Abstract
Background:
Cysteinyl leukotrienes (CysLTs) are potent pro-phlogistic mediators in asthma; however, inhibition of CysLT1 receptors is not a consistently effective treatment, suggesting additional regulatory mechanisms. Other cysteinyl-containing lipid mediators derived from docosahexaenoic acid (DHA), namely maresin conjugates of tissue regeneration (MCTRs), were recently discovered. Their production and actions in the lung are therefore of considerable interest.
Objective:
To determine MCTR production, bioactions and mechanism in human lung and in experimental allergic airway inflammation.
Methods:
Lipid mediator metabololipidomics profiling of lung was performed using liquid chromatography with tandem mass spectrometry. Donor-derived human precision cut lung slices (hPCLS) were exposed to leukotriene D4 (LTD4), MCTRs, or both, before determination of airway contraction. The actions of exogenous MCTRs on murine allergic host responses were determined in ovalbumin and house-dust-mite induced lung inflammation.
Results:
Lipidomic profiling showed that the most abundant cysteinyl lipid mediators in healthy human lung were MCTRs whereas CysLTs were most prevalent in disease. MCTRs blocked LTD4-initiated airway contraction in hPCLS. In mouse allergic lung inflammation, MCTRs were present with temporally regulated production With ovalbumin-induced inflammation, MCTR1 was most potent for promoting resolution of eosinophils and MCTR3 potently decreased airway hyperreactivity to methacholine, bronchoalveolar lavage fluid albumin, and serum IgE levels. MCTR1 and MCTR3 inhibited lung eosinophilia after house dust mite-induced inflammation.
Conclusion:
These results identified lung MCTRs that when added exogenously blocked human LTD4-induced airway contraction and promoted the resolution of murine allergic airway responses. Together, these findings uncover pro-resolving mechanisms for lung responses that may be disrupted in disease.
Keywords: Resolution, inflammation, lung, asthma
Graphical Abstract
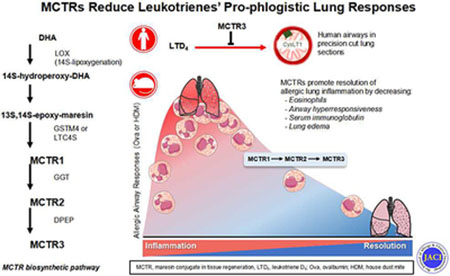
Capsule Summary
Cysteinyl-leukotrienes are potent pro-phlogistic mediators in asthma. Here, metabololipidomic profiling showed that maresin conjugates in tissue regeneration (MCTRs) were abundant in lung, blocked LTD4-initiated human airway contraction, and accelerated murine allergic lung resolution of inflammation.
Introduction
The resolution of the acute inflammatory response is ideally self-limited and an active host response that is mediated in part by a novel superfamily of endogenous molecules termed specialized proresolving mediators (SPM) that includes lipoxins, resolvins, protectins, and maresins 1. SPM convey potent counter-regulation of pro-phlogistic signals, including cytokines such as a TNFα and IL-1β, and lipid mediators such as prostaglandins and leukotrienes, and help orchestrate the return to homeostasis 1. The absence of SPM results in tissue injury, failed resolution, and chronic inflammation, raising the potential that SPMs and their circuits may be harnessed for therapies 2,3.
In asthma, arachidonic acid (AA) is converted to leukotriene C4, D4, and E4 that serve as highly potent pro-phlogistic mediators for airway smooth muscle contraction, tissue edema and airway secretions 4–7. These cysteinyl leukotrienes (CysLTs) exert their bioactions by binding and activating specific receptors that transduce contraction of human airway smooth muscle and vascular permeability 8. Selective CysLT1 receptor antagonists are drugs in clinical use for asthma treatment, yet with surprisingly limited clinical effectiveness 9 In view of these findings, additional regulatory mechanisms and chemical mediators are likely to be involved in the pathobiology of asthma and other diseases where unresolved inflammation is pivotal.
A new family of mediators in tissue regeneration, namely SPM sulfido-conjugates (SPM-SC), were recently discovered and their stereochemical structures established 10–15. These chemical mediator pathways give rise to unique bioactive structures that include maresin conjugates in tissue regeneration (MCTRs, Fig. 1A), protectin conjugates in tissue regeneration (PCTRs), and resolvin conjugates in tissue regeneration (RCTRs) 10–12, 16, 17. Of interest, recent results uncovered that MCTRs compete with LTD4 binding on recombinant CysLT1 receptors 18, thus warranting their investigation in mammalian tissues. Here, we report that MCTRs are major bioactive chemical mediators produced by human lung that counter-regulate CysLTs in tissue homeostasis and the resolution of complex immune responses.
