Abstract
Cranial electrical stimulation (CES) is a noninvasive brain stimulation technique that has been shown to improve pain. However, few studies have investigated the potential benefits associated with remotely supervised CES in older adults with knee osteoarthritis (OA). The aim of this study was to examine the feasibility and preliminary efficacy of remotely supervised CES via secure videoconferencing software on clinical pain severity, experimental pain sensitivity, and pain-related cortical response in older adults with knee OA. Thirty participants with symptomatic knee OA pain were randomly assigned to receive 10 daily sessions (60 minutes each) of remotely supervised CES (n=15) or sham CES (n=15) over two weeks. We measured clinical pain severity via a Numeric Rating Scale, experimental pain sensitivity (e.g., heat pain sensitivity, pressure pain sensitivity, and conditioned pain modulation) using quantitative sensory testing, and pain-related cortical response via functional near-infrared spectroscopy imaging. We also measured participant satisfaction with treatment using the Client Satisfaction Questionnaire. Active CES significantly reduced scores on the Numeric Rating Scale and increased heat pain threshold, pressure pain thresholds, and conditioned pain modulation. We also found significant changes in pain-related cortical hemodynamic activity after CES. Participants tolerated CES well without serious adverse effects and were satisfied with the treatment. Our findings demonstrate promising clinical efficacy of remotely supervised CES for older adults with knee OA.
Keywords: cranial electrical stimulation, knee osteoarthritis, quantitative sensory testing, conditioned pain modulation, quantitative sensory testing
1. Introduction
Chronic pain affects 100 million people in the United States, and its treatment costs up to $635 billion annually [1]. Osteoarthritis (OA) is one of the most prominent causes of chronic pain, functional impairment, and disability in older adults [2–4]. OA predominantly involves weight-bearing joints, with knees being the most affected location of pain [5, 6]. Current treatment modalities for knee OA are aimed toward reducing symptom burden and largely comprise pharmacologic agents, including local and systemic analgesics [7–9]. However, managing OA pain is particularly challenging for older adults, because existing pharmacologic approaches often produce significant adverse effects (e.g., drowsiness, constipation, and falls) [7–9]. Moreover, recent literature suggests alterations in pain-related brain mechanisms are associated with OA-related clinical pain and symptoms [10–14]. These findings have prompted increased attention toward novel nonpharmacologic pain management approaches targeting pain-related brain function for patients with knee OA.
Noninvasive brain stimulation, such as cranial electrical stimulation (CES), has received notable consideration for managing pain in patients with chronic conditions owing to its neuromodulatory effects in the central nervous system. CES delivers a low-amplitude alternating electric current to the brain via electrodes applied to the earlobes in a noninvasive and painless manner and augments the brain’s response to painful stimuli by facilitating the reversal of maladaptive cortical reorganization within the brain [15]. CES, which is a safe modulation of brain activity, has been approved by the U.S. Food and Drug Administration for treating pain.
As a nonpharmacologic intervention for OA pain, portable CES that can be used at home offers several advantages in that only a brief training is needed for use; it is minimally burdensome; and it can be used as an adjunct therapy to other interventions as needed. Home-based interventions are especially critical, as older adults have great limited mobility and difficulty attending clinic-based sessions over several days [16–18]. Furthermore, recent technological advances have strengthened the potential applicability of home interventions with real-time monitoring through a secure videoconferencing platform, thus facilitating high adherence. Therefore, the purpose of this pilot clinical study was to examine the feasibility and preliminary efficacy of remotely supervised CES via secure videoconferencing software on clinical pain severity, experimental pain sensitivity, and pain-related cortical response in older adults with knee OA.
2. Methods
The study protocol was approved by the UTHealth Institutional Review Board prior to commencement and is registered with ClinicalTrials.gov (NCT04016259). Interested individuals received a brief phone pre-screening to verify basic eligibility, and then were scheduled for a full in-person screening, at which time we obtained written informed consent. All participants were given detailed information about the protocol and notified that they could receive either sham or active CES.
2.1. Participants
Participants with symptomatic knee OA pain were recruited in the Houston metropolitan area between August 2019 and November 2019 using advertisements in local institutions and communities. Individuals 50–85 years old were considered eligible if they had self-reported unilateral or bilateral knee OA pain, according to American College of Rheumatology criteria [19]; had knee OA pain in the past 3 months with an average rating of at least 30 on a 0–100 Numeric Rating Scale (NRS) for pain; had a smartphone that could be used for secure videoconferencing software (e.g., WebEX); and had no plan to change medication regimens for pain throughout the trial. According to American College of Rheumatology criteria [19], participants must meet at least 3 of 6 criteria for symptomatic knee OA, such as age over 50 years, less than 30 minutes of morning stiffness, crepitus on active motion, bony tenderness, bony enlargement, and no palpable warmth of synovium. Participants were excluded if they had any concurrent medical conditions that could pose a safety risk for any of the assessments or preclude the successful completion of the protocol, including a history of brain surgery, brain tumor, seizure, stroke, or intracranial metal implantation; uncontrolled hypertension, heart failure, or myocardial infarction; alcohol/substance abuse; cognitive impairment; and a history of psychiatric illness.
