standardized data collection form, and all suspected events were centrally adjudicated using pre-specified criteria. Although exploratory, our analysis suggests that extended thromboprophylaxis with rivaroxaban in medically ill patients may lead to reductions in arterial thromboembolic events (primarily ischemic stroke) as well as a reduction in symptomatic VTE. Such a finding is in line with the results of the APEX trial (12), which in post hoc analyses revealed a ∼30% reduction in fatal and irreversible ischemic events (including PE, MI, nonfatal ischemic stroke, or CV death) without an increase in fatal or intracranial hemorrhage, and a ∼50% reduction in ischemic stroke with betrixaban compared with placebo (6,7). A recent pooled analysis of both the MARINER and MAGELLAN trials of extended thromboprophylaxis with the 10 mg dose of rivaroxaban also revealed a significant 22% reduction in all-cause mortality and major thromboembolic events, including symptomatic VTE, MI, and nonhemorrhagic stroke (1.80% vs. 2.31%; HR: 0.78; 95% CI: 0.63 to 0.97; p = 0.024), without an increase in critical site or fatal bleeding (13).
The association between VTE and arterial thromboembolic disease has been well-established (5,14). Underlying common mechanisms of both VTE and arterial thromboembolism suggest that primary thromboprophylaxis using an established anticoagulant regimen, possibly against a background of antiplatelet therapy and given for a sufficient duration, can be effective in reducing both types of events. An analysis of MARINER data in the subset of patients receiving rivaroxaban plus aspirin, although not randomized for aspirin use and hypothesis generating, suggested greater efficacy with rivaroxaban plus aspirin in reducing symptomatic VTE and VTE-related death (15). In the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease; NCT01776424) trial, a very large study that compared 2.5 mg twice daily of rivaroxaban plus 100 mg aspirin daily with 100 mg aspirin daily alone in patients with chronic coronary artery disease or peripheral artery disease, rivaroxaban plus aspirin significantly reduced the incidence of CV death, stroke, and MI by 24% (16). This dose of rivaroxaban would be predicted to produce trough levels similar to those achieved with 10 mg once daily (17,18); this finding was supported by data from the MARINER pharmacokinetic substudy in patients receiving the 10 mg dose (9).
Our findings may have important implications for the population of medically ill patients now that rivaroxaban is approved for extended post-hospital discharge thromboprophylaxis in at-risk medically ill patients in the United States. A previous benefit/risk assessment of a low-bleed risk subpopulation of the MAGELLAN trial revealed that extended thromboprophylaxis with the 10 mg dose of rivaroxaban would lead to 21 fewer fatal and major thromboembolic events per 10,000 patients at the expense of 9 more critical site or fatal bleeds, with a number needed to treat of 481 and number needed to harm of 1,061 (19). In the large population of at-risk medically ill patients each year, this strategy would have the potential to prevent 12,000 fatal and major thromboembolic events annually at the cost of one-fourth to one-half that number of major or fatal bleeds (19).
Study limitations
Limitations of this study include its exploratory nature, because the MARINER study failed to meet its primary efficacy endpoint. We cannot exclude potential under-reporting of arterial thromboembolic events as the trial was powered to assess the risk of VTE, although this is unlikely because a standardized case report form was used with careful study oversight of outcomes and central adjudication of all of these events. In addition, the composite endpoint was driven primarily by a reduction in symptomatic VTE and ischemic stroke. Although event rates may be considered low (<2.0%), the time-to-event curves suggest that events continued to accumulate after the study period of 45 days such that we cannot exclude the possibility that rivaroxaban continues to reduce these events over a longer period of time. Our study also excluded subjects with moderate renal insufficiency because the reduced dose chosen (7.5 mg) was deemed inadequate. However, a recent study has demonstrated efficacy in this population with 10 mg daily of rivaroxaban with a favorable benefit risk profile (9). As approximately 50% of our study population had baseline aspirin use, an important antithrombotic synergy between prophylactic dose rivaroxaban and antiplatelet therapy cannot be excluded, and there are mechanistic implications of such a synergistic antithrombotic strategy that may lead to reductions in CV outcomes (15,16).
