Abstract
Context
Armadillo repeat containing 5 (ARMC5) on chromosome 16 is an adrenal gland tumor suppressor gene associated with primary aldosteronism, especially among African Americans (AAs). We examined the association of ARMC5 variants with aldosterone, plasma renin activity (PRA), blood pressure, glucose, and glycosylated hemoglobin A1c (HbA1c) in community-dwelling AAs.
Methods
The Jackson Heart Study is a prospective cardiovascular cohort study in AAs with baseline data collection from 2000 to 2004. Kernel machine method was used to perform a single joint test to analyze for an overall association between the phenotypes of interest (aldosterone, PRA, systolic and diastolic blood pressure [SBP, DBP], glucose, and HbA1c) and the ARMC5 single nucleotide variants (SNVs) adjusted for age, sex, BMI, and medications; followed by Baysian Lasso methodology to identify sets of SNVs in terms of associated haplotypes with specific phenotypes.
Results
Among 3223 participants (62% female; mean age 55.6 (SD ± 12.8) years), the average SBP and DBP were 127 and 76 mmHg, respectively. The average fasting plasma glucose and HbA1c were 101 mg/dL and 6.0%, respectively. ARMC5 variants were associated with all 6 phenotypes. Haplotype TCGCC (ch16:31476015-31476093) was negatively associated, whereas haplotype CCCCTTGCG (ch16:31477195-31477460) was positively associated with SBP, DBP, and glucose. Haplotypes GGACG (ch16:31477790-31478013) and ACGCG (ch16:31477834-31478113) were negatively associated with aldosterone and positively associated with HbA1c and glucose, respectively. Haplotype GCGCGAGC (ch16:31471193-ch16:31473597(rs114871627) was positively associated with PRA and negatively associated with HbA1c.
Conclusions
ARMC5 variants are associated with aldosterone, PRA, blood pressure, fasting glucose, and HbA1c in community-dwelling AAs, suggesting that germline mutations in ARMC5 may underlie cardiometabolic disease in AAs.
Keywords: ARMC5, aldosterone, renin, blood pressure, glucose, African Americans
Cardiovascular disease (CVD) remains the most common cause of morbidity and mortality in the United States among US racial/ethnic minority populations (1). CVD affects African Americans (AAs) at an earlier age than non-Hispanic white (NHW) Americans, and AAs with coronary heart disease have lower long-term survival rates than NHWs, which may be mediated by a higher prevalence of risk factors (2, 3). Hypertension is a major risk factor for CVD and is influenced by the renin-angiotensin-aldosterone system (RAAS), which is involved in the regulation of fluid, electrolyte, and blood pressure homeostasis (4-8). AAs have more resistant and severe hypertension, possibly secondary to a genetically determined predisposition to salt and water retention (9). Inappropriate RAAS activation among AAs has been hypothesized to explain some of the racial disparities observed in the incidences of hypertension, left ventricular hypertrophy, and heart failure (5, 10). In particular, the higher rate of hypertension-related complications seen in AAs, including heart failure and death, may be attributed to greater activity of downstream mediators of the RAAS, including angiotensin II and aldosterone (10, 11).
A less-recognized relationship is the role of the RAAS system in glucose metabolism. The 2 most critical factors in the pathophysiology of type 2 diabetes—insulin sensitivity and insulin secretion—can both be impaired by aldosterone (12-14). Type 2 diabetes is more prevalent in primary aldosteronism (high aldosterone, low angiotensin II, and low plasma renin activity [PRA]) with hypertension than in hypertensive controls (15). The association of RAAS with hyperglycemia and diabetes in AAs and multi-ethnic populations has been assessed in the Jackson Heart Study (JHS) and Multi-Ethnic Study of Atherosclerosis (16, 17). Higher levels of aldosterone and renin were associated with greater insulin resistance, hyperglycemia, and incident diabetes in African Americans. Thus, the RAAS is potentially critical to the development of cardiometabolic disease in AAs.
Limited data exist on the genetics of RAAS in the context of blood pressure and glycemia in cohorts spanning from normotensive to hypertensive. In the pathophysiological condition of primary aldosteronism due to aldosterone producing adenomas, there are identified somatic mutations in CACNA1D, KCNJ5, ATP1A1, and ATP2B3 among AAs (18). Armadillo repeat containing 5 (ARMC5) is a tumor suppressor gene expressed in the adrenal cortex. Knockdown (decreased gene expression) of ARMC5 in human adrenocortical cells (H295R) leads to decreased expression of CYP11B2, the gene encoding aldosterone synthase. Recently, Zilbermint et al (19) identified germline ARMC5 gene variants in 10.7% of patients with primary hyperaldosteronism (6 of 56 patients) and notably, all subjects with predicted damaging ARMC5 variants were AA. This was the first report showing that germline ARMC5 gene variants may play a significant role in the development of primary hyperaldosteronism.