2.2. Study design
The study was conducted in a single-center, double-blind, randomized, sham-controlled clinical design to evaluate the feasibility and preliminary efficacy of 10 daily sessions of remotely supervised CES for 60 minutes over two weeks (Monday through Friday) for pain in older adults with knee OA. Neither the participants nor experimenters were aware of the intervention condition. Participants who gave informed consent were randomly assigned to either the active CES (n = 15) or sham CES group (n = 15), and allocation concealment was ensured. The randomization balanced the allocation of participants to the study arms by a statistician with no clinical involvement in this trial with respect to distributions of age, sex, and race using the method of minimization [20]. Participants received in-person training at the baseline visit for the remotely supervised CES intervention, and all intervention sessions were remotely supervised via secure videoconferencing software (e.g., WebEx) by trained research staff to ensure optimal protocol adherence.
2.3. Remotely supervised CES intervention
CES was applied for 60 minutes per session daily for 2 weeks (Monday through Friday) via the Alpha-Stim M Stimulator (Electromedical Products International, Inc., Mineral Wells, TX). The electrode pads of the ear clips were moisturized using a conduction solution provided by the manufacturer before applying the ear clips to the earlobes. The CES devices were preset at 0.1 mA at a frequency of 0.5 Hz by the manufacturer (only the on/off button was operable by the study participants; they were not able to adjust the device settings). After the participant turned on the CES device, the screen on the device showed a timer that counted down the minutes until the end of the session. At the completion of 60 minutes, the device shut down automatically, and study staff instructed the participant to remove the ear clips and safely store all materials for the next session.
2.4. Clinical pain severity and experimental pain sensitivity measure
Clinical pain was measured via an NRS, whereby participants rated their current knee pain from 0 (no pain) to 100 (worst pain imaginable). In addition, a multimodal quantitative sensory testing was completed to measure experimental pain sensitivity, including heat pain sensitivity (i.e., threshold and tolerance), pressure pain threshold (PPT), and conditioned pain modulation (CPM). First, thermal stimuli were delivered using a computer-controlled TSA-II NeuroSensory Analyzer (Medoc Ltd., Ramat Yishai, Israel) to measure heat pain threshold and heat pain tolerance on the index knee using an ascending method of limits. From a baseline of 32°C, the thermode temperature increased by 0.5°C per second until the participants responded by pressing a button to stop heat stimuli. Participants were instructed to press the button when the sensation “first becomes painful” to assess the heat pain threshold and when they “no longer feel able to tolerate the pain” to assess their heat pain tolerance.
Following the assessment of heat pain sensitivity, a handheld digital pressure algometer (Wagner, Greenwich, CT) was applied at a constant rate of 0.3 kgf/cm2 per second to measure the PPT on the index knee. For assessing the PPT, participants were asked to notify the experimenter when the pressure sensation “first becomes painful,” and the pressure was recorded. Five minutes following the assessment of the PPT, CPM was assessed as a measure of descending pain inhibition. CPM was assessed by determining the change in PPT on the trapezius immediately after the immersion of the contralateral hand up to the wrist in a cold-water bath (Neslab, Portsmouth, NH) at 12°C for one minute. We selected this temperature based on previous studies in older adults with knee OA, and this approach is commonly used in clinical pain studies [21–23]. These quantitative sensory testing measures were obtained twice (at baseline and after the final intervention session).
2.5. Pain-related cortical response
We measured pain-related cortical response using a continuous-wave, multichannel functional near-infrared spectroscopy (fNIRS) imaging system (LIGHTNIRS, Shimadzu, Kyoto, Japan) with three semiconductor lasers at 780, 805, and 830 nm. The illumination and detection optodes were arranged in a geometrical layout that covered the prefrontal cortex regions bilaterally, consistent with the locations investigated in previous studies [24, 25]. For evoked pain scans, moderate-intensity thermal stimuli was applied to the right arm for 20 seconds during multiple scanning runs, followed by an approximately equivalent interval of no stimulation, which is commonly used in pain-related neuroimaging studies [24–27].
