Abstract
Neuroimaging manifestations of COVID-19 are being reported with increasing frequency with recent reports of associated atypical leukoencephalopathies. We add to this literature by describing a COVID-19 + patient who demonstrated imaging findings typical for posterior reversible encephalopathy syndrome (PRES). The inflammatory syndrome associated with novel corona virus infection has shown markedly increased levels of cytokines and inflammatory markers. This has also been described in a proposed mechanism for PRES, where elevated inflammatory markers result in endothelial injury causing interstitial fluid extravasation typical of PRES. We expect that other cases of PRES will be observed in this population given the scope of the Covid-19 pandemic.
A novel β-coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2), emerged as a respiratory illness in December 2019 [1]. Similar to other coronaviruses, including severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS-Cov), this COVID-19 virus primarily manifests as a respiratory illness characterized by cough and sore throat that may progress to acute respiratory distress syndrome given its effect on the lower airways [2], [3]. As of April 21, 2020 the World Health Organization reported 2,319,066 confirmed cases of COVID-19 worldwide [4].
We describe imaging findings of posterior reversible encephalopathy syndrome (PRES) in a 59-years old man without significant past medical history who initially presented to the hospital with fever, respiratory distress and tested positive for COVID-19. On admission the oxygen-saturation was 93% on 4L oxygen by nasal cannula. On the second day of admission, he developed acute respiratory failure requiring intubation and was transfer to the intensive care unit. The patient received a seven-day course of Ceftriaxone and three doses of azithromycin for persistent fever during his admission. He was also enrolled in a trial utilizing the antiviral remdesivir.
During the hospital course, his BUN level fluctuated however eGFR remained within normal range (>60 cc/min). Blood pressure lability was recorded during the hospital course never exceeding a one day maximum of 173/96 mmHg, that had decreased prior to the time of imaging. Despite treatment and maintenance of oxygen saturation, progressive respiratory compromise occurred. With cessation of paralytics, the patient was found to be encephalopathic so brain imaging was requested. Non-contrast head CT was performed on hospital admission day #12 and demonstrated symmetric hypoattenuation of the posterior subcortical cerebral white matter and external capsules (Fig. 1 ). Diagnoses of PRES, toxic leukoencephalopathy and extrapontine myelinolysis were considered and MRI was requested for further assessment. MR imaging performed the following day showed extensive FLAIR-hyperintensity with increased diffusivity in the subcortical greater than deep cerebral white matter, internal and external capsules and cerebellar white matter (Fig. 2 ). Hyperintense signal without restricted diffusion was also present in the deep grey matter. There was no post-contrast enhancement. Overall these imaging findings were highly concordant with the imaging pattern attributed to PRES [5], [6]. The patient succumbed to the coronavirus infection on day 14 of admission. His family declined autopsy.
Fig. 1.
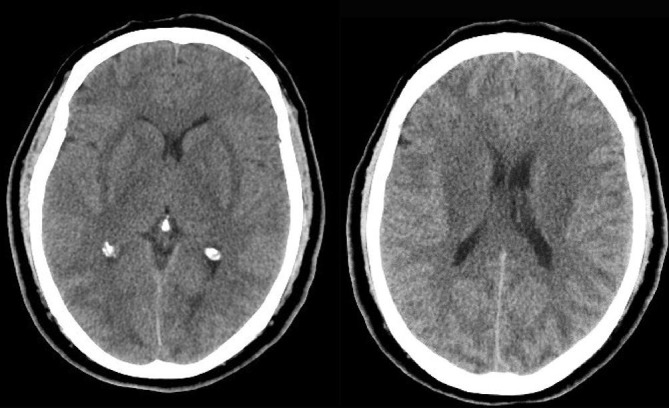
Axial non-contrast CT images show decreased attenuation diffusely involving subcortical WM (posterior > anterior) and bilateral external capsules.
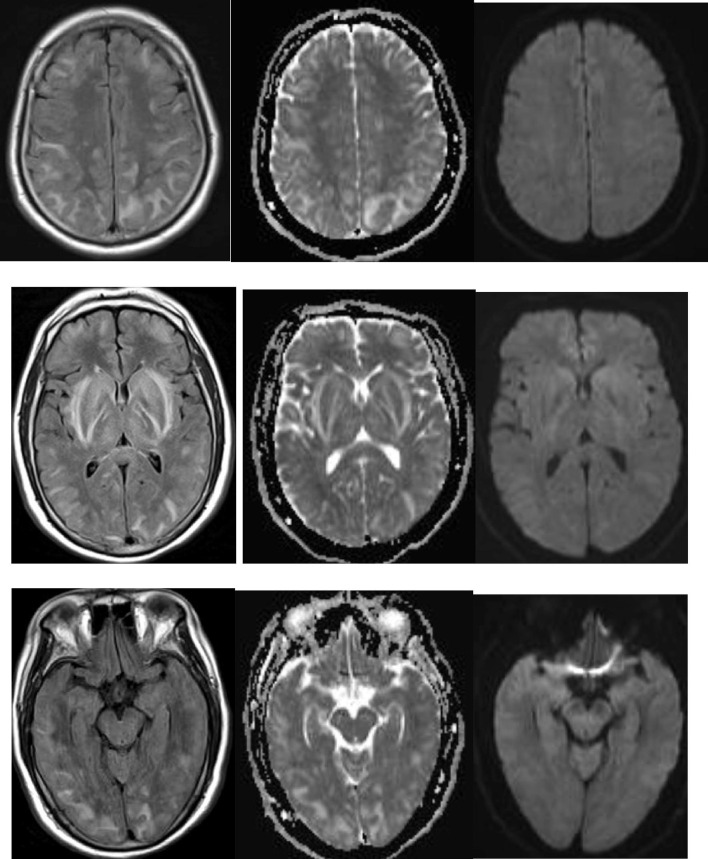
Fig. 2.