Primary hyperaldosteronism is a pathophysiological condition, but it is unknown whether ARMC5 may play a role across the spectrum of blood pressure and glycemia, which is a distinct possibility given our previous dose-dependent findings linking RAAS to type 2 diabetes and CVD among AAs. Identification of ARMC5 genetic variants associated with the RAAS, blood pressure, and glycemia would provide a potential pathway for genetic screening focusing on personalized medicine with usage of medications that target the RAAS system. Thus, it is paramount to investigate the role of ARMC5 with regards to RAAS and cardiometabolic disease. Therefore, we examined: (1) the associations of ARMC5 variants with renin and aldosterone among AAs in the JHS; (2) the association of ARMC5 variants with blood pressure; and (3) the association of ARMC5 variants with hyperglycemia (fasting plasma glucose [FPG] and glycosylated hemoglobin A1c [HbA1c]). We hypothesized that ARMC5 variants will be associated with: (1) higher serum aldosterone and lower plasma renin activity; and (2) higher blood pressure and measures of hyperglycemia, among AAs in the JHS.
Methods
Study participants
The Jackson Heart Study (JHS) is a prospective study of the development and progression of CVD in a cohort of 5306 AA adults, aged 21 to 94 years from the tri-county area of metropolitan Jackson, Mississippi. Enrollment and baseline examinations were performed from 2000 to 2004. There were 2 subsequent in-person follow-up examinations from 2005-2008 and 2009-2013. Details about the study design, recruitment and methods used have been described elsewhere (20). For the cross-sectional analysis, participants were excluded for missing the following data at baseline: 5306 subjects completed the baseline exam in the JHS; we excluded 2082 participants without genetic data and 1 participant with missing phenotypic data for a total analytic cohort of 3223 participants. The JHS was approved by the institutional review boards of University of Mississippi Medical Center, Jackson State University, and Tougaloo College, and the participants gave written informed consent.
Exposure: ARMC5 Whole Exome Sequence
ARMC5 gene on chromosome 16 from deep coverage whole genome sequencing
Whole genome sequencing was performed through the National Heart, Lung, and Blood Institute (NHLBI) Trans-Omics for Precision Medicine (TOPMed) project (www.nhlbiwgs.org). Briefly, ~30× whole genome sequencing was performed at Northwest Genomics Center at the University of Washington. The sequencing reads were transferred to the TOPMed Informatics Research Center (IRC), where they were aligned to build GRCh37 and joint genotype calling was performed on all TOPMed samples in the October 2016 release. Quality control was performed by the Sequencing Centers, the IRC and the TOPMed Data Coordinating Center (DCC) prior to release to dbGAP.
Outcome: Aldosterone, PRA, FPG, hbA1c, systolic and diastolic blood pressure
Fasting blood samples were drawn in the supine position and processed using a standardized protocol; plasma and serum were prepared from samples by sedimentation in a refrigerated centrifuge within 2 hours of blood collection, stored at −70°C and sent to central laboratories (University of Minnesota) (20, 21). Serum aldosterone was measured by radioimmunoassay (Siemens) and the intra-assay coefficients of variation for low and high concentrations were 8.7% and 6.2%, respectively. PRA was measured at baseline using immunoradiometric assays in ng/ml/h (n = 2252) with intra-assay coefficients of variation of 8.0%.
FPG concentrations were measured on either a Vitros 950 or 250 Ortho-Clinical Diagnostics analyzer (Raritan, NJ) using standard procedures that met the College of American Pathologists’ accreditation requirement (21). A high-performance liquid chromatography system (Tosoh Corporation, Tokyo, Japan) was used to measure glycosylated A1c concentrations. Diabetes was defined as HbA1c ≥6.5% (48 mmol/mol) FPG ≥126 mg/dL, taking diabetes medications, or a self-reported physician diagnosis (22).
Resting seated blood pressure was measured twice at 5-minute intervals using an appropriately sized cuff with standard Hawksley random-zero instruments, and measurements were averaged for analysis. Hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg, diastolic blood pressure (DBP) ≥90 mmHg, or the use of antihypertensive therapy.