2.6. Feasibility and Tolerability
We collected data on participants’ acceptability of treatment via a 10-item questionnaire, adapted from Gillick et al. [28] and Cha et al. [29], after the conclusion of the CES intervention on a 0 (strongly disagree) to 10 (strongly agree) scale. In addition, we measured participants’ satisfaction with the CES intervention using the Client Satisfaction Questionnaire (CSQ-8) [30, 31]. The CSQ-8 comprises eight items that are summed to yield an overall score of 8–32, with higher scores indicating greater satisfaction. Furthermore, we evaluated the presence and severity of possible side effects of CES, including itching, burning, headache, fatigue, nervousness, dizziness, and difficulty concentrating, at the end of each session on a 0 (not at all) to 10 (highest degree) scale. This approach was commonly used in previous studies [23, 32].
2.7. Statistical analysis
SAS 9.4 was employed to conduct all statistical analyses in this study. Data were screened for outliers using median absolute deviation. The Shapiro-Wilk test was used to examine the normality assumption of data distribution. Descriptive statistics were generated to summarize the demographics of participants. Two sample t-tests (or a Wilcoxon-Mann-Whitney test when appropriate) and Chi-square tests were used for continuous variables (e.g., age, weight) and categorical variables (e.g., gender, race), respectively, to compare the active CES group with the sham CES group.
To adjust for participants’ baseline heterogeneity, the differences in related pain scores between the baseline and the end of the treatment were calculated. A two-sample t-test (or Wilcoxon-Mann-Whitney test for non-normal data) was then employed to examine the significance of such differences in clinical pain measurements. Bonferroni correction was not applied for multiple testing, given that this was a feasibility study. Moreover, Cohen’s d effect size was computed for each pain score to quantify the standardized difference between the two groups.
To obtain cortical hemodynamic responses to the pain stimulation, raw optical signals of each optical channel (i.e., source-detector pairing) were converted to changes in concentrations of oxygenated hemoglobin according to the Modified Beer-Lambert law [33, 34]. The quality of the recording of each channel was assessed using a quantitative method that estimates the strength of the physiological cardiac pulsation, which directly indicates a good optical coupling between the scalp and the fNIRS optode. At the individual level, the cortical hemodynamic activity in response to thermal stimulation was evaluated with a general linear model based on an autoregressive iteratively reweighted least squares approach, as described in the previous study [35]. At the group level, we used a linear mixed model to assess the relationship between the estimated coefficient of each optical channel and the interaction of time and treatment (fixed effect), considering the subject-specific intercept value as a random effect. Conversion from raw data to hemodynamic parameters and individual and group-level analyses were performed using the software AnalyzIR [36]. All code was executed in MATLAB (Natick, MA, USA).
3. Results
3.1. Participants
The participant flow diagram is shown in Figure 1. Of 40 individuals who were assessed for eligibility, 10 declined to participate for personal reasons. Therefore, 30 participants were randomly assigned to receive either active CES (n=15) or sham CES (n=15). No participant withdrew from the study, and all 30 participants who continued in the study completed all sessions and assessments. The demographic and clinical characteristics of the participants at baseline are summarized in Table 1. Of the 30 participants, the majority (66.67%) were female, and the mean age was 59.43 years (standard deviation [SD] = 6.27). The mean body mass index in the sample was 33.11 kg/m2 (SD = 8.13), and the mean duration of OA was 81.03 months (SD = 80.86). The demographic and clinical characteristics did not differ significantly between the active and sham groups.
Figure 1.
