Abstract
Purpose:
Psychological stress is a significant health problem in veterans and their family members. Traumatic brain injury (TBI) and stress lead to onset, progression, and worsening of several inflammatory and neurodegenerative diseases in the veterans and civilians. Alzheimer’s disease (AD) is a progressive irreversible neuroinflammatory disease that causes problems with memory, thinking, and behavior. TBI and chronic psychological stress cause and accelerate the pathology of neuroinflammatory diseases such as AD. However, the precise molecular and cellular mechanisms governing neuroinflammation and neurodegeneration are currently unknown, especially in the veterans. Therefore, the purpose of this review article is to advance the hypothesis that stress and TBI-mediated immune response substantially contribute and accelerate the pathogenesis of AD in veterans and their close family members and civilians.
Methods:
The information in this article was collected and interpreted from the published articles in PubMed between 1985 and 2020 using the keywords stress, psychological stress, Afghanistan war, Operation Enduring Freedom (OEF), Iraq War, Operation Iraqi Freedom (OIF), Operation New Dawn (OND), traumatic brain injury, mast cell and stress, stress and neuroimmune response, stress and Alzheimer’s disease, traumatic brain injury and Alzheimer’s disease.
Findings:
Chronic psychological stress and brain injury induce the generation and accumulation of beta-amyloid (Aβ) peptide, amyloid plaques (APs), neurofibrillary tangles (NFTs) and phosphorylation of tau in the brain thereby contributing to AD pathogenesis. Active military personnel and veterans are under enormous psychological stress due to various war-related activities, including TBI, disabilities, fear, new environmental conditions, lack of normal life activities, insufficient communications, explosions, military-related noise, and health hazards. Brain injury, stress, mast cell, and other immune cell activation can induce headache, migraine, dementia, and upregulate neuroinflammation and neurodegeneration in the OEF, OIF, and OND veterans. TBI, post-traumatic stress disorder (PTSD), psychological stress, pain, glial activation, and dementia in the active military personnel, veterans or in their family members can cause AD several years into the future in their late lives. We suggest that an increased number of veterans with TBI and stress, and they may develop AD in their late lives if there is no appropriate therapeutic intervention available.
Implications:
The published reports indicate the fact that TBI and psychological stress can accelerate the pathogenesis of AD should be recognized. Active military personnel, veterans, and their close family members should be regularly evaluated for the stress symptoms to prevent the pathogenesis of neurodegenerative diseases, including AD.
Keywords: Afghanistan war, Alzheimer’s disease, amyloid plaque, Iraq war, psychological stress, traumatic brain injury
INTRODUCTION
The Afghanistan war Operation Enduring Freedom (OEF, 2001-2014) was started in 2001. This was followed by Operation Freedom’s Sentinel (January 2015-present) with US troops that focuses on training, advising, and assisting Afghanistan security forces. In 2014, US troops were withdrawn from Afghanistan, although some remained there as advisors. The Iraq war Operation Iraqi Freedom (OIF) was started in March 2003 by the U.S. led coalition forces. The total number of US troops in Iraq peaked at a major combat operation. Subsequently, the number varied over time, depending on the events. The transition to Operation New Dawn (OND), September 2010, marked the official end to OIF (August 31, 2010) and combat operations by the US forces in Iraq. During OND, service members have conducted stability operations in Iraq.
Nine years of Iraq war claimed the lives of nearly 4500 US troops. Both OEF and OIF caused traumatic brain injury (TBI), psychological stress, and post-traumatic stress disorder (PTSD) that are considered as the risk factor for neurodegenerative diseases in the veterans and their family members. Long-term health effects, including mental health in OEF and OIF veterans, are currently of more significant concern [1]. Neurocognition Deployment Health Study and VA cooperative Studies Program data indicate post-deployment stressors linked to mental problems and that post-deployment social support reduced the risk of mental problems in OEF and OIF veterans [2]. Veterans from the Persian Gulf War showed Gulf War illness (GWI) associated with neuropsychiatric, brain, and immune system abnormalities [3]. Various stress conditions are known to exacerbate the severity of inflammatory diseases. Therefore, there is an urgent need to identify and treat psychological stress in the active war-zone military personnel, veterans, and their family members to prevent the onset and progression of irreversible neuroinflammatory diseases including Alzheimer’s disease (AD) in later life.
TBI is an injury from outside force to the head that causes alteration or loss of consciousness [4]. TBI is a significant health problem in military personnel and civilians since this can cause long-term neuropsychiatric and dementia-associated problems [5, 6]. About 1.7 million TBI incidents are reported annually in the USA [7]. Several previous studies have shown that blast exposure and TBI are the risk factors for the pathogenesis of neuroinflammatory diseases including traumatic encephalopathy and AD [8–10]. AD is a progressive neuroinflammatory and neurodegenerative disease associated with cognitive dysfunction, synaptic loss, increased intracellular neurofibrillary tangles (NFTs), and extracellular amyloid plaques (APs) in the brain. About 5.8 million Americans are currently living with AD, and this number will increase to about 14 million by 2050 (Alzheimer’s Association, Chicago, IL; alz.org). Interestingly about two-thirds of Americans with AD are women. Aged African-Americans are more susceptible to have AD or other dementias than aged whites (Alzheimer’s Association, Chicago, IL). Currently, there is no effective prevention strategy or therapeutic option for AD [11]. A recent report indicates about 40% blunt injury and 37% explosive injury in 156 TBI patients from OEF [6]. Over the last two decades, blast injury due to war is the leading cause of about 383,000 TBIs. Severe TBI may cause long term disabilities, including cognitive impairment, stroke, posttraumatic epilepsy, psychological illness such as stress, neuroinflammation, and neurodegenerative diseases [6]. One study investigated whether psychological distress (PTSD, depressive, and anxiety disorders) impacted communication of 228 male service members from the Iraq and Afghanistan wars in one year with their non-deployed female partners. This study concluded that the psychological stress in service members affected the interactions with their intimate partners that indicated the significance of treating such psychological stress in the service members [12].
