Abstract
Rationale
Stress is related to cognitive impairments which are observed in most major brain diseases. Prior studies showed that the brain concentration of the tryptophan metabolite kynurenic acid (KYNA) is modulated by stress, and that changes in cerebral KYNA levels impact cognition. However, the link between these phenomena has not been tested directly so far.
Objectives
To investigate a possible causal relationship between acute stress, KYNA and fear discrimination.
Methods
Adult rats were exposed to one of three acute stressors - restraint, predator odor, or inescapable foot shocks (ISS) - and KYNA in the prefrontal cortex was measured using microdialysis. Corticosterone was analyzed in a subset of rats. Another cohort underwent a fear discrimination procedure immediately after experiencing stress. Different auditory conditioned stimuli (CSs) were either paired with foot shock (CS+) or non-reinforced (CS−). One week later, fear was assessed by re-exposing rats to each CS. Finally, to test whether stress-induced changes in KYNA causally impacted fear discrimination, a group of rats that received ISS were pre-treated with the selective KYNA synthesis inhibitor PF-04859989.
Results
ISS caused the greatest increase in circulating corticosterone levels and raised extracellular KYNA levels by ~85%. The two other stressors affected KYNA much less (<25% increase). Moreover, only rats that received ISS were unable to discriminate between CS+ and CS−. PF-04859989 reduced the stress-induced KYNA increase and also prevented the impairment in fear discrimination in animals that experienced ISS.
Conclusions
These findings demonstrate a causal connection between stress-induced KYNA increases and cognitive deficits. Pharmacological manipulation of KYNA synthesis therefore offers a novel approach to modulate cognitive processes in stress-related disorders.
Keywords: Acute stress, Inescapable shock, Kynurenine aminotransferase, Tryptophan
Introduction
Severe stress can have harmful health consequences and has a key role in the pathophysiology of various mental illnesses (Musazzi et al. 2018). Cognitive dysfunction is one of the major and impactful consequences of stress in patients (Paul et al. 2015; Bora 2016; Misiak et al. 2018; Flanagan et al. 2018). Investigating the molecular mechanisms involved in stress-induced phenomena is therefore not only critical for understanding the pathophysiology of psychiatric disorders but may also lead to new strategies to prevent or normalize stress-related cognitive deficits. A variety of rodent stress models are available to study the domains and constructs of mental disorders in this context (Schöner et al. 2017). Although differing in nature and duration, they share face validity and often cause similar cognitive dysfunctions in experimental animals.
The role of the essential amino acid tryptophan in stress-related phenomena has been thoroughly investigated and documented in both humans and experimental animals (Ruddick et al. 2006; Miura et al. 2008). Stress-induced activation of thehypothalamic-pituitary-adrenal (HPA) axis raises the levels of corticosterone (CORT) and increases inflammatory processes, and these effects, in turn, stimulate the conversion of tryptophan to the pivotal metabolite kynurenine by activating tryptophan-2,3-dioxygenase (TDO) and indolamine-2,3-dioxygenases 1 and 2 (IDO1, IDO2), respectively (Sitaramam and Ramasarma 1975; Mándi and Vécsei 2012). This process diverts tryptophan metabolism away from producing serotonin, providing a conceptual link between stress and the pathophysiology of depression (Kiank et al. 2010; Gibney et al. 2014; Nold et al. 2019). Based on this theoretical construct, and on chemical analyses of blood and cerebrospinal fluid of depressed patients (Gabbay et al. 2010; Oxenkrug et al. 2014; Bradley et al. 2015; Serafini et al. 2017), and studies in relevant animal models (Laugeray et al. 2010; Fuertig et al. 2016; Ohta et al. 2017; Martín-Hernández et al. 2018; Duda et al. 2019), investigators also began to consider a possible role of kynurenine and its downstream metabolites (“kynurenines”) in the etiology of major depressive disorders. This connection was attributed mostly to the excessive production of compounds such as 3-hydroxykynurenine (3-HK) and quinolinic acid (QUIN), in the so-called “neurotoxic” branch of the metabolic cascade, following repeated or prolonged stress (Müller and Schwarz 2007; Raison et al. 2010; Krause et al. 2012; Myint et al. 2012; Erhardt et al. 2013; Parrott et al. 2016; Laumet et al. 2017).
So far, only little attention has been paid to kynurenic acid (KYNA), a neuroinhibitory compound that is produced from kynurenine in a sidearm of the pathway, though acute stress in rodents is known to result in prompt increases in cerebral KYNA levels in the fetus (Notarangelo and Schwarcz 2017) and in adulthood (Pawlak et al. 2000). KYNA is an astrocyte-derived metabolite which influences cognitive functions bi-directionally (Pocivavsek et al. 2016). Thus, experimental increases in brain KYNA levels caused by systemic administration of kynurenine lead to impairments in prefrontal cortex (PFC)-mediated set-shifting (Alexander et al. 2012), spatial contextual memory (Pocivavsek et al. 2011), fear learning (Chess et al. 2009), and working memory (Chess et al. 2007). Conversely, reductions in cerebral KYNA, effected by either pharmacological inhibition or genetic deletion of kynurenine aminotransferase II (KAT II), the main enzyme responsible for the synthesis of readily mobilizable KYNA in the mammalian brain, improves cognitive function (Potter et al. 2010; Kozak et al. 2014; Wu et al. 2014; Pocivavsek et al. 2019). Presumably, these modulatory effects of endogenous KYNA are directly related to the fact that the metabolite interferes with the function of α7 nicotinic cholinergic and NMDA receptors (Kessler et al. 1989; Hilmas et al. 2001), both of which are critical for learning and memory (Levin et al. 2006; Robbins and Murphy 2006). Notably, KYNA levels are elevated in the PFC and cerebrospinal fluid of patients with schizophrenia, a disorder in which cognitive dysfunction is a core feature (Heinrichs and Zakzanis 1998; Schwarcz et al. 2001; Erhardt et al. 2001; Sathyasaikumar et al. 2011; Linderholm et al. 2012; Kindler et al. 2019).
To date, the possible causal relationship between stress, KYNA, and cognitive function has not been directly examined. In a first attempt to investigate this connection, the present study was designed to determine the effect of three acute stressors of different severity on extracellular KYNA levels in the PFC and on PFC-dependent fear discrimination in rats. Causality was examined by using the selective, brain-penetrable KAT II inhibitor PF-04859989 (Kozak et al. 2014), which allowed us to test whether blockade of KYNA neosynthesis prevents the stress-induced KYNA increase seen in the PFC and affects associated impairments in fear discrimination.