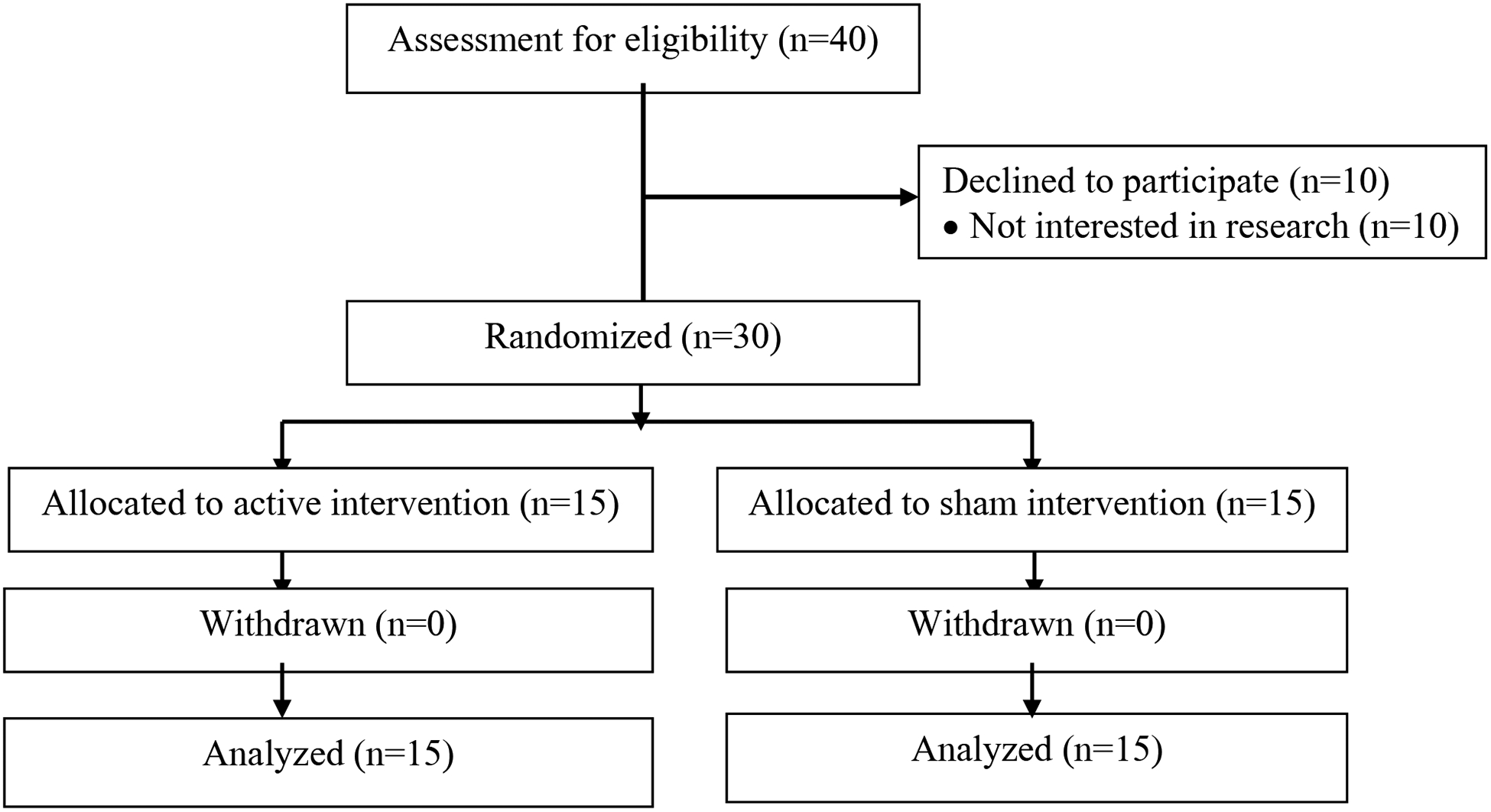
Participant flow diagram
Table 1.
Baseline demographic and clinical characteristics of the participants
| Variables | Total (n = 30) |
Sham group (n = 15) |
Active group (n = 15) |
df | p-value |
|---|---|---|---|---|---|
| Age, years, M ± SD | 59.43 ± 6.27 | 59.13 ± 5.83 | 59.77 ± 6.88 | 28 | 1.00 |
| Sex, n (%) | 1 | 1.00 | |||
| Male | 10 (33.30) | 5 (33.30) | 5 (33.30) | ||
| Female | 20 (66.67) | 10 (66.67) | 10 (66.67) | ||
| Race, n (%) | 3 | 0.43 | |||
| Asian | 2 (6.67) | 0 (0.00) | 2 (13.33) | ||
| White | 15 (50.00) | 7 (46.67) | 8 () | ||
| African American | 8 (26.67) | 5 (33.33) | 3 (20.00) | ||
| Hispanic | 5 (16.67) | 3 (20.00) | 2 (13.33) | ||
| Education, n (%) | 4 | 0.51 | |||
| < High school | 11 (36.67) | 5 (33.33) | 6 (40.00) | ||
| 2-year college | 5 (16.67) | 1 (6.67) | 4 (26.67) | ||
| 4-year college | 7 (23.30) | 5 (33.33) | 2 (13.33) | ||
| Master’s degree | 5 (16.67) | 3 (20.00) | 2 (13.33) | ||
| Doctoral degree | 2 (6.67) | 1 (6.67) | 1 (6.67) | ||
| OA duration, month, M (SD) | 81.03 ± 80.86 | 55.87 ± 40.64 | 108 ± 103.87 | 28 | 0.10 |
| BMI, kg/m2, M ±SD | 33.11 ± 8.13 | 33.66 ± 8.33 | 32.56 ± 8.17 | 28 | 0.72 |
| NRS, M ± SD | 39.50 ± 26.92 | 34.67 ± 32.48 | 44.33 ± 19.90 | 28 | 0.33 |
| HPTH, °C, M ± SD | 39.23 ± 3.16 | 40.12 ± 2.69 | 38.34 ± 3.44 | 28 | 0.05 |
| HPTO, °C, M ± SD | 43.54 ± 3.16 | 43.85 ± 3.26 | 43.22 ± 3.15 | 28 | 0.60 |
| PPT, kgf, M ± SD | 1.73 ± 1.14 | 1.86 ± 1.32 | 1.60 ± 0.95 | 28 | 0.51 |
| CPM, M ± SD | 2.61 ± 1.06 | 2.70 ± 1.26 | 2.53 ± 0.86 | 28 | 0.67 |
Note. df, degree of freedom; M, mean; SD, standard deviation; OA, Osteoarthritis, BMI, body mass index; NRS, Numeric Rating Scale (range, 0–100); HPTH, heat pain threshold; HPTO, heat pain tolerance; PPT, pressure pain threshold; CPM, conditioned pain modulation.