Chronic pain is a common problem in both male and female Iraq as well as Afghanistan period veterans that causes stress and affects the quality of life. In general, women veterans report high pain scores. Iraq and Afghanistan era women veterans increasingly using Veterans Affairs (VA) health care system as well as civilian female populations are at higher risk for chronic pain disorders [13]. Chronic pain and chronic headache are important contributors to psychological stress [7, 14]. AD patients show chronic pain that associate with the extent of cognitive decline. Recent study links chronic pain and AD and that pain can accelerate AD pathogenesis [15]. Exposure of soldiers to extreme heat or cold during deployment also causes stress. During 2014-2018, about 325-heat illnesses were reported in the service members of Iraq and Afghanistan, indicating environmental stressors. One study report that veterans (Swedish) deployed in OEF show increased incidence of divorce and reduced rate of marry again after the deployment period when compared to the individuals that were not deployed indicating psychological stress [16]. The elevated suicide rate was reported in the TBI population [17]. The suicidal rate in active-service members in the US military has significantly increased after 2004. The suicide rate also increased during the Afghanistan and Iraq wars, and the range was 29.7/100,000 in 2012 and 20.2-29.7/100,000 from 2008 to 2019 [18]. War zone stressors include personal threat conditions (injured in combat, attacked, surrounded or ambushed by enemies), witnessing situations (seen, processed, transported dead bodies or parts of dead bodies, informed or seen someone critically injured or killed, seen brutality on civilians, captured enemies or prisoners civilians) and moral challenges (seen morally reprehensible occurrences). Adverse childhood emotional and behavioral changes were associated with paternal PTSD due to deployment in Iraq and Afghanistan [19]. Afghanistan war deployment significantly increased mental disorders in the Canadian Armed forces [20]. Our previous review articles have reported how mast cell activation, chronic stress, and TBI can cause or exacerbate immune response, neuroinflammation, and neurodegenerative diseases, including AD [21–24]. However, still, there is no strong experimental and clinical evidence to prove this. The present review article focusses on how psychological stress-mediated immune response in the veterans and their close family members may contribute and accelerate the pathogenesis of AD.
Methods:
The information in this article was collected and interpreted from the published articles between years 1985 to 2020 using the keywords stress, psychological stress, Afghanistan war, Operation Enduring Freedom, Iraq war, Operation Iraqi Freedom, traumatic brain injury, mast cell and stress, stress and neuroimmune response, stress and Alzheimer’s disease, traumatic brain injury and Alzheimer’s disease. The relevant articles were identified for this study, and then the reference list in those papers was manually searched for additional relevant articles related to this paper.
War Associated Stress, Immune Response, Neuroinflammation and Alzheimer’s Disease
Though stress reaction is a protective response of the body, too much and especially chronic stress is harmful to the body. Stress induces chronic disease, and chronic disease can cause psychological stress in a vicious fashion (Fig. 1). Immune system and stress are intertwined even in the brain. Stress can generate amyloid precursor protein (APP), increase beta-amyloid (Aβ) peptide, APs, and NFTs formation that are relevant to the pathogenesis of AD [22]. Veterans and their family members are under enormous psychological stress due to various war-related activities, including TBI, disabilities, fear, new environmental conditions, lack of normal life activities, lack of communication, explosions, noise, and health hazards. OEF, OIF, and Servicemembers also experience family stressors that contribute to PTSD during deployment [25]. The extent of combat exposure and Gulf War Illness can increase the risk for AD [26]. However, the long-term effect of combat exposure on AD is not clearly known. Alhough several studies link stress and AD, the exact mechanisms involved in this are not clearly known, especially the role of the stress hormone cortisol. A recent preliminary study indicated no link to increased chronic traumatic encephalopathy severity and AD characteristics with the military service [27]. Therefore, clearly, more clinical and experimental research evidence is needed on the relationship between stress and AD pathogenesis [28].
Figure 1.