Conclusions
Our analysis suggests that in at-risk medically ill patients who are discharged from the hospital, extended-duration rivaroxaban at the 10 mg daily dose leads to a significant risk reduction in a composite of fatal and major thromboembolic events—including symptomatic VTE, MI, nonhemorrhagic stroke, and CV death—without a significant increase in major bleeding, compared with placebo. These data suggest that in properly selected patients at risk for VTE and at low risk for bleeding, that extended-duration rivaroxaban at 10 mg has a favorable benefit risk profile.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Hospitalized medically ill patients remain at risk of venous and arterial thromboembolism, especially within the first 6 weeks after discharge. Treatment with rivaroxaban (10 mg once daily) for up to 45 days beyond discharge of patients at higher risk of thromboembolism and lower risk of bleeding reduces the incidence of fatal and major thromboembolism without increasing major bleeding.
TRANSLATIONAL OUTLOOK: Prospective studies of patients hospitalized with coronavirus disease-2019 pneumonia are needed to confirm the benefit and safety of extended antithrombotic therapy in that specific subset of medically ill patients.
Acknowledgments
The authors thank the patients who participated in the MARINER trial. The authors also thank Traci Weber, who supported the writing of this manuscript.
Footnotes
The MARINER study is sponsored by Janssen Research & Development LLC. Dr. Spyropoulos has served as a consultant for Janssen Research & Development LLC, Bayer, Portola, Boehringer Ingelheim, and Bristol-Myers Squibb; has received research support from Boehringer Ingelheim and Janssen; has served on an Advisory Board for Daiichi-Sankyo; and has received a stipend from the ATLAS group. Dr. Ageno has received research grant support from Bayer; and has received honoraria for Advisory Board activity from Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Sanofi, Portola, Janssen, and Aspen. Dr. Albers has served as a consultant for Bayer and Janssen Research and Development, LLC. Dr. Elliott has served as a consultant for Bayer and Janssen Research and Development, LLC; and has received honoraria from the University of Cincinnati and Spectrum Health. Dr. Halperin has served as a consultant for Janssen Research & Development LLC, Johnson & Johnson, Ortho-McNeil-Janssen, Bayer, Abbott, Boehringer Ingelheim, National Institute of Health, and the ATLAS group. Dr. Hiatt has received research grants from Janssen Research & Development LLC, Bayer, Amgen, and the National Institutes of Health. Dr. Maynard has served on the Executive Committee of the MARINER trial for Janssen Research & Development LLC. Dr. Steg has received research grants from Amarin, Bayer, Sanofi, and Servier; and has served on clinical trials and served as a speaker or consultant for Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Idorsia, Novartis, Pfizer, Sanofi, and Servier. Dr. Weitz has received consultancy or honoraria fees from Janssen Research & Development LLC, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Ionis, Merck, Novartis, Pfizer, Portola, Anthos, and Servier. Drs. Lu and Barnathan are employees and shareholders of Janssen Research & Development LLC. Dr. Spiro was an employee of Bayer U.S. LLC. Dr. Raskob has served as a consultant for Janssen Research & Development LLC, Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Boehringer Ingelheim, Eli Lilly, Pfizer, Portola, Novartis, Anthos, Tetherex, and XaTek. Samuel Z. Goldhaber, MD served as Guest Associate Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
References
- 1.Piazza G., Fanikos J., Zayaruzny M., Goldhaber S.Z. Venous thromboembolic events in hospitalised medical patients. Thromb Haemost. 2009;102:505–510. doi: 10.1160/TH09-03-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Office of the Surgeon General; Rockville, MD: 2008. Office of the Surgeon General, National Heart, Lung, and Blood Institute. Publications and reports of the Surgeon General. [PubMed] [Google Scholar]