3.2. Clinical pain severity and experimental pain sensitivity
The Shapiro-Wilk test results showed that the normality assumption did not hold for certain pain score measurements (e.g., PPT at the medial knee at baseline); in such cases, the Wilcoxon-Mann-Whitney test was employed instead of a t-test. The baseline clinical pain scores were not significantly different between the two groups.
The changes in measurements from baseline to the end of the intervention were calculated and compared between the active and sham CES groups (Table 2). Most measurements of clinical pain severity and experimental pain sensitivity differed significantly between the active and sham groups (Cohen’s d = 1.43, P < 0.01 for NRS; d = 1.40, P < 0.01 for heat pain threshold; d = 1.50, P < 0.01 for PPT; and d = 1.95, P < 0.01 for CPM).
Table 2.
Comparison between groups on changes at the end of treatment from baseline
| Variables | Sham group (n = 15) |
Active group (n = 15) |
Effect size (Cohen’s d) |
P-value |
|---|---|---|---|---|
| NRS change | 5.73 ± 16.02 | −17.00 ± 15.79 | 1.43 | < 0.01 |
| HPTH change | −2.23 ± 1.82 | 2.69 ± 4.63 | 1.40 | < 0.01 |
| HPTO change | −0.80 ± 2.72 | 1.34 ± 3.16 | 0.73 | 0.06 |
| PPT change | −0.80 ± 1.08 | 0.99 ± 1.29 | 1.50 | < 0.01 |
| CPM change | −0.77 ± 0.67 | 1.11 ± 1.18 | 1.95 | < 0.01 |
Note. Mean ± standard deviation are presented in the first two columns. NRS, Numeric Rating Scale; HPTH, heat pain threshold; HPTO, heat pain tolerance; PPT, pressure pain threshold; CPM, conditioned pain modulation.
3.3. Pain-related cortical response
Figure 2 reports the difference in functional activation measured before and after CES treatment for each optical channel in the frontal cortex in response to moderate-intensity thermal stimulation. Active CES induced a significant decrease in oxygenated hemoglobin in the left frontal cortex (P < 0.05), whereas sham CES produced no differences in functional hemodynamic activity.
Figure 2.