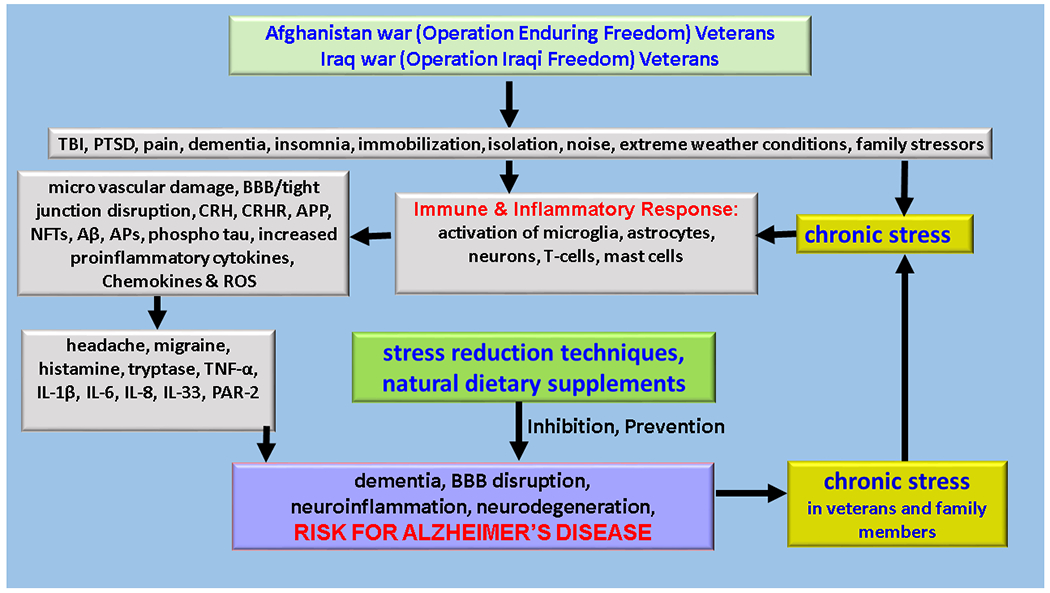
We present a schematic diagram showing war associated stress, immune response, and AD in the veterans. War associated disabilities, TBI, PTSD, and pain cause stress-mediated neuroimmune response. The excessive neuroimmune response causes the activation of immune and inflammatory cells, including microglia, astrocytes, neurons, mast cells, and T-cells in the brain and immune cells in the peripheral organs. Excessive immune activation leads to BBB dysfunction, neuroinflammation, and neurodegeneration. Neuroinflammation further induces cognitive impairments and neurodegenerative diseases such as AD several years after in the veterans and their family members. Stress reduction procedures such as with dietary supplements in active duty members, veterans, and their family members may be useful to prevent the risk of onset and progression of neurodegenerative diseases in these populations. Aβ = beta amyloid; APs = amyloid plaques; APP = amyloid precursor protein; IL = interleukin; NFTs = neurofibrillary tangles; PAR-2 = proteinase-activated receptor-2; ROS = reactive oxygen species; TBF - tumor necrosis factor.
Stress and inflammation are the main pathologic factors in many diseases [22, 29]. Bidirectional communication of microglia and mast cells in the hypothalamus and amygdala may mediate stress-induced inflammation, as shown in Fig. 1 [22, 30]. Histamine from activated mast cells in stress and brain injury can induce headaches, migraine, and upregulate neuroinflammation [31, 32]. Excessive immune response in the brain causes neuroinflammation and that immunotherapy may be useful for neurodegenerative diseases [33]. Neuroinflammation due to glia and immune cell activation is a clear risk factor for neurodegenerative diseases [34, 35]. Neuroinflammation is characterized by the presence of elevated levels of inflammatory cytokines, chemokines and other neurotoxic mediators in the central nervous system (CNS) that may cause neurodegenerative diseases such as AD. Neuroinflammation may be due to CNS damage or neurodegeneration. Mast cells play an important role in neuroinflammatory conditions by secreting several proinflammatory mediators that activate glial cells, neurons, and increase the permeability of the blood-brain barrier (BBB) [36–42]. Previous reports indicate that mast cells are implicated in AD [36, 40, 43–45]. Neuroinflammation may also occur due to systemic infection and environmental stressors through the hypothalamic pituitary adrenal (HPA) axis [46]. Exposure to stressors such as traumatic events in the veterans can increase immune reactivity to subsequent stressors called stress sensitization [47]. This stress sensitization causes the development of PTSD. Soldiers with high combat stress and increased innate cytokine production show increased PTSD symptoms. Thus, detecting and normalizing immune activation due to stress can prevent the progression of PTSD severity after the deployment period [47]. Dutch military personnel deployed to a combat-area showed increased cytokine release from T-cells until at least six months after return, and this could contribute to the depressive symptoms [48]. PTSD is a risk factor for late-onset dementia, and dementia is the risk factor for delayed-onset PTSD in veterans and civilians [49]. In fact, veterans affected with PTSD show higher risk (twice) for dementia and AD than veterans without PTSD [50]. Stress is known to reduce brain-derived neurotrophic factor (BDNF) in animals, and patients with depressive symptoms are predisposed to AD pathogenesis. AD patients show a decreased level of BDNF [51]. PTSD-like condition increased Aβlevel and activated stress response-associated corticotropin-releasing factor (CRF) neurons in the AD animal model [52]. Loss of Formin 2 (Fmn2) in PTSD like conditions leads to memory dysfunction [53]. These findings provide a novel mechanistic insight that might play a significant role in stress-induced neuroinflammation, neurodegeneration that may lead to AD.
