Abstract
Objective:
The roles of the large membrane surface of the oxygenator and the high mechanical shear stress (HMSS) of the pump in the ECMO circuit were examined under a pediatric support setting.
Methods:
A clinical centrifugal pump and a pediatric oxygenator were used to construct the ECMO circuit. An identical circuit without the oxygenator was constructed for comparison. Fresh human blood was circulated in the two circuits for 4 hours under the identical pump speed and flow. Blood samples were collected hourly for blood damage assessment, including platelet activation, generation of platelet-derived microparticles (PDMP), losses of key platelet hemostasis receptors (glycoprotein (GP) Ibα (GPIbα) and GPVI) and high molecular weight multimers (HMWM) of von Willebrand factor (VWF) and plasma free hemoglobin (PFH). Platelet adhesion on fibrinogen, VWF and collagen was further examined.
Results:
The levels of platelet activation and generation of PDMP and PFH exhibited an increasing trend with circulation time while the expression levels of GPIbα and GPVI receptors on the platelet surface decreased. Correspondingly, the platelets in the blood samples exhibited increased adhesion capacity to fibrinogen and decreased adhesion capacities on VWF and collagen with circulation time. Loss of HMWM of VWF occurred in both circuits. No statistically significant differences were found in all the measured parameters for blood damage and platelet adhesion function between the two circuits.
Conclusions:
The results indicate that HMSS from the pump played a dominant role in blood damage associated with ECMO and the impact of the large surface of the oxygenator on blood damage was insignificant.
Introduction
Extracorporeal membrane oxygenation (ECMO) has become a viable therapy for patients with respiratory failure and cardiopulmonary collapse refractory to medical therapy. The number of ECMO cases has increased dramatically over the last decade. According to the Extracorporeal Life Support Organization (ELSO) Registry Report in January 2019, 112,231 patients received Extracorporeal Life Support (ECLS) with survival to discharge or transfer rates of approximately 55% [1]. However, ECMO is considered as a last resort and associated with significant morbidity and mortality. Two major life-threatening complications are hemorrhagic (18–35%) and thromboembolic events (4–13%) [2, 3], which are closely related to ECMO-induced hemostatic abnormality in patients [4]. Many factors may contribute to the ECMO-induced hemostatic abnormality in patients, including hemodilution, blood-surface interaction, consumption of coagulation factors and platelets, platelet dysfunction, imbalance between coagulation and fibrinolysis, and systemic anticoagulation [5]. It is commonly believed that the exposure of blood to artificial surfaces of ECMO circuit components (pump, oxygenator, arterial/venous cannulas, and tubing) can alter the coagulation system and trigger activation of platelets, depending on types of artificial materials, anticoagulant therapy, and individual patient characteristics [5, 6]. Therefore, the membrane oxygenator in ECMO or cardiopulmonary bypass circuits has been a major concern to the circuit-induced blood damage because of its large surface area [7, 8].
High mechanical shear stresses (HMSS) at various levels have been shown to cause blood damage [9–15]. The high-speed rotation of an impeller in a typical centrifugal pump used in the ECMO circuit can generate HMSS at levels similar to those observed to cause blood damage [16], including hemolysis, activation of platelets and coagulation factors [9, 17, 18]. Since platelets and coagulation factors are essential for physiological hemostasis and pathological thrombosis, blood damage caused by the exposure to the large surface of the oxygenator and HMSS resulted from pumping blood in the ECMO circuit could potentially contribute to clinically observed bleeding and thrombotic complications. However, it is unclear which factor, large surface area or HMSS, plays a dominant role in blood damage. The aim of this study was to investigate these two perceived contributing factors to ECMO-associated blood damage relevant to hemostatic function. Platelet activation, PDMP generation, surface expression of key platelet hemostasis receptors (GPIbα and GPVI), altered platelet adhesion behavior on biological substrates, loss of HMWM of VWF and hemolysis were characterized in-vitro in a pediatric ECMO support setting.
Materials and methods
Blood collection
Blood was collected from six healthy donors who did not take any medication within 10 days prior to blood donation. All the donors were informed the aim of the study and gave their written informed consent. One unit of blood (450 ml) was drawn from an antecubital vein and directly collected into a sterilized collection bag (Teruflex b-600, Terumo Corporation, Tokyo, Japan) mixed with 3.2% buffered sodium citrate (50 ml) (Medicago AB, Uppsala, Sweden). The study was approved by the Institutional Review Board (IRB) of the University of Maryland, Baltimore and performed in compliance with the regulations of the Helsinki Declaration.