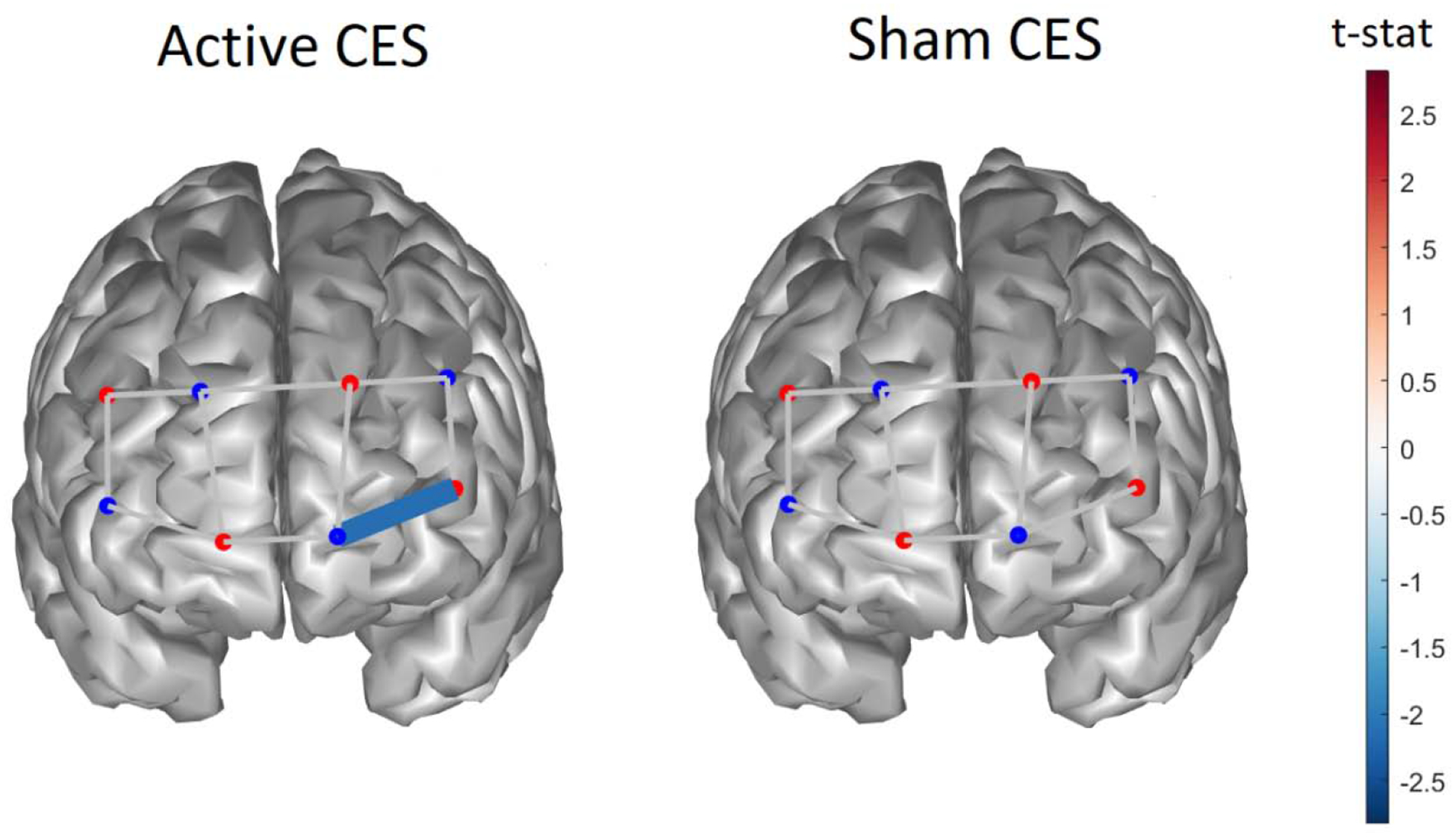
Difference in functional hemodynamic activity for oxygenated hemoglobin in response to thermal pain measured on the subject groups treated with active CES (left column) and sham CES (right). Only channels with statistically significant difference (P < 0.05) are shown before and after CES treatment.
3.4. Feasibility and Tolerability
All 30 participants completed the post-study acceptability and satisfaction questionnaire. A summary of participant acceptability is presented in Table 3. The average rating of the easiness of preparation of the device and accessories, easiness of the use of the device, helpfulness of remote supervision via videoconference platform, and confidence in the use of the device was 9.87 (SD = 0.43), 9.97 (SD = 0.18), 9.00 (SD = 2.24), and 9.83 (SD = 0.91), respectively. In addition, patient satisfaction rates were high (CSQ-8 = 28.37 ± 3.90). Furthermore, all participants tolerated CES well without experiencing any adverse effects. No participants reported any side effects associated with the treatment (e.g., itching, burning, headache, fatigue, nervousness, dizziness, or difficulty concentrating).
Table 3.
