To the Editor:
A 62-year-old man without notable medical history was admitted to a regional hospital with high fever, cough, and shortness of breath. Chest CT revealed bilateral ground-glass opacities consistent with viral pneumonia. SARS-CoV-2 infection was confirmed by positive RT-PCR on nasopharyngeal swab.
Initial treatment comprised atazanavir and ceftriaxone. Four days later, the patient's condition deteriorated and he required endotracheal intubation. He was then transferred to our tertiary care center for further management. Antimicrobial therapy was replaced by hydroxychloroquine and then remdesivir as well as by piperacillin-tazobactam (Fig. 1 ). Conventional lung protective ventilation with prone positioning was initiated. On day 7 after transfer, despite antithrombotic prophylaxis with standard-dose unfractionated heparin, bilateral segmental pulmonary embolism was diagnosed and therapeutic anticoagulation initiated. At that point, coagulation tests revealed slightly decreased prothrombin time (70%; reference range, 80–120%), explained by a mild constitutional isolated factor VII deficiency, and D-dimers of 10,620 ng/ml (cut-off for venous thromboembolism, <500 ng/ml) (Table S1). By day 20, plasma fibrinogen rose to 7.1 g/L (reference range, 2.0-4.0 g/L). The further course was complicated by ventilator-associated pneumonia treated with cefepime and then meropenem as well as by critical illness polyneuropathy. PCR for SARS-CoV-2 was negative on bronchoalveolar lavage performed on day 21. A tracheotomy was performed on day 24.
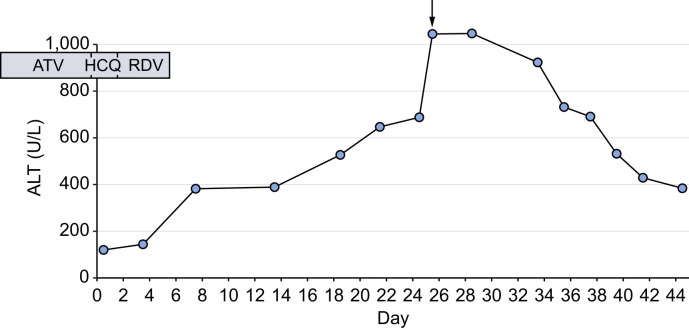
Fig. 1.
Evolution of ALT over time. The arrow denotes the liver biopsy.
ALT, alanine aminotransferase; ATZ, atazanavir; HCQ, hydroxychloroquine; RDV, remdesivir.
In parallel, the patient developed hepatitis. On the day of admission to our center, transaminases were moderately elevated (alanine aminotransferase [ALT] 137 U/L [reference range, 11–60 U/L], aspartate aminotransferase [AST] 111 U/L [reference range, 14-50 U/L]), with only slightly elevated alkaline phosphatase (135 U/L; reference range, 36-108 U/L) and normal total bilirubin. Subsequent analyses revealed a progressive increase of ALT to a peak of 1,048 U/L on day 25 (Fig. 1), with AST of 870 U/L, alkaline phosphatase of 196 U/L and total bilirubin of 26 μmol/L (reference range, <21 μmol/L) (Table S1). Synthetic liver cell function was preserved (factor V 140%; reference range, 70–180%). Antiviral medication and antibiotics had been stopped 20 days and 2 days prior to the peak of transaminases, respectively. The liver was normal on imaging, with patent portal and hepatic veins. Serologies and molecular testing for hepatitis B, C and E as well as herpes simplex, parvovirus B19, human herpesvirus 6, Epstein-Barr virus, and SARS-CoV-2 were negative. Blood PCR for cytomegalovirus (CMV) was positive at 50,800 copies/ml and ganciclovir at dose of 10 mg/kg/day was started, resulting in a drop of viremia to 2,100 copies/ml within 10 days.