Experimental circuits
Two in-vitro circuits were used in this study (Figure 1). One circuit consisted of a CentriMag centrifugal pump (Abbott, Pleasanton, CA, USA), a custom-made pediatric hollow fiber membrane oxygenator and 3/8 in ID × 1 foot arterial and venous tubing (Medtronic, Minneapolis, MN, USA). The other circuit included only a CentriMag pump and 3/8 in ID × 1 foot arterial and venous tubing without an oxygenator. A custom-made soft-shell bag (250 ml) served as a blood reservoir. The pediatric oxygenator features a cylindrical hollow fiber membrane bundle with an outside-in flow path. The hollow fiber membranes are made from Polymethylpentene (PMP) (Oxplus, 3M/Membrana, Wuppertal, Germany). Table 1 lists the specifications of the CentriMag centrifugal pump and the pediatric oxygenator.
Figure 1.

Photographs of the pediatric ECMO circuit with a CentriMag centrifugal pump and a pediatric oxygenator (left: circuit 1) and the comparison circuit with only the CentriMag pump (right, circuit 2).
Table 1.
Specifications of the circuit components.
| CentriMag Pump | Pediatric oxygenator | |
|---|---|---|
| Blood flow rate | 0–9.9 L/min | 0–2.5 L/min |
| Surface area | 0.014 m2 | 0.4 m2 |
| Rotational speed | 0–5500 rpm | - |
| Priming volume | 31 ml | 60 ml |
| Inlet & outlet ID | 3/8 inch | 3/8 inch |
The hematocrit of the donated collected blood was adjusted to 33.0 ± 2.0% by using phosphate-buffered saline (PBS) with 0.5% bovine serum albumin (BSA). About 300 ml of the blood was primed into each of the circuits. All air bubbles were carefully removed. The rotational speed of the CentriMag pump was adjusted to 3,100 rpm to generate a flow rate of 2.5 L/min against a back pressure of ~ 238 mmHg in the circuits by using a clamp placed at the arterial line. The pre- and post-pump pressures in the two circuits were nearly identical (Table 2). The blood in the circuits was circulated for 4 hours at room temperature. The flow rate was monitored with a clamp-on probe with a transonic flow meter (ME9PXL/T401, Transonic System, Ithaca, NY, USA). The pressures in the circuits were measured with calibrated pressure transducers (1502B01EZ5V10GPSI, PCB Piezotronic, Depew, NY, USA). Blood samples were collected for analysis before and hourly after the initiation of the pump-assisted circulation. Complete blood count was measured with a hematology analyzer (ABX Micros 60, Horiba, Irvine, CA, USA).
Table 2.
Experimental parameters in the two circuits
| Parameters | Circuit 1 (pump + oxygenator) | Circuit 2 (pump only) |
|---|---|---|
| Flow rate (L/min) | 2.5±0.01 | 2.5±0.01 |
| Pre-pump pressure (mmHg) | 29.4±3.5 | 28.3±6.2 |
| Post-pump pressure (mmHg) | 239.3±3.9 | 238.8±6.0 |
| Post-oxygenator pressure (mmHg) | 163.0±1.9 | - |
| Hematocrit (%) | 32.7±0.6 | 32.9± 0.7 |
Note: values are expressed as mean ± standard error
Flow cytometry assays of platelet activation, PDMP generation and shedding of GPIbα and GPVI receptors
Platelet activation in the blood samples was quantified with a whole blood flow cytometry assay. Fluorescein isothiocyanate (FITC)-labeled anti-human PAC-1 antibody, a monoclonal antibody against activated platelet GPIIb/IIIa, was used to detect platelet activation. FITC-labeled immunoglobulin IgMκ antibody served as the negative control for PAC-1. V450-labeled anti-human CD41a was used for platelet identification. All the antibodies were purchased from BD Biosciences (San Jose, CA, USA). 5 μl of whole blood was incubated with 25 μl 10 mM HEPES buffer (Mediatech, Inc., Manassas, VA, USA) containing antibody cocktails for 30 minutes in the dark at room temperature. After incubation, 1% paraformaldehyde (Affymetrix, Inc., Cleveland, OH, USA) in PBS (Quality Biological, Gaithersburg, MD, USA) was used to fix the samples for 30 minutes at 4°C in the dark. Then, flow cytometry data for platelet activation were acquired with a flow cytometer (FACS LSR II, BD Biosciences, San Jose, CA, USA) and analyzed with the FCS Express software (De Novo Software, Los Angeles, CA, USA).