Materials and Methods
Animals
A total of 200 adult, male Sprague-Dawley rats (180–200 g on arrival; Charles River Laboratories, Kingston, NY, USA) were maintained in a temperature- and humidity-controlled, AAALAC-approved animal facility on a 12/12 h light/dark cycle (lights on at 07:00 a.m.) and housed in pairs. Food and water were available ad libitum. Following delivery to the animal facility, the animals were adapted to the new environment for at least one week before experiments commenced. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore, and were in full accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chemicals/Drugs
KYNA was purchased from Sigma Chemical Co. (St. Louis, MO, USA). PF-04859989 was obtained from Pfizer (New York, NY, USA), dissolved in 0.9% saline, and administered subcutaneously (s.c.) for in vivo experimentation. All other chemicals were obtained from a variety of suppliers and were of the highest commercially available purity.
Stress models
Animals were assigned to one of three experimental conditions (exposure to predator odor in the home cage, physical restraint, or inescapable and unpredictable foot shock delivered in an operant chamber; see below), or to one of two control conditions (undisturbed and remaining in their home cage, or placed in the operant chamber but with no shock delivered). In all cohorts, the effects of the different experimental conditions were compared using biochemical analyses (CORT and KYNA levels) and behavior (fear discrimination) as outcome asures.
Predator odor stress
Rats were left in their home cage, and a beaker with ~30–40 ml of fox urine (Leg Up Enterprises, Lovell, ME, USA) was placed in the center of the cage for 2 h.
Restraint stress
Rats were placed in a clear plastic cylinder (diameter: 5.5 cm, with adjustable length) with the entire body tightly restrained for 2 h.
Inescapable shock stress (ISS)
Rats were placed in an operant chamber (Coulbourn Instruments, Holliston, MA, USA) where they received 1 mA foot shocks (30 sec in duration) with an inter-stimulus interval of 60 sec for a total of 2 h. The chamber (25.4 cm W x 25.4 cm H x 19.1 cm L) had aluminum sidewalls and ceiling, front and back walls made of plexiglass, and a removable grid floor (stainless steel columns 1 cm apart).
Microdialysis
On the day before microdialysis was performed, rats were anesthetized with isoflurane (2–5% in oxygen) and mounted onto a stereotaxic frame (David Kopf, Tujunga, CA, USA). After exposing the skull and drilling a burr hole (AP: 3.2 mm anterior to bregma, L: 0.8 mm from midline), a guide cannula (MAB 2.14.G, SciPro Inc., Sanborn, NY, USA) was positioned unilaterally over the medial PFC (2.0 mm below the dura; hemispheres counterbalanced) and secured to the skull with anchor screws and acrylic dental cement. On the next day, a microdialysis probe (MAB 9.14.2, membrane length: 2 mm, SciPro Inc.) was inserted through the guide cannula of the awake and freely-moving animal. The probe was then connected to a microinfusion pump set to a speed of 1 μl/min and perfused with Ringer solution containing 144 mM NaCl, 4.8 mM KCl and 1.7 mM CaCl2 (pH 6.7). After a 1-h stabilization period, microdialysate samples were collected every 30 min for up to 8 h. The first 2 h of collection provided baseline values.
Biochemical measures
Plasma corticosterone
To study CORT levels, rats from each of the 5 groups were euthanized by CO2 asphyxiation immediately after the 2-h period, and trunk blood was collected in EDTA-containing vials. Plasma was separated by centrifugation (6,000 × g, 10 min), and plasma CORT was determined by radioimmunoassay according to the protocol provided by the manufacturer (MP Biochemicals, Orangeburg, NY, USA).
Extracellular kynurenic acid
The concentration of KYNA was determined by high-performance liquid chromatography with fluorescence detection (excitation wavelength: 344 nm; emission wavelength: 398 nm). To this end, 30 μl of the microdialysate were applied to a BDS Hypersil C18 column (100 mm x 4.6 mm, particle size 3 μm; Thermo Fisher Scientific, Waltham, MA, USA), and KYNA was isocratically eluted at a flow rate of 1 ml/min using a mobile phase containing 250 mM zinc acetate, 50 mM sodium acetate, and 4.5% acetonitrile (pH 6.2), as previously described (Wu et al. 1992). The retention time of KYNA was ~6 min. Data were not corrected for recovery from the microdialysis probe.
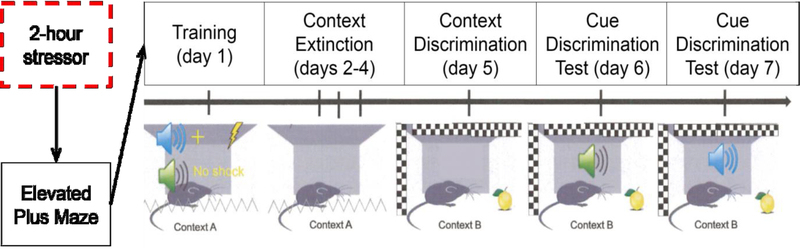
Behavioral studies (cf. Fig. 1)
Figure 1:
Schematic of experimental design for behavioral studies. Freezing behavior was recorded on days 6 and 7, with counterbalanced cues.
Elevated plus maze
A black plexiglass plus-shaped platform (74 cm above the floor) with two open arms and two closed arms (each 50 × 10 cm) was placed in a quiet, dimly lit room. The arms were connected by a central area (10 × 10 cm). Animals were placed in the central area facing a closed arm and were allowed to explore freely for 5 min. The time spent in each arm and the number of entries into each arm were recorded. An entry was counted when the front two paws of the rat were placed on an arm. The maze was cleaned with 70% ethanol between tests.
Fear discrimination
The fear discrimination procedure was performed in the operant chamber used for the ISS. A 1500 KHz tone and white noise were used as the auditory cues (CS+, CS−; counterbalanced). Rats underwent a week-long procedure consisting of daily sessions as follows (Fig 1): On the first day (conditioning session), rats were presented with three 10-sec pairings of the CS+ followed immediately by a 1 mA, 1-sec foot shock, intermixed with three 10-sec presentations of the CS−, with a 3-min interval between cue presentations. For the next three days (days 2–4), contextual fear to the training chamber was extinguished by re-exposing the animals to the training chamber (context A) for 10 min/day with no cues or shocks delivered. On day 5, rats were exposed to a new context (context B), created by changing to a square grid steel mesh floor, adding white paper to the walls of the chamber, and the presence of an odor (Pine-Sol). On the following two days (days 6 and 7), rats underwent the critical cue-discrimination test sessions, during which the animals were re-exposed to each CS three times in context B with no shocks delivered. One of the CSs was presented during the first test session (day 6) and the other CS during the second test session (day 7; counterbalanced). During the test sessions, freezing behavior (no movement except respiration; Fanselow 1980) was recorded only during the 10-sec cue presentations and was expressed as a percentage of total observations, as described previously (Keene and Bucci 2008; Bucci et al. 2016).