Baseline assessments
Baseline information was obtained during clinic visits or at home using standardized questionnaires, including medical conditions and current prescription medication usage from the medication survey form, which was derived from review of medication bottles brought in by participants. Calibrated devices were used by certified technicians and nurses to measure participants’ weight and height. Body mass index (BMI) was calculated as weight (kilograms)/ height squared (meters).
Statistical analysis
We performed several sets of analyses to thoroughly assess potential associations between the outcome variables and the single nucleotide variants (SNVs) in ARMC5. Although Zilbermint et al (19) have found several “damaging” variants, the sample size used was small, and thus we analyzed the JHS data without an a priori notion of damaging/benign/protective variants. Among the 99 variants identified in ARMC5, 95 of them are SNVs with at least one copy of minor allele among the individuals in JHS. Of these 95 variants, 3 have minor allele frequency (MAF; frequency of the less common allele) greater than 0.05 and are referred to as common; 10 are uncommon (MAF between 0.01 and 0.05), and the rest—the majority—are rare (MAF < 0.01). In fact, 44 of the variants are extremely rare, with only 1 copy of the minor allele among the 3223 individuals with genotype information. The full list of these 95 SNVs with their rs numbers, or genomic locations (in base pairs), is provided in Supplementary Table S1 (23). For those that have a rs number, their MAFs based on the 1000 Genomes Study are also listed in Supplementary Table S1 (23).
We first performed individual SNV analysis using the trend test as implemented in PLINK (https://www.cog-genomics.org/plink/1.9/). The results are shown in Supplementary Table S2 (23). Although 3 SNPs were detected at the nominal level of 0.05, none were still significant after adjusting for multiple testing. Because of concerns about the lack of statistical power with single SNV tests due to the need for multiple testing corrections for all SNV-phenotype pairs and especially for rare variants, we next used a kernel machine method in the GAMuT package (https://epstein-software.github.io/GAMuT/) to perform a single joint test to ascertain whether there was an overall association between all the phenotypes of interest and all the SNVs adjusted for age, sex, BMI, medications, and principal components of genome-wide SNVs (24). However, an overall significant result does not pinpoint the significant variants nor the specific associated phenotypes; therefore, we also applied the Baysian Lasso methodology (LBL (25) for binary traits and QBL (26) for quantitative traits [https://www.asc.ohiostate.edu/statistics/statgen/SOFTWARE/LBL/]) to identify sets of SNVs in terms of associated haplotypes with specific phenotypes. In this set of analyses, we included age, sex, BMI, medications (blood pressure medication for blood pressure analyses and diabetes medication for glycemia analyses), and the first principle component (PC1) of all genetic variants in the genome as covariates to address potential confounding. The most attractive feature of a Bayesian Lasso analysis is that rare associated haplotypes can be detected and the effect sizes with directionality (positive or negative) can be estimated more precisely than traditional analytical methods (25). Furthermore, haplotypes implicitly account for linkage disequilibrium, a feature that is critically important for identifying joint effects of multiple variants interacting in cis.
Results
Table 1 shows the profile of the 3223 individuals included in this analysis. 62% of participants were female, with a mean age of 55.6 (SD ± 12.8) years and a mean BMI of 32.0 (SD ± 7.4) kg/m2. The average SBP and DBP were 127 and 76 mmHg, respectively, and 57% of the participants had hypertension. The average FPG and HbA1c were 101 mg/dL and 6.0%, respectively, and 23% had diabetes.
Table 1.
Baseline Characteristics of Participants in the Jackson Heart Study
| Baseline Characteristics* | N = 3223 |
|---|---|
| Age (years) | 55.6 (12.8) |
| Female sex (%) | 62% |
| Body mass index (kg/m2) | 32.0 (7.4) |
| Systolic blood pressure (mmHg) | 127 (17) |
| Diastolic blood pressure (mmHg) | 76 (9) |
| Hypertension (%)a | 57% |
| Blood pressure medications (%) | 53% |
| Glucose (mg/dL) | 101 (34) |
| Hemoglobin A1c (%) | 6.0% (1.3) |
| Type 2 diabetes mellitus (%)b | 23% |
| Diabetes medications (%) | 17% |
| Non-parametric variables | |
| Aldosterone (ng/dL) | 4.40 (2.60, 7.30) |
| Plasma renin activity (PRA) (ng/mL/h)c | 0.50 (0.20, 1.20) |
*Mean (standard deviation), Median (interquartile range) or percentages are listed.
aHypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive therapy.
bDiabetes was defined based on the 2010 American Diabetes Association guidelines (HbA1c ≥6.5%, fasting blood glucose ≥7.0 mmol/L [126 mg/dL], taking diabetes medications or with a self-reported physician diagnosis) (22)
cn = 1509 participants with plasma renin activity at baseline
Multi-trait joint analysis
The GAMuT test, which considered the overall effect of all 95 SNVs on aldosterone, PRA, aldosterone/PRA ratio, SBP, DBP, FPG, and HbA1c, jointly led to a P value of 0.0076. Since only a single test was performed, this result is considered to be significant at α = 0.01.