PDMP generation in the blood samples was determined by the forward scatter (FSC) characteristics of all CD41 positive events according to the methods described in Hagberg et al. [19]. Briefly, CD41 positive events were divided into two subpopulations based on the FSC-corresponded sizes. Events in the smaller size population were defined as PDMP. The PDMP generation was expressed by the percentage of PDMP out of all CD41 positive events.
Shedding of platelet GPIbα and GPVI receptors in the blood samples was characterized by quantifying the expression of these two receptors on the platelet surface. BV-510 conjugated anti-CD42b antibody (BD Biosciences San Jose, CA, USA) and eFlour 660 conjugated anti-GPVI antibody (eBioScience, San Diego, CA, USA) were used to determine the surface expression levels of the platelet GPIbα and GPVI receptors, respectively. Platelet population was identified by V450-conjugated anti-CD41a antibody (BD Biosciences) and side scatter (SSC) characteristics. BV-510 and eFluor 660 conjugated IgG1K (IgG1K-BV510) and (IgG1K- eFluor 660) (both from BD Biosciences) were used for the negative controls for the expression of platelet GPIbα and GPVI receptors, respectively. Similar procedures for the blood sample preparation and flow cytometry data acquisition and analysis described above for platelet activation were utilized for determining the expression of platelet GPIbα and GPVI receptors.
Platelet Adhesion Assay
Glass capillary tubes (0.2mm × 2mm × 25mm, VitroCom, Mountain Lakes, NJ) were coated with collagen (1 mg/ml, Chrono-log, Havertown, PA, USA), VWF (100 μg/ml, Haematologic Technologies, Essex Junction, VT, USA) and fibrinogen (1 mg/ml, EMD Millipore), respectively, and incubated in a humidified box at 4°C overnight. The glass tubes were blocked with 1% BSA in PBS at room temperature for one hour. The coated glass capillary tubes were perfused at a shear rate of 500 s−1 in the dark with the blood samples wherein platelets had been fluorescently labeled with mepacrine (10 μM) (Sigma, St. Louis, MO, USA) using a syringe pump (NE 300, New Era Pump Systems, Inc., Farmingdale, NY, USA). After the perfusion, 3.7% paraformaldehyde (PFA) solution was used to fix platelets adhered on the surface. Platelets adhered on the coated glass tube were visualized by using a fluorescent microscope (IX71, Olympus, Tokyo, Japan) equipped with an Olympus DP80 digital camera. Ten digital images (1360 × 1024 pixels) along the center-line of each glass tube were acquired and recorded on a personal computer. The area coverage of the adhered platelets on each image was determined by a custom-written program in MATLAB (Mathworks, Natick, MA, USA).
Quantification of Multimeric Profile of VWF
The multimeric profile of VWF in the blood samples was analyzed by western blotting. Collected platelet poor plasma was mixed with Laemmli sample buffer (1:20) (Bio-Rad, Hercules, CA, USA). 0.6% Agarose gels (Sigma, St. Louis, MO, USA) were used for electrophoresis for separating VWF multimers. After the electrophoresis, the protein ladders were transferred to a 0.45 μm nitrocellulose membrane (Thermo Fisher Scientific, Waltham, MA, USA). The polyclonal rabbit anti-human VWF antibody conjugated with Horseradish Peroxidase (1:3000 dilution) (Agilent Dako, Santa Clara, CA, USA) was used to detect VWF. Blots were developed using Clarity Western ECL Blotting Substrates (Bio-Rad, Hercules, CA, USA) and scanned with a ChemiDoc Imaging System (Bio-Rad). The optical density of each band was quantitated using UN-SCAN-IT Gel 6.1 analysis software (Silk Scientific, Orem, UT, USA). Western blot bands from 1 (lowest molecular weight band) to 10 were classified as low to medium multimers and all those above 10 as HMWM [20]. The HMWM portion of VWF was calculated as the densitometric area of those HMWM bands. The HMWM VWF index was defined as the ratio of the HMWM portion to the densitometric area of total VWF.