Statistical analysis
All data are expressed as the mean ± S.E.M. Statistical analyses were performed as follows: plasma CORT levels were analyzed using a one-way analysis of variance (ANOVA). For analysis of microdialysis data, a two-way repeated measures ANOVA was used [factors: time (within subjects factor) and condition (between subjects factor)]. Elevated plus maze data were analyzed by Student’s t-test. Fear discrimination data were analyzed by paired t-test comparing CS+ vs. CS−. Unless stated otherwise, all analyses included a Bonferroni’s post-hoc test. A p value of <0.05 was considered significant in all cases.
Results
Effect of acute stress on plasma CORT levels
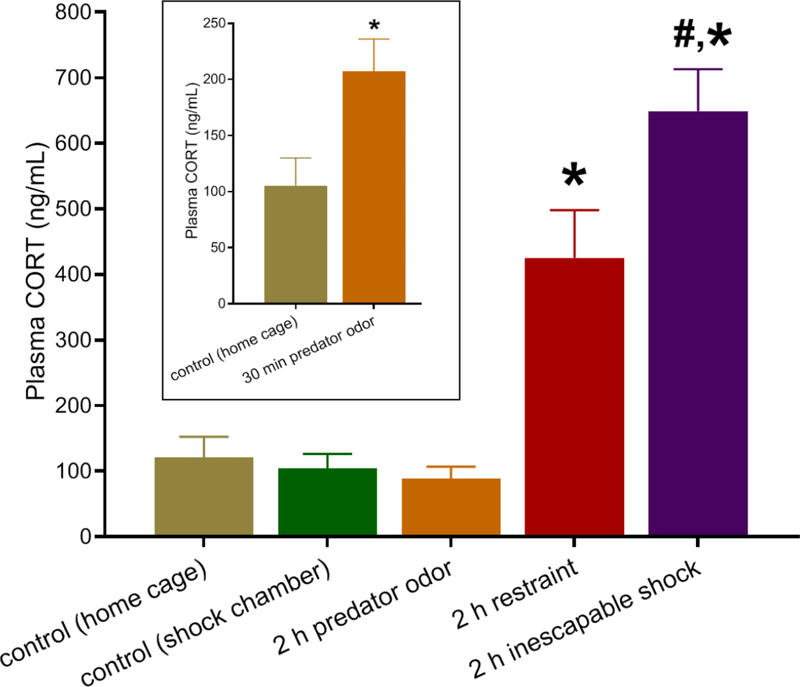
We first tested the effect of the three acute stressors on plasma CORT levels (Fig. 2). Acute stress significantly affected CORT [F(4,41) = 20.56, p<0.0001]. Specifically, 2 h of restraint stress (n = 11) and ISS (n = 12) increased CORT levels by 352 ± 61% and 622 ± 61%, respectively (Bonferroni post-hoc test, p<0.01 and p<0.001, respectively) compared to control rats. Indicating higher stress severity, ISS raised CORT levels nearly twice as much as restraint (Bonferroni post-hoc test, p<0.05).
Figure 2:
Plasma corticosterone (CORT) levels immediately after termination of a 2-h stress. Controls were left undisturbed in their home cage or in the shock chamber for 2 h. Inset: plasma CORT levels after 30 min exposure to fox urine. Data are the mean ± SEM (n = 7–12 per group; inset: n = 4–8 per group). *p<0.05 vs. control, #p<0.05 vs. 2-h restraint.
Although measurement of circulating CORT after a 2-h exposure to predator odor (n = 7) did not reveal significant differences from control (n = 9) levels (Fig. 2; Bonferroni post-hoc test, p>0.05), 30 min of predator odor (n = 4), assessed in a separate cohort, significantly increased CORT levels to 197 ± 27% of controls (n = 8) (Fig. 2 inset; Student’s t-test, t(10) = 0.031, p<0.05).
Effect of acute stress on extracellular KYNA levels in the PFC
Next, we examined the effect of the stressors on extracellular KYNA levels in the PFC (Fig. 3). The average basal concentration of the metabolite in all rats studied was 2.1 ± 0.1 nM (n = 31). Stress significantly affected KYNA levels (main effect of stress: F(9,52) = 3.611, p<0.01). However, of the three stressors tested, only ISS raised extracellular KYNA levels significantly compared to baseline at each timepoint starting 1 h after the initiation of the stress (n = 6; Bonferroni’s post-hoc test, p<0.05), with a maximal increase (194 ± 10%) 1.5 h after ISS termination. In contrast, no significant increases (p>0.05) compared to basal levels were caused by restraint stress (n = 6) or exposure to predator odor (n = 7).
Figure 3:
Effect of various stressors on extracellular KYNA levels in the rat PFC. Basal levels (2.1 ± 0.1 nM) are the average of all animals used in the microdialysis studies. See text for experimental details. Data are the mean ± SEM (n = 6–7 per group). *p<0.05 vs. basal levels.
Behavioral effects of acute stress
Anxiety-like behavior
Separate cohorts of animals were tested on the elevated plus maze immediately after the termination of stress. Irrespective of the stressor used (ISS, restraint or predator odor), none of the rats spent a significantly different amount of time in the open arms compared to control animals (n = 8 per group; each p>0.05 vs. controls).
Fear discrimination
Immediately following evaluation in the elevated plus maze, rats were trained in the fear discrimination paradigm. Baseline freezing behavior before fear conditioning training (Suppl. Fig. 1a) and freezing to each of the cues during training (Suppl. Fig. 1b) were not significantly different between groups. Additionally, regardless of the stressor, rats extinguished at similar freezing rates across the three-day sessions and had similar freezing rates when introduced to a novel context (data not shown). As illustrated in Fig. 4, freezing following re-exposure to CS+ in control rats was significantly more pronounced than after re-exposure to CS− [t(7) = 4.753, p<0.01]. On average, control animals froze 73 ± 6% of the time to CS+, while only freezing to CS− for 46 ± 6% of the time during re-exposure sessions. This discrimination behavior was also seen in rats that had experienced restraint [t(7) = 3.391, p<0.05] or predator odor stress [t(7) = 2.567, p<0.05]. However, rats in the ISS group failed to freeze significantly more often to CS+ vs. CS− [t(7) = 0.859, p=0.42], indicating impaired fear learning. These results indicate that the cue discrimination impairment found in rats exposed to ISS was not due to freezing differences during the training session, differences in context extinction, or pre-exposure effects to the US.
Figure 4:
Effect of various stressors on fear cue discrimination. See text for experimental details. Data are the mean ± SEM (n = 8 per group). *p<0.05 CS+ vs. CS−. **p<0.01 CS+ vs. CS−. ns: not significant.