As early as in May 2012, a meeting was organized by Alzheimer’s Association and the Veterans Health Research Institute (NCIRE) with specialists from the US military to brainstorm the hypothesis linking TBI, PTSD, and AD [4]. TBI and PTSD are considered “invisible wounds” and “signature injuries” of 21st-century wars [4]. Veterans returning from recent OEF, OIF, and OND show co-occurrence of blast-related mild TBI (mTBI) and PTSD [54]. TBI mediated cognitive disorders can be active chronically for many years after the original injury [55]. Mild cognitive impairment (MCI) can progress into AD [56]. The mechanism of cognitive decline in the military personnel with stress and TBI is not clearly understood. Both physical and psychologic chronic stress during extensive military training can be a risk factor for accelerated cognitive decline or behavioral abnormalities or early-onset dementia (EOD) [9]. Similarly, chronic stress and TBI is a risk factor for EOD/cognitive decline in civilians [9]. More effective body protection systems in the recent wars prevent or reduce mortality but may increase long-term disabilities associated with neurologic and psychiatric disorders, including neuroinflammation, cortical lesions, and suicidal tendencies in OEF/OIF/OND veterans [9]. This study reports that chronic stress is clearly a risk factor for developing AD and dementia [9, 57, 58]. TBI, with or without PTSD, can increase tau deposition in the brain in the veterans [59]. PTSD and TBI are strongly associated with neurocognitive dysfunction in OIF veterans [60]. Even childhood stress can cause late-life dementia indicating stress can affect even in late-life, especially during age-dependent dementia and AD [61]. A study with 160 OEF and OIF war veterans with mTBI indicates the association of TBI with decreased cortical thickness in AD specific areas [55]. Both TBI and PTSD increase cognitive decline and increase AD-associated Aβ deposition in different pathways in the veterans [62]. Imaging analysis and biomarkers analysis were used to analyze the link between TBI/PTSD and AD risk in the veterans [63]. It is known that the presence of APOE ε4 is a significant risk factor for AD. Additionally, the presence of APOE ε4 causes poor prognosis indicating a risk factor for AD [4, 64]. TBI, due to close range blast exposure, causes white matter abnormalities in Apolipoprotein (APOE) ε4 carriers. However, the mechanisms involved in this are not yet understood. Psychiatric problems such as anxiety and irritability are associated with increased Aβ deposition leading to AD [65]. Chronic traumatic encephalopathy (CTE) is due to repetitive TBI. CTE in the veterans shows accelerated Aβ deposition, severe pathology and worse clinical outcome [66]. CTE also shows a massive deposition of phosphorylated tau in the frontal cortex that is distinct from seen in AD [4]. CTE increases stress levels and further increases the risk of AD in the veterans. Oxidative stress and inflammatory response induce neurodegeneration, vascular vertigo, and BBB disruption through reactive oxygen species (ROS) and other inflammatory mediators [67–69]. TBI leads neurovascular vertigo, ischemia, neurodegeneration, dementia and AD pathogenesis [70, 71].
Delaying or Prevention of AD Risk
Though many studies show a link between TBI and PTSD with AD, some reports indicate that TBI and PTSD is not a risk factor for AD based upon amyloid PET and suggest additional mechanistic studies are needed to confirm this link [72]. Chronic stress is a significant risk factor associated with AD pathogenesis [22, 51]. Stress reduction techniques such as yoga and dietary supplements/natural compounds may help to decrease stress, mast cell activation, neuroinflammation, and AD risk though the exact mechanism are not yet clearly known [28, 73–75]. Individuals with high risk for AD may use natural food supplements/nutraceuticals [76]. Thus, Veterans of OEF, OIF, and OND with high risk for AD may use an acceptable dose of nutritional supplements to halt or prevent AD in later life. However, it is not clearly known whether they are safe and effective for chronic diseases [77]. Natural compounds Luteolin with Ashwagandha may be useful to treat the diseases associated with stress and inflammation [30]. A combination of polyphenols instead of single polyphenol, may be more beneficial. Some natural plant hormones, such as Brassinosteroids, provide resistance and protect plants from biotic and abiotic stress conditions [78]. These plant hormones may use useful to reduce stress response in humans. Plant Ocimum sanctum leaf extract show stress relieving and anti-depressant effects, neuroprotection and enhance cognitive functions in animals and humans [79–82]. Yoga may be useful to reduce the severity of stress and ultimately prevent or postpone AD pathogenesis. One study reported that yoga intervention effectively suppressed PTSD symptoms in OEF, OIF, and OND veterans [83].
Conclusions
War can cause TBI and PTSD that can induce severe psychological stress in the military personnel, veterans, and family members. Stress can cause excessive and abnormal neuroimmune response leading to neuroinflammation, neurodegeneration, dementia, and mental illness. Excessive stress is harmful and can generate AD pathogenesis specific NFTs, tau phosphorylation, and APs in the brain. Stress and TBI can accelerate and shorten the duration required for the pathogenesis of AD. The fact that psychological stress can accelerate the development of AD should be recognized, and more research efforts will enable us to decipher the precise underlying novel molecular and cellular mechanisms. Extended social, family, economic, medical, and psychiatric support may reduce stress and AD risk in our veterans. Active military personnel in the war zone, veterans, and their family members should be evaluated for stressors to prevent or delay the onset, progression, and the severity of neurodegenerative diseases, including AD. We conclude that significantly increased number of veterans with TBI and stress may develop AD in their late lives if there is no appropriate therapeutic intervention available.
ACKNOWLEDGMENTS
This research was supported by NIH grant AG048205, Veterans Affairs Merit Award I01BX002477, and VA Research Career Scientist Award to Asgar Zaheer.