Haplotype analysis
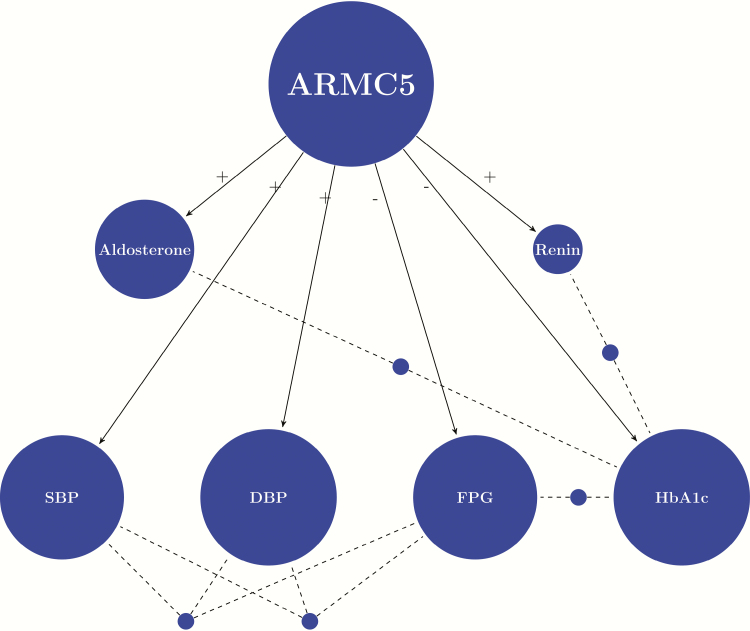
With the significant result from GAMuT, we ran LBL and QBL to identify specific haplotypes (both common and rare) that have a significant association with the phenotypes of interest. First, we observed that PC1 was not significant in any of the analyses, indicating there is little population stratification among our study participants. Other covariates, including age, sex, BMI, and medications, may be significant for some particular phenotypes. The full results are provided in Supplementary Table S3 (23), where all significant haplotypes and effects of the covariates for each of the phenotypes considered are listed. We summarized the results into a network plot (Fig. 1) to show the phenotypes that were found to be significantly influenced by haplotypes (in blocks of SNVs) of the ARMC5 gene. In all, the ARMC5 gene has significant effects on 6 phenotypes: aldosterone, PRA, SBP, DBP, FPG, and HbA1c. There are haplotypes that commonly influence multiple (at least 2) phenotypes (also summarized in Fig. 1), with details provided in Table 2. For example, the haplotype spanning SNVs 48 to 52 (over a 79-bp region: 31476015 to 31476093) is associated with 3 phenotypes: aldosterone, DBP, and FPG. A further summary of the haplotype blocks (with at least one significantly associated haplotype with any of the phenotypes) together with the Linkage Disequilibrium (LD) plot provides a clear visualization of all the associated haplotypes (Fig. 2). It shows that, although many of the SNVs in the ARMC5 gene are involved in significant haplotypes, those in the middle of the gene (from SNV 48 to 52; 31476015 to 31476093) appear to be the most important in terms of influencing a variety of inter-related phenotypes, leading to a number of associated haplotypes.
Figure 1.
Network plot indicating phenotype-haplotype relationships. The size of a phenotype node is proportional to the number of significant haplotypes in all blocks of the SNVs in the ARMC5 gene. A (+) from the ARMC5 node to a phenotype node indicates that the majority and most significant associations are positive; while a (-) indicates that the majority and the most significant associations are negative. Phenotypes that are connected by dashed lines originated from a blue dot are those that share at least one common significant haplotype. Abbreviations: ARMC5 (Armadillo Repeat Containing 5); Renin (plasma renin activity); SBP (systolic blood pressure); DBP (diastolic blood pressure); FPG (fasting plasma glucose); and HbA1c (hemoglobin A1c).
Table 2.
