Abstract
The discovery that β-propiolactone (BPL), once a commercially important chemical, causes various tumors in experimental animals has led to a significant decrease in its use. However, owing to its efficacy this possible human carcinogen remains to be utilized in vaccines for inactivation of viruses. The focus of the current study was to uncover the mechanisms of β-propiolactone reactions with both nucleobases and glutathione (GSH) through computer simulations based on quantum chemical methods. Our results, in accordance with in vitro studies, show that among all nucleobases guanine most readily forms adducts with BPL through SN2 reaction mechanism. Acquired activation energies with incorporated solvent effects reveal that alkylation represents an energetically more favorable reaction than acylation for all nucleobases. Comparison of activation free energies of glutathione and guanine reactions with BPL suggest that glutathione may represent an efficient natural scavenger of BPL. Therefore, glutathione present in the organism may provide protection to the DNA and thus prevent BPL’s genotoxicity, mutagenicity, and possibly even carcinogenicity.
1. Introduction
As β-propiolactone is reported to cause tumors in experimental animals in various different tissues and by several different routes of exposure, International Agency For Research On Cancer has listed it in group 2A as a possible human carcinogen.1−3 In the 1950s, BPL represented a commercially important chemical in the manufacture of acrylic acid.1−3 Additionally, it was used for sterilization of surgical instruments, blood plasma, tissue grafts, milk, water, nutrient broth, and enzymes.2−5 BPL’s sporicidal action was also used against vegetative bacteria, pathogenic fungi, and viruses.2−5 Even though its use strongly decreased since it was listed as a possible human carcinogen, BPL, owing to its efficiency, continues to be used in vaccines for inactivation of viruses.1,5−11 On the basis of experimental trials, it has been concluded that propiolactone represents a human carcinogen.12 The results have shown the generation of tumors in several tissues and from different administration routes.12 Therefore, several health care workers may be exposed to this dangerous chemical along with the employees in various organic synthetic industries.1,12
Direct exposure to BPL, in addition to probable carcinogenicity, brings severe irritations to several systems, including skin burns and permanent damage to the eye, liver, and kidney.5 Even at a single administered dose, BPL is shown to be highly tumorigenic, genotoxic, and carcinogenic in experimental animals.12 At higher doses it even causes death.12 Acute exposure to BPL needs to be limited to 0.1 mg/L as higher doses, can cause toxicity.12 However, the threshold value is set to 0.5 ppm for daily work time (8 h) as chronic exposure to higher values may cause severe irritation.13,14 On the other hand, the Henry Ford Hospital transfusion tests reported absolutely no chronic toxic effects from their infusion of β-propiolactone-pretreated plasma into human volunteers.5 In mammals, BPL is fortunately quickly hydrolyzed to 3-hydroxy-propionic acid that shows no carcinogenic effects.5,12 Moreover, with chloride ions, it is metabolized, especially in blood plasma, to 3-chloropropionic acid that similarly shows no tumor-initiating activities.12 Thus, there is a large probability that in the case of transfusion tests BPL reacted with plasma proteins ahead of the infusion of pretreated plasma to the volunteers and consequentially in its metabolized form could not cause carcinogenic damage to the organism. Carcinogenicity of BPL seems to be strongly connected with its reactivity as a (mono)alkylating agent.12 That is presumably why hydrolytic and metabolic products show no carcinogenic effects. The only exception is an adduct of BPL with cysteine that shows carcinogenic activity, although a very weak one.15 Carcinogenic activity of BPL starts with alkylations or acylations of nucleobases that can lead to DNA damage like depurination mutations and resulting A to T transversions.
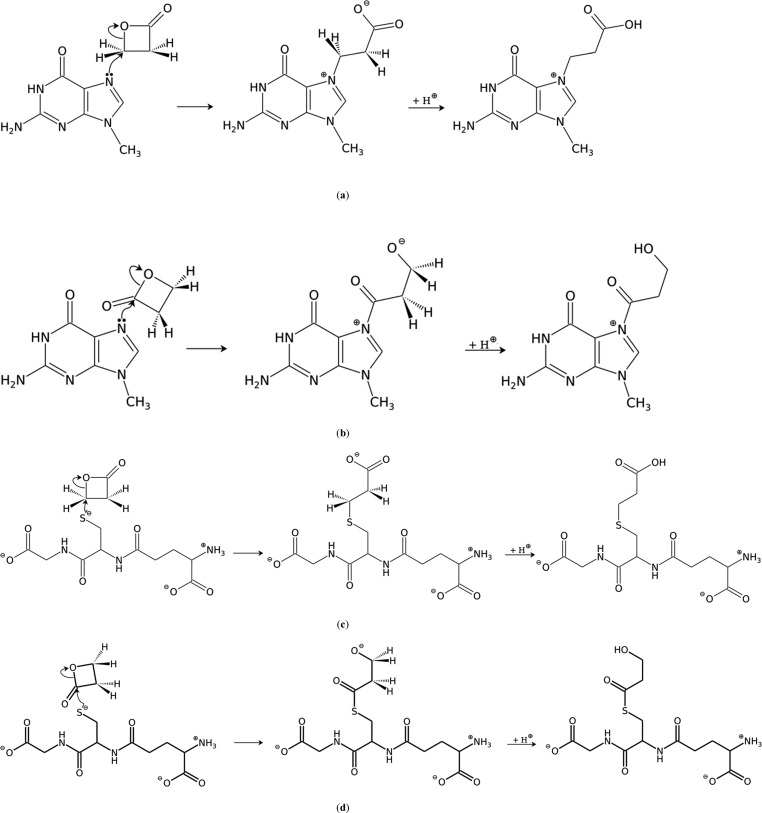
The half-life of BPL in water at 25 °C is 3 h; fortunately, in the body (where various nucleophiles are present) it is presumably much shorter.2,16 BPL preferentially reacts with the extracellular water and nucleophiles, with only a small portion reaching the intracellular macromolecules, particularly the nuclear components.15 However, if it reaches the biopolymers, then BPL was shown in vitro and in vivo to bind to the proteins, DNA as well as RNA of mouse skin.10,17 The SN2 reaction mechanism is proposed for the reactions of nucleophilic centers with BPL.16,18 Several in vitro studies have shown that the main product of BPL’s reaction with DNA is 7-(2-carboxyethyl)guanine (7-CEG) represented in Scheme 1(a), although adducts of other nucleobases are formed as well.16,19−21 Even though the majority of the monoalkylating agents cause only point mutations, BPL is in addition capable of cross-linking the DNA and linking the proteins to the DNA22 as well as of inflicting chromosomal changes.23 All listed reactions could possibly play a role in the oncogenesis.22
Scheme 1. Proposed Mechanism of (a) the Alkylation Reaction between β-Propiolactone and Methylguanine Giving Rise to the Main 7-(2-Carboxyethyl)methylguanine Adduct; (b) the Acylation Reaction between β-Propiolactone and Methylguanine; (c) the Alkylation Reaction between β-Propiolactone and the Ionized Form of Glutathione; and (d) the Acylation Reaction between β-Propiolactone and the Ionized Form of Glutathione.
After Roe and Glendenning17 discovered that BPL exerts carcinogenesis when in contact with mouse skin, investigation of its reactivity and genotoxicity increased tremendously. Roberts and Warwick24 performed in vitro reactions of BPL with guanosine, deoxyguanylic acid, and RNA. The same product was isolated in all those reactions; 7-(2-carboxyethyl)-guanine (7-CEG). Colburn et al.25 subsequently isolated an additional product 7,9-di(2-carboxyethyl)-guanine, a dialkylated product which formed in the same manner as 7-CEG, but at longer reaction times. In vivo experiments16 confirmed that reacting β-propiolactone with both DNA and RNA yields 7-CEG in the enol form. Later Mate et colleagues additionally isolated 1-(2-carboxyethyl)-adenine (l-CEA),19 3-(2-carboxyethyl)-thymine (3-CET),20 and 3-(2-carboxyethyl)-cytosine (3-CEC)21 in the reactions of calf thymus or mouse liver DNA with BPL.
Since only alkylation products were isolated in the reactions of BPL with nucleic acids, Helmminki26 investigated the alkylation reactions of several additional lactones with nucleic acids. BPL proved to be the most reactive lactone, about 50–100 times more reactive than the second in reactivity (β-butyrolactone). The reaction rate decreased from the smallest residues of nucleic acids (nucleobases) toward the largest (double-stranded DNA). Like that in previous studies the main alkylation sites were N-1 of adenosine, N-3 of cytidine, and N-7 of guanosine. The author26 thus suggested that the carcinogenic potency of lactones correlates with their reactivity rather than with the specificity of the adducts formed.
Findings of Uittenbogaard et al.7 were very similar to the exception of the reaction with guanine, where the second observed adduct was deoxyguanosine-N-7-carboxyethylated with hydrolyzed imidazole ring; and the reaction of BPL with cytidine, which resulted also in the formation of N-(2-carboxyethyl) cytidine. These inconsistencies with previous studies the authors7 attributed to the low BPL concentration that is normally used for inactivation of viruses.