Effects of KYNA synthesis inhibition
To examine a possible causal role of KYNA in the impaired fear discrimination seen in rats that experienced ISS, animals received a s.c. injection of the specific KAT II inhibitor PF-04859989 (30 mg/kg) or its vehicle 1 h before stress initiation. Separate groups were then examined with the same experimental designs and outcome measures used in the experiments described above.
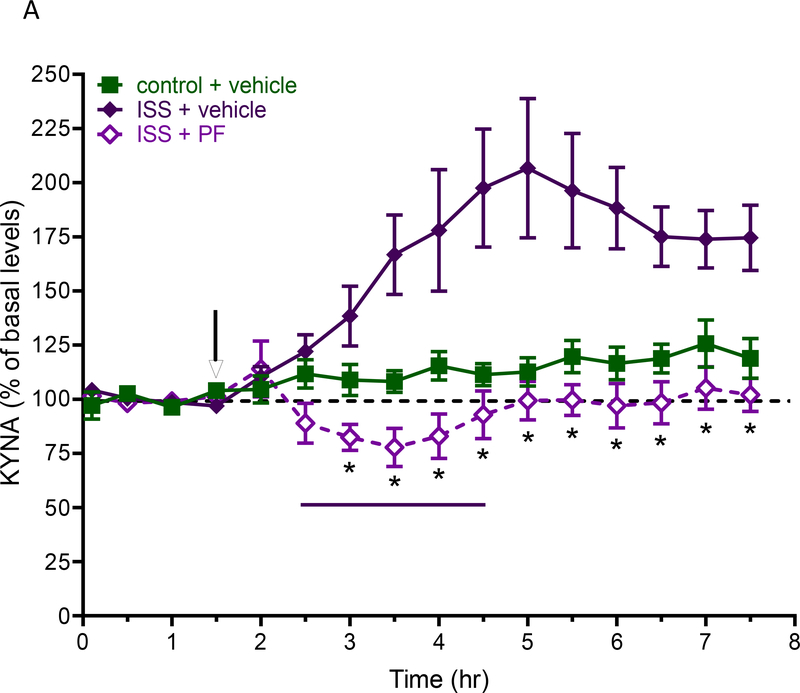
As illustrated in Fig. 5a, pre-treatment with PF-04859989 totally abolished the KYNA increase in animals that had been exposed to ISS compared to vehicle-treated animals (n = 6–7 per group; Bonferroni’s post-hoc test, p<0.05).
Figure 5.
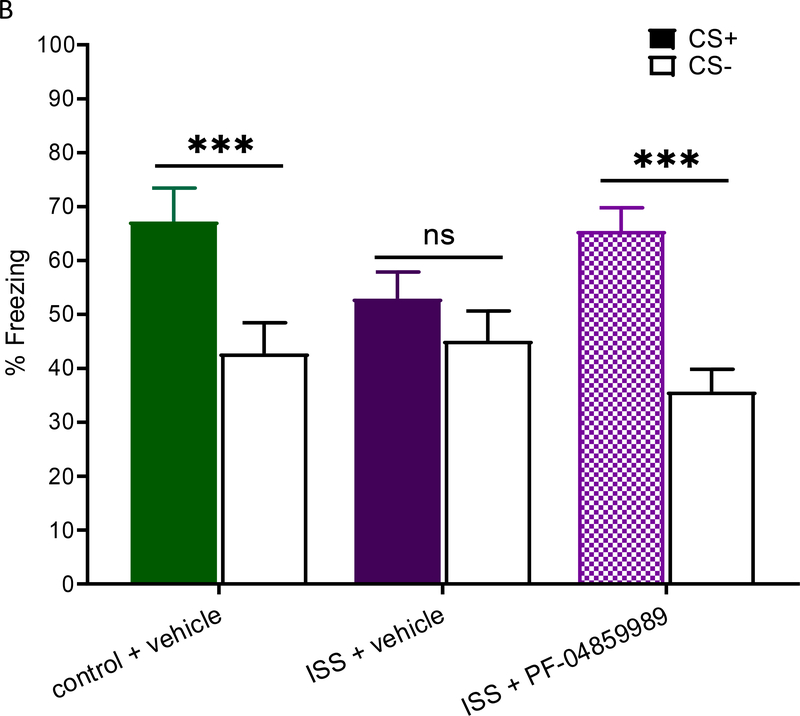
a: Effect of the KYNA synthesis inhibitor PF-04859989 (PF) on extracellular KYNA levels in the PFC of rats receiving inescapable shocks (ISS) for 2 h (bar). PF-04859989 (30 mg/kg, s.c.) was administered 1 h before the initiation of ISS (arrow). See text for experimental details. Data are the mean ± SEM (n = 6–7 per group). *p<0.05 (ISS + vehicle vs. ISS + PF-04859989). b: Effect of PF-04859989 on fear cue discrimination. Data are the mean ± SEM (n = 12–17 per group). ***p<0.001 CS+ vs. CS−. ns: not significant.
Behavioral tests were performed in separate animals (n = 12–17 per group). Unexpectedly, and possibly related to the additional systemic injection the animals received prior to being stressed, both vehicle-pre-treated [t(27) = 3.069, p<0.01] and PF-04859989-pre-treated [t(27) = 3.162, p<0.01] ISS rats showed anxiolytic behavior in the elevated plus maze compared to controls. Of note, this effect was unrelated to KYNA since it was observed after pre-treatment with either vehicle or PF-04859989 [t(32) = 0.009, p=0.99].
Baseline freezing behavior before fear conditioning training (Suppl. Fig. 2a) and freezing to each of the cues during training (Suppl. Fig. 2b) were not significantly different between groups. Moreover, PF-04859989 had no impact on extinction as all three groups froze at similar rates across the three-day sessions and froze similarly when introduced to a novel context (data not shown). In the subsequent cue discrimination test, rats treated with PF-04859989 before receiving ISS were able to distinguish between CS+ and CS− (Fig. 5b; t(16) = 6.313, p<0.001), whereas ISS rats pre-treated with vehicle showed the expected abnormal fear discrimination behavior [t(16) = 1.340, p=0.20; cf. above]. Thus, KAT II inhibition prevented the abnormal behavior seen after ISS, indicating a causal connection between the observed KYNA increase and the impairment in cue discrimination in these animals.
Discussion
In the present study, we tested the effect of acute stressors of varying degrees of severity on brain KYNA and behavior. Our results revealed that the most severe stress we applied, i.e. ISS, raised extracellular KYNA levels in the PFC for a sustained period and led to impairments when animals had to discriminate between a cue that predicts the occurrence of an aversive stimulus and a cue that predicts safety. Importantly, there were no differences in the acquisition of fear condition between the 5 groups used in the study. Moreover, as re-exposure to CS+ and CS− occurred in a different context than acquisition and the ISS, all freezing to CS+ and CS− was due to the cues themselves and not to robust contextual fear. Causality between the neurochemical and behavioral effects of acute ISS was established using the systemically active KYNA synthesis inhibitor PF-04859989, i.e. animals treated with PF-04859989 before receiving ISS were able to differentiate between the auditory cues that were either paired with foot shock or non-reinforced. In other words, the impairment in fear learning observed after a severe acute stress could be averted by preventing the stress-related increase in KYNA levels. As elaborated below, these results may have significant ramifications for both the pathophysiology and the treatment of several brain diseases, including post-traumatic stress disorder (PTSD).