Footnotes
CONFLICTS OF INTERESTS
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Declarations of Interest
None
REFERENCES
- 1.Slatore CG, Falvo MJ, Nugent S, Carlson K: Afghanistan and Iraq War Veterans: Mental Health Diagnoses are Associated with Respiratory Disease Diagnoses. Mil Med 2018, 183:e249–e257. [DOI] [PubMed] [Google Scholar]
- 2.Ciarleglio MM, Aslan M, Proctor SP, Concato J, Ko J, Kaiser AP, Vasterling JJ: Associations of Stress Exposures and Social Support With Long-Term Mental Health Outcomes Among U.S. Iraq War Veterans. Behav Ther 2018, 49:653–667. [DOI] [PubMed] [Google Scholar]
- 3.Georgopoulos AP, James LM, Carpenter AF, Engdahl BE, Leuthold AC, Lewis SM: Gulf War illness (GWI) as a neuroimmune disease. Exp Brain Res 2017, 235:3217–3225. [DOI] [PubMed] [Google Scholar]
- 4.Weiner MW, Friedl KE, Pacifico A, Chapman JC, Jaffee MS, Little DM, Manley GT, McKee A, Petersen RC, Pitman RK, Yaffe K, Zetterberg H, Obana R, Bain LJ, Carrillo MC: Military risk factors for Alzheimer’s disease. Alzheimers Dement 2013, 9:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasoon J: Blast-associated traumatic brain injury in the military as a potential trigger for dementia and chronic traumatic encephalopathy. US Army Med Dep J 2017:102–105. [PubMed] [Google Scholar]
- 6.Patel P, Taylor D, Park MS: Characteristics of traumatic brain injury during Operation Enduring Freedom-Afghanistan: a retrospective case series. Neurosurg Focus 2019, 47:E13. [DOI] [PubMed] [Google Scholar]
- 7.Hanas JS, Hocker JRS, Lerner MR, Couch JR: Distinguishing and phenotype monitoring of traumatic brain injury and post-concussion syndrome including chronic migraine in serum of Iraq and Afghanistan war veterans. PLoS One 2019, 14:e0215762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan DR, Logue MW, Wolf EJ, Hayes JP, Salat DH, Fortier CB, Fonda JR, McGlinchey RE, Milberg WP, Miller MW: Close-Range Blast Exposure Is Associated with Altered White Matter Integrity in Apolipoprotein varepsilon4 Carriers. J Neurotrauma 2019, 36:3264–3273. [DOI] [PubMed] [Google Scholar]
- 9.Iacono D, Lee P, Edlow BL, Gray N, Fischl B, Kenney K, Lew HL, Lozanoff S, Liacouras P, Lichtenberger J, Dames-O’connor K, Cifu D, HInds SR, Peri DP : Early-Onset Dementia in War Veterans: Brain Polypathology and Clinicopathologic Complexity. J Neuropathol Exp Neurol 2019, 79:144–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z, Wang ZH, Liu X, Zhang Z, Gu X, Yu SP, Keene CD, Cheng L, Ye K: Traumatic brain injury triggers APP and Tau cleavage by delta-secretase, mediating Alzheimer’s disease pathology. Prog Neurobiol 2019, 185:101730. [DOI] [PubMed] [Google Scholar]
- 11.Vann AS: War on Alzheimer’s? J Am Geriatr Soc 2014, 62:1819–1820. [DOI] [PubMed] [Google Scholar]
- 12.Zamir O, Gewirtz AH, Cheng CH, Zhang N, Lavee Y: Psychological distress and communication quality in military couples after deployment to war. J Fam Psychol 2019. doi: 10.1037/fam0000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naylor JC, Wagner HR, Johnston C, Elbogen EE, Brancu M, Marx CE, Group VAM- AMW, Group VAM-AMWVW, Strauss JL: Pain Intensity and Pain Interference in Male and Female Iraq/Afghanistan-era Veterans. Womens Health Issues 2019, 29 Suppl 1:S24–S31. [DOI] [PubMed] [Google Scholar]
- 14.Nordstrand AE, Boe HJ, Holen A, Reichelt JG, Gjerstad CL, Hjemdal O: Danger- and non-danger-based stressors and their relations to posttraumatic deprecation or growth in Norwegian veterans deployed to Afghanistan. Eur J Psychotraumatol 2019, 10:1601989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao S, Fisher DW, Yu T, Dong H: The link between chronic pain and Alzheimer’s disease. J Neuroinflammation 2019, 16:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pethrus CM, Reutfors J, Johansson K, Neovius K, Soderling J, Neovius M, Bruze G: Marriage and divorce after military deployment to Afghanistan: A matched cohort study from Sweden. PLoS One 2019, 14:e0207981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadhawan A, Stiller JW, Potocki E, Okusaga O, Dagdag A, Lowry CA, Benros ME, Postolache TT: Traumatic Brain Injury and Suicidal Behavior: A Review. J Alzheimers Dis 2019, 68:1339–1370. [DOI] [PubMed] [Google Scholar]
- 18.Smith JA, Doidge M, Hanoa R, Frueh BC: A Historical Examination of Military Records of US Army Suicide, 1819 to 2017. JAMA Netw Open 2019, 2:e1917448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fear NT, Reed RV, Rowe S, Burdett H, Pernet D, Mahar A, Iversen AC, Ramchandani P, Stein A, Wessely S: Impact of paternal deployment to the conflicts in Iraq and Afghanistan and paternal post-traumatic stress disorder on the children of military fathers. Br J Psychiatry 2018, 212:347–355. [DOI] [PubMed] [Google Scholar]