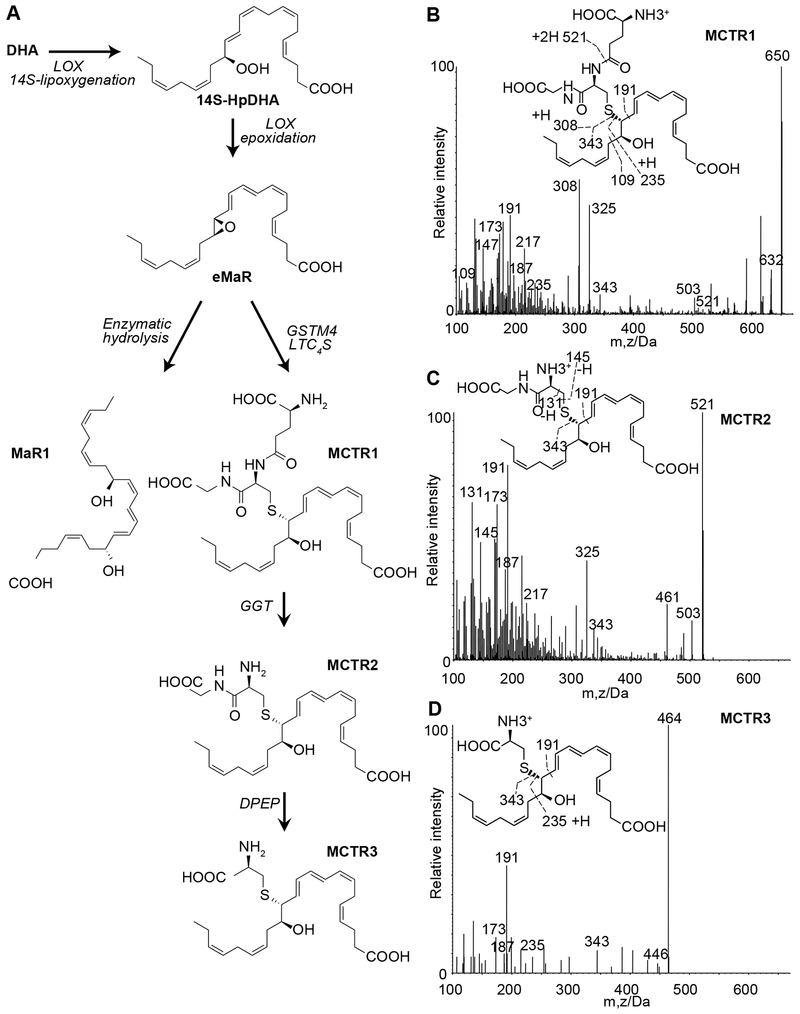
Fig. 1. Human lung SPM-SCs in health and disease.
(A) MCTRs biosynthetic pathway. Healthy and diseased lung tissues were extracted and lipid mediators along with internal standards for quantitation. Representative MS/MS spectra of MCTR1 (B), MCTR2 (C), and MCTR3 (D) (m/z, mass/charge ratio) identified using characteristic diagnostic ions.
Methods
Materials:
Maresin 1 (MaR1), MCTR1, MCTR2, and MCTR3 were each synthesized via total organic synthesis and validated by tandem liquid chromatography with tandem mass spectrometry (LC-MS/MS) immediately prior to use. LTD4, montelukast, and MCTRs were also obtained from Cayman Chemical (Ann Arbor, MI). Isotope-containing MCTRs were prepared by total organic synthesis by Prof. Nicos Petasis for the NIH program project grant #P01-GM095467 organic synthesis core and their synthesis will be reported separately. These were used for identification and quantitative recoveries. These were 13C2,15N-MCTR1, 13C2,15N-MCTR2, and 13C2,15N-MCTR3. Ovalbumin (OVA; Grade III) was purchased from Millipore Sigma (St. Louis, MO). Methacholine (MCh) chloride was purchased from MP Biochemicals, LLC (Solon, OH). Fluorescent activated cell sorting (FACS) antibodies were obtained from Biolegend (San Diego, CA): CD45 (30-F11), F4/80 (BM8), and CD3 (17A2); BD Biosciences (San Diego, CA): Siglec F (E50-2440).
LC-MS/MS-based metabololipidomics of human and murine lung:
Lung tissue samples were snap frozen in liquid nitrogen and stored at −80°C. Samples were then taken to solid-phase extraction (SPE) using Isolute C18 (100 mg) SPE 3 mL cartridges (Biotage, USA) 11, 12, 17. Briefly, deuterium-labeled internal standards (d8-5-HETE, d5-RvD2, d5-RvD3, d5-LXA4, d4-LTB4, d4-PGE2, d5-RvE1, d5-MaR1, d5-LTC4, d5-LTD4; 500 pg each; Cayman Chemicals, USA) and 13C2,15N-MCTR1, 13C2,15N-MCTR2, and 13C2,15N-MCTR3 were added along with 1 mL of methanol before SPE. Tissue was gently dispersed with a glass dounce and placed on ice for 30 minutes to allow for protein precipitation and then reconstituted to 10% methanol; 90% double distilled water; pH 3.5. During SPE, samples were loaded onto SPE cartridges and each cartridge was washed by elution of 3 mL double distilled water, followed by 3 mL hexane. Methyl formate (3 mL) fractions and methanol (3 mL) fractions were collected for two separate LC-MS/MS-based analyses. The first methyl formate fractions from SPE were assessed using a liquid chromatography-tandem mass spectrometry system, QTrap 5500 (AB Sciex) in the negative ion mode to identify eicosanoids and SPMs and was equipped with a Shimadzu LC-20AD HPLC (Tokyo, Japan). A Poroshell 120 EC-18 column (100 mm × 4.6 mm × 2.7 μm; Agilent Technologies, USA) was kept in a column oven maintained at 50°C, and materials were eluted in a gradient of methanol/water/acetic acid from 55:45:0.01 (v/v/v) to 98:2:0.01 at 0.5 mL/min flow rate. The second system, methanol fractions from SPE containing CysLTs and SPM-SCs were identified and profiled as in (11, 14) using a second LC-MS/MS QTrap 5500 (AB Sciex) operated in positive ion mode and equipped with a Shimadzu LC-20AD HPLC (Tokyo, Japan). A Poroshell 120 EC-18 column (100 mm × 4.6 mm × 2.7 μm; Agilent Technologies, USA) was kept in a column oven maintained at 50°C, and lipid mediators were eluted in a gradient of methanol/water/acetic acid from 55:45:0.1 (v/v/v) to 98:2:0.1 at 0.6 mL/min flow rate. To monitor and quantify the levels of targeted lipid mediators, multiple reaction monitoring (MRM) was used with MS/MS matching signature ion fragments for each molecule (at least six diagnostic ions; ~0.1 pg limits of detection) and standard curves (r2>0.98 for each lipid mediator and pathway marker) for both systems. SPM pathway analyses were carried out using Cytoscape software, version 3.4.0 (The Cytoscape Consortium, New York, NY). List of abbreviations and stereochemical assignment for each lipid mediator is in the supplementary material.