- 3.Amin A.N., Varker H., Princic N., Lin J., Thompson S., Johnston S. Duration of venous thromboembolism risk across a continuum in medically ill hospitalized patients. J Hosp Med. 2012;7:231–238. doi: 10.1002/jhm.1002. [DOI] [PubMed] [Google Scholar]
- 4.Piazza G., Goldhaber S.Z. Venous thromboembolism and atherothrombosis: an integrated approach. Circulation. 2010 May 18;121:2146–2150. doi: 10.1161/CIRCULATIONAHA.110.951236. [DOI] [PubMed] [Google Scholar]
- 5.Prandoni P., Bilora F., Marchiori A. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;348:1435–1441. doi: 10.1056/NEJMoa022157. [DOI] [PubMed] [Google Scholar]
- 6.Gibson C.M., Chi G., Halaby R., for the APEX Investigators Extended-duration betrixaban reduces the risk of stroke versus standard-dose enoxaparin among hospitalized medically ill patients: an APEX trial substudy (Acute Medically Ill Venous Thromboembolism Prevention With Extended duration Betrixaban) Circulation. 2017;135:648–655. doi: 10.1161/CIRCULATIONAHA.116.025427. [DOI] [PubMed] [Google Scholar]
- 7.Gibson C.M., Korjian S., Chi G., for the APEX Investigators Comparison of fatal or irreversible events with extended-duration betrixaban versus standard dose enoxaparin in acutely ill medical patients: an APEX trial substudy. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spyropoulos A.C., Ageno W., Albers G.W. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med. 2018;379:1118–1127. doi: 10.1056/NEJMoa1805090. [DOI] [PubMed] [Google Scholar]
- 9.Weitz J.I., Raskob G.E., Spyropoulos A.C. Thromboprophylaxis with rivaroxaban in acutely ill medical patients with renal impairment: insights from the MAGELLAN and MARINER trials. Thromb Haemost. 2020;120:515–524. doi: 10.1055/s-0039-1701009. [DOI] [PubMed] [Google Scholar]
- 10.Raskob G.E., Spyropoulos A.C., Zrubek J. The MARINER trial of rivaroxaban after hospital discharge for medical patients at high risk of VTE. Design, rationale, and clinical implications. Thromb Haemost. 2016;115:1240–1248. doi: 10.1160/TH15-09-0756. [DOI] [PubMed] [Google Scholar]
- 11.Squizzato A., Lussana F., Ageno W., Cattaneo M. Effect of thromboprophylaxis with anticoagulant drugs on the incidence of arterial thrombotic events in medical inpatients: a systematic review. Intern Emerg Med. 2016;11:467–476. doi: 10.1007/s11739-016-1427-5. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A.T., Harrington R.A., Goldhaber S.Z., for the APEX Investigators Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534–544. doi: 10.1056/NEJMoa1601747. [DOI] [PubMed] [Google Scholar]
- 13.Raskob G.E., Spyropoulos A.C., Cohen A.T. Rivaroxaban for extended thromboprophylaxis after hospitalization for medical illness: pooled analysis of mortality and major thromboembolic events in 16,496 patients from the MAGELLAN and MARINER trials. Circulation. 2019;140:A12863. [Google Scholar]
- 14.Ageno W., Becattini C., Brighton T., Selby R., Kamphuisen P.W. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 15.Tomkowski W.Z., Davidson B.L. Thromboprophylaxis by rivaroxaban, aspirin, both, or placebo after hospitalization for medical illness. Thromb Res. 2019;180:62–63. doi: 10.1016/j.thromres.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Eikelboom J.W., Connolly S.J., Bosch J., for the COMPASS Investigators Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L., Yan X., Nandy P. Influence of model-predicted rivaroxaban exposure and patient characteristics on efficacy and safety outcomes in patients with acute coronary syndrome. Ther Adv Cardiovasc Dis. 2019;13:1–15. doi: 10.1177/1753944719863641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willmann S., Zhang L., Frede M. Integrated population pharmacokinetic analysis of rivaroxaban across multiple patient populations. CPT Pharmacometrics Syst Pharmacol. 2018;7:309–320. doi: 10.1002/psp4.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spyropoulos A.C., Lipardi C., Xu J. Improved benefit risk profile of rivaroxaban in a subpopulation of the MAGELLAN Study. Clin Appl Thromb Hemost. 2019;25 doi: 10.1177/1076029619886022. [DOI] [PMC free article] [PubMed] [Google Scholar]