- 20.Boulos D, Zamorski MA: Contribution of the Mission in Afghanistan to the Burden of Past-Year Mental Disorders in Canadian Armed Forces Personnel, 2013. Can J Psychiatry 2016, 61:64S–76S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempuraj D, Ahmed ME, Selvakumar GP, Thangavel R, Dhaliwal AS, Dubova I, Mentor S, Premkumar K, Saeed D, Zahoor H, Raikwar SP, Zaheer S, Iyer SS, Zaheer A: Brain Injury-Mediated Neuroinflammatory Response and Alzheimer’s Disease. Neuroscientist 2019:1073858419848293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kempuraj D, Mentor S, Thangavel R, Ahmed ME, Selvakumar GP, Raikwar SP, Dubova I, Zaheer S, Iyer SS, Zaheer A: Mast Cells in Stress, Pain, Blood-Brain Barrier, Neuroinflammation and Alzheimer’s Disease. Front Cell Neurosci 2019, 13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kempuraj D, Thangavel R, Selvakumar GP, Zaheer S, Ahmed ME, Raikwar SP, Zahoor H, Saeed D, Natteru PA, Iyer S, Zaheer A: Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration. Front Cell Neurosci 2017, 11:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H, Zaheer S, Iyer SS, Zaheer A: Neuroinflammation Induces Neurodegeneration. J Neurol Neurosurg Spine 2016, 1 pii: 1003. [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders W, Smith BN, Fox AB, Vogt D: Five-Year Impacts of Family Stressors and Combat Threat on the Mental Health of Afghanistan and Iraq War Veterans. J Trauma Stress 2019, 32:724–732. [DOI] [PubMed] [Google Scholar]
- 26.Veitch DP, Friedl KE, Weiner MW: Military risk factors for cognitive decline, dementia and Alzheimer’s disease. Curr Alzheimer Res 2013, 10:907–930. [DOI] [PubMed] [Google Scholar]
- 27.Tripathy A, Shade A, Erskine B, Bailey K, Grande A, deLong JJ, Perry G, Castellani RJ: No Evidence of Increased Chronic Traumatic Encephalopathy Pathology or Neurodegenerative Proteinopathy in Former Military Service Members: A Preliminary Study. J Alzheimers Dis 2019, 67:1277–1289. [DOI] [PubMed] [Google Scholar]
- 28.Ashford JW, Mahoney L, Burkett T: A Role for Complementary and Integrative Medicine in Alzheimer’s Disease Prevention. J Alzheimers Dis 2015, 48:13–14. [DOI] [PubMed] [Google Scholar]
- 29.Karagkouni A, Alevizos M, Theoharides TC: Effect of stress on brain inflammation and multiple sclerosis. Autoimmun Rev 2013, 12:947–953. [DOI] [PubMed] [Google Scholar]
- 30.Theoharides TC, Kavalioti M: Stress, inflammation and natural treatments. J Biol Regul Homeost Agents 2018, 32:1345–1347. [PubMed] [Google Scholar]
- 31.Yuan H, Silberstein SD: Histamine and Migraine. Headache 2018, 58:184–193. [DOI] [PubMed] [Google Scholar]
- 32.Meng ID, Cao L: From migraine to chronic daily headache: the biological basis of headache transformation. Headache 2007, 47:1251–1258. [DOI] [PubMed] [Google Scholar]
- 33.Ciccocioppo F, Bologna G, Ercolino E, Pierdomenico L, Simeone P, Lanuti P, Pieragostino D, Del Boccio P, Marchisio M, Miscia S: Neurodegenerative diseases as proteinopathies-driven immune disorders. Neural Regen Res 2020, 15:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung YJ, Tweedie D, Scerba MT, Greig NH: Neuroinflammation as a Factor of Neurodegenerative Disease: Thalidomide Analogs as Treatments. Front Cell Dev Biol 2019, 7:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Shen Y, Chuang H, Chiu C, Ye Y, Zhao L: Neuroinflammation in Alzheimer’s Disease: Microglia, Molecular Participants and Therapeutic Choices. Curr Alzheimer Res 2019, 16:659–674. [DOI] [PubMed] [Google Scholar]
- 36.Jones MK, Nair A, Gupta M: Mast Cells in Neurodegenerative Disease. Front Cell Neurosci 2019, 13:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittal A, Sagi V, Gupta M, Gupta K: Mast Cell Neural Interactions in Health and Disease. Front Cell Neurosci 2019, 13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta K, Harvima IT: Mast cell-neural interactions contribute to pain and itch. Immunol Rev 2018, 282:168–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kempuraj D, Thangavel R, Selvakumar GP, Ahmed ME, Zaheer S, Raikwar SP, Zahoor H, Saeed D, Dubova I, Giler G, et al. : Mast Cell Proteases Activate Astrocytes and Glia-Neurons and Release Interleukin-33 by Activating p38 and ERK1/2 MAPKs and NF-kappaB. Mol Neurobiol 2019, 56:1681–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kempuraj D, Selvakumar GP, Thangavel R, Ahmed ME, Zaheer S, Raikwar SP, Iyer SS, Bhagavan SM, Beladakere-Ramaswamy S, Zaheer A: Mast Cell Activation in Brain Injury, Stress, and Post-traumatic Stress Disorder and Alzheimer’s Disease Pathogenesis. Front Neurosci 2017, 11:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conti P, D’Ovidio C, Conti C, Gallenga CE, Lauritano D, Caraffa A, Kritas SK, Ronconi G: Progression in migraine: Role of mast cells and pro-inflammatory and anti-inflammatory cytokines. Eur J Pharmacol 2019, 844:87–94. [DOI] [PubMed] [Google Scholar]
- 42.Elieh Ali Komi D, Wohrl S, Bielory L: Mast Cell Biology at Molecular Level: a Comprehensive Review. Clin Rev Allergy Immunol 2019. doi: 10.1007/s12016-019-08769-2 [DOI] [PubMed] [Google Scholar]
- 43.Shaik-Dasthagirisaheb YB, Conti P: The Role of Mast Cells in Alzheimer’s Disease. Adv Clin Exp Med 2016, 25:781–787. [DOI] [PubMed] [Google Scholar]
- 44.Skaper SD, Facci L, Zusso M, Giusti P: An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front Cell Neurosci 2018, 12:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hendriksen E, van Bergeijk D, Oosting RS, Redegeld FA: Mast cells in neuroinflammation and brain disorders. Neurosci Biobehav Rev 2017, 79:119–133. [DOI] [PubMed] [Google Scholar]