Evidence that stress stimulates tryptophan metabolism along the kynurenine pathway both in the periphery and in the brain has been provided in a considerable number of studies (Laugeray et al. 2010; Gibney et al. 2014; Vecchiarelli et al. 2016; Fuertig et al. 2016; Ohta et al. 2017). Mechanistically, this connection is generally attributed to the fact that stress induces the formation of CORT and pro-inflammatory cytokines, which in turn activate TDO and IDO, respectively (Miura et al. 2008). The interest of neurobiologists in these findings was initially driven by the concept that the shunting of tryptophan degradation toward the formation of kynurenine and its downstream metabolites decreases serotonin production and may, therefore, play a role in the pathophysiology of depressive illness. However, emphasis gradually shifted to the idea that increased levels of kynurenines are directly involved in the pathophysiology of major depressive disorders. To study causality, the animal models used to examine an etiologically relevant link between kynurenines and depression involved either repeated exposure to stressors or prolonged activation of the immune system. The results of these preclinical studies, which in several cases included selective genetic or pharmacological manipulations of kynurenine pathway enzymes, suggested that an extended increase in the activity of the “neurotoxic” branch of the cascade, resulting in more 3-HK and/or QUIN formation at the expense of KYNA, could indeed be of special significance in the pathophysiology of chronic depressive disorders (Arnone et al. 2018; Ogyu et al. 2018).
Some of the most compelling data in this regard pointed to a critical involvement of kynurenine 3-monooxygenase (KMO), which plays a pivotal role in the regulation of the kynurenine pathway (Laumet et al. 2017; Tashiro et al. 2017). In line with this hypothesis, a number of independent studies reported elevated serum levels of 3-HK and QUIN, and an increased ratio between these compounds and KYNA, in the serum of depressed patients (Savitz et al. 2015; Meier et al. 2016; Veen et al. 2016; Aarsland et al. 2019). Complementary evidence came from studies showing increased circulating levels of KYNA following exercise or other antidepressant interventions (Maciejak et al. 2013; Agudelo et al. 2014; Guloksuz et al. 2015; Zhou et al. 2018). However, data from serum measurements in depressed patients are far from consistent (Arnone et al. 2018; Ogyu et al. 2018), and no supportive results were obtained from post-mortem analyses of kynurenines and kynurenine pathway enzymes in the brain of depressed individuals (Miller et al. 2008; Clark et al. 2016). In view of the remaining ambiguities regarding the role of immune and genetic risk factors, and with persisting uncertainties concerning the relationship between peripheral and central kynurenines, the role of kynurenine pathway dysfunction in depressive disorders must therefore still be considered unresolved at this time (see Savitz 2019 for review).
In contrast to the extensive focus on the consequences of repeated or prolonged stress (Laugeray et al. 2010; Dugan et al. 2015; Fuertig et al. 2016; Nold et al. 2019), only very few studies have so far assessed the short-term effects of acute stress on cerebral kynurenine pathway metabolism. Thus, though the rapid response of brain tryptophan levels to immobilization stress or to systemically applied hydrocortisone has been known for decades (Curzon and Green 1971; Young 1981), reports on kynurenines have so far been limited to the rapid effects of repeated electric foot shocks (causing elevated 3-HK and KYNA levels; Pawlak et al. 2000) and of a 2-h restraint during pregnancy, which leads to increased kynurenine and KYNA levels in the fetal brain (Notarangelo and Schwarcz 2017) and to brain region-specific changes in the expression of kynurenine pathway enzymes in adulthood (Vecchiarelli et al. 2016). Focusing on the mechanism of stress-induced impairment specifically in fear discrimination in rats, the present study showed that extracellular concentrations of KYNA in the PFC rise promptly when adult animals are exposed to severe stress. Notably, this increase, which lasted for several hours following the termination of the stress, was only observed in rats which were exposed to the strongest of the 3 stressors used (i.e. ISS), as validated by the largest increase in circulating CORT levels. Thus, the observed acute increase in brain KYNA depends on the severity of the stressor and, possibly, on the developmental status of the affected animal (Notarangelo and Schwarcz 2017).
The fear discrimination impairment shown here is in line with the well-established concept that an increase in brain KYNA levels adversely impacts multiple dimensions of learning and memory, including fear learning (see Introduction). The fact that an acute, stress-induced KYNA surge in the PFC prevents rats from discriminating between a cue that is paired with a foot shock (CS+) and a cue that should indicate safety (CS−) also has considerable translational ramifications. Thus, fear over-generalization is a major component of many stress-related disorders, including PTSD, and discrimination between safe and dangerous environmental cues is evolutionarily critical for survival in the wild (Asok et al. 2019). As this type of cognitive flexibility is strongly controlled by the PFC (Broersen 2000; McKlveen et al. 2015), our findings are in agreement with the fact that executive processes are impaired when the PFC is dysfunctional (Watanabe 2017). Understanding the molecular mechanisms that cause animals to over-generalize a fear response is therefore highly relevant for developing interventions to prevent or alleviate cognitive impairments that are triggered by stressful experiences.
Pre-administration of PF-04859989 normalized the impairment of fear discrimination in all rats experiencing ISS.The observation that selective prevention of the ISS-induced KYNA increase preserved normal fear learning indicates that the elevation in extracellular KYNA participated directly in the behavioral dysfunction seen in these stressed animals. Although the role of brain circuits and clinical implications remain to be clarified, the present findings are both mechanistically and conceptually in line with the fact that a reduction in cortical or hippocampal KYNA levels by genetic (Potter et al. 2010) or pharmacological (Pocivavsek et al. 2011, 2019; Kozak et al. 2014; Wu et al. 2014) means has pronounced pro-cognitive consequences in several established experimental models. Inhibition of KYNA neosynthesis by specifically targeting KAT II may, therefore, have a broader range of clinical implications than previously assumed.