Human precision-cut lung sections (hPCLS) preparation:
Healthy lungs that were unsuitable for transplantation were obtained from Brigham and Women’s Hospital (Boston, MA). From these lungs, hPCLS were prepared, cryopreserved, and thawed for use as previously described 19. Thawed lung slices were cultured in DMEM/F-12 at 5% CO2, 37 °C for 12 hours before the start of each experiment. For all hPCLS experiments, viable airways were chosen based on the presence of beating airway cilia and the robustness of smooth muscle surrounding the airway. Diseased human lung tissues along with patient information were purchased from Cooperative Human Tissue Network (Philadelphia, PA).
Mice:
All mice studies were reviewed and approved by the Institutional Animal Care and Use Committee at Brigham and Women’s Hospital (protocol #2016N000356). Six to eight week old FVB male mice (initial body weight 20-25g; Charles River Laboratory, Wilmington, MA) were housed in isolated cages in pathogen-free facilities on a 12-hour light-dark cycle at 25°C. Mice were fed a standard diet (Laboratory Rodent Diet 5001; PMI Nutrition International, St Louis, MO), containing 4.5% total with 0.3 ω-3 fatty acids and less than 0.01% C20:4, and were provided water ad libitum. By the end of the allergen sensitization and challenge protocol (3-4 weeks), the mice weighed 31.9 ± 0.1 g (mean ± s.e.m.). Ovalbumin-induced and house dust mite (HDM)-induced allergic lung models were carried out as in 20, 21, see supplemental information for additional experimental details.
Statistical analysis
All data are expressed as mean ± s.e.m. Comparisons between groups were conducted using ANOVA and Student’s t-test as appropriate. A level of p ≤ 0.05 was considered to indicate statistical significance. Statistics were performed using Graphpad Prism 7.0 (San Diego, CA).
SI Methods details ex vivo conversion of MCTR1 by hPCLS, determination of human airway contraction, mice experiments, murine model of allergic lung inflammation, histology, measurements of airway resistance, bronchoalveolar lavage and serum collection, immunophenotyping of BALF cells, and analysis of molecules in collected serum and BALF.
Results:
MCTRs are produced in human lung.
Lipid mediator metabololipidomics using LC-MS/MS based profiling of healthy human lung tissue identified 14S-lipoxygenation products derived from DHA (Fig. 1A), namely the SPM-SCs maresins (MCTR1, MCTR2, and MCTR3, Figs 1B–D, S1A). Each were identified using characteristic diagnostic ions, retention times, coelution with authentic standards, and compared to synthetic molecules (Fig. S1B and see Methods). Classic CysLTs 7, 8 were also identified (LTC4, LTD4 and LTE4, Figs. S1A and S1C–D). Relative to CysLTs, the summation of SPM-SCs were more abundant in healthy lung (SPM-SCs:CysLTs ≈ 10:3, Table S1). The levels of these mediators and their quantitative relationship changed in diseased lung tissues with a marked increase in CysLTs levels relative to SPM-SCs (SPM-SCs:CysLTs ≈ 10:[100-300], [min – max], Table S1). In addition, the amounts of MCTR3 relative to MCTR1 and MCTR2 increased (Table S1), a pattern also present in human trachea (Table S1). Because these findings suggested MCTR biosynthetic pathways present in human lung, we next incubated authentic MCTR1 with human precision cut lung sections. There was conversion of MCTR1 to MCTR2 and MCTR3 that were identified by MS-MS. MCTR1 decreased by 50% (T50) in 33.5 ± 8.6 sec (mean ± s.e.m, Fig S1E). In the same lung sections, the LTC4 T50 was approximately twice as fast as MCTR1 T50 (14.9 ± 2.9 sec, mean ± s.e.m, Fig. S1F). Together, these findings establish the presence and production of SPM-SCs, namely MCTR biosynthesis, in human lung and suggest changes in this pathway with lung disease.
MCTRs reduced LTD4-stimulated airway contraction.
CysLTs carry potent contractile properties for human bronchi 7, we therefore investigated the airway actions of MCTRs in live human precision cut lung sections. These lung sections contain airways composed of live human ciliated epithelial cells 19 and smooth muscle cells that contract with LTD4 (Fig. 2A), resulting in narrowing of the airway diameter (Fig 2A–B) and subsequent decrease in airflow which characterizes human asthma. MCTR1, MCTR2 and MCTR3 (1-100 μM) were each tested to determine whether they stimulate changes in airway caliber and were directly compared to LTD4 (1-100 μM). None of the MCTRs significantly impacted airway caliber (Fig. 2B). LTD4 stimulated contraction of airways of several different diameters, leading to a 20.6% ± 3.3% (mean ± s.e.m.) reduction from unstimulated baseline airway cross-sectional area (Figs. 2A and 2C). LTD4 contractions were partially inhibited by a CysLT1 receptor antagonist (i.e., montelukast (ML), 100 nM) (Fig. 2C).