- 46.O’Callaghan JP, Miller DB: Neuroinflammation disorders exacerbated by environmental stressors. Metabolism 2019, 100S:153951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smid GE, van Zuiden M, Geuze E, Kavelaars A, Heijnen CJ, Vermetten E: Cytokine production as a putative biological mechanism underlying stress sensitization in high combat exposed soldiers. Psychoneuroendocrinology 2015, 51:534–546. [DOI] [PubMed] [Google Scholar]
- 48.van Zuiden M, Heijnen CJ, van de Schoot R, Amarouchi K, Maas M, Vermetten E, Geuze E, Kavelaars A: Cytokine production by leukocytes of military personnel with depressive symptoms after deployment to a combat-zone: a prospective, longitudinal study. PLoS One 2011, 6:e29142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desmarais P, Weidman D, Wassef A, Bruneau MA, Friedland J, Bajsarowicz P, Thibodeau MP, Herrmann N, Nguyen QD: The Interplay Between Post-traumatic Stress Disorder and Dementia: A Systematic Review. Am J Geriatr Psychiatry 2020, 28:48–60. [DOI] [PubMed] [Google Scholar]
- 50.Tasseff TL, Nies MA: Alzheimer’s and Dementia: The Next War for Rural Veterans and Their Families? Am J Nurs 2017, 117:11. [DOI] [PubMed] [Google Scholar]
- 51.Bisht K, Sharma K, Tremblay ME: Chronic stress as a risk factor for Alzheimer’s disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiol Stress 2018, 9:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Justice NJ, Huang L, Tian JB, Cole A, Pruski M, Hunt AJ Jr., Flores R, Zhu MX, Arenkiel BR, Zheng H: Posttraumatic stress disorder-like induction elevates beta-amyloid levels, which directly activates corticotropin-releasing factor neurons to exacerbate stress responses. J Neurosci 2015, 35:2612–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agis-Balboa RC, Pinheiro PS, Rebola N, Kerimoglu C, Benito E, Gertig M, Bahari-Javan S, Jain G, Burkhardt S, Delalle I, Jatzko A, Dettenhofer M, Zunszain PA, Schmitt A, Falkai P, Pape JC, Binder EB, Mulle C, Fischer A, Sananbenesi F: Formin 2 links neuropsychiatric phenotypes at young age to an increased risk for dementia. EMBO J 2017, 36:2815–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez-Garcia G, Gama Sosa MA, De Gasperi R, Tschiffely AE, McCarron RM, Hof PR, Gandy S, Ahlers ST, Elder GA: Blast-induced “PTSD”: Evidence from an animal model. Neuropharmacology 2019, 145:220–229. [DOI] [PubMed] [Google Scholar]
- 55.Hayes JP, Logue MW, Sadeh N, Spielberg JM, Verfaellie M, Hayes SM, Reagan A, Salat DH, Wolf EJ, McGlinchey RE, et al. : Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain 2017, 140:813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor JL, Hambro BC, Strossman ND, Bhatt P, Hernandez B, Ashford JW, Cheng JJ, Iv M, Adamson MM, Lazzeroni LC, McNerney MW: The effects of repetitive transcranial magnetic stimulation in older adults with mild cognitive impairment: a protocol for a randomized, controlled three-arm trial. BMC Neurol 2019, 19:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Escher CM, Sannemann L, Jessen F: Stress and Alzheimer’s disease. J Neural Transm (Vienna) 2019, 126:1155–1161.. [DOI] [PubMed] [Google Scholar]
- 58.Snyder HM, Carare RO, DeKosky ST, de Leon MJ, Dykxhoorn D, Gan L, Gardner R, Hinds SR 2nd, Jaffee M, Lamb BT, Landau S, Manley G, McKee A, Perl D, Schneider JA, Weiner M, Wellington C, Yaffe K, Bain L, Pacifico AM, Carrillo MC: Military-related risk factors for dementia. Alzheimers Dement 2018, 14:1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohamed AZ, Cumming P, Gotz J, Nasrallah F, Department of Defense Alzheimer’s Disease Neuroimaging I: Tauopathy in veterans with long-term posttraumatic stress disorder and traumatic brain injury. Eur J Nucl Med Mol Imaging 2019, 46:1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasterling JJ, Aslan M, Lee LO, Proctor SP, Ko J, Jacob S, Concato J: Longitudinal Associations among Posttraumatic Stress Disorder Symptoms, Traumatic Brain Injury, and Neurocognitive Functioning in Army Soldiers Deployed to the Iraq War. J Int Neuropsychol Soc 2018, 24:311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Donley GAR, Lonnroos E, Tuomainen TP, Kauhanen J: Association of childhood stress with late-life dementia and Alzheimer’s disease: the KIHD study. Eur J Public Health 2018, 28:1069–1073. [DOI] [PubMed] [Google Scholar]
- 62.Mohamed AZ, Cumming P, Srour H, Gunasena T, Uchida A, Haller CN, Nasrallah F, Department of Defense Alzheimer’s Disease Neuroimaging I: Amyloid pathology fingerprint differentiates post-traumatic stress disorder and traumatic brain injury. Neuroimage Clin 2018, 19:716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiner MW, Veitch DP, Hayes J, Neylan T, Grafman J, Aisen PS, Petersen RC, Jack C, Jagust W, Trojanowski JQ, Shaw LM, Saykin AJ, Green RC, Harvey D, Toga AW, Friedl KE, Pacifico A, Sheline Y, Yaffe K, Mehlenoff B: Effects of traumatic brain injury and posttraumatic stress disorder on Alzheimer’s disease in veterans, using the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement 2014, 10:S226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou W, Xu D, Peng X, Zhang Q, Jia J, Crutcher KA: Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J Neurotrauma 2008, 25:279–290. [DOI] [PubMed] [Google Scholar]