An in vitro study by Dijkstra27 showed surprising results; BPL produced equal amounts of the alkylation and acylation products with glutathione. This directed us to study the kinetics of both acylation and alkylation reactions of BPL with nucleobases, focusing on ΔG⧧, the activation free energy of the rate-limiting step of the reaction, that is directly related to the carcinogenicity of β-propiolactone. Moreover, we investigated the reactions of BPL with glutathione that represents one of the major compounds involved in the cellular detoxification process, protecting the cells against xenobiotic agents generating oxidative stress.28−30 Glutathione can scavenge free radicals, reduce peroxides, or be conjugated with electrophilic compounds.28 Thus, glutathione provides the cells with multiple defense mechanisms not only against the reactive oxygen species but also against their toxic products.28 However, glutathione and its catabolites may eventually lead to the formation of reactive oxygen species and free radicals, as seen in the case of metal ion-mediated reactions.29 All in all, we must regard GSH as a major factor in the regulation of cell cycle, proliferation, and apoptosis, therefore various mechanisms serve to accomplish its biological roles.29 Consequently, we decided to explore the kinetics of alkylation and acylation reactions of BPL with glutathione as well. We wanted to see (i) whether alkylation and acylation reactions are indeed energetically similar and thus equally plausible and (ii) if these reactions are more favorable than the competing genotoxic reactions of BPL with different nucleobases. The proposed mechanisms for the alkylation and acylation reactions of BPL with either guanine or glutathione are depicted in Scheme 1.
In this study, the activation free energies for the reactions of BPL with either nucleobases or glutathione were obtained using computer simulations based on the quantum chemical methods. The calculations were performed on the methylated nucleobases as the methyl group represents a good replacement for the deoxyribose part of the DNA backbone.31−34 Our objective was to determine which of the reactions (acylation or alkylation) is more favorable and whether the reaction of BPL with glutathione is more favorable than the competing reaction of BPL with the most reactive nucleobase. The latter can provide an answer to whether glutathione represents a successful natural scavenger of BPL and is, therefore, able to prevent BPL’s genotoxicity and carcinogenicity.
2. Computational Methods
All our calculations were carried out on the CROW computer cluster located at the National Institute of Chemistry in Ljubljana.35,36 The Born–Oppenheimer hypersurfaces of the alkylation and acylation reactions of β-propiolactone with nucleobases and with glutathione were obtained by performing several ab initio, DFT, and semiempirical MO calculations with the Gaussian 09 suite of programs.37
From ab initio calculations, the Hartree–Fock (HF) and the Møller–Plesset perturbation theory of the second-order (MP2) level of theory, were employed. Among DFT methods the B3LYP, M06-2X, and mPWPW91 were chosen. Both, ab initio and DFT calculations applied flexible basis sets 6-31G(d), 6-31+G(d,p), and 6-311++G(d,p). Finally, the semiempirical MO methods AM1 and PM3 were used.
In order to obtain the activation energy of the reactions, we had to locate the reactant and transition state structures. The structure of BPL was obtained from ChemSpider38 and subsequently geometrically optimized in Gaussian 09 suite of programs37 at the HF 6-31G(d) level of theory. Whereas the structures of nucleobases were built in Molden39 and subsequently geometrically optimized in Gaussian 09 suite of programs37 at the HF 6-31G(d) level of theory. For the starting reactant structure we combined the optimized reactive species structures so that the distance between the bond-forming atoms was around 3 Å. Subsequently, geometry optimization was performed in order to obtain the structure lying in the true minimum of the potential energy surface. For the evaluation of the located reactant-state structure the vibrational analysis in the harmonic approximation was performed, ensuring only real frequencies had been obtained. This obtained optimized structure of the reactants was then subjected to a relaxed potential surface scan along the reaction coordinate to uncover an approximate structure of the transition state. Among scanned structures, the one with the highest energy was chosen as the starting point for the Berny algorithm40 to locate the optimized transition state structure. A vibrational analysis in the harmonic approximation was performed, ensuring a single imaginary frequency was obtained for each transition state. For the proposed SN2 reaction mechanism this coordinate coincides with the bond formation between the reactive species and with the bond cleavage in the BPL ring. The activation energy represents the energy difference between the obtained transition and reactant states.
To include the solvent effects in the form of hydration free energies of reactant and transition states the self-consistent reaction field–polarizable continuum model (SCRF- PCM) method of Tomasi and co-workers,41 as well as the Langevin dipoles (LD) model of Florian and Warshel,42 were employed. For the semiempirical MO methods AM1 and PM3, the AM1 SM1 and PM3 SM3 hydration models43 were used. Merz–Kollman partial atomic charges were obtained by the restrained electrostatic potential fit (RESP) procedure provided in Gaussian 09 suite of programs37 on the electrostatic potentials obtained by ab initio and DFT methods and served as an input for the LD model implemented in the ChemSol program.44 The calculations for AM1 SM1 and PM3 SM3 hydration models were performed by the AMSOL 5.4.1. program of Truhlar and colleagues.43
In order to evaluate the validity of our computational results, a comparison with the experimental free energy of BPL alkylation was made. Manso and colleagues40 investigated the alkylation reaction of BPL with 4-(p-nitrobenzyl)pyridine (NBP) that is used as a substitute for guanine nucleobase. From the reaction rate constant based on the Eyring transition state theory, they calculated the activation parameters for NBP alkylation by BPL. The experimental estimate of the corresponding activation free energy ΔG⧧ amounts to 20.8 ± 0.5 kcal/mol.
3. Results and Discussion
3.1. Alkylation of Guanine
Several in vitro studies have shown that among nucleobases guanine most readily forms adducts with BPL.19−21,24,25 The calculated activation energies for the alkylation of methylguanine with BPL in the gas phase and solvated with the SCRF, LD, and AMSOL models, lowest vibrational frequencies of reactant states, imaginary vibrational frequencies of transition states, and corresponding distances between the reactive centers are collected in Table 1.
Table 1. Activation Free Energies for the Formation of the Main 7-(2-Carboxyethyl)methylguanine Adduct Calculated with Different Methodsa.
| method/basis set | ΔGgas⧧ (kcal/mol)b | ΔΔGhydrSCRF (kcal/mol)c | ΔGSCRF⧧ (kcal/mol)d | ΔGLD⧧ (kcal/mol)e | ΔGAMSOL⧧ (kcal/mol)f | ωTS (I cm–1)g | ωR (cm–1)h | dTS (Å)i | dR (Å)j |
|---|---|---|---|---|---|---|---|---|---|
| AM1 | 51.86 | 34.57 | 772 | 7 | 1.88 | 4.99 | |||
| PM3 | 52.17 | 19.38 | 793 | 6 | 1.84 | 4.34 | |||
| HF/6-31 G(d) | 45.10 | –19.8 | 25.30 | 15.56 | 551 | 19 | 1.99 | 3.42 | |
| HF/6-31+ G(d,p) | 42.72 | –20.66 | 22.06 | 11.83 | 533 | 17 | 2.02 | 3.50 | |
| HF/6-311++ G(d,p) | 43.31 | –20.86 | 22.45 | 12.06 | 542 | 16 | 2.03 | 3.50 | |
| B3LYP/6-31 G(d) | 37.07 | –18.53 | 18.54 | 8.90 | 469 | 20 | 1.90 | 3.34 | |
| B3LYP/6-31+ G(d,p) | 33.53 | –19.21 | 14.32 | 1.75 | 470 | 7 | 1.95 | 3.45 | |
| B3LYP/6-311++ G(d,p) | 33.32 | –18.00 | 14.33 | 2.04 | 463 | 10 | 1.95 | 3.44 | |
| MP2/6-31 G(d) | 44.12 | –24.31 | 19.81 | 16.78 | 634 | 10 | 1.85 | 3.46 | |
| MP2/6-31+ G(d,p) | 42.08 | –26.59 | 15.49 | 11.69 | 632 | 13 | 2.03 | 3.44 | |
| MP2/6-311++G(d,p) | 42.81 | –25.21 | 21.96 | 11.11 | 652 | 10 | 1.88 | 3.44 | |
| M06-2X/6-31 G(d) | 42.63 | –16.98 | 25.65 | 14.05 | 625 | 26 | 1.88 | 3.42 | |
| M06-2X/6-31+ G(d,p) | 39.98 | –19.62 | 20.36 | 8.26 | 631 | 19 | 1.91 | 3.15 | |
| M06-2X/6-311++ G(d,p) | 40.14 | –19.39 | 20.75 | 8.34 | 638 | 20 | 1.91 | 3.17 | |
| mPWPW91/6-31 G(d) | 40.53 | –18.52 | 22.01 | 12.03 | 609 | 19 | 1.89 | 3.27 | |
| mPWPW91/6-31+G(d,p) | 38.06 | –19.07 | 18.99 | 6.75 | 586 | 16 | 1.92 | 3.33 | |
| mPWPW91/6-311++ G(d,p) | 38.20 | –18.98 | 19.22 | 7.20 | 587 | 15 | 1.92 | 3.32 |
Experimental value for 4-(p-nitrobenzyl)pyridine alkylation by BPL: ΔG⧧ = 20.8 ± 0.5 kcal/mol.