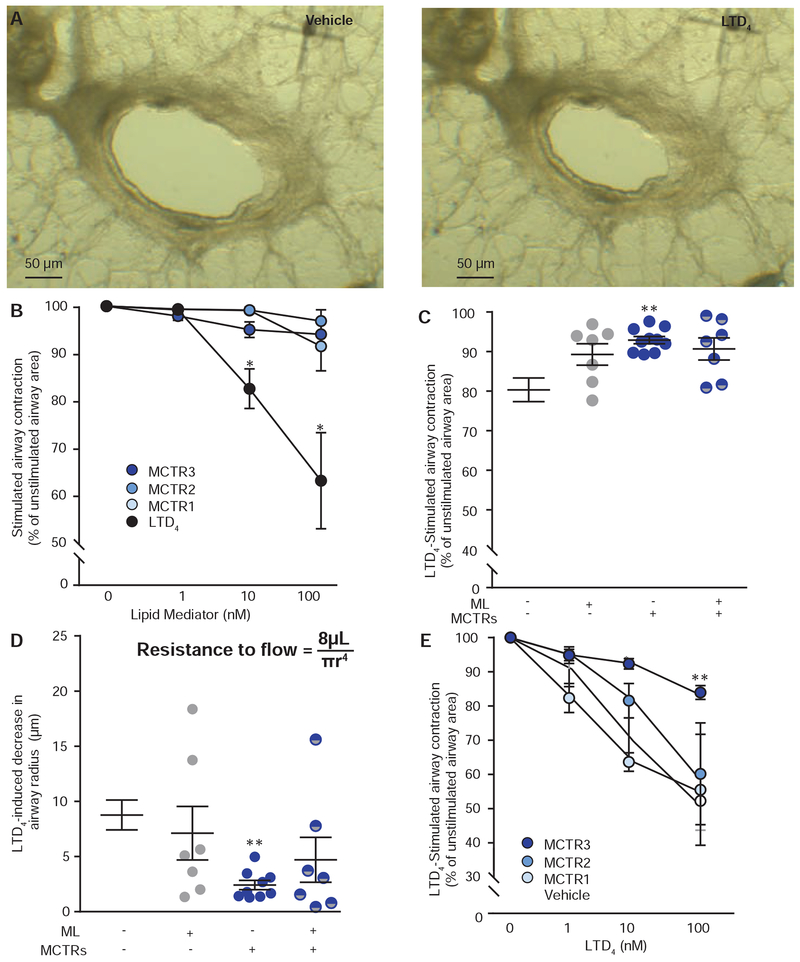
Fig. 2. MCTR inhibition of LTD4-induced human airway contraction.
(A) Representative images of airways in live human precision-cut lung sections exposed to LTD4 (10nM) or vehicle (ethanol < 0.01%). (B) Dose-response relationship for individual MCTRs and LTD4. (C) Impact of MCTRs (MCTR1, MCTR2, and MCTR3 at 10nM each) and montelukast (ML, 100nM) on LTD4-induced change in airway area, and (C) changes in measured airway radii (Poiseuille’s law, inset). (D) Dose-response relationship for LTD4-induced airway contraction for human lung sections exposed to MCTR1, MCTR2, MCTR3, or vehicle. Values represent the mean +/− s.e.m. for n ≥ 4, *P<0.05 and **P<0.005 relative to MCTRs at the equivalent concentration (B) or vehicle (C-E) by 2-way ANOVA.
MCTR1, MCTR2, and MCTR3 (10 nM) each inhibited LTD4 contractions when used together (Fig. 2C). The extent of the MCTR inhibition of LTD4 was dependent in part on airway caliber (Fig. 2D); moreover, considering the Hagen-Poiseuille equation for resistance to flow with radii to the fourth power in the denominator (Resistance to flow = 8μL / πr4, Fig. 2D inset), these relative changes in airway radius are expected to have significant functional impact on airflow obstruction in the intact organ. Of note, MCTR inhibition of LTD4 was partially antagonized by the receptor antagonist (Fig. 2C–D), results in line with MCTR interactions with CysLT receptors in model systems 18. LTD4-induced airway contraction was concentration dependent [1-100 nM] (Fig. 2E). Individual MCTRs blocked LTD4-mediated contraction with rank order potency of MCTR3>MCTR2~MCTR1 (Fig. 2E).
MCTRs in mouse lung during allergic airway inflammation.
Using a murine model of allergic airway responses where the initiation and resolution phases of inflammation are characterized (cf. 20 and Fig. 3A), we defined endogenous production of both SPM-SCs and CysLTs. Lungs were collected at baseline (day 0), peak lung inflammation (day 18) and during the resolution phase (day 21) (Fig. 3A), to perform lipid mediator (LM) metabololipidomics (Table S2). From liquid chromatography with tandem LC-MS-MS based LM identification, results were submitted for unsupervised principal component analysis (PCA). The resulting two-dimensional score plot gave clear separation between days 0, 18 and 21, consistent with temporally regulated production of lipid mediators during these complex tissue responses (Fig. 3B). The loading plot for individual LMs and biosynthetic pathway intermediates demonstrated the appearance of MCTRs in the resolution phase (Fig. S2A). Network analyses were carried out for the biosynthetic pathways and quantities for each mediator (Figs. 3C and S2B–C).