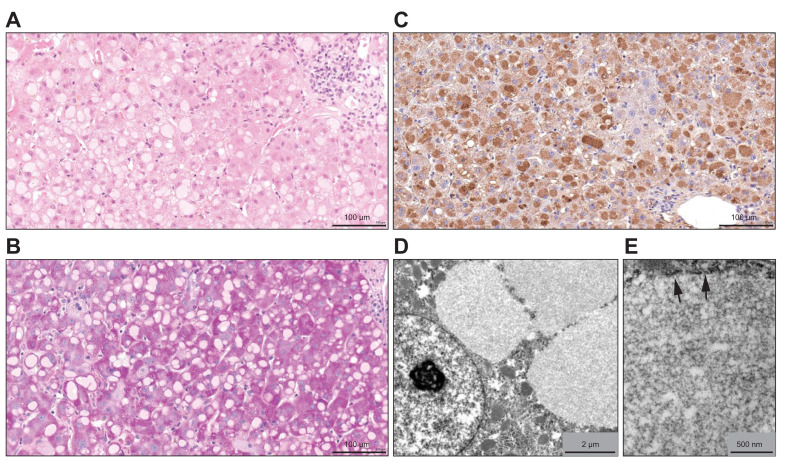
Liver biopsy performed on day 25 revealed a mild lymphoplasmocytic infiltrate in the portal tracts, without interface hepatitis or fibrosis, together with a few apoptotic hepatocytes scattered throughout the lobules. The presence of some hepatocyte mitoses and of numerous ceroid macrophages indicated that hepatitis had been ongoing for a while. There was no evidence of endotheliitis or hemophagocytosis, and there was no sinusoidal fibrin deposition. The more striking histological feature was the presence of numerous ground-glass hepatocytes with weakly eosinophilic cytoplasmic inclusions of various size, showing round or reniform shape and sharp edges (Fig. 2 A). Periodic Acid Schiff (PAS) stain was negative (Fig. 2B), as well as immunochemistry for hepatitis B surface antigen (HBsAg, not illustrated). The cytoplasmic inclusions strongly reacted with an anti-fibrinogen antibody (Fig. 2C), demonstrating that they were composed mainly of fibrinogen. They were also positive, in a patchy pattern, for C-reactive protein (not illustrated). Immunochemistry for CMV was negative; PCR for CMV in the tissue was only weakly positive (200 copies/ml). At electron microscopy, the inclusions contained a homogenous, moderately electron dense granular material (Fig. 2D). They were delineated, at least focally, by a membrane, arguing in favor of dilated endoplasmic reticulum (Fig. 2E). Hence, the morphological picture suggested hepatocellular type II fibrinogen inclusions.1
Fig. 2.
Liver biopsy findings.
Pale hyaline ground-glass inclusions are present in the cytoplasm of numerous hepatocytes (A, hematoxylin-eosin). They are negative for Periodic Acid Schiff staining (B), while exhibiting strong immunohistochemical reactivity for fibrinogen (C). At electron microscopy, they contain a faintly granular amorphous electron dense material (D, E) and appear as membrane-bound inclusions (E, arrowheads).
Genetic analysis did not reveal any known mutations responsible for fibrinogen storage disease in exons 8 and 9 of the FGG gene.
The patient's condition progressively improved and he could be successfully weaned from mechanical ventilation on day 37. On day 44, at the time of writing, ALT has dropped to 384 U/L, with AST of 166 U/L, alkaline phosphatase of 323 U/L, and normal total bilirubin.