ARMC5 Haplotypes Associated With at Least 2 Phenotypes in the Jackson Heart Study
| SNV # | ARMC5 Haplotype | Aldosterone | Renin | Systolic Blood Pressure | Diastolic Blood Pressure | Fasting Plasma Glucose | Hemoglobin A1c |
|---|---|---|---|---|---|---|---|
| 14 to 21 | GCGCGAGC | o | + | o | o | o | - |
| 27 to 35 | CGGCTCTAG | o | o | o | o | - | - |
| 48 to 52 | TCGCC | o | o | - | - | - | o |
| 67 to 75 | CCCCTTGCG | o | o | + | + | + | o |
| 91 to 95 | ACGCG | - | o | o | o | + | o |
A positive association is denoted by a (+), a negative association is denoted by (-), and no association is denoted by (o). Covariate adjusted for age, sex (male=0, female=1), blood pressure medication (systolic and diastolic blood pressure analyses) or diabetes medication (glycemic analyses), body mass index, and the first principal component.
Figure 2.
Linkage disequilibrium (LD) plot among the SNVs studied and the histogram of the number of significant haplotypes over the blocks. The LD plot is based on both D′ for all 95 SNVs, although LD was not calculated for SNVs with MAF <0.001. For the histogram above the LD plot, only blocks with at least one significant haplotype are shown. The height of the bar indicates the number of significant haplotypes over the block across all phenotypes studied. Also, note that the blocks may overlap one another. Finally, the SNVs are positioned at equal distance rather than drawn according to their actual physical distances. The LD plot should be interpreted with caution: neither D′ nor R2, the traditional measures of LD, can accurately capture LD information (43). In fact, LD information involving SNVs with MAF smaller than 0.001 was not provided in the plot.
Discussion
In this first report of the association of ARMC5 variants with cardiometabolic outcomes in a large population of AAs, we found an overall significant association between the ARMC5 variants and a collection of phenotypes of interest. Further haplotype-based analysis found significant associations of ARMC5 haplotypes with blood pressure and glycemia that were of consistent directionality. For example, in the region that spans from SNV 48 to 52; 31476015 to 31476093, haplotype TCGCC is negatively associated with SBP, DBP, and FPG; in the region between SNV 67 and 75; 31477195 to 31477460, haplotype CCCCTTGCG exhibits positive association with SBP, DBP, and FPG. For glycemic measures, this is the first study to report the novel association of the effect of ARMC5 variants on hyperglycemia. In contrast to our hypothesis and the blood pressure association, we did not see consistent directionality between haplotypes, aldosterone/renin, and hyperglycemia. We found that often the haplotype had an opposite association with aldosterone or renin compared to the effect on glycemia. For instance, in the region that spans from SNV 91 to 95; 31477834 to 31478113, haplotype ACGCG is negatively associated with aldosterone and positively associated with glucose levels. These results suggest that the effect of ARMC5 on glycemia may be modulated/mediated by other genes/pathways, leading to an indirect impact on hyperglycemia that is opposite of the direct effect of ARMC5 on aldosterone.
ARMC5, RAAS, and blood pressure
Our findings of an association of ARMC5 variants with aldosterone and blood pressure in a population of AAs ranging from normotensive to hypertensive are novel. ARMC5 has been significantly associated with primary hyperaldosteronism, using both clinical and cell-based models (19). Interestingly, among patients, the findings were only significant among AAs with primary aldosteronism, thus potentially indicating a major role for ARMC5 in aldosterone production among AAs. Recently, Zilbermint et al showed one common SNP of the ARMC5 gene that was associated with the risk of hypertension in AAs and a set of 16 rare variants associated with hypertension in AAs in the Minority-Health Grid consortium (27). Our analysis corroborated these findings, with significant associations of ARMC5 variants with aldosterone and blood pressure.
ARMC5, RAAS, and glycemia
Recently, aldosterone-producing cell clusters (APCCs) with high expression of aldosterone synthase (CYP11B2) have been found in both normal and primary aldosteronism adrenal tissue (28). Given the association of germline ARMC5 mutations with primary aldosteronism, we could hypothesize that ARMC5 germline variants may predispose to APCC accumulation. However, only 1 ARMC5 benign variant has been identified out of the 5 adrenals with the highest APCC score sequenced, suggesting that they were not predisposing to APCC accumulation (28). Therefore, the consequence of ARMC5 mutations may differ in the zona glomerulosa (aldosterone) and zona fasciculata (cortisol production), as ARMC5 was initially found to have a role in macronodular hyperplasia leading to hypercortisolism (29). It is possible that the ARMC5 mutations positively associated with glycemia may be functioning through other hormones known to cause hyperglycemia (30).