Spectrophotometric assay of PFH
PFH concentration was measured by using a hemoglobin assay kit (Sigma-Aldrich, St. Louis, MO, USA) with a SpectraMax M3 Spectrophotometer (Molecular Devices, CA, USA) following the manufacturer’s instruction. Normalized index of hemolysis (NIH) was calculated according to the ASTM standard formula [21].
Statistical analysis
The data were presented as mean ± SE (standard error of the mean). The comparisons of the data at the baseline and post-circulation time points between the two circuits were carried out using the two-way repeated measures ANOVA with a Fisher’s Least Significant Difference (LSD) post hoc test (Prism, GraphPad Software, San Diego, CA). The blood sampling time and the circuit type were the main factors. Regression analysis was performed to determine and compare the rates of change in measured parameters with respect to the blood circulation time between the two circuits. A p-value of less than 0.05 was considered to be statistically significant.
Results
Hematological data
The experimental conditions of the two circuits are listed in Table 2. The priming volume was 322 ± 14 ml in the two circuits. The hematocrit of the primed blood was 32.7 ± 0.6% and 32.9 ± 0.7% in the circuits, respectively. During the 4-hour circulation, the flow rate was 2.50 ± 0.01 L/min in the two circuits. The post-pump pressures were similar in the two circuits (239.3±3.9 mmHg vs. 238.8±6.0 mmHg). The pressure drop across the pediatric oxygenator was 76.3 mmHg. The measured hematocrit, RBC and platelet counts over the 4-hour circulation were similar in the two circuits (Figure 2). There was a slight decrease in both the hematocrit and RBC count in the blood samples collected at the first hour after the pump-assisted circulation. Thereafter, the hematocrit and RBC counts were stable. There were slight increases in the measured platelet counts over the circulation time in the two circuits. The increase might be caused by counting fragments from other larger cells (RBC and leukocytes) as the platelet population since the hematology analyzer counts each platelet in whole blood based on the size of each particle. There was no overall statistical difference in these measured hematological parameters over the 4-hour circulation between the two circuits.
Figure 2.



Measured hematological parameters of the blood in the two circuits over time: (a) Hematocrit, (b) RBC count, and (c) platelet count.
Platelet activation and PDMP generation
The levels of platelet activation in the blood in the circuits over time, as indicated as the percentage of PAC-1 positive platelets in the platelet population, are shown in Figure 3 (a), and PDMP generation as indicated as the percentage of CD41 positive small particles in the population of all CD-41 positive events in Figure 3 (b). Both the platelet activation and PDMP generation significantly increased after the first hour after initiation of the circulation in the two circuits. However, there was no statistical difference in the levels of platelet activation and PDMP generation in the blood samples at any time points between the two circuits. The regression analysis indicated that the slopes of the trending lines for platelet activation and PDMP generation are not statistically different between the two circuits.
Figure 3.

Levels of platelet activation and generation of platelet derived microparticles (PDMP) in the hourly collected blood samples from the two circuits over time.
Loss of platelet GPIbα and GPVI receptors
The expression levels of GPIbα and GPVI receptors on the platelet surface in the blood in the circuits over time are shown in Figure 4. The data are presented as the normalized mean flow cytometric fluorescence intensity to the baseline blood sample. The fluorescence intensity can represent the relative quantity of the GPVI and GPIbα receptors on the platelet surface since the intensity of the fluorescent light is proportional to the fluorophores-conjugated to the antibody bound to the available receptors on the platelet surface. The data indicate that there was a progressive decrease in the expression of GPIbα and GPVI on the platelet surface with increasing circulation time in the two circuits, suggesting the loss of these receptors. The decreases in the expression levels of GPIbα and GPVI receptors on the platelet surface became statistically significant at one hour and two hours after the pump-assisted circulation, respectively. However, there is no statistical difference in the expression levels of GPIbα and GPVI receptors on the platelet surface in the blood samples collected at any time points between the two circuits. The regression analysis indicated that the slopes of the decreasing trending lines for the expression levels of the two key platelet hemostasis receptors (GPIbα and GPVI) between the two circuits are not statistically different.
Figure 4.

Expression levels of platelet GPIbα and GPVI receptors on the platelet surface in the hourly collected blood samples from the two circuits over time.