Gas-phase activation energy.
Hydration free energy of the transition state minus hydration free energy of the reactant state obtained by the SCRF-PCM method.
Activation free energy obtained by the SCRF-PCM method.
Activation free energy obtained by the LD method.
Activation free energy obtained by the AM1-SM1 and PM3-SM3 methods.
The imaginary frequency corresponding to the transition state.
The lowest vibrational frequency corresponding to the reactant state.
The distance between the reacting N7 atom of methylguanine and the BPL β carbon atom in the transition state structure.
The distance between the reacting N7 atom of methylguanine and the BPL β carbon atom in the reactant state structure.
From the acquired gas-phase activation energies presented in Table 1, we can conclude that the convergence in terms of basis set size was reached at all levels of theory. Variation among the acquired reactant and transition state structures was relatively low as can be seen through highly similar distances between the reactive centers. Larger values of dR compared to dTS are consistent with the much weaker intermolecular forces in the reactant state and with the consequently much shallower potential hypersurface. Activation barrier in the gas-phase ranges from 43 kcal/mol using the Hartree–Fock theory level and lowered to around 38 kcal/mol using DFT levels, with the exception of B3LYP method that provided as low as 33 kcal/mol in conjunction with the flexible basis set 6-311++G(d,p) level. On the contrary, the results of semiempirical methods AM1 and PM3 yielded the highest activation energies of about 52 kcal/mol.
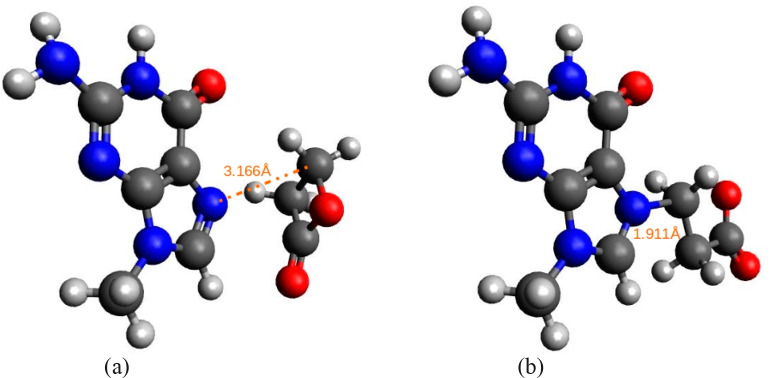
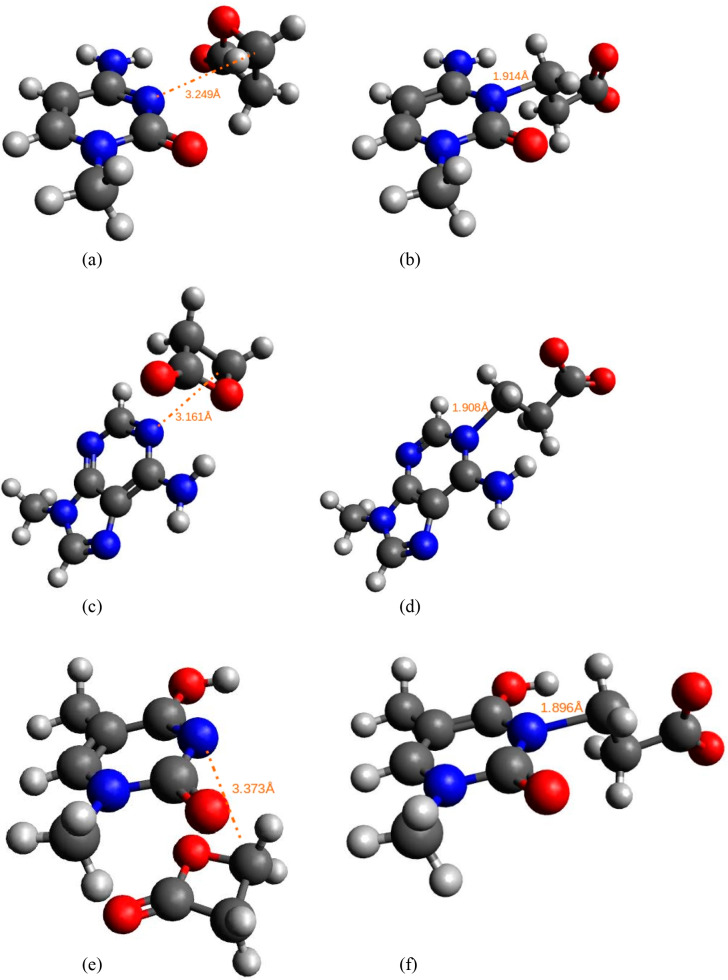
As biological processes take place in a solution and the experiment chosen for the evaluation of our results also proceeds in a solution, we performed another series of calculations where the solvation effects were incorporated. For all the solvation models and all the theory levels, ΔΔGhydr is negative, meaning that the transition state is better solvated than the reactant state. Consequently, solvent lowers the activation barrier and thus accelerates the reaction in accordance with the zwitterionic-like nature of the transition state and only polar nature of the reactant state. According to the SCRF-PCM model, activation free energies in water medium are around 23 kcal/mol using the Hartree–Fock theory level and lower to around 20 kcal/mol using DFT levels. Similarly, as in the gas phase, the result of the B3LYP method is the lowest, yielding approximately 14 kcal/mol in conjunction with the flexible 6-311++G(d,p) basis set. The result acquired by M06-2X method is identical to the experimentally determined one. Therefore, for all future comparisons we have focused on the results obtained with this theory level. Hartree–Fock and MP2 theory levels slightly overestimate the activation barrier, whereas DFT levels slightly underestimate. Our results are in accordance with the tests performed by the developers of the M06-class (and, earlier, M05-class) functionals as they recommend M06-2X for the main group thermochemistry and kinetics.45 The structures of the reactant and the transition state for the alkylation of guanine with BPL are depicted in Figure 1.
Figure 1.
Structure of (a) the reactant state and (b) the transition state for the methylguanine alkylation by BPL as predicted using the M06-2X/6-311++G(d,p) level of theory. Carbon atoms are depicted in gray, oxygen atoms in red, nitrogen atoms in blue, and hydrogen atoms in white color. The orange dotted lines and the values represent the distances between reactive centers (dR and dTS) in angstroms.
The values of activation free energies obtained by the Langevine dipoles implicit solvation model are significantly underestimated for about 10 kcal/mol compared to those provided by the SCRF-PCM model, regardless of the theory level. Solvation model AMSOL for semiempirical methods resumed in Table 1 provided two very different results. The AM1 method gave a significantly overestimated activation free energy (more than 10 kcal/mol higher compared to the ones obtained by the SCRF-PCM method), however, the PM3 method gave a result very similar to those obtained with DFT methods using the SCRF-PCM model. Its value fits very well to the experimentally determined one.
3.2. Alkylation of other DNA Bases Compared to Guanine
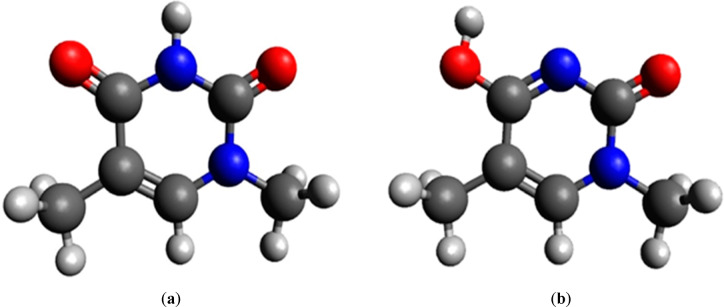
Previous in vitro studies have isolated adducts of BPL with all DNA bases. Therefore, we investigated the reactions of BPL with all nucleobases (adenine, cytosine, guanine, and thymine) in their most abundant tautomeric forms and with the methyl group bound to the site where in the DNA deoxyribose is placed. For simulations of thymine reactions with BPL we primarily applied the most abundant lactam tautomeric form presented as (a) in Figure 2. However, this form posed a sterical hindrance for the BPL alkylation due to the presence of hydrogen at the N3 atom, therefore, we used another lactim tautomer presented as (b) in Figure 2. Later, the difference in free energy between both tautomeric forms was incorporated, so the results provided in Table 2 correspond to the most abundant lactam tautomeric form of (methyl)thymine. In the Supporting Information (SI) in Table S10 the activation free energies for BPL alkylation of both thymine tautomeric forms are provided. The comparison of the results presented in Table 2 with the yield of nucleobase adducts isolated from the in vitro reactions is presented later in this subsection.
Figure 2.
Structure of (a) the most abundant lactam tautomeric form of (methyl)thymine and (b) the lactim tautomeric form used in our simulations. Carbon atoms are depicted in gray, oxygen atoms in red, nitrogen atoms in blue, and hydrogen atoms in white color.