Experiments currently under way in our laboratories are focused on both mechanistic questions and the translational significance of our results. These studies are designed, for example, to determine the nature of the apparent link between circulating CORT and stress-induced brain KYNA formation and to explore anxiety-related behaviors of stressed rats in this context in greater depth. Because of obvious relevance in the clinical realm, we are also testing the longevity of the ISS-induced increase in KYNA levels in the PFC, possible brain region-specificity of the effect and, in particular, the biochemical and behavioral consequences of delayed KAT II inhibition. Additional plans involve the analysis of kynurenine pathway metabolism in humans at various timepoints after experiencing acute stress. Notably, only one study has so far assessed kynurenines in the context of PTSD. This investigation, which compared (90% male) patients with alcohol use disorder with and without PTSD, revealed no significant differences in the kynurenine/tryptophan ratio (a somewhat controversial indicator of kynurenine pathway metabolism; Badawy and Guillemin 2019) in plasma (Neupane et al. 2017).
In conclusion, we show here that sustained elevation of extracellular KYNA levels in the PFC resulting from severe acute stress leads to impaired fear discrimination. Causality between biochemical changes and behavioral outcome was shown by pharmacological means, and implications of these findings for individuals afflicted with stress-related disorders were pointed out. In both humans and in model organisms, future studies should examine KYNA-related mechanisms at different stages of development (Popov et al. 2008; Yoshida et al. 2012; Babenko et al. 2015; Notarangelo and Pocivavsek 2017), the relationship between the immune system and phenomena involving both stress and KYNA (Mándi and Vécsei 2012), and a possible role of kynurenine pathway metabolism in the effects of drugs that are currently used to remedy stress-related disorders (Kim et al. 2019).
Supplementary Material
Acknowledgements
This work was supported by NIMH grant MH103222 (R. Schwarcz, PI) and a Dartmouth College Scholarly Innovation Award (D. Bucci). The authors express their gratitude to Dr. Ana Pocivavsek for her valuable contributions to data interpretation and to Dr. Travis Todd and Nicole DeAngeli for their assistance with the behavioral procedures.
Footnotes
Conflict of interest statement
The authors report no financial or other conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Aarsland TI, Leskauskaite I, Midttun Ø, et al. (2019) The effect of electroconvulsive therapy (ECT) on serum tryptophan metabolites. Brain Stimul 12:1135–1142. 10.1016/j.brs.2019.05.018 [DOI] [PubMed] [Google Scholar]
- Agudelo LZ, Femenía T, Orhan F, et al. (2014) Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159:33–45. 10.1016/j.cell.2014.07.051 [DOI] [PubMed] [Google Scholar]
- Alexander KS, Wu H-Q, Schwarcz R, Bruno JP (2012) Acute elevations of brain kynurenic acid impair cognitive flexibility: Normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berl) 220:627–637. 10.1007/s00213-011-2539-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Saraykar S, Salem H, et al. (2018) Role of kynurenine pathway and its metabolites in mood disorders: A systematic review and meta-analysis of clinical studies. Neurosci Biobehav Rev 92:477–485. 10.1016/j.neubiorev.2018.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A, Kandel ER, Rayman JB (2019) The neurobiology of fear generalization. Front Behav Neurosci 12:1–15. 10.3389/fnbeh.2018.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko O, Kovalchuk I, Metz GAS (2015) Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev 48:70–91. 10.1016/j.neubiorev.2014.11.013 [DOI] [PubMed] [Google Scholar]
- Badawy AA-B, Guillemin G (2019) The plasma [kynurenine]/[tryptophan] ratio and indoleamine 2,3-dioxygenase: time for appraisal. Int J Tryptophan Res 12:117864691986897 10.1177/1178646919868978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E (2016) Differences in cognitive impairment between schizophrenia and bipolar disorder: Considering the role of heterogeneity. Psychiatry Clin Neurosci 70:424–433. 10.1111/pcn.12410 [DOI] [PubMed] [Google Scholar]
- Bradley KAL, Case JAC, Khan O, et al. (2015) The role of the kynurenine pathway in suicidality in adolescent major depressive disorder. Psychiatry Res 227:206–12. 10.1016/j.psychres.2015.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broersen LM (2000) Attentional processes and learning and memory in rats: The prefrontal cortex and hippocampus compared. Prog Brain Res 126:79–94. 10.1016/S0079-6123(00)26008-1 [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Deangeli N, Herrington K, et al. (2016) Alternations in fear behavior following acute stress in adrenalectomized rats: Involvement of kynurenic acid and implications for PTSD. In: Society for Neuroscience Abstract. p 456:05 [Google Scholar]
- Chess AC, Landers AM, Bucci DJ (2009) L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav Brain Res 201:325–331. 10.1016/j.bbr.2009.03.013 [DOI] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE, Bucci DJ (2007) Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull 33:797–804. 10.1093/schbul/sbl033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Pocivavsek A, Nicholson JD, et al. (2016) Reduced kynurenine pathway metabolism and cytokine expression in the prefrontal cortex of depressed individuals. J Psychiatry Neurosci 41:. 10.1503/jpn.150226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon G, Green AR (1971) Regional and subcellular changes in the concentration of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat brain caused by hydrocortisone, DL-α-methyltryptophan l-kynurenine and immobilization. Br J Pharmacol 43:39–52. 10.1111/j.1476-5381.1971.tb07155.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda W, Curzytek K, Kubera M, et al. (2019) Interaction of the immune-inflammatory and the kynurenine pathways in rats resistant to antidepressant treatment in model of depression. Int Immunopharmacol 73:527–538. 10.1016/j.intimp.2019.05.039 [DOI] [PubMed] [Google Scholar]
- Dugan AM, Parrott JM, Redus L, et al. (2015) Low-level stress induces production of neuroprotective factors in wild-type but not BDNF+/− mice: Interleukin-10 and kynurenic acid. Int J Neuropsychopharmacol 19:1–5. 10.1093/ijnp/pyv089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, et al. (2001) Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 313:96–98. 10.1016/S0304-3940(01)02242-X [DOI] [PubMed] [Google Scholar]
- Erhardt S, Lim CK, Linderholm KR, et al. (2013) Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 38:743–752. 10.1038/npp.2012.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS (1980) Conditional and unconditional components of post-shock freezing. Pavlov J Biol Sci Off J Pavlov 15:177–182. 10.1007/BF03001163 [DOI] [PubMed] [Google Scholar]