- 65.Bensamoun D, Guignard R, Furst AJ, Derreumaux A, Manera V, Darcourt J, Benoit M, Robert PH, David R: Associations between Neuropsychiatric Symptoms and Cerebral Amyloid Deposition in Cognitively Impaired Elderly People. J Alzheimers Dis 2016, 49:387–398. [DOI] [PubMed] [Google Scholar]
- 66.Stein TD, Montenigro PH, Alvarez VE, Xia W, Crary JF, Tripodis Y, Daneshvar DH, Mez J, Solomon T, Meng G, Kubilus CA, Cormier KA, Meng S, Babcocl K, Kiernan P, Murphy L, Nowinski CJ, Martin B, Dixon D, Stern RA, Cantu RC, Kowall NW, McKee CA: Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol 2015, 130:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eastman CL, D’Ambrosio R, Ganesh T: Modulating neuroinflammation and oxidative stress to prevent epilepsy and improve outcomes after traumatic brain injury. Neuropharmacology 2019:107907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lutton EM, Farney SK, Andrews AM, Shuvaev VV, Chuang GY, Muzykantov VR, Ramirez SH: Endothelial Targeted Strategies to Combat Oxidative Stress: Improving Outcomes in Traumatic Brain Injury. Front Neurol 2019, 10:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian SX, Gu JX, Guan QB, Zhang XL, Wang YP: Serum oxidative stress, inflammatory response and platelet activation in patients with vascular vertigo. J Biol Regul Homeost Agents 2019, 33:499–504. [PubMed] [Google Scholar]
- 70.Shulman A, Strashun AM: Fluid dynamics vascular theory of brain and inner-ear function in traumatic brain injury: a translational hypothesis for diagnosis and treatment. Int Tinnitus J 2009, 15:119–129. [PubMed] [Google Scholar]
- 71.Hicks AJ, James AC, Spitz G, Ponsford JL: Traumatic Brain Injury as a Risk Factor for Dementia and Alzheimer Disease: Critical Review of Study Methodologies. J Neurotrauma 2019, 36:3191–3219. [DOI] [PubMed] [Google Scholar]
- 72.Weiner MW, Harvey D, Hayes J, Landau SM, Aisen PS, Petersen RC, Tosun D, Veitch DP, Jack CR Jr., Decarli C, et al. : Effects of traumatic brain injury and posttraumatic stress disorder on development of Alzheimer’s disease in Vietnam Veterans using the Alzheimer’s Disease Neuroimaging Initiative: Preliminary Report. Alzheimers Dement (N Y) 2017, 3:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGrattan AM, McEvoy CT, McGuinness B, McKinley MC, Woodside JV: Effect of dietary interventions in mild cognitive impairment: a systematic review. Br J Nutr 2018, 120:1388–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kempuraj D, Tagen M, Iliopoulou BP, Clemons A, Vasiadi M, Boucher W, House M, Wolfberg A, Theoharides TC: Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell-dependent stimulation of Jurkat T cells. Br J Pharmacol 2008, 155:1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Theoharides TC, Stewart JM, Panagiotidou S, Melamed I: Mast cells, brain inflammation and autism. Eur J Pharmacol 2016, 778:96–102. [DOI] [PubMed] [Google Scholar]
- 76.Spence J, Chintapenta M, Kwon HI, Blaszczyk AT: A Brief Review of Three Common Supplements Used in Alzheimer’s Disease. Consult Pharm 2017, 32:412–414. [DOI] [PubMed] [Google Scholar]
- 77.Costa C, Tsatsakis A, Mamoulakis C, Teodoro M, Briguglio G, Caruso E, Tsoukalas D, Margina D, Dardiotis E, Kouretas D, Fenga C: Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem Toxicol 2017, 110:286–299. [DOI] [PubMed] [Google Scholar]
- 78.Ali MM, Ihsan MA, Zafar H, Rauf QA, Akhtar MK: Brassinosteroid biosynthesis, stress resistance in plants, and application of brassinosteroids in plant biotechnology. J Biol Regul Homeost Agents 2018, 32:1457–1459. [PubMed] [Google Scholar]
- 79.Sampath S, Mahapatra SC, Padhi MM, Sharma R, Talwar A: Holy basil (Ocimum sanctum Linn.) leaf extract enhances specific cognitive parameters in healthy adult volunteers: A placebo controlled study. Indian J Physiol Pharmacol 2015, 59:69–77. [PubMed] [Google Scholar]
- 80.Jothie Richard E, Illuri R, Bethapudi B, Anandhakumar S, Bhaskar A, Chinampudur Velusami C, Mundkinajeddu D, Agarwal A: Anti-stress Activity of Ocimum sanctum: Possible Effects on Hypothalamic-Pituitary-Adrenal Axis. Phytother Res 2016, 30:805–814. [DOI] [PubMed] [Google Scholar]
- 81.Cohen MM: Tulsi - Ocimum sanctum: A herb for all reasons. J Ayurveda Integr Med 2014, 5:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmad A, Khan MM, Raza SS, Javed H, Ashafaq M, Islam F, Safhi MM, Islam F: Ocimum sanctum attenuates oxidative damage and neurological deficits following focal cerebral ischemia/reperfusion injury in rats. Neurol Sci 2012, 33:1239–1247. [DOI] [PubMed] [Google Scholar]
- 83.Cushing RE, Braun KL, Alden CISW, Katz AR: Military-Tailored Yoga for Veterans with Post-traumatic Stress Disorder. Mil Med 2018, 183:e223–e231. [DOI] [PMC free article] [PubMed] [Google Scholar]