Summary of participants’ acceptability
| Questionnaires | Total (n = 30) |
Sham group (n = 15) |
Active group (n = 15) |
p-value |
|---|---|---|---|---|
| 1. It was easy to prepare the device and accessories. | 9.87 ± 0.43 | 9.80 ± 0.56 | 9.93 ± 0.26 | 0.41 |
| 2. The device was unnecessarily complex. | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.00 |
| 3. The device was easy to use. | 9.97 ± 0.18 | 9.93 ± 0.29 | 10.00 ± 0.00 | 0.33 |
| 4. I felt the video conferences with a technical person were helpful. | 9.00 ± 2.24 | 8.73 ± 2.55 | 9.27 ± 1.94 | 0.52 |
| 5. I would imagine that most people would learn to use this device quickly. | 9.77 ± 0.57 | 9.67 ± 0.62 | 9.87 ± 0.52 | 0.34 |
| 6. The device was cumbersome to use. | 0.17 ± 0.53 | 0.27 ± 0.70 | 0.07 ± 0.26 | 0.32 |
| 7. I felt confident using the device. | 9.83 ± 0.91 | 9.67 ± 1.29 | 10.00 ± 0.00 | 0.33 |
| 8. I needed to learn a lot of things before I could get going with this device. | 0.37 ± 1.03 | 0.53 ± 1.36 | 0.20 ± 0.56 | 0.39 |
| 9. The effectiveness of the treatment increased over the course of treatment. | 6.07 ± 3.90 | 6.47 ± 3.50 | 5.67 ± 4.35 | 0.58 |
| 10. I felt that transcranial electrical stimulation treatment benefited me. | 6.07 ± 3.78 | 5.80 ± 3.55 | 6.33 ± 4.10 | 0.70 |
Note. Mean ± standard deviation are presented in the first three columns. The scale is 0 (strongly disagree) to 10 (strongly agree).
4. Discussion
To our knowledge, this pilot study is the first to assess the feasibility and efficacy of remotely supervised CES in older adults experiencing pain associated with knee OA using a randomized, sham-controlled design. We found that remotely supervised CES was feasible and well tolerated by older adults with knee OA. In addition, we demonstrated that remotely supervised CES reduced clinical pain severity and increased heat pain threshold, PPT, and CPM. Also, we observed marginal improvement in heat pain tolerance. Moreover, using fNIRS imaging, we found that active CES significantly altered hemodynamic activity in the left prefrontal cortex in response to painful thermal stimulation. Finally, participants were notably satisfied with remotely supervised CES treatment.
The findings of our study are consistent with previous clinical studies evaluating the efficacy of CES on pain in other conditions. For example, Taylor et al. [37] found that CES significantly reduced pain among patients with fibromyalgia in a randomized, controlled, double-blind study. In addition, Tan et al. [38] reported that CES significantly decreased pain among patients with spinal cord injury in a multicenter, double-blinded, sham-controlled study. Furthermore, Yennurajalingam et al. [39] demonstrated that CES reduced clinical pain in patients with advanced cancer in a pre - and post-intervention study.
While the precise mechanism of action surrounding the analgesic effects elicited by CES is not fully understood, our findings support the notion that brain stimulation produces palliative effects by modifying central pain processing pathways. CES is thought to excite specific neurons in the brain that increase its ability to produce neurotransmitters (e.g., serotonin, norepinephrine, and dopamine), which are necessary for biochemical homeostasis after disruption caused from pain, and to induce a relaxed state by increasing plasma beta endorphins and decreasing plasma cortisol levels, which may decrease pain perception and stress response [40]. Our study adds to the growing literature regarding brain-related responses after CES treatment. To date, no studies have assessed the effect of CES on the cortical response to pain in a quantitatively rigorous manner, and this study used state-of-the-art fNIRS methods to demonstrate pain-evoked cortical hemodynamic activity.
Our findings should be interpreted with consideration of the study’s limitations. First, we analyzed a small sample of older adults with knee OA, which restricts the generalizability of the study findings. The outcomes may not translate to patients with other chronic pain etiologies. Second, despite our preference to maintain consistency with the timing of each stimulation session, we had to accommodate each participant’s varying daily schedule. This variance resulted in slight deviations among the intervals between daily stimulation sessions; however, it represents actual situations that may occur due to fluctuations in daily activities. Finally, this study did not include follow-up evaluation and appraisal and cannot establish long-term efficacy of CES intervention.
Despite the aforementioned limitations, the findings of this study provide an important foundation for future clinical research. First, future research is needed with a larger sample and a well-designed randomized, blinded, controlled method, while incorporating appropriate follow-up evaluations and appraisal. Our study outcomes regarding the feasibility and clinical efficacy provide validation for such future studies to replicate and extend these results. Second, the underlying physiopsychological pain processes that contribute to the efficacy of remotely supervised CES need to be further assessed. Finally, remotely supervised CES with other biobehavioral pain interventions is needed since CES may potentiate the effect of other pain interventions that stimulate adaptive changes in the brain.
5. Conclusion
In conclusion, we demonstrated that CES with real-time remote supervision is feasible and beneficial in alleviating clinical pain severity and experimental pain sensitivity without adverse side effects in older adults with knee OA. Our study contributes to the growing body of literature supporting nonpharmacologic interventions aimed at pain-related brain function and reducing the symptom burden of knee OA.
Highlights.
Remotely-supervised cranial electrical stimulation reduced clinical pain.