In a patient with severe COVID-19, we describe an unusual form of liver disease, characterized by a ground-glass appearance of the hepatocytes resulting from the pathological cytoplasmic accumulation of fibrinogen. The differential diagnosis of ground-glass hepatocytes includes first the presence of HBsAg in chronic hepatitis B infection that can be identified by specific immunohistochemistry.2 Then, most of the other types of ground glass inclusions are linked to the accumulation of abnormal glycogen granules and are therefore PAS positive.2 They are observed in Lafora’s disease, type IV glycogenosis, and cyanamide aversion therapy in alcoholic patients,2 and have also been more recently described as “polyglucosan-like” hepatocellular inclusions in patients under polypharmacotherapy.3 In our patient, the ground glass inclusions were PAS negative, which prompted us to think of the possibility of abnormal fibrinogen accumulation, confirmed by the strong immunohistochemical reaction with an anti-fibrinogen antibody, and by the electron microscopy feature of membrane-bound inclusions.1 , 2
Fibrinogen is a large, oligomeric glycoprotein complex produced in the liver and secreted into the blood. Fibrinogen α, β and γ chains are encoded by the FGA, FGB and FGG genes, respectively. These are located on chromosome 4 and expressed almost exclusively in hepatocytes. Fibrinogen is converted by thrombin to fibrin, the most abundant component of a blood clot.4 , 5 Plasma fibrinogen levels are increased by mediators of the acute-phase inflammatory response, e.g. IL-6, or may be decreased as a result of consumption in disseminated intravascular coagulation. Mutations in fibrinogen genes cause congenital disorders that are typically associated with a-, hypo-, and/or dys-fibrinogenemia.6 In rare cases, a few mutations clustered in exons 8-9 of FGG result in the intracellular accumulation of misfolded fibrinogen in hepatocytes, chronic liver disease of various severity, and hypofibrinogenemia.7 Of note, fibrinogen storage disease without hypofibrinogenemia, which corresponds to the clinical picture presented by our patient, has rarely been associated with acute infections in patients without any hereditary defect of fibrinogen.8 , 9
Our patient presented very high plasma fibrinogen levels, making the presence of a known FGG mutation very unlikely, as confirmed by genetic testing. Increased fibrinogen production likely played a key role in the hypercoagulable state and pulmonary embolism.10 Increased fibrin formation and lysis can account for the very high levels of D-dimers observed in our patient. This has been previously associated with worse outcomes in patients with COVID-19.[11], [12], [13]
Information on liver involvement in COVID-19 is limited to date.[14], [15], [16] Elevated transaminases have been noted in up to 53% of patients with COVID-19. Liver injury in patients with SARS-CoV-2 infection may be caused by direct viral effects or indirectly by the systemic inflammatory response, drug toxicity, hemodynamic alterations or other factors. It appears to be more prevalent in severe compared to mild cases of COVID-19.
Only few reports have assessed liver histology in COVID-19.[17], [18], [19] Observed lesions include sinusoidal dilatation, mild portal and lobular inflammation, microvesicular steatosis or patchy necrosis. To our knowledge, a manifestation similar to that documented in our patient has not been reported to date in the setting of SARS-CoV-2 infection.
The histopathological substrate in our patient was an acute mostly lobular hepatitis with hepatocellular type II fibrinogen inclusions9 associated with high plasmatic fibrinogen levels in a context of severe systemic inflammation. Based on the temporal relationships, none of the administered drugs could be unequivocally linked to ALT increase. However, it is possible that one of them or another as yet unidentified extrinsic or intrinsic factor impaired fibrinogen secretion and contributed to intrahepatic accumulation as a “second hit”. Of note, hydroxychloroquine impacts on lysosomal function, autophagy and the Golgi apparatus.20 , 21 Although our patient had been treated with hydroxychloroquine for only 2 days, one may speculate that this may have contributed to pathological hepatic accumulation of fibrinogen. A direct viral effect is less likely given the negative PCR results on nasopharyngeal swabs and bronchoalveolar lavage performed 10 days and 4 days prior to liver biopsy, respectively. In addition, SARS-CoV-2 does not appear to circulate systemically at relevant levels.22
Experimental studies will have to confirm a cascade linking SARS-CoV-2-induced severe inflammation, hyperfibrinogenemia, and an as yet unidentified additional factor impairing hepatic fibrinogen secretion with acquired fibrinogen storage disease and hepatitis.
Financial support
The authors received no financial support to produce this manuscript.
Authors' contributions
All authors were involved in the clinical management of the described patient. MF, DM, LA and CS wrote the manuscript. All authors revised the manuscript for important intellectual content.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The authors gratefully acknowledge C. Chapuis for electron microscopy, as well as H. Goubin, D. Maison and N. Piazzon for immunohistochemistry.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2020.06.021.