Table 2. Comparison of SCRF-PCM and LD Activation Free Energies for BPL Alkylation of Methylated Nucleobases.
| guanined (kcal/mol) | adenine(kcal/mol) | cytosine(kcal/mol) | thymine(kcal/mol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| method/basis set | ΔGgas⧧a | ΔGSCRF⧧b | ΔGLD⧧c | ΔGgas⧧a | ΔGSCRF⧧b | ΔGLD⧧c | ΔGgas⧧a | ΔGSCRF⧧b | ΔGLD⧧c | ΔGgas⧧a | ΔGSCRF⧧b | ΔGLD⧧c |
| AM1 | 51.86 | 51.68 | 52.98 | 66.96 | ||||||||
| PM3 | 52.17 | 54.29 | 56.03 | 94.60 | ||||||||
| HF/6-31 G(d) | 45.10 | 25.30 | 15.56 | 47.14 | 29.62 | 22.71 | 49.18 | 27.74 | 19.29 | 71.71 | 47.52 | 41.51 |
| HF/6-31+ G(d,p) | 42.72 | 22.06 | 11.83 | 44.72 | 26.42 | 19.16 | 46.84 | 24.53 | 13.14 | 66.91 | 41.82 | 34.01 |
| HF/6-311++ G(d,p) | 43.31 | 22.45 | 12.06 | 45.12 | 26.98 | 19.54 | 47.11 | 25.15 | 13.87 | 67.16 | 42.61 | 34.25 |
| MP2/6-31 G(d) | 44.12 | 19.81 | 16.78 | 46.75 | 25.68 | 21.97 | 49.74 | 24.47 | 17.09 | 72.23 | 42.32 | 40.25 |
| MP2/6-31+ G(d,p) | 42.08 | 15.49 | 11.69 | 44.10 | 21.53 | 17.58 | 47.29 | 20.08 | 12.36 | 66.98 | 34.91 | 33.09 |
| MP2/6-311++ G(d,p) | 42.81 | 21.96 | 10.69 | 44.23 | 23.16 | 18.29 | 47.88 | 21.76 | 13.73 | 67.61 | 35.84 | 34.69 |
| M06-2X/6-31 G(d) | 42.63 | 25.65 | 14.05 | 42.26 | 26.98 | 19.26 | 45.74 | 26.76 | 17.46 | 64.34 | 43.51 | 37.05 |
| M06-2X/6-31+ G(d,p) | 39.98 | 20.36 | 8.26 | 39.28 | 23.54 | 15.24 | 42.94 | 23.18 | 12.37 | 59.54 | 37.96 | 29.66 |
| M06-2X/6-311++ G(d,p) | 40.14 | 20.75 | 8.34 | 39.08 | 23.33 | 15.01 | 42.58 | 23.08 | 12.53 | 59.58 | 38.35 | 28.62 |
| mPWPW91/6-31 G(d) | 40.53 | 22.01 | 12.03 | 41.10 | 25.60 | 18.07 | 44.15 | 24.69 | 15.25 | 64.52 | 44.77 | 36.33 |
| mPWPW91/6-31+ G(d,p) | 38.06 | 18.99 | 6.75 | 38.43 | 22.53 | 13.71 | 41.65 | 21.44 | 10.49 | 59.89 | 39.33 | 29.05 |
| mPWPW91/6-311++ G(d,p) | 38.20 | 19.22 | 7.20 | 38.27 | 22.53 | 14.00 | 41.37 | 21.43 | 10.31 | 59.76 | 39.61 | 27.97 |
The gas-phase activation energy of the alkylation reaction of a specific methylated nucleobase with β-propiolactone.
Activation free energies of the alkylation reaction between a specific methylated nucleobase and β-propiolactone obtained by the SCRF-PCM method.
Activation free energies of the alkylation reaction between a specific methylated nucleobase and β-propiolactone obtained by the LD method.
Experimental value: ΔG⧧ = 20.8 ± 0.5 kcal/mol.
According to the gas-phase activation energies, guanine seems to be the most reactive base toward BPL with the exception of DFT method M06-2X and the semiempirical method AM1 that give preference to adenine. The second in the overall reactivity seems to be adenine, followed by cytosine. Thymine seems to be by far the least reactive base with its activation energies higher for about 20 kcal/mol compared to other bases. However, the differences between other nucleobases range only from 1 to 4 kcal/mol.
Activation free energies of BPL alkylation of methylated nucleobases with incorporated solvent effects suggest that the most reactive nucleobase represents guanine as its activation barriers are the lowest regardless of the method or solvation model. Even though gas phase activation barriers of adenine are lower than those of cytosine, the ones with incorporated solvent effects show exactly the opposite. Therefore, we believe that at biological conditions cytosine is more reactive toward BPL than adenine. Thymine shows the highest reaction barriers up to 38 kcal/mol at the most accurate M06-2X/6-311++G(d,p) SCRF-PCM level of theory. Hence, the reaction of BPL with thymine is very unlikely or it could happen only to a very low extent.
Consistently with our results, Mate et al.19 discovered that when the BPL-reacted DNA was hydrolyzed for shorter time (1 h) and at physiological temperature (37 °C), the ratio of alkylated purines 7-CEG to l-CEA was 3:1, however, when the hydrolyzation lasted longer (16 h) at a higher temperature (100 °C) the ratio was reversed pointing to the instability of the 7-CEG adduct.
Previous in vitro studies were unable to detect alkylation adducts of BPL with thymine, confirming our results that the reactivity of thymine with BPL is very low. Segal et al.,20 however, successfully isolated the thymine adduct, although, the yield was considerably low from 100A units of BPL-reacted DNA only 3A units of adduct 3-CET were isolated. A year later it was reported21 that the reaction of BPL with calf thymus DNA yielded adducts with all DNA bases. Two methods were employed;21 by the first method the alkylation reaction was conducted in phosphate buffer at 0–5 °C and pH 7.5 and provided 0.23, 1.00, 0.39, and 0.41 molar ratios of 1-CEA, 7-CEG, 3-CET, and 3-CEC isolated from BPL-reacted DNA following perchloric acid hydrolysis. By the second method, the alkylation reaction was conducted in the water at 37 °C and pH maintained at 7.0–7.5 by adding NaOH, the isolated molar ratios were 0.10, 1.00, 0.29, and 0.28 respectively. Surprisingly high thymine adduct yields could possibly be attributed to the reaction of some other thymine tautomer as opposed to the one used in our study. With the mentioned exception of thymine, all other in vitro results are consistent with the findings of our study. This confirms that guanine represents the most reactive base toward the BPL, followed by cytosine and adenine. On the basis of our calculations, thymine is the least reactive nucleobase with BPL, at least the examined lactim tautomeric form of thymine. The precise tautomeric form of thymine that gets alkylated at biological conditions remains to be investigated in future studies. In Figure 3, the reactant and transition state structures for the alkylation of cytosine, adenine, and thymine with BPL obtained by the M06-2X method and the 6-311++G(d,p) basis set, are presented.
Figure 3.
Structure of (a) the reactant state and (b) the transition state for the nucleophilic attack (alkylation) of BPL by the N3 atom of methylcytosine as predicted at the M06-2X/6-311++G(d,p) level of theory. The structure of (c) the reactant state and (d) the transition state for the nucleophilic attack (alkylation) of BPL by the N1 atom of methyladenine as predicted at the M06-2X/6-311++G(d,p) level of theory. The structure of (e) the reactant state and (f) the transition state for the nucleophilic attack (alkylation) of BPL by the N3 atom of methylthymine as predicted at the M06-2X/6-311++G(d,p) level of theory. Carbon atoms are depicted in gray, oxygen atoms in red, nitrogen atoms in blue, and hydrogen atoms in white color. The orange dotted lines and the values represent the distances between reactive centers (dR and dTS) in angstroms.
3.3. Alkylation vs Acylation Reactions
It was observed that only those lactones reactive enough to undergo hydrolysis in the neutral medium (like BPL) are also able to react with biological nucleophiles (nucleobases or glutathion) in vivo.40,46,47 Of great importance is also the knowledge through which mechanism (alkylation or acylation) those reactions occur. Considering that the amino bond is harder to cleave than the β-ketoamide bond, the accepted notion is that lactones which follow the alkylation reaction (β-lactones such as BPL, BBL) afford stable DNA adducts and therefore represent effective carcinogens.46,47 On the contrary, the lactones undergoing acylation reaction (β-lactone diketene) that are readily hydrolyzed cannot act as strong carcinogens.40,46,47 In the following Table 3, the activation free energies of alkylation and acylation reactions of methylated nucleobases with BPL are presented.