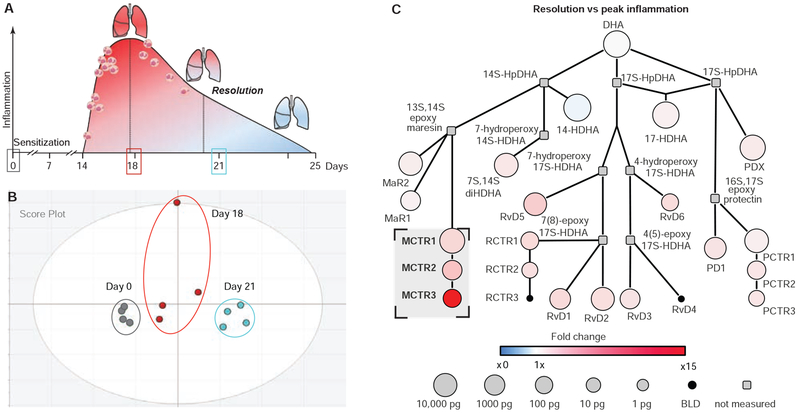
Fig. 3. Metabololipidomic profiling of lipid mediators in murine allergic lung inflammation and resolution.
(A) Murine model of self-limited allergic lung inflammation induced by aerosolized ovalbumin. (B) Two-dimensional score plot from a multivariate principal component analysis of murine lung lipid mediator metabololipidomics (see corresponding loading plot in Fig. S2A). (C) Network illustration of the lung DHA metabolome including biosynthetic intermediates and lipid mediators during resolution relative to peak inflammation. Colors represent the magnitude of fold change (red = increased, blue = decreased), and the size of the circle correlates with abundance. n ≥ 4 per time point.
For the DHA-derived bioactive metabolome, direct comparisons of peak inflammation to baseline uncovered increases in MCTRs, as well as PCTRs, and RCTRs (Fig. S2B), and AA conversion to CysLTs (Fig. S2C). Comparisons between resolution and peak inflammation showed increased MCTR conversion to MCTR3 (Fig. 3C) and decreased CysLTs (Fig. S2D). Together, these results using a targeted metabolomics approach demonstrate distinct temporal regulation of SPM-SC and CysLT pathways during complex inflammatory responses and their resolution.
MCTRs promote the resolution of allergic lung inflammation.
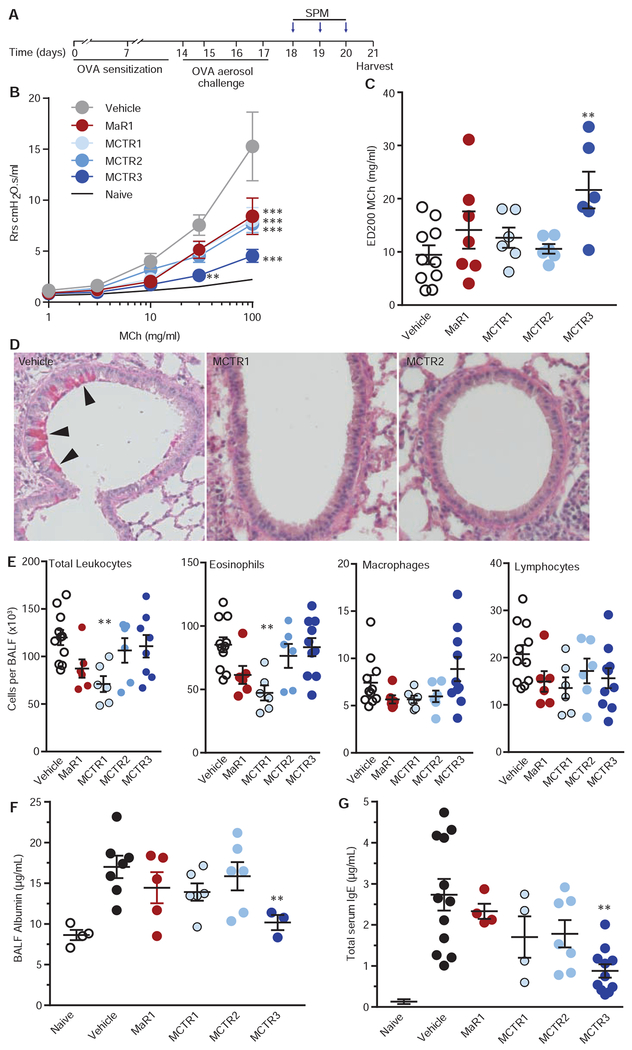
To assess the potential bioactions of MCTRs, animals were treated with either MCTR1, MCTR2, MCTR3, MaR1 (10 ng, intravenous i.v.) or vehicle at peak inflammation and then the mice were phenotyped during resolution (Fig. 4A). Methacholine-stimulated resistance of the respiratory system (Rrs) was determined 24 hrs after the last treatment (protocol day 21, see Methods), and mice given MCTR3 displayed marked protection (Fig. 4B) with an approximate doubling of the ED200 for methacholine (Fig. 4C). MCTR1, MCTR2 and MaR1 gave partial inhibition of the Rrs (Fig. 4B–C). Methacholine-stimulated airway hyper-reactivity in this model was significantly decreased from protocol day 21 to 23 (Fig. S3A), which was 6 days after the last allergen challenge. Significant differences between MCTR3 and vehicle were no longer apparent on protocol day 23 (Fig. S3B–C), which was 72 hrs after the last exposure to MCTR3. When allergen-naïve mice were exposed to MCTR3, there were no substantial changes in lung physiology (Fig S3D) and there was no significant change in the methacholine ED200 for MCTR3 compared to vehicle (Fig S3E). Animals receiving MCTR1 and MCTR2 had significant protection for airway epithelial mucous cell metaplasia (Fig. 4D). MCTR1 also significantly decreased bronchoalveolar lavage (BAL) total cell counts, in particular eosinophils, indicating reduced inflammation, and MCTR2 and MaR1 also gave trends for decreased BAL eosinophils (Fig. 4E). MCTR3 decreased BAL fluid albumin, consistent with enhanced restitution of airway barrier integrity (Fig. 4F), and MCTR3 accelerated resolution of serum total IgE levels (Fig. 4G). MCTR3 did not impact airway eosinophil numbers at this time interval (Fig. 4E).