- Flanagan JC, Hand A, Jarnecke AM, et al. (2018) Effects of oxytocin on working memory and executive control system connectivity in posttraumatic stress disorder. Exp Clin Psychopharmacol 26:391–402. 10.1037/pha0000197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertig R, Azzinnari D, Bergamini G, et al. (2016) Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behaviour: Both effects are reversed by inhibition of indoleamine 2,3-dioxygenase. Brain Behav Immun 54:59–72. 10.1016/j.bbi.2015.12.020 [DOI] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Katz Y, et al. (2010) The possible role of the kynurenine pathway in adolescent depression with melancholic features. J Child Psychol Psychiatry 51:935–43. 10.1111/j.1469-7610.2010.02245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney SM, Fagan EM, Waldron A-MM, et al. (2014) Inhibition of stress-induced hepatic tryptophan 2,3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour. Int J Neuropsychopharmacol 17:917–928. 10.1017/S1461145713001673 [DOI] [PubMed] [Google Scholar]
- Guloksuz S, Arts B, Walter S, et al. (2015) The impact of electroconvulsive therapy on the tryptophan-kynurenine metabolic pathway. Brain Behav Immun 48:48–52. 10.1016/j.bbi.2015.02.029 [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK (1998) Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12:426–45. 10.1016/j.knosys.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EFR, Alkondon M, et al. (2001) The Brain Metabolite Kynurenic Acid Inhibits α7 Nicotinic Receptor Activity and Increases Non-α7 Nicotinic Receptor Expression: Physiopathological Implications. J Neurosci 21:7463–7473. 10.1523/JNEUROSCI.21-19-07463.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ (2008) Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behav Neurosci 122:89–97. 10.1037/0735-7044.122.1.89 [DOI] [PubMed] [Google Scholar]
- Kessler M, Terramani T, Lynch G, Baudry M (1989) A glycine site associated with N methyl-d-aspartic acid receptors: Characterization and identification of a new class of antagonists. J Neurochem 52:1319–1328. 10.1111/j.1471-4159.1989.tb01881.x [DOI] [PubMed] [Google Scholar]
- Kiank C, Zeden J-P, Drude S, et al. (2010) Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS One 5:e11825 10.1371/journal.pone.0011825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Amidfar M, Won E (2019) A review on inflammatory cytokine-induced alterations of the brain as potential neural biomarkers in post-traumatic stress disorder. Prog Neuro-Psychopharmacology Biol Psychiatry 91:103–112. 10.1016/j.pnpbp.2018.06.008 [DOI] [PubMed] [Google Scholar]
- Kindler J, Lim CK, Weickert CS, et al. (2019) Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry 10.1038/s41380-019-0401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak R, Campbell BM, Strick CA, et al. (2014) Reduction of brain kynurenic acid improves cognitive function. J Neurosci 34:10592–10602. 10.1523/JNEUROSCI.1107-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DL, Riedel M, Müller N, et al. (2012) Effects of antidepressants and cyclooxygenase-2 inhibitor on cytokines and kynurenines in stimulated in vitro blood culture from depressed patients. Inflammopharmacology 20:169–176. 10.1007/s10787-011-0112-6 [DOI] [PubMed] [Google Scholar]
- Laugeray A, Launay JM, Callebert J, et al. (2010) Peripheral and cerebral metabolic abnormalities of the tryptophan-kynurenine pathway in a murine model of major depression. Behav Brain Res 210:84–91. 10.1016/j.bbr.2010.02.014 [DOI] [PubMed] [Google Scholar]
- Laumet G, Zhou W, Dantzer R, et al. (2017) Upregulation of neuronal kynurenine 3-monooxygenase mediates depression-like behavior in a mouse model of neuropathic pain. Brain Behav Immun 66:94–102. 10.1016/j.bbi.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH (2006) Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 184:523–539. 10.1007/s00213-005-0164-7 [DOI] [PubMed] [Google Scholar]
- Linderholm KR, Skogh E, Olsson SK, et al. (2012) Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull 38:426–432. 10.1093/schbul/sbq086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejak P, Szyndler J, Turzyńska D, et al. (2013) The kynurenine pathway: A missing piece in the puzzle of valproate action? Neuroscience 234:135–145. 10.1016/j.neuroscience.2012.12.052 [DOI] [PubMed] [Google Scholar]
- Mándi Y, Vécsei L (2012) The kynurenine system and immunoregulation. J Neural Transm 119:197–209. 10.1007/s00702-011-0681-y [DOI] [PubMed] [Google Scholar]
- Martín-Hernández D, Tendilla-Beltrán H, Madrigal JLM, et al. (2018) Chronic mild stress alters kynurenine pathways changing the glutamate neurotransmission in frontal cortex of rats. Mol Neurobiol 1–12. 10.1007/s12035-018-1096-7 [DOI] [PubMed] [Google Scholar]
- McKlveen JM, Myers B, Herman JP (2015) The medial prefrontal cortex: Coordinator of autonomic, neuroendocrine and behavioural responses to stress. J Neuroendocrinol 27:446–456. 10.1111/jne.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Drevets WC, Wurfel BE, et al. (2016) Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun 53:39–48. 10.1016/j.bbi.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Cwik M, et al. (2008) Alterations in kynurenine precursor and product levels in schizophrenia and bipolar disorder. Neurochem Int 52:1297–1303. 10.1016/j.neuint.2008.01.013 [DOI] [PubMed] [Google Scholar]
- Misiak B, Beszłej JA, Kotowicz K, et al. (2018) Cytokine alterations and cognitive impairment in major depressive disorder: From putative mechanisms to novel treatment targets. Prog Neuro-Psychopharmacology Biol Psychiatry 80:177–188. 10.1016/j.pnpbp.2017.04.021 [DOI] [PubMed] [Google Scholar]
- Miura H, Ozaki N, Sawada M, et al. (2008) A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress 11:198–209. 10.1080/10253890701754068 [DOI] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ (2007) The immune-mediated alteration of serotonin and glutamate: Towards an integrated view of depression. Mol Psychiatry 12:988–1000. 10.1038/sj.mp.4002006 [DOI] [PubMed] [Google Scholar]
- Musazzi L, Tornese P, Sala N, Popoli M (2018) What acute stress protocols can tell us about PTSD and stress-related neuropsychiatric disorders. Front Pharmacol 9:1–13. 10.3389/fphar.2018.00758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint A-M, Schwarz MJ, Müller N (2012) The role of the kynurenine metabolism in major depression. J Neural Transm 119:245–251. 10.1007/s00702-011-0741-3 [DOI] [PubMed] [Google Scholar]
- Neupane SP, Bramness JG, Lien L (2017) Comorbid post-traumatic stress disorder in alcohol use disorder: Relationships to demography, drinking and neuroimmune profile. BMC Psychiatry 17:1–10. 10.1186/s12888-017-1479-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold V, Sweatman C, Karabatsiakis A, et al. (2019) Activation of the kynurenine pathway and mitochondrial respiration to face allostatic load in a double-hit model of stress. Psychoneuroendocrinology 107:148–159. 10.1016/j.psyneuen.2019.04.006 [DOI] [PubMed] [Google Scholar]