Table 3. Activation Free Energies Obtained Using the SCRF-PCM Solvation Model for the Alkylation and Acylation Reactions of Methylated Nucleobases with BPL.
| guanine[kcal/mol] |
adenine[kcal/mol] |
cytosine[kcal/mol] |
thymine[kcal/mol] |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| alkylation |
acylation |
alkylation |
acylation |
alkylation |
acylation |
alkylation |
acylation |
|||||||||
| method/basis set | ΔGgas⧧a | ΔGSCRF⧧b | ΔGgas⧧c | ΔGSCRF⧧d | ΔGgas⧧a | ΔGSCRF⧧b | ΔGgas⧧c | ΔGSCRF⧧d | ΔGgas⧧a | ΔGSCRF⧧b | ΔGgas⧧c | ΔGSCRF⧧d | ΔGgas⧧a | ΔGSCRF⧧b | ΔGgas⧧c | ΔGSCRF⧧d |
| AM1 | 51.86 | 46.16 | 51.68 | 41.41 | 52.98 | 39.92 | 66.96 | 75.32 | ||||||||
| PM3 | 52.17 | 36.24 | 54.29 | 35.91 | 56.03 | 35.68 | 94.60 | 96.20 | ||||||||
| HF/6-31 G(d) | 45.10 | 25.30 | 59.20 | 48.20 | 47.14 | 29.62 | 53.77 | 49.38 | 49.18 | 27.74 | 55.28 | 48.75 | 71.71 | 47.52 | 104.01 | 87.60 |
| HF/6-31+ G(d,p) | 42.72 | 22.06 | 58.24 | 46.37 | 44.72 | 26.42 | 52.99 | 48.45 | 46.84 | 24.53 | 54.85 | 48.03 | 66.91 | 41.82 | 100.53 | 82.47 |
| HF/6-311++ G(d,p) | 43.31 | 22.45 | 58.65 | 47.11 | 45.12 | 26.98 | 53.23 | 48.66 | 47.11 | 25.15 | 54.88 | 48.60 | 67.16 | 42.61 | 100.17 | 82.46 |
| MP2/6-31 G(d) | 44.12 | 19.81 | 57.67 | 38.20 | 46.75 | 25.68 | 52.54 | 28.88 | 49.74 | 24.47 | 57.25 | 28.30 | 72.23 | 42.32 | 108.61 | 69.14 |
| MP2/6-31+ G(d,p) | 42.08 | 15.49 | 55.40 | 33.30 | 44.10 | 21.53 | 50.96 | 26.91 | 47.29 | 20.08 | 55.73 | 25.63 | 66.98 | 34.91 | 104.82 | 62.22 |
| MP2/6-311++ G(d,p) | 42.81 | 21.96 | 54.62 | 38.83 | 44.23 | 23.16 | 51.84 | 27.47 | 47.88 | 21.76 | 56.35 | 25.88 | 67.61 | 35.84 | 104.66 | 61.78 |
| M06-2X/6-31 G(d) | 42.63 | 25.65 | 48.29 | 38.86 | 42.26 | 26.98 | 32.79 | 29.57 | 45.74 | 26.76 | 34.56 | 29.97 | 64.34 | 43.51 | 80.98 | 70.64 |
| M06-2X/6-31+ G(d,p) | 39.98 | 20.36 | 46.86 | 36.17 | 39.28 | 23.54 | 31.60 | 28.05 | 42.94 | 23.18 | 33.75 | 28.65 | 59.54 | 37.96 | 78.45 | 67.24 |
| M06-2X/6-311++ G(d,p) | 40.14 | 20.75 | 47.32 | 36.44 | 39.08 | 23.33 | 32.30 | 28.69 | 42.58 | 23.08 | 33.91 | 29.46 | 59.58 | 38.34 | 78.64 | 67.19 |
| mPWPW91 6-31 G(d) | 40.53 | 22.01 | 48.08 | 38.89 | 41.10 | 25.60 | 33.93 | 30.39 | 44.15 | 24.69 | 37.47 | 31.56 | 64.52 | 44.77 | 85.50 | 76.22 |
| mPWPW91/6-31+ G(d,p) | 38.06 | 18.99 | 46.67 | 36.50 | 38.43 | 22.53 | 32.75 | 29.25 | 41.65 | 21.44 | 36.42 | 30.20 | 59.89 | 39.33 | 82.85 | 72.73 |
| mPWPW91/6-311++ G(d,p) | 38.20 | 19.22 | 46.67 | 36.36 | 38.27 | 22.53 | 33.39 | 29.88 | 41.37 | 21.43 | 36.45 | 30.51 | 59.76 | 39.61 | 82.62 | 72.30 |
Gas-phase activation energy of alkylation.
Activation free energy of alkylation of specific methylated nucleobase with BPL obtained by the SCRF-PCM method.
Gas-phase activation energy of acylation.
Activation free energy of acylation of specific methylated nucleobase with BPL obtained by the SCRF-PCM method.
Details of obtained activation barriers for the alkylation and acylation reactions of nucleic bases with BPL in the gas phase and solvated with the SCRF-PCM, LD, and AMSOL models, lowest vibrational frequencies of reactant states, imaginary vibrational frequencies of transition states, and corresponding distances between the reactive centers like in the case of guanine alkylation are provided in the SI (Tables S1–S7).
The gas-phase activation energies for guanine and thymine undoubtedly show that alkylation represents the more favorable reaction and is thus more plausible than acylation. In the case of adenine and cytosine, various methods give different preferences toward acylation and alkylation reactions. HF and MP2 methods suggest that alkylation is more favorable, however, all the DFT and semiempirical methods predict exactly the contrary.
However, the acquired activation free energies with incorporated solvation effects confirm that alkylation is indeed energetically more favorable reaction than acylation for all nucleobases. Although, activation free energies for the acylation reactions are low enough to occur naturally as well.
Visual inspection of the transition state structures for both reactions (alkylations and acylations), we observed a trend (for all theory levels except MP2) that β-propiolactone prefers to attack the nucleobases perpendicular to the plane of the specific nucleobase, with the notable exception of guanine alkylation, where both reactive species (in the transition state) lie in the same plane. This observed difference may well be the reason why the alkylation reaction of BPL with guanine possesses the lowest activation barrier among all simulated reactions regardless of the theory level. The structure of guanine, of course, enables such an attack whereas in all other nucleobases large neighboring groups provide steric hindrance through the repulsion between the electron clouds. In the DNA environment guanine’s transition state structure may be additionally favored while the steric effects induced by the presence of the stacked nucleobases hinder perpendicular attacks of BPL on remaining nucleobases. This may represent an additional reason for guanine to form the preferred adduct.
Consistently with a plethora of experimental studies, our results show that BPL represents an effective chemical carcinogen as it is very reactive toward all nucleobases especially through the alkylation mechanism that provides stable DNA adducts. Consequently, we decided to investigate the potential anticarcinogenic mechanisms as well by reacting BPL with glutathione—the most abundant natural scavenger of chemical carcinogens in human cells.
3.4. Alkylation vs Acylation of Glutathione Compared to Guanine
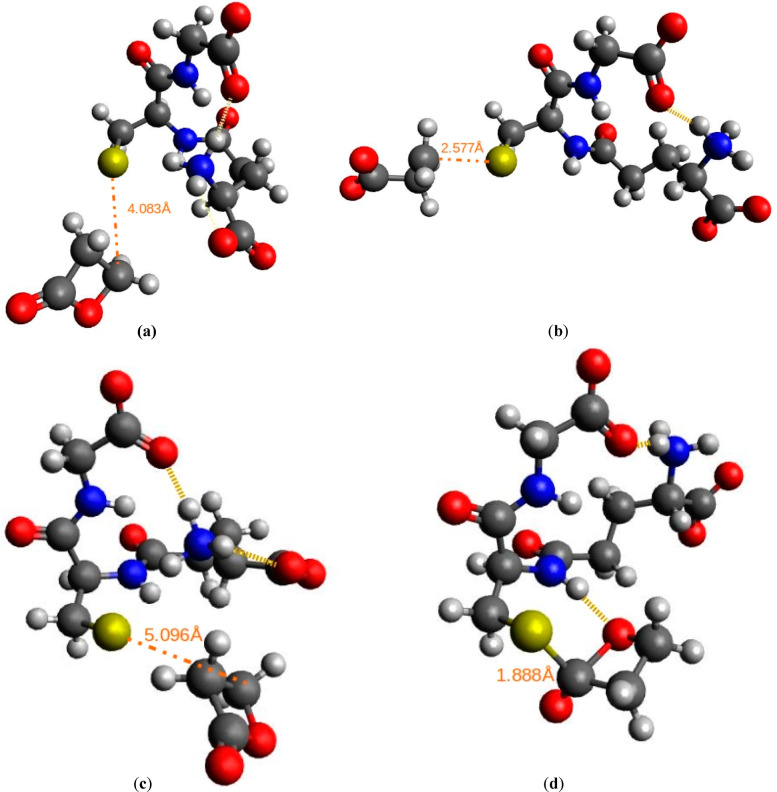
From Figure 4, one can see that in the transition state of alkylation reaction glutathione rearranges circularly as a result of hydrogen bond formation. For acylation reaction, one can observe that in the transition state amino acid residues and the two reactive structures are also closer than in the reactant state due to a number of hydrogen bonds formed. Although, this may be an artifact due to the absence of explicit water molecules in our calculations. In a true aqueous solution, there would be alternatives to form hydrogen bonds. Such artificially increased compactness when comparing the reactant and the transition states could then render the transition state free energies too favorable. In Table 4 the activation free energies of alkylation and acylation reactions of methylated guanine and glutathione with BPL are presented.
Figure 4.