Strengths and Limitations
Strengths of our study include a large AA cohort with comprehensive measurement of biological factors, such as SBP, paired with standardized assessment of laboratory measures, including FPG and HbA1c and a large genetic database. In GWAS studies among AAs, ARMC5 variants have not been identified to be associated with blood pressure (31-35) or glucose (36-38). To our knowledge, there are no GWAS of aldosterone or renin in AAs, but in European Americans there were no ARMC5 variants identified (39, 40). Zilbermint et al (41), using target single-variant and gene-based association analyses, identified 1 common variant (rs116201073) and 16 rare variants located in the ARMC5 gene that were associated with decreased risk of hypertension in a sample set of 1377 AAs from the MH-GRID study. This study takes a different approach by considering the joint effects of multiple SNVs, which has the advantage of increasing statistical power and taking the underlying linkage disequilibrium into account, although the estimates may have large variabilities for rare haplotypes due to the limited sample size.
Furthermore, haplotype analysis using the state-of-the-art methodology enables one to detect the effects of individual rare haplotypes, leading to the possibility of better understanding of the causal effects. Despite these and other strengths, there are some potential limitations. First, the participants in the JHS are from one geographic area in the southeastern United States and may not be representative of all AAs. Second, we did not have a measure of 24-hour urinary sodium in the cohort and thus were unable to assess the impact of dietary salt intake on aldosterone. Third, since the estimated frequencies of some of the haplotypes are extremely small, care should be exercised in interpreting some of the results from the haplotype analyses. Although there are data from more than 3000 individuals and thus it is deemed a large AA cohort, this sample size is still very small for studying the effects of extremely rare variants. For instance, when analyzing the DBP trait without adjusting for blood pressure medications, we found a significant association with haplotype CCGTC in the block spanned by SNV 48 and 52. On the other hand, although the effect size estimate when adjusting for blood pressure medications is similar to that without adjusting, the lower bound of the credible interval falls just below zero, rendering the result insignificant from a purely statistical point of view. This is an example where, due to the small sample size, the estimated parameters, including the effect sizes and haplotype frequencies, are expected to be sensitive. A more definitively positive result is expected with additional data. Lastly, the mean BMI in this analysis of the JHS was 32.0 kg/m2 (62% female). In 2018, the US average BMIs for AA men and women were 29.0 kg/m2 and 31.9 kg/m2, respectively (42), similar to the BMI in this analysis. The mean BMIs for NHW men and women were 29.1 kg/m2 and 29.1kg/m2, respectively (42). The BMI is similar to other populations used in GWAS studies of AAs (32). BMI was adjusted for in the analyses, and the results (Supplementary Table S3) show that, when BMI is associated with a trait, the association is positive. As such, a significant association between a haplotype and the trait is unlikely to be due to the confounding effect of BMI. Additionally, by adjusting for BMI, the chances of finding spurious associations are reduced.
In conclusion, we found significant positive and negative associations of ARMC5 haplotypes in different regions of the ARMC5 gene with aldosterone, PRA, blood pressure, and glycemia in this large population of AAs. These findings suggest germline mutations in ARMC5 may be partially responsible for underpinning cardiometabolic disease in AAs. Given the disparities in cardiometabolic diseases, further research in several directions may help define genetic testing that could be used for personalized approaches to both prevention and management of RAAS-mediated hypertension and diabetes. These include replicating the research in another AA sample; a genome-wide analysis to consider other genes/pathways (although doing so would require a larger sample size to ensure adequate statistical power) and identification of potential epigenetic modifiers.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the JHS for their valuable contributions.