Fig. 4. Proresolving bioactions of MCTRs in murine allergic lung inflammation.
(A) Timing for once daily SPM treatment (Maresin 1 (MaR1), MCTR1, MCTR2, or MCTR3 (10 ng), or vehicle i.v.) after allergen sensitization and aerosol challenge. (B) Dose-response relationships for methacholine (MCh)-induced changes in total respiratory system resistance on day 21. **P<0.01 and ***P<0.001 relative to vehicle by 2-way ANOVA. (C) Impact on the effective dose of MCh that increased resistance by 200% (ED200). (D) Representative lung histology stained with periodic acid-Schiff for detection of airway mucous cell metaplasia (arrowheads) in samples collected from animals given MCTR1, MCTR2 or vehicle. (E) Bronchoalveolar lavage fluid (BALF) differential leukocyte counts and (F) albumin concentration, and (G) serum total IgE. Values represent the mean +/− s.e.m. for n ≥ 5. *P<0.05 and **P<0.005 by one-way ANOVA for comparison of SPM to vehicle.
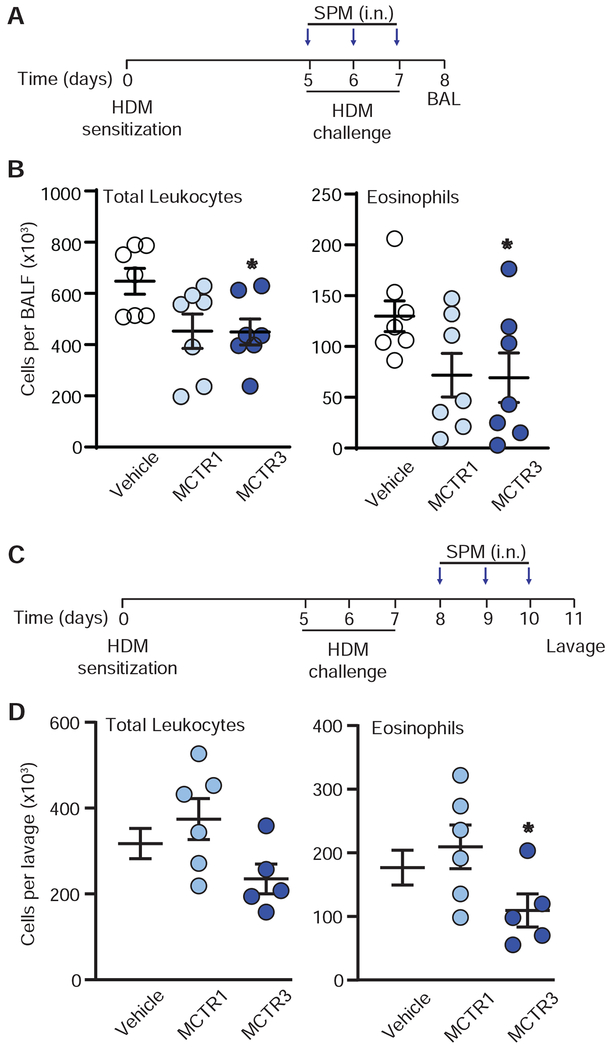
MCTR actions were next investigated in a second model of allergen-induced lung inflammation that is designed for direct sensitization and challenge in the airway. Mice sensitized then challenged with intra-nasal house dust mite (HDM) were exposed to MCTR1, MCTR3, or vehicle (i.n.), 30 minutes before allergen challenge or daily for 3 days after the last HDM administration. MCTR3 significantly decreased lung eosinophil accumulation (Fig 5B). For investigation of MCTR activity after allergen challenge, MCTR1, MCTR3 or vehicle were given (i.n.) 24 hrs after the last administration of HDM daily for three consecutive days (protocol days 8-10). Compared to vehicle, MCTR3 significantly decreased eosinophils in lung lavage performed 24 hrs later on protocol day 11 (Fig. 5D).
Fig. 5. Protective bioactions of MCTRs in house dust mite-induced murine allergic lung inflammation.
(A) Timing for MCTR1 or MCTR3 (10 ng, i.n.) or vehicle at time of house dust mite (HDM) intranasal sensitization and challenge, and (B) bronchoalveolar lavage fluid (BALF) total leukocyte and eosinophil counts. P value for BALF eosinophils in MCTR1 vs vehicle is 0.06. (C) Timing for once daily MCTR1 or MCTR3 (10 ng, i.n.) or vehicle after HDM intranasal sensitization and challenge, and (D) left lung lavage total leukocyte and eosinophil counts. P = 0.08 for lavage total leukocytes in MCTR3 vs vehicle. P < 0.05 relative to vehicle. In all experiments, N ≥ 5.
Discussion:
Here, we report that MCTRs are produced in human lung. These SPM-SCs were more abundant than CysLTs in health and were within their bioactive ranges 10; in contrast, CysLTs appear to be increased relative to SPM-SCs in disease. MCTR3 regulated LTD4-initiated airway contraction at an equimolar concentration [10 nM] and was ~10 times more potent than ML, a clinically available CysLT receptor antagonist. MCTRs and PCTRs were temporally regulated with increased levels concomitant with the resolution phase of murine allergen-driven lung inflammation. MCTRs were also potent immunoresolvents, in that they reduce airway hyperreactivity, mucous cell metaplasia, leukocyte accumulation, barrier breach and serum IgE levels.