Structure of (a) the reactant state and (b) the transition state for the nucleophilic attack of the sulfur atom of deprotonated glutathione on BPL through alkylation reaction as predicted at the HF/6-311++G(d,p) level of theory. The structure of (c) the reactant state and (d) the transition state for the nucleophilic attack of the sulfur atom of deprotonated glutathione on BPL through acylation reaction as predicted at the HF/6-311++G(d,p) level of theory. Carbon atoms are depicted in gray, oxygen atoms in red, nitrogen atoms in blue, sulfur atoms in yellow, and hydrogen atoms in white color. The orange dotted lines and the values represent the distances between reactive centers (dR and dTS) in angstroms. Yellow horizontal lines represent hydrogen bonds.
Table 4. Activation Energies of Alkylation and Acylation Reactions of BPL with Glutathione and Methylguanine.
| glutathione (kcal/mol) |
guanine (kcal/mol) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| alkylation |
acylation |
alkylation |
acylation |
|||||||||
| method/basis set | ΔGgas⧧a | ΔGSCRF⧧b | ΔGLD⧧c | ΔGgas⧧d | ΔGSCRF⧧e | ΔGLD⧧f | ΔGgas⧧a | ΔGSCRF⧧b | ΔGLD⧧c | ΔGgas⧧d | ΔGSCRF⧧e | ΔGLD⧧f |
| AM1 | 5.37 | 11.96 | 51.86 | 46.16 | ||||||||
| PM3 | 16.97 | 16.94 | 52.17 | 36.24 | ||||||||
| HF/6-31 G(d) | 22.55 | 22.20 | 22.78 | 37.44 | 32.88 | 29.86 | 45.10 | 25.30 | 15.56 | 59.20 | 48.20 | 41.57 |
| HF/6-31+ G(d,p) | 20.37 | 18.93 | 19.51 | 35.21 | 29.86 | 27.07 | 42.72 | 22.06 | 11.83 | 58.24 | 46.37 | 38.94 |
| HF/6-311++ G(d,p) | 20.85 | 19.24 | 18.78 | 35.14 | 29.91 | 25.90 | 43.31 | 22.45 | 12.06 | 58.65 | 47.11 | 39.96 |
| MP2/6-31 G(d) | 23.16 | 23.33 | 25.80 | 36.30 | 13.97 | 31.66 | 44.12 | 19.81 | 16.78 | 57.67 | 38.20 | 40.37 |
| MP2/6-31+ G(d,p) | 22.55 | 18.91 | 19.75 | 35.37 | 7.66 | 24.96 | 42.08 | 15.49 | 11.69 | 55.40 | 33.30 | 35.04 |
| MP2/6-311++ G(d,p)g | 22.00 | 24.01 | 22.84 | 35.16 | 9.82 | 24.91 | 42.81 | 17.60 | 10.69 | 54.62 | 38.83 | 34.00 |
| M06-2X/6-31 G(d) | 23.57 | 25.72 | 27.63 | 25.97 | 20.98 | 19.81 | 42.63 | 25.65 | 14.05 | 48.29 | 38.86 | 34.86 |
| M06-2X/6-31+ G(d,p) | 20.62 | 21.62 | 30.00 | 22.99 | 16.33 | 22.99 | 39.98 | 20.36 | 8.26 | 46.86 | 36.17 | 29.84 |
| M06-2X/6-311++ G(d,p) | 20.37 | 21.74 | 22.79 | 22.92 | 16.36 | 15.26 | 40.14 | 20.75 | 8.34 | 47.32 | 36.44 | 29.95 |
| mPWPW91/6-31 G(d) | 20.44 | 21.25 | 22.54 | 25.13 | 20.20 | 17.35 | 40.53 | 22.01 | 12.03 | 48.08 | 38.89 | 34.28 |
| mPWPW91/6-31+ G(d,p) | 18.16 | 17.84 | 18.51 | 9.72 | 8.58 | 7.86 | 38.06 | 18.99 | 6.75 | 46.67 | 36.50 | 30.02 |
| mPWPW91/6-311++ G(d,p) | 18.38 | 17.94 | 19.80 | 9.91 | 8.86 | 8.05 | 38.20 | 19.22 | 7.20 | 46.67 | 36.36 | 29.81 |
Gas-phase activation energy of the alkylation reaction of glutathione or guanine with β-propiolactone.
Activation free energy of alkylation obtained by the SCRF-PCM method.
Activation free energy of alkylation obtained by the LD method.
Gas-phase activation energy of the acylation reaction of glutathione or guanine with β-propiolactone.
Activation free energy of acylation obtained by the SCRF-PCM method.
Activation free energy of acylation obtained by the LD method.
Activation free energy of alkylation obtained by the SCRF-PCM method with single point calculation from MP2 6-31+G(d,p) structure.
Details of obtained activation barriers for the glutathione alkylation and acylation with BPL in the gas phase and solvated with the SCRF-PCM, LD, and AMSOL models, lowest vibrational frequencies of reactant states, imaginary vibrational frequencies of transition states, and corresponding distances between the reactive centers like in the case of guanine alkylation are provided in the SI (Tables S8 and S9).
According to the gas-phase activation energies of the reactions of glutathione with BPL, the alkylation reaction is more favorable and therefore more plausible than acylation reaction. Comparison between the reaction of glutathione with BPL and methylguanine with BPL reveals that glutathione is significantly more reactive toward BPL than guanine.
On the contrary to the results of the reactions in gas-phase (vacuum), taking into account solvent effects makes our results consistent with the Dijkstra’s in vitro study.27 Alkylation and acylation reactions between BPL and glutathione seem to be equally probable. As Hartree–Fock and B3LYP favor alkylation, while other DFT methods, including M06-2X that best fits the experimental results, favor acylation. Therefore, the assumption that all carcinogenic lactones (like BPL) exclusively alkylate nucleophiles (like glutathione) and that all inactive lactones exclusively acylate nucleophiles is refuted once more.40,46
Comparison of activation free energies of glutathione’s and guanine’s alkylation and acylation reactions with BPL suggest that glutathione may represent an efficient scavenger of BPL as both reactions have lower activation barriers, with the notable exception being the results of MP2 and M06-2X methods for the alkylation reaction which give somewhat higher values for glutathione than guanine. However, the acylation reaction of glutathione is still energetically more favorable by these methods than the alkylation of guanine. All in all, glutathione may react with BPL through either of the alkylation or acylation reactions before it reaches the genetic material. Therefore, glutathione present in the organism can provide protection to the DNA and thus prevent BPL’s genotoxicity, mutagenicity, and possibly carcinogenicity.
4. Conclusions
To the best of our knowledge this is the first study where not only mechanisms of alkylation were revealed but acylation reactions as well under identical conditions facilitating a direct comparison between the two. Thereby we revealed several transition state structures to provide additional insight into molecular mechanisms of BPL’s carcinogenicity. In addition to reactions of BPL with nucleobases the same types of reactions with a possible natural scavenger molecule glutathione were simulated.
In accordance with several in vitro studies our results confirm that guanine represents the most reactive nucleobase toward BPL, followed by cytosine and adenine. Our calculations conclusively regard lactim tautomeric form of thymine as the least reactive nucleobase, in agreement with a majority of in vitro studies, where the alkylation adducts of BPL with thymine were only minor or could even not be detected. However, the study of Segal21 reported notably higher thymine adduct yields, leading to the presumption that some other thymine tautomer would have reacted with BPL instead of the lactim form.
The acquired activation free energies with incorporated solvation effects suggest that alkylation is indeed an energetically more favorable reaction than acylation and thus more probable for all nucleobases. However, the acylation reactions possess activation barriers low enough to occur naturally as well.
The geometry of guanine enables β-propiolactone to attack in the same plane, as opposed to other nucleobases where sterical hindrance is exerted by neighboring groups which force BPL to attack perpendicular to the plane of a nucleobase. This may be the main reason why guanine exhibits the lowest activation free energies among all nucleobases regardless of the theory level chosen.
In agreement with Dijkstra’s in vitro study,27 our results with incorporated solvent effects confirm that alkylation and acylation reactions between BPL and glutathione seem to be equally plausible. Moreover, the activation barriers of glutathione’s reactions with BPL regardless of the mechanism are lower than the ones for guanine alkylation reaction with BPL suggesting that glutathione may represent an efficient scavenger of BPL as it can react with BPL before it damages the genetic material. Glutathione proved to be reactive enough for the role of natural scavenger of BPL. However, to represent a good natural scavenger we must consider other aspects as well. Presence of glutathione in the nucleus cannot be a hurdle as it represents the most abundant nonprotein thiol in mammalian cells,48 present at millimolar concentrations (up to 10 mM) in most cell types.30,49 Its concentration, therefore, not only reaches that of nucleobases (averaging to 0,2 until 5 mM)50 but even exceeds it. As glutathione is synthesized in the cytosol,49 it could even scavenge BPL before it diffuses into the nucleus. However, glutathione concentrates in the nucleus in the early phases of cell growth and redistributes uniformly between nucleus and cytoplasm only when cells reach confluence.48 To conclude, in all aspects glutathione represents an excellent candidate for a natural scavenger of BPL. Glutathione present in the organism, therefore, possesses the ability to protect the genetic material and hence prevent BPL’s genotoxicity, mutagenicity, and possibly even carcinogenicity.