Financial Support: The JHS is supported and conducted in collaboration with Jackson State University (HHSN268201800013I) Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I/HHSN26800001) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). The authors also wish to thank the staffs and participants of the JHS. J.J.J. received additional support from K23DK117041 from the National Institute of Diabetes and Digestive and Kidney Diseases (USA) and The Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program ID# 76236 (USA). The study was also in part supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), NIH. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Glossary
Abbreviations
- AA
African American
- APCC
aldosterone-producing cell cluster
- BMI
body mass index
- CVD
cardiovascular disease
- GWAS
genome-wide association study
- JHS
Jackson Heart Study
- NHW
non-Hispanic white
- MAF
minor allele frequency
- PRA
plasma renin activity
- RAAS
renin-angiotensin-aldosterone system
- SNV
single nucleotide variant
Additional Information
Disclosure Summary: Authors J.J.J., X.Z., M.B.L., A.B., J.G.W., W.A.H., S.H.G., and S.L. have nothing to disclose. M.Z. is a consultant for Guidepoint. C.A.S. holds patent on the PRKAR1A, PDE11A and GPR101 genes and/or their function and his laboratory has received research funding from Pfizer Inc. F.R.F. holds patents on the GPR101 gene and/or its function.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References:
- 1. Carnethon MR, Pu J, Howard G, et al. Cardiovascular health in African Americans: a Scientific Statement From the American Heart Association. Circulation. 2017;136(21):e393-e423. [DOI] [PubMed] [Google Scholar]
- 2. Feinstein M, Ning H, Kang J, et al. Racial Differences in Risks for First Cardiovascular Events and Non-Cardiovascular Death: the Atherosclerosis Risk in Communities Study (ARIC), the Cardiovascular Health Study (CHS), and the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2012; 126(1):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomas KL, Honeycutt E, Shaw LK, Peterson ED. Racial differences in long-term survival among patients with coronary artery disease. Am Heart J. 2010;160(4):744-751. [DOI] [PubMed] [Google Scholar]
- 4. Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 Suppl B):9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grim CE, Cowley AW Jr, Hamet P, et al. Hyperaldosteronism and hypertension: ethnic differences. Hypertension. 2005;45(4):766-772. [DOI] [PubMed] [Google Scholar]
- 6. Rifkin DE, Khaki AR, Jenny NS, et al. Association of renin and aldosterone with ethnicity and blood pressure: the Multi-Ethnic Study of Atherosclerosis. Am J Hypertens. 2014;27(6):801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kidambi S, Kotchen JM, Grim CE, et al. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension. 2007;49(3):704-711. [DOI] [PubMed] [Google Scholar]
- 8. Musani SK, Vasan RS, Bidulescu A, et al. Aldosterone, C-reactive protein, and plasma B-type natriuretic peptide are associated with the development of metabolic syndrome and longitudinal changes in metabolic syndrome components: findings from the Jackson Heart Study. Diabetes Care. 2013;36(10):3084-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spence JD, Rayner BL. Hypertension in blacks: individualized therapy based on renin/aldosterone phenotyping. Hypertension. 2018;72(2):263-269. [DOI] [PubMed] [Google Scholar]
- 10. Michel FS, Norton GR, Majane OH, et al. Contribution of circulating angiotensinogen concentrations to variations in aldosterone and blood pressure in a group of African ancestry depends on salt intake. Hypertension. 2012;59(1):62-69. [DOI] [PubMed] [Google Scholar]
- 11. Joseph JJ, Echouffo-Tcheugui JB, Kalyani RR, et al. Aldosterone, renin, cardiovascular events, and all-cause mortality among African Americans: the jackson heart study. JACC Heart Fail. 2017;5(9):642-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corry DB, Tuck ML. The effect of aldosterone on glucose metabolism. Curr Hypertens Rep. 2003;5(2):106-109. [DOI] [PubMed] [Google Scholar]
- 13. Underwood PC, Adler GK. The renin angiotensin aldosterone system and insulin resistance in humans. Curr Hypertens Rep. 2013;15(1):59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo C, Ricchiuti V, Lian BQ, et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117(17):2253-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reincke M, Meisinger C, Holle R, et al. ; Participants of the German Conn’s Registry Is primary aldosteronism associated with diabetes mellitus? Results of the German Conn’s Registry. Horm Metab Res. 2010;42(6):435-439. [DOI] [PubMed] [Google Scholar]
- 16. Joseph JJ, Echouffo-Tcheugui JB, Kalyani RR, et al. Aldosterone, Renin, and Diabetes Mellitus in African Americans: the Jackson Heart Study. J Clin Endocrinol Metab. 2016;101(4):1770-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joseph JJ, Echouffo Tcheugui JB, Effoe VS, Hsueh WA, Allison MA, Golden SH. Renin-Angiotensin-aldosterone system, glucose metabolism and incident type 2 diabetes mellitus: MESA. J Am Heart Assoc. 2018;7(17):e009890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nanba K, Omata K, Gomez-Sanchez CE, et al. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension. 2019;73(4):885-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zilbermint M, Xekouki P, Faucz FR, et al. Primary Aldosteronism and ARMC5 Variants. J Clin Endocrinol Metab. 2015;100(6):E900-E909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor HA Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6-S4. [PubMed] [Google Scholar]