MCTR1 was the most abundant SPM-SC in healthy lung tissue. Decreased MCTR1 and increased MCTR3 in diseased lung suggested enzymatic conversion of MCTR1 to MCTR3. In severe asthma, CysLTs production is increased and lipoxin production is decreased 22. Airway mucosal biopsies show decreased DHA and increased AA in asthma 23. Two enzymes from the GST family, GSTM4 and LTC4S, can catalyze formation of MCTR1 and LTC4 16, 17 from their respective allylic epoxide intermediates, 13S,14S-epoxy-maresin and LTA4. Of interest, LTC4S displays higher affinity for LTA4, and GSTM4 displays higher affinity for 13S,14S-epoxy-maresin10, 11. Thus, in addition to substrate availability, the expression of these biosynthetic enzymes can determine tissue LTC4 and MCTR1. In addition, further conversion of LTC4 to LTD4 and LTE4 as well as further conversion of MCTR1 to MCTR2 and MCTR3 may affect lung levels. Human lung rapidly metabolizes LTC4 to LTD4 and LTE4 24 (Fig S1F) while MCTR1 conversion to MCTR2 and MCTR3 was slower, suggesting distinct temporal regulation between CysLTs and MCTRs in the lung.
The CysLTs bind specific receptors, including CysLT1 receptors to transduce their potent bioactions 7, 8. As demonstrated recently in specific binding studies, MCTR1, MCTR2 and MCTR3 compete for specific [3H]-LTD4 binding to human recombinant CysLT1 receptors with apparent lower affinity than LTD4 18 and reduce LTD4-mediated intracellular signaling 18. The relatively lower affinity of MCTRs to CysLT1 compared to LTD4 suggests that MCTRs may signal via other CysLT-specific receptors such as GPR99, a LTE4 receptor 25,26, and high-affinity MCTR-specific receptors that are yet to be uncovered. MCTRs [0.1-10 nM] also stimulate human macrophage phagocytosis; a pro-resolving action blocked by CysLT receptor antagonism 18. The present results are the first for human lung where MCTRs each reduced LTD4-stimulated human airway narrowing.
Using murine self-limited allergic airway inflammation to model a complex tissue response in vivo, temporal regulation of SPM-SC production was established with increased levels during the tissue resolution phase. Treatment with MCTRs beginning after the development of peak inflammation showed a rank order potency of MCTR3>MCTR2>MCTR1 for limiting airway hyperreactivity, consistent with MCTR inhibition of LTD4-initiated human airway contraction. Interestingly, the kinetic actions of MCTR3 on airway responses in vivo were present 24 hrs after the last administration but did not extend to 72 hrs. In ovalbumin-induced inflammation, MCTR3 proved to be the most potent MCTR for restoring barrier integrity, whereas MCTR1 was most potent in regulating mucous cell metaplasia and lung eosinophil accumulation. In HDM-induced inflammation, MCTR1 and MCTR3 gave similarly potent regulation of lung eosinophil accumulation. In these models, MCTR1 and MCTR3 were bioactive when delivered systemically or topically. In sum, these MCTRs counter-regulated and promoted the resolution of airway leukocytosis and eosinophilia with direct protective and proresolving actions in two different experimental models of allergic lung inflammation (i.e., ovalbumin or HDM). The range of bioactions here supports multipronged mechanisms for MCTRs in resolving tissue events in allergic airway responses such as eosinophilia, IgE production, mucous metaplasia, and airway hyperreactivity, all characteristics of human asthma27, 28.
For over 100 years, decoding the puzzle of asthma heterogeneity has been central to translational research on this widely prevalent condition 29. From the initial characterization of the slow-reacting substance of anaphylaxis (SRS-A) 30, 31, lipid mediators have proven to play critical roles in lung biology 32 which are exemplary of their broad actions in many other human organs. However, inconsistent clinical responses to anti-leukotrienes are reported despite clear evidence for the potent bioactions of CysLTs, exemplifying asthma heterogeneity 33. In the present study, on their own in the lung, MCTRs appear to be present without provocation. Our results establish the lung production of MCTRs and their potent counter-regulatory actions after exogenous administration, suggesting a broader tissue range of sulfido-conjugated chemical mediators derived from DHA than CysLTs, in line with protective outcomes in human asthma and lower respiratory tract infections 34. The protective actions for MCTRs in airway responses raise the possibility for broader counter-regulatory roles for SPM-SCs in promoting resolution, tissue regeneration, and homeostasis.
Supplementary Material
Key Messages:
Maresin conjugates in tissue regeneration (MCTR) are abundant cysteinyl lipid mediators in healthy human lung
MCTRs block LTD4-initiated airway contraction in lung sections.
MCTRs selectively promoted the resolution of several airway responses, including hyper-reactivity to methacholine, inflammation and mucosal barrier permeability.
Acknowledgments:
The authors acknowledge the contributions of Ian Riley with the network analyses of the AA and DHA bioactive metabolome and Prof. Bernd Spur for synthetic MCTR3 and PCTR1, PCTR2 and PCTR3.
The work was supported by P01GM095467 (C.N.S. and B.D.L.), R01HL122531 (B.D.L.) and K08HL130540 (R.E.A.).