Alteration of platelet adhesion capacities
The changes in the platelet adhesion capacities to VWF, collagen and fibrinogen of the blood in the circuits over time are showed in Figures 5 and 6. Figure 5 shows representative fluorescent images of adherent platelets on VWF, collagen and fibrinogen for the blood samples collected baseline and three hours after circulation in the two circuits. It can be seen in the images that there were fewer adherent platelets on VWF and collagen for the blood samples collected at three hours after circulation in the circuits compared with the baseline blood samples. In contrast, there were more adherent platelets on fibrinogen in the blood samples collected at three hours after circulation compared with the baseline blood sample. The area coverages of platelets on VWF, collagen and fibrinogen by platelets for the baseline and four hourly collected blood samples are shown in Figure 6. The area coverages of platelets on VWF and collagen progressively decreased over time. In contrast, the area coverage of platelets on fibrinogen increased over time. The platelet adhesion capacities to VWF, collagen, and fibrinogen were significantly altered after the first hour of the pump-assisted circulation in both the two circuits. However, there were no statistical differences in the changes in the platelet adhesion capacities on the three hemostasis-relevant biological substrates between the two circuits. The regression analysis indicated that there were no statistical differences in the rates (slopes) of the increase or decrease in the platelet adhesion capacities between the circuits.
Figure 5.

Representative fluorescent images of adherent platelets on VWF, collagen, and fibrinogen of the blood samples at baseline and three hours after circulation in the two circuits (magnification, X400).
Figure 6.


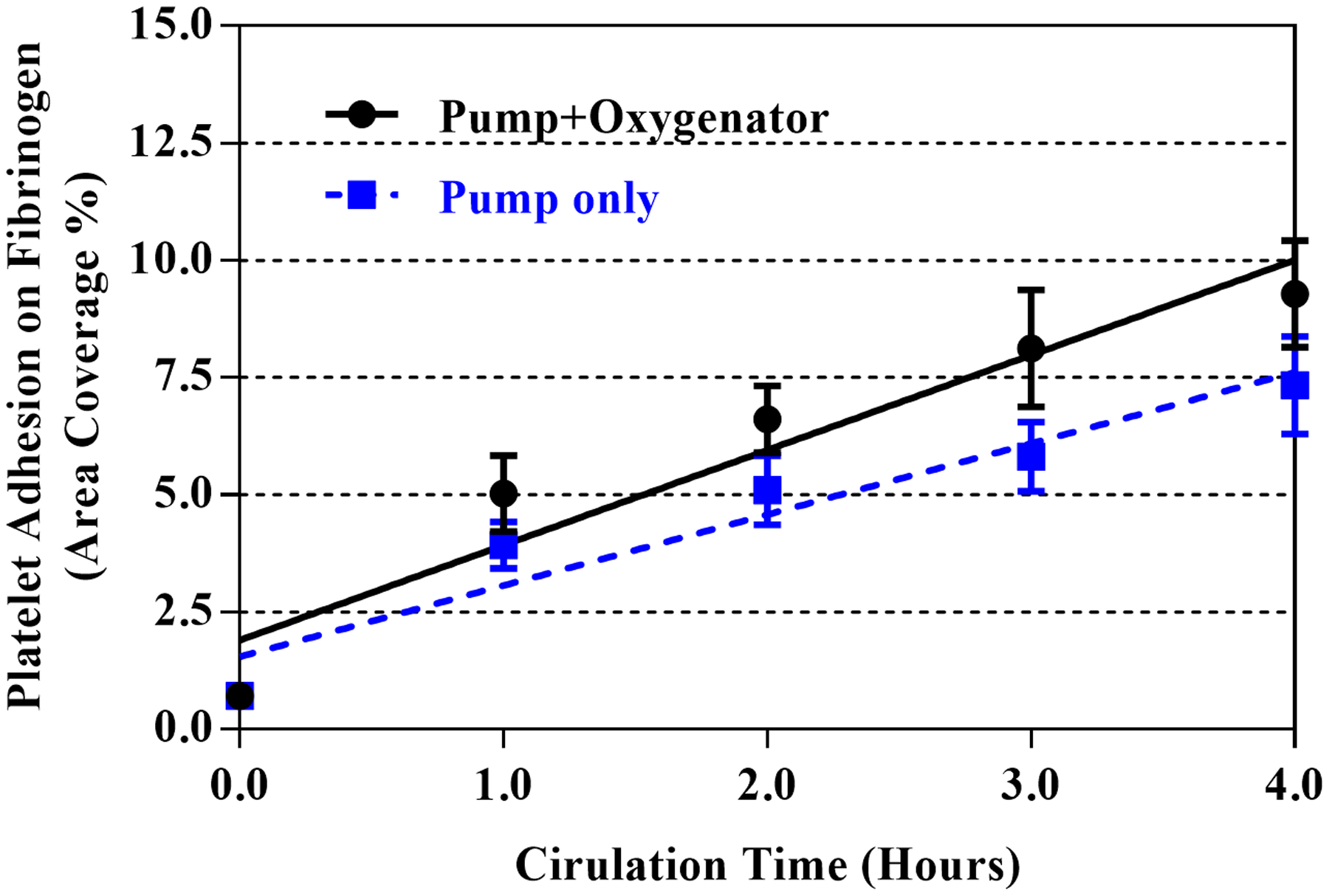
Changes in platelet adhesion capacities on three biological substrates of the hourly collected blood samples from the two circuits over time: (a) VWF, (b) collagen, and (c) fibrinogen.