The high reactivity of BPL toward all nucleobases revealed by our study, in accordance with numerous in vitro studies, especially through the alkylation mechanism that on top provides stable DNA adducts, confirms that BPL represents an effective chemical carcinogen. BPL is a monoalkylating agent meaning that when alkylation reaction occurs its reactivity decreases to a level where it is no longer carcinogenic. As glutathione represents not only the crucial low molecular weight redox buffer that shields nuclear processes against oxidative stress but also a flexible regulator of genetic and epigenetic functions,51 it could in certain cellular conditions as well happen that the concentration of free glutathione would be too low to sufficiently protect the genetic material. It would, therefore, be sensible to explore using identical computational methodology also other potential natural scavengers for example from the family of polyphenols52−57 that could provide additional protection from BPL and related carcinogenic agents. This could lead to the discovery of novel anticancer drugs that could effectively prevent carcinogenesis.
Glossary
Abbreviations
- l-CEA
1-(2-carboxyethyl)-adenine
- 3-CEC
3-(2-carboxyethyl)-cytosine
- 3-CET
3-(2-carboxyethyl)-thymine
- 7-CEG
7-(2-carboxyethyl)-guanine
- BPL
β-propiolactone
- BBL
β-butyrolactone
- DFT
density functional theory
- GSH
glutathione
- HF
Hartree–Fock
- HSDB
Hazardous Substances Data Bank
- IARC
International Agency for Research on Cancer
- LD
Langevin dipoles
- MO
molecular orbitals
- MP2
Møller–Plesset perturbation theory of the second order
- NBP
4-(p-nitrobenzyl)pyridine
- SCRF-PCM
self-consistent reaction field–polarizable continuum model
- SN2
nucleophilic substitution 2.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.chemrestox.9b00389.
Tables with the calculated activation parameters in the gas phase and solvated with the SCRF-PCM, LD, and AMSOL models, lowest vibrational frequencies of reactant states, imaginary vibrational frequencies of transition states, and corresponding distances between the reactive centers for alkylation and acylation reactions of all combinations of nucleic bases with BPL, reactive species, quantum mechanical methods, and basis sets and for the acylation and alkylation reactions of glutathione with BPL (Tables S1–S9); comparison of activation free energies for BPL alkylation of the two thymine tautomeric forms (Table S10); and tables with the absolute energies and Cartesian atomic coordinates for alkylation and acylation reactions of nucleobases and glutathione with BPL at the M06-2X/6-311++G(d,p) level of theory (Tables S11–S20) (PDF)
Financial support from the Slovenian Research Agency through project grants J1–6736 and J1–5448 as well as from the Slovenian Ministry of Education, Science and Sports through program grants F4F and AB FREE is gratefully acknowledged.
The authors declare no competing financial interest.
Supplementary Material
References
- NTP (National Toxicology Program) .β-Propiolactone CAS No. 57–57–8, in Report on Carcinogens, 13th ed.; US Department of Health and Human Services, Public Health Service: Research Triangle Park, NC, U.S, Available online: http://ntp.niehs.nih.gov/go/roc13 , 2014. (accessed on 14th of June 2016). [Google Scholar]
- IARC . β-Propiolactone, inSome Aromatic Amines, Hydrazine and Related Substances, N-Nitroso Compounds and Miscellaneous Alkylating Agents, IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans; International Agency for Research on Cancer: Lyon, France, 1974, Vol. 4., pp 259–269. [Google Scholar]
- IARC . β-Propiolactone, in Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide, IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans; Vol. 71; International Agency for Research on Cancer: Lyon, France, 1999; pp 1110–1126. [PMC free article] [PubMed] [Google Scholar]
- NTP (National Toxicology Program) . β-Propiolactone, in Report on Carcinogens; U.S. Department of Health and Human Services, Public Health Service: Research Triangle Park, NC, U.S, 14th. ed., Available online: https://ntp.niehs.nih.gov/go/roc14, 2016. (accessed on 23rd of May 2019). [Google Scholar]
- The National Academies . Health Effects Of Project Shad Chemical Agent: Betapropiolactone; The Center for Research Information, Inc.: Silver Spring, MD, USA, 2004. [Google Scholar]
- Kon T. C.; Onu A.; Berbecila L.; Lupulescu E.; Ghiorgisor A.; Kersten G. F.; Cui Y.-Q.; Amorij J.-P.; Van der Pol L. (2016) Influenza Vaccine Manufacturing: Effect of Inactivation, Splitting and Site of Manufacturing. Comparison of Influenza Vaccine Production Processes. PLoS One 11 (3), e0150700. 10.1371/journal.pone.0150700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uittenbogaard J. P.; Zomer B.; Hoogerhout P.; Metz B. (2011) Reactions of β-Propiolactone with Nucleobase Analogues, Nucleosides, and Peptides. J. Biol. Chem. 286 (42), 36198–36214. 10.1074/jbc.M111.279232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refaie F. M.; Esmat A. Y.; Mohamed A. F.; Mohamed W. A. (2004) The effect of chemical inactivation of bovine viral diarrhea virus with β-propiolactone and binary ethyleneimine on plasma proteins and coagulation factors. Egypt J. Immunol. 11 (2), 9–20. [PubMed] [Google Scholar]
- Bonnafous P.; Nicolaï M.-C.; Taveau J.-C.; Chevalier M.; Barrière F.; Medina J.; Le Bihan O.; Adam O.; Ronzon F.; Lambert O. (2014) Treatment of influenza virus with β-propiolactone alters viral membrane fusion. Biochim. Biophys. Acta, Biomembr. 1838, 355–363. 10.1016/j.bbamem.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Olayan E., El-Khadragy M., Mohamed A. F., Mohamed A. K., Shebl R. I., and Yehia H. M.. Evaluation of Different Stabilizers and Inactivating Compounds for the Enhancement of Vero Cell Rabies Vaccine Stability and Immunogenicity: In Vitro Study. Biomed Res. Int. 2019, Article ID 4518163, 9 pages, DOI: 10.1155/2019/4518163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S.; Gao X.; Sun Y.; Yu X.; Zhao L. (2018) Gas chromatography-mass spectrometry method for determination of β-propiolactone in human inactivated rabies vaccine and its hydrolysis analysis. J. Pharm. Anal. 8 (8), 373–377. 10.1016/j.jpha.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information . PubChem Database. β-Propiolactone, CID = 2365, Available online: https://pubchem.ncbi.nlm.nih.gov/compound/beta-Propiolactone, (accessed on 10th of February 2020).
- ACGIH . Threshold Limit Values for Chemical Substances and Biological Exposure Indices, American Conference of Governmental Industrial Hygienists, Cincinnati, Ohio, USA, 2005; p 24.