- 21. Carpenter MA, Crow R, Steffes M, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328(3):131-144. [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33:S62-S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joseph J, Zhou X, Zilbermint M, et al. The association of ARMC5 with the Renin-Angiotensin-Aldosterone system, blood pressure and glycemia in African Americans - Supplementary Tables. figshare. 2020. Deposited April 22, 2020; doi: 10.6084/M9.FIGSHARE.12173694.V2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Broadaway KA, Cutler DJ, Duncan R, et al. A Statistical approach for testing cross-phenotype effects of rare variants. Am J Hum Genet. 2016;98(3):525-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biswas S, Xia S, Lin S. Detecting rare haplotype-environment interaction with logistic Bayesian LASSO. Genet Epidemiol. 2014;38(1):31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou X, Wang M, Zhang H, Stewart WCL, Lin S. Logistic Bayesian LASSO for detecting association combining family and case-control data. BMC Proc. 2018;12(Suppl 9):54. doi: 10.1186/s12919-018-0139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zilbermint M, Gaye A, Berthon A, et al. ARMC5 variants and risk of hypertension in African Americans: Minority Health-GRID study. Endocr Abstr [Internet]. 2018; 56:OC10.3. Proceedings of the 20th European Congress of Endocrinology 2018. doi:10.1530/endoabs.56.OC10.3 [Google Scholar]
- 28. Nishimoto K, Tomlins SA, Kuick R, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A. 2015;112(33):E4591-E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Assié G, Libé R, Espiard S, et al. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing’s syndrome. N Engl J Med. 2013;369(22):2105-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2017;1391(1):20-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adeyemo A, Gerry N, Chen G, et al. A genome-wide association study of hypertension and blood pressure in African Americans. Plos Genet. 2009;5(7):e1000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fox ER, Young JH, Li Y, et al. ; International Consortium for Blood Pressure Genome-wide Association Studies (ICBP-GWAS) Association of genetic variation with systolic and diastolic blood pressure among African Americans: the Candidate Gene Association Resource study. Hum Mol Genet. 2011;20(11):2273-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Franceschini N, Fox E, Zhang Z, et al. ; Asian Genetic Epidemiology Network Consortium Genome-wide association analysis of blood-pressure traits in African-ancestry individuals reveals common associated genes in African and non-African populations. Am J Hum Genet. 2013;93(3):545-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liang J, Le TH, Edwards DRV, et al. Single-trait and multi-trait genome-wide association analyses identify novel loci for blood pressure in African-ancestry populations. Plos Genet. 2017;13(5):e1006728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Understanding Society Scientific Group, International Consortium for Blood Pressure, Blood Pressure-International Consortium of Exome Chip Studies, Million Veteran Program, Giri A, Hellwege JN, Keaton JM, et al. Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat Genet. 2019;51:51-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramos E, Chen G, Shriner D, et al. Replication of genome-wide association studies (GWAS) loci for fasting plasma glucose in African-Americans. Diabetologia. 2011;54(4):783-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ng MC, Shriner D, Chen BH, et al. ; FIND Consortium; eMERGE Consortium; DIAGRAM Consortium; MuTHER Consortium; MEta-analysis of type 2 DIabetes in African Americans Consortium Meta-analysis of genome-wide association studies in African Americans provides insights into the genetic architecture of type 2 diabetes. Plos Genet. 2014;10(8):e1004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rotimi CN, Bentley AR, Doumatey AP, Chen G, Shriner D, Adeyemo A. The genomic landscape of African populations in health and disease. Hum Mol Genet. 2017;26(R2):R225-R236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lieb W, Chen MH, Teumer A, et al. ; CKDGen Consortium; ICBP; EchoGen Consortium Genome-wide meta-analyses of plasma renin activity and concentration reveal association with the kininogen 1 and prekallikrein genes. Circ Cardiovasc Genet. 2015;8(1):131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ji LD, Li JY, Yao BB, Cai XB, Shen QJ, Xu J. Are genetic polymorphisms in the renin-angiotensin-aldosterone system associated with essential hypertension? Evidence from genome-wide association studies. J Hum Hypertens. 2017;31(11):695-698. [DOI] [PubMed] [Google Scholar]
- 41. Zilbermint M, Gaye A, Berthon A, et al. ARMC 5 Variants and Risk of Hypertension in Blacks: MH- GRID Study. J Am Heart Assoc. 2019;8(14):e012508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. National Health Statistics Reports, Number 122, December 20, 2018. 2018;16. [Google Scholar]
- 43. Turkmen A, Lin S. Are rare variants really independent? Genet Epidemiol. 2017;41(4):363-371. [DOI] [PubMed] [Google Scholar]