Loss of HMWM-VWF
Representative western blots of the multimeric profile of VWF in the blood samples collected prior to and after the initiation of the pump-assisted circulation in the two circuits were depicted in Figure 7 (a). It can be clearly seen that the highest molecular weight bands of VWF disappeared in the blood after the first hour of the pump-assisted circulation. The remaining higher molecular weight bands (>10) of VWF had relatively slow degradation thereafter. The quantitative comparison of the HMWM portion of VWF in the baseline and four hourly blood samples after the blood pump-assisted circulation in the two circuits are shown in Figure 7 (b). The data were fitted well to a quadratic function (R2> 0.62). The overall trend of the loss of the HMWM of VWF with the circulation and fit coefficients were almost identical between the two circuits.
Figure 7.

Representative images of multimeric bands of VWF (a) and quantification of the loss of HMWM of VWF (b) in the hourly collected blood samples from the two circuits over time.
Hemolysis – PFH Generation
After the 4-hour pump-assisted circulation in the two circuits, the PFH generation was relatively low in both the circuits, indicating low hemolysis. The mean NIH values were 0.0045±0.0034 g/100 L in the circuit with the CentriMag pump and the pediatric oxygenator and 0.0052±0.0040 g/100L in the circuit with the CentriMag pump only. No statistical difference was found between the two circuits.
Discussion
The effects of the oxygenator surface and the HMSS on blood damage related to hemostatic dysfunction in a pediatric ECMO circuit setting were carefully examined in this study. The results from this study showed that both the ECMO circuit with the CentriMag pump and the pediatric oxygenator, and the circuit with the CentriMag pump only caused platelet activation and PDMP generation with an increasing trend over time. Correspondingly, platelet adhesion on fibrinogen increased with the circulation time. The results also showed that the expression levels of the two important hemostasis-relevant platelet receptors (GPIbα and GPVI) in the blood decreased with the circulation time. Corresponding to the decreased expression of GPIbα and GPVI receptors, it was found that platelet adhesion capacities on VWF and collagen decreased with the circulation time, suggesting that the pump-assisted circulation in the two circuits caused shedding (loss) of these receptors on the platelet surface.
As implicated by the above results, the pump-assisted circulation-induced platelet activation and receptor shedding could result in two opposite effects on the platelet hemostatic function. The platelet activation enhances the adhesion of platelets to fibrinogen, which can lead to an elevated risk of thrombosis. When activated platelets circulate through the patient’s vasculature and the ECMO extracorporeal circuit, it is easier for them to aggregate on the artificial surface of the circuit with deposited fibrinogen or in low shear regions of the vasculature or at sites of vascular injury. At the same time, the loss of the platelet GPIbα and GPVI receptors can compromise the platelet adhesion capacities on related proteins (VWF and collagen), which may lead to hemostasis dysfunction and elevated propensity of bleeding. When platelets with lower adhesion capacities circulate in the patient’s vasculature, it is more likely to lead to the weakened binding of platelets with VWF and collagen at sites of vascular injury. Thrombosis and bleeding are two clinically-significant complications in patients on ECMO support. Concurrent thrombosis and bleeding indeed occur in many patients [2, 3, 22]. Our data suggest that platelet activation and loss of key platelet receptors may be the underlying reason for the current risk for bleeding and thrombosis in addition to inadequate anticoagulation therapies.