Abbreviations:
- SPM
specialized proresolving mediators
- SPM-SC
specialized proresolving mediators sulfido-conjugates
- AA
arachidonic acid
- CysLT
cysteinyl leukotrienes
- MCTR
include maresin conjugates in tissue regeneration
- LC-MS-MS
liquid chromatography with tandem mass spectrometry
- LM
lipid mediator
- hPCLS
human precision-cut lung sections
- BALF
bronchoalveolar lavage fluid
- GSTM4
glutathione S-transferase Mu 4
- LTC4S
leukotriene C4 synthase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A list of abbreviations and chemical structures of lipid mediators can be found in supplementary information
The authors have declared that no conflict of interest exists
References:
- 1.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014; 510:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perretti M, Leroy X, Bland EJ, Montero-Melendez T. Resolution Pharmacology: Opportunities for Therapeutic Innovation in Inflammation. Trends Pharmacol Sci 2015; 36:737–55. [DOI] [PubMed] [Google Scholar]
- 3.Vachier I, Bonnans C, Chavis C, Farce M, Godard P, Bousquet J, et al. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. J Allergy Clin Immunol 2005; 115:55–60. [DOI] [PubMed] [Google Scholar]
- 4.Peebles RS Jr. Prostaglandins in asthma and allergic diseases. Pharmacol Ther 2019; 193:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis RA, Wasserman SI, Goetzi EJ, Austen KF. Formation of slow-reacting substance of anaphylaxis in human lung tissue and cells before release. J Exp Med 1974; 140:1133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasserman SI. Mast cells and airway inflammation in asthma. Am J Respir Crit Care Med 1994; 150:S39–41. [DOI] [PubMed] [Google Scholar]
- 7.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 1987; 237:1171–6. [DOI] [PubMed] [Google Scholar]
- 8.Yokomizo T, Nakamura M, Shimizu T. Leukotriene receptors as potential therapeutic targets. J Clin Invest 2018; 128:2691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med 1999; 130:487–95. [DOI] [PubMed] [Google Scholar]
- 10.Dalli J, Chiang N, Serhan CN. Identification of 14-series sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc Natl Acad Sci U S A 2014; 111:E4753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalli J, Ramon S, Norris PC, Colas RA, Serhan CN. Novel proresolving and tissue-regenerative resolvin and protectin sulfido-conjugated pathways. FASEB J 2015; 29:2120–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Rosa X, Norris PC, Chiang N, Rodriguez AR, Spur BW, Serhan CN. Identification and Complete Stereochemical Assignments of the New Resolvin Conjugates in Tissue Regeneration in Human Tissues that Stimulate Proresolving Phagocyte Functions and Tissue Regeneration. Am J Pathol 2018; 188:950–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez AR, Spur BW. First total synthesis of pro-resolving and tissue-regenerative Maresin sulfido-conjugates. Tetrahedron Letters 2015; 56:3936–40. [Google Scholar]
- 14.Rodriguez AR, Spur BW. Total synthesis of pro-resolving and tissue-regenerative Protectin sulfido-conjugates. Tetrahedron Letters 2015; 56:5811–5. [Google Scholar]
- 15.Rodriguez AR, Spur BW. First total synthesis of pro-resolving and tissue-regenerative resolvin sulfido-conjugates. Tetrahedron Letters 2017; 58:1662–8. [Google Scholar]
- 16.Haeggstrom JZ. Leukotriene biosynthetic enzymes as therapeutic targets. J Clin Invest 2018; 128:2680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalli J, Vlasakov I, Riley IR, Rodriguez AR, Spur BW, Petasis NA, et al. Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc Natl Acad Sci U S A 2016; 113:12232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang N, Riley IR, Dalli J, Rodriguez AR, Spur BW, Serhan CN. New maresin conjugates in tissue regeneration pathway counters leukotriene D4-stimulated vascular responses. FASEB J 2018; 32:4043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai Y, Krishnamoorthy N, Patel KR, Rosas I, Sanderson MJ, Ai X. Cryopreserved Human Precision-Cut Lung Slices as a Bioassay for Live Tissue Banking. A Viability Study of Bronchodilation with Bitter-Taste Receptor Agonists. Am J Respir Cell Mol Biol 2016; 54:656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol 2008; 9:873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnamoorthy N, Douda DN, Bruggemann TR, Ricklefs I, Duvall MG, Abdulnour RE, et al. Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci Immunol 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med 2005; 172:824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med 2004; 350:560–9. [DOI] [PubMed] [Google Scholar]
- 24.Kumlin M, Dahlén S-E. Characteristics of formation and further metabolism of leukotrienes in the chopped human lung. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism 1990; 1044:201–10. [DOI] [PubMed] [Google Scholar]
- 25.Kanaoka Y, Maekawa A, Austen KF. Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J Biol Chem 2013; 288:10967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bankova LG, Lai J, Yoshimoto E, Boyce JA, Austen KF, Kanaoka Y, et al. Leukotriene E4 elicits respiratory epithelial cell mucin release through the G-protein-coupled receptor, GPR99. Proc Natl Acad Sci U S A 2016; 113:6242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khatri SB, Erzurum SC. Asthma. Clin Chest Med 2019; 40:xiii–xiv. [DOI] [PubMed] [Google Scholar]
- 28.Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Nat Rev Dis Primers 2015; 1:15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rackemann FM. A clinical study of 150 cases of bronchial asthma. . Boston Med Surg J 1918; 178:770–2. [Google Scholar]
- 30.Austen KF. Biochemical characteristics and pharmacologic modulation of the antigen-induced release of the chemical mediators of immediate hypersensitivity. 1973.
- 31.Wasserman SK Frank Austen MD, and slow-reacting substance of anaphylaxis in the sulfidopeptide leukotriene era. Journal of Allergy and Clinical Immunology 2006; 118:978–80. [Google Scholar]
- 32.Piper PJ. SRS-A and Leukotrienes In: Piper PJ, ed. Proceedings of the Institute of Basic Medical Sciences Royal College of Surgeons of England: Research Studies Press, 1980. [Google Scholar]
- 33.Fanta CH. Asthma. N Engl J Med 2009; 360:1002–14. [DOI] [PubMed] [Google Scholar]
- 34.Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, Schoos AM, et al. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N Engl J Med 2016; 375:2530–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.