Supplementary data
References
- 1.Callea F., de Vos R., Togni R., Tardanico R., Vanstapel M.J., Desmet V.J. Fibrinogen inclusions in liver cells: a new type of ground-glass hepatocyte. Immune light and electron microscopic characterization. Histopathology. 1986;10:65–73. doi: 10.1111/j.1365-2559.1986.tb02461.x. [DOI] [PubMed] [Google Scholar]
- 2.Vázquez J.J. Ground-glass hepatocytes: light and electron microscopy. Characterization of the different types. Histol Histopathol. 1990;5:379–386. [PubMed] [Google Scholar]
- 3.Lefkowitch J.H., Lobritto S.J., Brown R.S., Jr., Edmond J.C., Schilsky M.L., Rosenthal L.A. Ground-glass, polyglycosan-like hepatocellular inclusions: a “new” diagnostic entity. Gastroenterology. 2006;131:713–718. doi: 10.1053/j.gastro.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Fish R.J., Neerman-Arbez M. Fibrinogen gene regulation. Thromb Haemost. 2012;108:419–426. doi: 10.1160/TH12-04-0273. [DOI] [PubMed] [Google Scholar]
- 5.Arbustini E., Narula N., D'Armini A.M. Fibrinogen: a circulating factor in search of its genetics architecture. Circulation. 2013;128:1276–1280. doi: 10.1161/CIRCULATIONAHA.113.005125. [DOI] [PubMed] [Google Scholar]
- 6.De Moerloose P., Neerman-Arbez M. Congenital fibrinogen disorders. Semin Thromb Hemost. 2009;35:356–366. doi: 10.1055/s-0029-1225758. [DOI] [PubMed] [Google Scholar]
- 7.Callea F., Giovannoni I., Sari S., Aksu A.U., Esendagly G., Dalgic B. Fibrinogen gamma chain mutations provoke fibrinogen and apolipoprotein B plasma deficiency and liver storage. Int J Mol Sci. 2017;18(12) doi: 10.3390/ijms18122717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marucci G., Morandi L., Macchia S., Betts C.M., Tardio M.L., dal Monte P.R. Fibrinogen storage disease without hypofibrinogenemia associated with acute infection. Histopathology. 2003;42:22–25. doi: 10.1046/j.1365-2559.2003.01551.x. [DOI] [PubMed] [Google Scholar]
- 9.Zen Y., Nishigami T. Rethinking fibrinogen storage disease of the liver: ground glass and globular inclusions do not represent a congenital metabolic disorder but acquired collective retention of proteins. Hum Pathol. 2020;100:1–9. doi: 10.1016/j.humpath.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Van Hylckama Vlieg A., Rosendaal F.R. High levels of fibrinogen are associated with the risk of deep venous thrombosis mainly in the elderly. J Thromb Haemost. 2003;1:2677–2678. doi: 10.1111/j.1538-7836.2003.0543b.x. [DOI] [PubMed] [Google Scholar]
- 11.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spieza L., Boscolo A., Poletto F., Cerruti L., Tiberio I., Campello E. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L., Liu J., Lu M., Yand D., Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian S., Xiong Y., Liu H., Niu L., Guo J., Liao M. Pathological study of the 2019 novel coronavirus disease (COVID-19) through post mortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagana S.M., De Michele S., Lee M.J. COVID-19 associated hepatitis complicating recent living donor liver transplantation. Arch Pathol Lab Med. 2020 Apr 17 doi: 10.5858/arpa.2020-0186-SA. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy S., Yu S., Banerjee D., Overton O., Mukhopadhyay G., Oddoux C. Assembly and secretion of fibrinogen. Degradation of individual chains. J Biol Chem. 1992;267:23151–23158. [PubMed] [Google Scholar]
- 21.Mauthe M., Orhon I., Rocchi C., Zhou X., Luhr M., Hijlkema K.J. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14:1435–1455. doi: 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wölfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.