Our data also showed that the loss of extreme HMWM (bands > 17) of VWF occurred rapidly, mostly within the first hour of the assisted circulation while the remaining HMWM (bands between 10 to 16) of VWF experienced relatively slow degradation. The trend of circuit-induced damage to VWF was different from those of platelet activation and receptor shedding (see Figures 3, 4 and 6). Both the levels of platelet activation and receptor shedding, as well as the platelet adhesion capacities on fibrinogen, vWF and collagen continued to increase or decrease after the first hour of the circulation. This observation may underscore the importance of circuit-induced platelet dysfunction relative to the loss of HMWM of VWF. Our data also showed that the hemolysis levels in the two circuits were relatively low, comparable to the levels produced by contemporary implantable VADs [23]. This is consistent with the reported clinical observation that marked hemolysis is not common in ECMO patients with modern devices and clinically obvious hemolysis often is caused by pump head thrombosis [24].
The large surface area of the blood oxygenator in an ECMO circuit has been perceived to be a major contributor to the ECMO-related blood cell damage, leading to hemostatic dysfunction [5, 6, 20]. In our study, we did not find statistically significant differences between the circuit with the CentriMag pump plus the pediatric oxygenator and the circuit with the CentriMag pump only with respect to all the above measured parameters relevant to hemostatic function. This finding indicates that the impact of the large surface of the oxygenator on blood damage was insignificant compared with HMSS.
Study Limitation
The experiments were performed in-vitro using the blood from healthy donors. The results may not represent real clinical scenarios for patients receiving ECMO support. Blood from healthy donors might be less susceptible to HMSS than patients with cardiopulmonary diseases. Platelet count did not significantly change after the 4-hour circulation because of the lack of platelet clearance and production that occur in the human body. Further in-vivo studies shall be performed to investigate ECMO circuit-induced blood damage by HMSS.
Conclusion
Pump-assisted circulation in ECMO circuits can induce platelet activation and receptor shedding which have opposite effects on platelet hemostatic function. Activated platelets have enhanced adhesion capacity on fibrinogen, which increases the risk of thrombosis. The loss of hemostasis related receptors (GPVI and GPIbα) weakens the platelet adhesion capabilities to collagen and VWF, which compromises platelet hemostasis function and increases the risk of bleeding. The data from this study may explain the concurrent risks of thrombosis and bleeding in patients on ECMO support. The results from this comparative study indicate that HMSS generated from the pumping action in the ECMO circuit played a dominant role in blood damage relevant to hemostatic function in mechanically assisted circulation and the impact of the surface of the oxygenator on blood damage was insignificant.
Acknowledgments
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (Award Numbers: R01HL118372, R01HL124170, and R01HL141817). The authors would like to acknowledge that Mr. Zachary Berk participated in some experiments.
Abbreviations and Acronyms
- BSA
bovine serum albumin
- ELSO
Extracorporeal Life Support Organization
- ECLS
extracorporeal life support
- ECMO
extracorporeal membrane oxygenation
- FITC
fluorescein isothiocyanate
- HMSS
high mechanical shear stress
- HMWM
high molecular weight multimer
- PBS
phosphate-buffered saline
- PDMP
platelet-derived microparticles
- PFH
plasma free hemoglobin
- VWF
von Willebrand factor
Footnotes
Conflict of Interest Statement
B.P. Griffith and Z.J. Wu disclose intellectual property and ownership interests in Breethe, Inc. J. Zhang discloses intellectual property interest related to blood oxygenators. All other authors declare that they have no conflict of interest in the subject matter or materials discussed in this study.