- New Jersey Department of Health and Senior Services (2002) Hazardous Substance Fact Sheet, Beta-Propiolactone; New Jersey Department of Health and Senior Services: Trenton, New Jersey, USA. [Google Scholar]
- Dickens F.; Jones H. E. H. (1961) Carcinogenic Activity of a Series of Reactive Lactones and Related Substances. Br. J. Cancer 15 (1), 85–100. 10.1038/bjc.1961.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutwell R. K.; Colburn N. H.; Muckerman C. C. (1969) In vivo reactions of β-propiolactone. Ann. N. Y. Acad. Sci. 163, 751–763. 10.1111/j.1749-6632.1969.tb24893.x. [DOI] [Google Scholar]
- Roe F. J. C.; Glendenning O. M. (1956) The Carcinogenicity Of β-Propiolactone For Mouse Skin. Br. J. Cancer 10 (2), 357–362. 10.1038/bjc.1956.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett P. D.; Small G. Jr. (1950) β-Propiolactone. IX. The Kinetics of Attack by Nucleophilic Reagents upon the Alcoholic Carbon of β-Propiolactone. J. Am. Chem. Soc. 72 (11), 4867–4869. 10.1021/ja01167a006. [DOI] [Google Scholar]
- Mate U.; Solomon J. J.; Segal A. (1977) In vitro binding of β-propiolactone to calf thymus DNA and mouse liver DNA to form l-(2carboxyethyl)adenine. Chem.-Biol. Interact. 18, 327–336. 10.1016/0009-2797(77)90018-7. [DOI] [PubMed] [Google Scholar]
- Segal A.; Solomon J. J.; Mate U. (1980) Isolation Of 3-(2-Carboxyethyl)Thymine Following In Vitro Reaction β-Propiolactone With Calf Thymus DNA. Chem.-Biol. Interact. 29, 335–346. 10.1016/0009-2797(80)90152-0. [DOI] [PubMed] [Google Scholar]
- Segal A.; Solomon J.; Mignano J.; Dino J. (1981) The isolation and characterization of 3-(2-carboxyethyl)cytosine following in vitro reaction of β-propiolactone with calf thymus DNA. Chem.-Biol. Interact. 35 (3), 349–361. 10.1016/0009-2797(81)90010-7. [DOI] [PubMed] [Google Scholar]
- Van Duuren B. L. (1969) Carcinogenic Epoxides, Lactones, and Halo-Ethers and Their Mode of Action, Part II: Chemical Carcinogenesis. Ann. N. Y. Acad. Sci. 163, 633–651. 10.1111/j.1749-6632.1969.tb24883.x. [DOI] [Google Scholar]
- Popescu N. C.; Amsbaugh S. C.; Milo G.; DiPaolo J. A. (1986) Chromosome Alterations Associated with in Vitro Exposure of Human Fibroblasts to Chemical or Physical Carcinogens. Cancer Res. 46, 4720–4725. [PubMed] [Google Scholar]
- Roberts J. J.; Warwick G. P. (1963) The reaction of BPL with guanosine, deoxyguanylic acid and RNA. Biochem. Pharmacol. 12, 1441–1442. 10.1016/0006-2952(63)90216-8. [DOI] [PubMed] [Google Scholar]
- Colburn N. H.; Richardson R. G.; Boutwell R. K. (1965) Studies of the Reaction of β-propiolactone with Deoxyguanosine and Related Compounds. Biochem. Pharmacol. 14, 1113–l118. 10.1016/0006-2952(65)90040-7. [DOI] [PubMed] [Google Scholar]
- Hemminki K. (1981) Reactions of β-propiolactone, β-butyrolactone and γ-butyrolactone with nucleic acids. Chem.-Biol. Interact. 34, 323–331. 10.1016/0009-2797(81)90104-6. [DOI] [PubMed] [Google Scholar]
- Dijkstra J. (1975) In Vitro Reaction Of β-Propiolactone with Glutathione and Cysteine and γ-Butyrolactone. Chem.-Biol. Interact. 10, 115–121. 10.1016/0009-2797(75)90105-2. [DOI] [PubMed] [Google Scholar]
- Hayes J. D.; Mclellan L. I. (1999) Glutathione and Glutathione-dependent Enzymes Represent a Co-ordinately Regulated Defence Against Oxidative Stress. Free Radical Res. 31, 273–300. 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- Pompella A.; Visvikis A.; Paolicchi A.; De Tata V.; Casini A. F. (2003) The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 66, 1499–1503. 10.1016/S0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- Dringen R.; Brandmann M.; Hohnholt M. C.; Blumrich E.-M. (2015) Glutathione-Dependent Detoxification Processes in Astrocytes. Neurochem. Res. 40, 2570–2582. 10.1007/s11064-014-1481-1. [DOI] [PubMed] [Google Scholar]
- Lajovic A.; Nagy L. D.; Guengerich F. P.; Bren U. (2015) Carcinogenesis of Urethane: Simulation versus Experiment. Chem. Res. Toxicol. 28, 691–701. 10.1021/tx500459t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bren U.; Zupan M.; Guengerich P. F.; Mavri J. (2006) Chemical Reactivity as a Tool to Study Carcinogenicity: Reaction between Chloroethylene Oxide and Guanine. J. Org. Chem. 71, 4078–4084. 10.1021/jo060098l. [DOI] [PubMed] [Google Scholar]
- Galeša K.; Bren U.; Kranjc A.; Mavri J. (2008) Carcinogenicity of Acrylamide: A Computational Study. J. Agric. Food Chem. 56, 8720–8727. 10.1021/jf800965y. [DOI] [PubMed] [Google Scholar]
- Bren U.; Guengerich P. F.; Mavri J. (2007) Guanine Alkylation by the Potent Carcinogen Aflatoxin B1: Quantum Chemical Calculations. Chem. Res. Toxicol. 20, 1134–1140. 10.1021/tx700073d. [DOI] [PubMed] [Google Scholar]
- Borštnik U.; Hodošček M.; Janežič D. (2004) Improving the performance of molecular dynamics simulations on parallel clusters. J. Chem. Inf. Comput. Sci. 44, 359–364. 10.1021/ci034261e. [DOI] [PubMed] [Google Scholar]
- Borštnik U.; Janežič D. (2005) Symplectic molecular dynamics simulations on specially designed parallel computers. J. Chem. Inf. Model. 45, 1600–1604. 10.1021/ci050216q. [DOI] [PubMed] [Google Scholar]
- Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., and Fox D. J.. Gaussian 09, revision E.01; Gaussian, Inc.: Wallingford, CT, 2009.
- ChemSpider, β-Propiolactone, Available online: http://www.chemspider.com/Chemical-Structure.2275.html?rid=9797aaf5-60b1-4342-9db9-3088d8ccceaf, (accessed on 10th of November 2015).
- MOLDEN a Visualization Program of Molecular and Electronic structure. Available online: http://cheminf.cmbi.ru.nl/molden/ (accessed on 2nd of January 2020).
- Manso J. A.; Perez-Prior M. T.; Garcia-Santos M. d. P.; Calle E.; Casado J. (2005) A Kinetic Approach to the Alkylating Potential of Carcinogenic Lactones. Chem. Res. Toxicol. 18, 1161–1166. 10.1021/tx050031d. [DOI] [PubMed] [Google Scholar]
- Miertus S.; Scrocco E.; Tomasi J. (1981) Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 55, 117–129. 10.1016/0301-0104(81)85090-2. [DOI] [Google Scholar]
- Florián J.; Warshel A. (1997) Langevin dipoles model for ab initio calculations of chemical processes in solution: Parametrization and application to hydration free energies of neutral and ionic solutes and conformational analysis in aqueous solution. J. Phys. Chem. B 101, 5583–5595. 10.1021/jp9705075. [DOI] [Google Scholar]
- Hawkins G. D., Lynch G. C., Giesen D. J., Rossi I., Storer J. W., Liotard D. A., Cramer C. J., and Truhlar D. G.. AMSOL, version 5.4.1.; University of Minnesota: Minneapolis, 1996. [Google Scholar]
- Florián J.; Warshel A. (1999) Calculations of hydration entropies of hydrophobic, polar, and ionic solutes in the framework of the Langevin dipoles solvation model. J. Phys. Chem. B 103, 10282–10288. 10.1021/jp992041r. [DOI] [Google Scholar]
- Zhao Y.; Truhlar D. G. (2008) Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 41 (2), 157–167. 10.1021/ar700111a. [DOI] [PubMed] [Google Scholar]
- Gómez-Bombarelli R.; González-Pérez M.; Pérez-Prior M. T.; Manso J. A.; Calle E.; Casado J. (2008) Chemical Reactivity and Biological Activity of Diketene. Chem. Res. Toxicol. 21, 1964–1969. 10.1021/tx800153j. [DOI] [PubMed] [Google Scholar]
- Gómez-Bombarelli R.; Calle E.; Casado J. (2013) Mechanisms of Lactone Hydrolysis in Neutral and Alkaline Conditions. J. Org. Chem. 78, 6868–6879. 10.1021/jo400258w. [DOI] [PubMed] [Google Scholar]
- Markovic J.; Borrás C.; Ortega Á.; Sastre J.; Viña J.; Pallardó F. V. (2007) Glutathione Is Recruited into the Nucleus in Early Phases of Cell Proliferation*. J. Biol. Chem. 282 (28), 20416–20424. 10.1074/jbc.M609582200. [DOI] [PubMed] [Google Scholar]
- Chakravarthi S.; Jessop C. E.; Bulleid N. J. (2006) The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep. 7 (3), 271–275. 10.1038/sj.embor.7400645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut T. W. (1994) Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 140 (1), 1–22. 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- Diaz-Vivancos P.; de Simone A.; Kiddle G.; Foyer C. H. (2015) Glutathione - linking cell proliferation to oxidative stress. Free Radical Biol. Med. 89, 1154–1164. 10.1016/j.freeradbiomed.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Brglez Mojzer E.; Knez-Hrnčič M.; Škerget M.; Knez Ž.; Bren U. (2016) Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 21, 901. 10.3390/molecules21070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knez- Hrnčič M.; Španinger E.; Košir I. J.; Knez Ž.; Bren U. (2019) Hop Compounds: Extraction Techniques, Chemical Analyses, Antioxidative, Antimicrobial, and Anticarcinogenic Effects. Nutrients 11, 257. 10.3390/nu11020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kores K.; Lešnik S.; Bren U.; Janežič D.; Konc J. (2019) Discovery of Novel Potential Human Targets of Resveratrol by Inverse Molecular Docking. J. Chem. Inf. Model. 59 (5), 2467–2478. 10.1021/acs.jcim.8b00981. [DOI] [PubMed] [Google Scholar]
- Furlan V.; Konc J.; Bren U. (2018) Inverse Molecular Docking as a Novel Approach to Study Anticarcinogenic and Anti-Neuroinflammatory Effects of Curcumin. Molecules 23 (12), 3351. 10.3390/molecules23123351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan V.; Bren U. (2020) Protective effects of [6]-gingerol against chemical carcinogens: mechanistic insights. Int. J. Mol. Sci. 21 (3), 695. 10.3390/ijms21030695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostnik G.; Gladović M.; Bren U. (2019) Tannin basic building blocks as potential scavengers of chemical carcinogens: a computational study. J. Nat. Prod. 82 (12), 3279–3287. 10.1021/acs.jnatprod.9b00435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.