- Notarangelo FM, Pocivavsek A (2017) Elevated kynurenine pathway metabolism during neurodevelopment: Implications for brain and behavior. Neuropharmacology 112:275–285. 10.1016/j.neuropharm.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo FM, Schwarcz R (2017) Restraint stress during pregnancy rapidly raises kynurenic acid levels in mouse placenta and fetal brain. Dev Neurosci 38:458–468. 10.1159/000455228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogyu K, Kubo K, Noda Y, et al. (2018) Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci Biobehav Rev 90:16–25. 10.1016/j.neubiorev.2018.03.023 [DOI] [PubMed] [Google Scholar]
- Ohta Y, Kubo H, Yashiro K, et al. (2017) Effect of water-immersion restraint stress on tryptophan catabolism through the kynurenine pathway in rat tissues. J Physiol Sci 67:361–372. 10.1007/s12576-016-0467-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug G, Turski WA, Zgrajka W, et al. (2014) Disturbances of tryptophan metabolism and risk of depression in HCV patients treated with IFN-alpha. J Infect Dis Ther 2:1–7. 10.4172/2332-0877.1000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JM, Redus L, Santana-Coelho D, et al. (2016) Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry 6:e918 10.1038/tp.2016.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Jeon WK, Bizon JL, Han JS (2015) Interaction of basal forebrain cholinergic neurons with the glucocorticoid system in stress regulation and cognitive impairment. Front Aging Neurosci 7:1–11. 10.3389/fnagi.2015.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak D, Takada Y, Urano T, Takada A (2000) Serotonergic and kynurenic pathways in rats exposed to foot shock. Brain Res Bull 52:197–205. 10.1016/S0361-9230(00)00252-5 [DOI] [PubMed] [Google Scholar]
- Pocivavsek A, Elmer GI, Schwarcz R (2019) Inhibition of kynurenine aminotransferase II attenuates hippocampus-dependent memory deficit in adult rats treated prenatally with kynurenine. Hippocampus 29:73–77. 10.1002/hipo.23040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Notarangelo FM, Wu H-Q, et al. (2016) Astrocytes as pharmacological targets in the treatment of schizophrenia: Focus on kynurenic acid. Handb Behav Neurosci 23:423–443. 10.1016/B978-0-12-800981-9.00025-0 [DOI] [Google Scholar]
- Pocivavsek A, Wu H-Q, Potter MC, et al. (2011) Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology 36:2357–2367. 10.1038/npp.2011.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov AV, Peresleni AI, Savvateeva-Popova EV, Riederer P (2008) Effect of mutation-induced excess brain concentration of intermediates of the kynurenine pathway of tryptophan metabolism on stress resistance of courtship behavior and communicative sound production in male Drosophila melanogaster. Russ J Genet 44:1061–1069. 10.1134/S1022795408090081 [DOI] [PubMed] [Google Scholar]
- Potter MC, Elmer GI, Bergeron R, et al. (2010) Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology 35:1734–42. 10.1038/npp.2010.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, et al. (2010) CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry 15:393–403. 10.1038/mp.2009.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Murphy ER (2006) Behavioural pharmacology: 40+ Years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci 27:141–148. 10.1016/j.tips.2006.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddick JP, Evans AK, Nutt DJ, et al. (2006) Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med 8:1–27. 10.1017/s1462399406000068 [DOI] [PubMed] [Google Scholar]
- Sathyasaikumar KV, Stachowski EK, Wonodi I, et al. (2011) Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull 37:1147–1156. 10.1093/schbul/sbq112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J (2019) The kynurenine pathway: a finger in every pie. Mol Psychiatry. 10.1038/s41380-019-0414-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Wurfel BE, et al. (2015) Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav Immun 46:55–59. 10.1016/j.bbi.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöner J, Heinz A, Endres M, et al. (2017) Post-traumatic stress disorder and beyond: an overview of rodent stress models. J Cell Mol Med 21:2248–2256. 10.1111/jcmm.13161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu H-Q, et al. (2001) Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 50:521–530. 10.1016/S0006-3223(01)01078-2 [DOI] [PubMed] [Google Scholar]
- Serafini G, Adavastro G, Canepa G, et al. (2017) Abnormalities in kynurenine pathway metabolism in treatment-resistant depression and suicidality: a systematic review. CNS Neurol Disord - Drug Targets 16:440–453. 10.2174/1871527316666170413110605 [DOI] [PubMed] [Google Scholar]
- Sitaramam V, Ramasarma T (1975) Nature of induction of tryptophan pyrrolase in cold exposure. J Appl Physiol 38:245–249. 10.1152/jappl.1975.38.2.245 [DOI] [PubMed] [Google Scholar]
- Tashiro T, Murakami Y, Mouri A, et al. (2017) Kynurenine 3-monooxygenase is implicated in antidepressants-responsive depressive-like behaviors and monoaminergic dysfunctions. Behav Brain Res 317:279–285. 10.1016/j.bbr.2016.09.050 [DOI] [PubMed] [Google Scholar]
- Vecchiarelli HA, Gandhi CP, Hill MN (2016) Acute psychological stress modulates the expression of enzymes involved in the kynurenine pathway throughout corticolimbic circuits in adult male rats. Neural Plast 2016:1–12. 10.1155/2016/7215684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen C, Myint A-M, Burgerhout KM, et al. (2016) Tryptophan pathway alterations in the postpartum period and in acute postpartum psychosis and depression. J Affect Disord 189:298–305. 10.1016/j.jad.2015.09.064 [DOI] [PubMed] [Google Scholar]
- Watanabe M (2017) The prefrontal cortex as an executive, emotional, and social brain [Google Scholar]
- Wu H-Q, Okuyama M, Kajii Y, et al. (2014) Targeting kynurenine aminotransferase II in psychiatric diseases: Promising effects of an orally active enzyme inhibitor. Schizophr Bull 40:S152–S158. 10.1093/schbul/sbt157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-Q, Ungerstedt U, Schwarcz R (1992) Regulation of kynurenic acid synthesis studied by microdialysis in the dorsal hippocampus of unanesthetized rats. Eur J Pharmacol 213:375–380. 10.1016/0014-2999(92)90626-F [DOI] [PubMed] [Google Scholar]
- Yoshida J, Tomonaga S, Ogino Y, et al. (2012) Intracerebroventricular injection of kynurenic acid attenuates corticotrophin-releasing hormone-augmented stress responses in neonatal chicks. Neuroscience 220:142–148. 10.1016/j.neuroscience.2012.06.041 [DOI] [PubMed] [Google Scholar]
- Young SN (1981) Mechanism of decline in rat brain 5-hydroxytryptamine after induction of liver tryptophan pyrrolase by hydrocortisone: roles of tryptophan catabolism and kynurenine synthesis. Br J Pharmacol 74:695–700. 10.1111/j.1476-5381.1981.tb10480.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zheng W, Liu W, et al. (2018) Antidepressant effect of repeated ketamine administration on kynurenine pathway metabolites in patients with unipolar and bipolar depression. Brain Behav Immun 74:205–212. 10.1016/j.bbi.2018.09.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.