References
- 1.Extracorporeal Life Support Organization. ECLS Registry Report, International Summary. Ann Arbor, MI: Extracorporeal Life Support Organization, January 2019;1–37. [Google Scholar]
- 2.Sy E, Sklar MC, Lequier L, Fan E, and Kanji HD. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: A systematic review and meta-analysis. J Crit Care 2017; 39: 87–96. [DOI] [PubMed] [Google Scholar]
- 3.Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017; 63: 60–67. [DOI] [PubMed] [Google Scholar]
- 4.Balle CM, Jeppesen AN, Christensen S, and Hvas AM. Platelet Function During Extracorporeal Membrane Oxygenation in Adult Patients: A Systematic Review. Front Cardiovasc Med 2018; 5: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muntean W. Coagulation and anticoagulation in extracorporeal membrane oxygenation. Artif Organs 1999; 23: 979–83. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimoto Y, Hasebe T, Takahashi K, et al. Ultrastructural characterization of surface-induced platelet activation on artificial materials by transmission electron microscopy. Microsc Res Tech 2013; 76: 342–9. [DOI] [PubMed] [Google Scholar]
- 7.Vroman L, Adams AL, and Klings M. Interactions among human blood proteins at interfaces. Fed Proc 1971; 30: 1494–502. [PubMed] [Google Scholar]
- 8.Weerasinghe A and Taylor KM. The platelet in cardiopulmonary bypass. Ann Thorac Surg 1998; 66: 2145–52. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Mondal NK, Zheng S, et al. High shear induces platelet dysfunction leading to enhanced thrombotic propensity and diminished hemostatic capacity. Platelets 2019; 30: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leytin V, Allen DJ, Mykhaylov S, et al. Pathologic high shear stress induces apoptosis events in human platelets. Biochem Biophys Res Commun 2004; 320: 303–10. [DOI] [PubMed] [Google Scholar]
- 11.Holme PA, Orvim U, Hamers MJ, et al. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler Thromb Vasc Biol 1997; 17: 646–53. [DOI] [PubMed] [Google Scholar]
- 12.Blackshear PL, Mechanical hemolysis in flowing blood In: Fung YC, Perrone N, Anliker M, Editors. Biomechanics: Its Foundation and Objectives. Englewood Cliffs, NJ; Prentice-Hall; 1972. p. 501–528. [Google Scholar]
- 13.Bluestein D. Yin W, Affeld K, Jesty J. Flow-induced Platelet Activation in Mechanical Heart Valves. J. Heart Valve Disease 2004; 13: 501–508. [PubMed] [Google Scholar]
- 14.Tsai HM. von Willebrand factor, shear stress, and ADAMTS13 in hemostasis and thrombosis. ASAIO J 2012; 58: 163–9. [DOI] [PubMed] [Google Scholar]
- 15.Ding J, Chen Z, Niu S, et al. Quantification of Shear-Induced Platelet Activation: High Shear Stresses for Short Exposure Time. Artif Organs 2015; 39: 576–83. [DOI] [PubMed] [Google Scholar]
- 16.Fraser KH, Zhang T, Taskin ME, Griffith BP, and Wu ZJ. A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. J Biomech Eng 2012; 134: 081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs G, Berg N, Broman LM, and Prahl Wittberg L. Flow-induced platelet activation in components of the extracorporeal membrane oxygenation circuit. Sci Rep 2018; 8: 13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horobin JT, Sabapathy S, and Simmonds MJ. Repetitive Supra-Physiological Shear Stress Impairs Red Blood Cell Deformability and Induces Hemolysis. Artif Organs 2017; 41: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 19.Hagberg IA and Lyberg T. Blood platelet activation evaluated by flow cytometry: optimised methods for clinical studies. Platelets 2000; 11: 137–50. [DOI] [PubMed] [Google Scholar]
- 20.Sakatsume K, Saito K, Akiyama M, et al. Association between the severity of acquired von Willebrand syndrome and gastrointestinal bleeding after continuous-flow left ventricular assist device implantation. Eur J Cardiothorac Surg 2018; 54: 841–846. [DOI] [PubMed] [Google Scholar]
- 21.ASTM International. The Standard Practice for Assessment of Hemolysis in Continuous Blood Flow Pumps - ASTM F1841 – 97, 2017.
- 22.Murphy DA, Hockings LE, Andrews RK, et al. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev 2015; 29: 90–101. [DOI] [PubMed] [Google Scholar]
- 23.Berk ZBK, Zhang J, Chen Z, Tran D, Griffith BP, and Wu ZJ. Evaluation of in vitro hemolysis and platelet activation of a newly developed maglev LVAD and two clinically used LVADs with human blood. Artif Organs 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehle K, Philipp A, Zeman F, et al. Technical-Induced Hemolysis in Patients with Respiratory Failure Supported with Veno-Venous ECMO - Prevalence and Risk Factors. PLoS One 2015; 10: e0143527. [DOI] [PMC free article] [PubMed] [Google Scholar]