MRI Flair, ADC, DWI a,b,c (centrum semiovale level), d,e,f (basal ganglia level), g,h,I (occipital level) showing diffuse but predominant posterior WM, subcortical FLAIR hyperintensity, with increased ADC. No contrast enhancement was present (not shown).
PRES may present as a manifestation of systemic hypertension, toxemia of pregnancy, uremia or chemotherapy. It has also been reported with infection and sepsis [5]. Although infections with gram-positive bacteria predominate, PRES has been reported with influenza A, varicella zoster and parainfluenza viral infections in case reports. Neurologic manifestations of COVID-19 disease may include dizziness and headache [7], anosmia [8], [9] and hypogeusia [7].
Acute and post-infectious encephalitis have been reported in other coronaviruses, including MERS-CoV [10], [11]. Recently two reports have been published describing atypical leukoencephalopathy patterns associated with the novel Corona virus. Poyiadji et al. described findings suggestive of acute hemorrhagic necrotizing encephalitis related to the COVID-19 infection and ascribed their findings to the related “cytokine storm” seen with the infection [12]. Also recently described is a report of a non-specific confluent posterior predominant white matter leukoencephalopathy, with scattered micro-hemorrhage most notably at the corpus callosum. The considered differential diagnosis included ADEM/AHEM and PRES with associated microhemorrhage. Notably their patient described showed no evidence for preexisting hypertension or other risk factors for PRES [13]. In our case, the imaging features were highly typical for PRES, showing confluent predominantly posterior subcortical and external capsule edema signal, with increased diffusivity and no associated contrast enhancement. Although our reported patient had labile hypertension during his hospital course, that could have contributed to the pathophysiology of PRES, his blood pressure had stabilized and was reduced by the time that imaging occurred.
The pathophysiology of PRES is controversial, originally attributed to autoregulation breakthrough or vasospasm-induced ischemia [14]. As reported etiologies expanded, a more widely inclusive pathophysiological mechanism has achieved consensus. In this concept PRES is thought to be initiated by a combination of endothelial injury and hypoperfusion of brain tissue that is often the result a systemic process in which increased levels of cytokines and inflammatory markers cause endothelial damage via upregulation of endothelial surface antigens and increased leukocyte adherence [14], [15], [16]. Indeed, increased levels of serum cytokines have been found in the highly pathogenic coronaviruses, SARS-CoV, MERS-CoV, and SARS-CoV-2, and correlate with the severity of the clinical disease [3]. In addition to the Th1 T-cell-secreted cytokines, high levels of cytokines produced by Th2 T-cells, which include IL-4 and IL-10, are specific to SARS-CoV-2 [3], [17]. Multiple studies have linked the severity of COVID-19 symptoms and progression to ARDS to increased level of cytokines and inflammatory markers including IL-2R, IL-6, IP-10, MCP-1, TNF-α, granulocyte colony-stimulating factor, and macrophage inflammatory protein-1A [17], [18], [19].
Therefore, in this context, the typical manifestations of extensive PRES in a patient infected with Covid-19 is not unexpected and is demonstrated in the presented case. We posit that PRES in this instance is primarily secondary to the “cytokine storm” associated with the pathogenesis of SARS-CoV-2 infections. We expect that other cases of PRES will be observed in this population given the scope of the Covid-19 pandemic.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bogoch I.I., Watts A., Thomas-Bachli A., Huber C., Kraemer M.U.G., Khan K. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J Travel Med. 2020;27(2) doi: 10.1093/jtm/taaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infection. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO COVID-19 Dashboard. https://covid19.who.int/. Accessed April 20, 2020.
- 5.Bartynski W.S. Posterior reversible encephalopathy syndrome, Part 1: Fundamental imaging and clinical features. Am. J. Neuroradiol. 2008;29(6):1036–1042. doi: 10.3174/ajnr.A0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartynski W.S., Boardman J.F. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. Am. J. Neuroradiol. 2007;28(7):1320–1327. doi: 10.3174/ajnr.A0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L., Wang M., Chen S. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: A Retrospective Case Series Study. Infectious Diseases (except HIV/AIDS) 2020 doi: 10.1101/2020.02.22.20026500. [DOI] [Google Scholar]
- 8.Gane S.B., Kelly C., Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020 doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins C., Surda P., Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020 doi: 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- 10.Nath A. Neurologic complications of coronavirus infections. Neurology. 2020 doi: 10.1212/WNL.0000000000009455. [DOI] [PubMed] [Google Scholar]
- 11.Arabi Y.M., Harthi A., Hussein J. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43(4):495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020:201187. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachs J., Gibbs K., Dionne E. COVID-19 associated leukoencephalopathy. Radiology. 2020 doi: 10.1148/radiol.2020201753. pubs.rsna.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartynski W.S. Posterior reversible encephalopathy syndrome, Part 2: Controversies surrounding pathophysiology of vasogenic edema. Am. J. Neuroradiol. 2008;29(6):1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantovani A., Bussolino F., Dejana E. Cytokine regulation of endothelial cell function. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1992;6(8):2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- 16.Maemura K., Kurihara H., Morita T., Oh-hashi Y., Yazaki Y. Production of endothelin-1 in vascular endothelial cells is regulated by factors associated with vascular injury. Gerontology. 1992;38(Suppl. 1):29–35. doi: 10.1159/000213360. [DOI] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond. Engl. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G., Wu D., Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L., Liu H.G., Liu W. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi Zhonghua Jiehe He Huxi Zazhi Chin. J. Tuberc. Respir. Dis. 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]