Remotely-supervised cranial electrical stimulation was feasible.
Remotely-supervised cranial electrical stimulation was well tolerated.
Funding:
This study was supported by in part by Theodore J. and Mary E. Trumble Endowment from The University of Texas Health Science Center at Houston and NINR grant R15NR018050.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflicts of interest or other disclosures.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–24. [DOI] [PubMed] [Google Scholar]
- [2].Chu CR, Millis MB, Olson SA. Osteoarthritis: From Palliation to Prevention: AOA Critical Issues. J Bone Joint Surg Am. 2014;96:e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013;39:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthr Cartil. 2015;23:507–15. [DOI] [PubMed] [Google Scholar]
- [6].Wallace IJ, Worthington S, Felson DT, Jurmain RD, Wren KT, Maijanen H, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci U S A. 2017;114:9332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].O’Neil CK, Hanlon JT, Marcum ZA. Adverse effects of analgesics commonly used by older adults with osteoarthritis: focus on non-opioid and opioid analgesics. Am J Geriatr Pharmacother. 2012;10:331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170:1968–76. [DOI] [PubMed] [Google Scholar]
- [9].Reid MC, Henderson CR Jr., Papaleontiou M, Amanfo L, Olkhovskaya Y, Moore AA, et al. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Med. 2010;11:1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–34. [DOI] [PubMed] [Google Scholar]
- [11].Hiramatsu T, Nakanishi K, Yoshimura S, Yoshino A, Adachi N, Okamoto Y, et al. The dorsolateral prefrontal network is involved in pain perception in knee osteoarthritis patients. Neurosci Lett. 2014;581:109–14. [DOI] [PubMed] [Google Scholar]
- [12].Sofat N, Smee C, Hermansson M, Howard M, Baker EH, Howe FA, et al. Functional MRI demonstrates pain perception in hand osteoarthritis has features of central pain processing. J Biomed Graph Comput. 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Parks EL, Geha PY, Baliki MN, Katz J, Schnitzer TJ, Apkarian AV. Brain activity for chronic knee osteoarthritis: dissociating evoked pain from spontaneous pain. Eur J Pain. 2011;15:843 e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lluch Girbes E, Nijs J, Torres-Cueco R, Lopez Cubas C. Pain treatment for patients with osteoarthritis and central sensitization. Phys Ther. 2013;93:842–51. [DOI] [PubMed] [Google Scholar]
- [15].Stock V, Knotkova H, Nitsche M. Principles of Neuromodulation In: Knotkova H, Rasche D, editors. Textbook of Neuromodulation. New York, NY: Springer; 2012. [Google Scholar]
- [16].Thibaut A, O’Brien AT, Fregni F. Strategies for replacing non-invasive brain stimulation sessions: recommendations for designing neurostimulation clinical trials. Expert Rev Med Devices. 2017;14:633–49. [DOI] [PubMed] [Google Scholar]
- [17].Probst JC, Laditka SB, Wang JY, Johnson AO. Effects of residence and race on burden of travel for care: cross sectional analysis of the 2001 US National Household Travel Survey. BMC Health Serv Res. 2007;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Witham MD, McMurdo MET. How to get older people included in clinical studies. Drugs Aging. 2007;24:187–96. [DOI] [PubMed] [Google Scholar]
- [19].Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- [20].Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–15. [PubMed] [Google Scholar]
- [21].Ahn H, Suchting R, Woods AJ, Miao H, Green C, Cho RY, et al. Bayesian analysis of the effect of transcranial direct current stimulation on experimental pain sensitivity in older adults with knee osteoarthritis: randomized sham-controlled pilot clinical study. J Pain Res. 2018;11:2071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].King CD, Sibille KT, Goodin BR, Cruz-Almeida Y, Glover TL, Bartley E, et al. Experimental pain sensitivity differs as a function of clinical pain severity in symptomatic knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ahn H, Zhong C, Miao H, Chaoul A, Park L, Yen IH, et al. Efficacy of combining home-based transcranial direct current stimulation with mindfulness-based meditation for pain in older adults with knee osteoarthritis: A randomized controlled pilot study. J Clin Neurosci. 2019;70:140–5. [DOI] [PubMed] [Google Scholar]
- [24].Yennu A, Tian F, Gatchel RJ, Liu H. Prefrontal hemodynamic mapping by functional near-infrared spectroscopy in response to thermal stimulations over three body sites. Neurophotonics. 2016;3:045008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yucel MA, Aasted CM, Petkov MP, Borsook D, Boas DA, Becerra L. Specificity of hemodynamic brain responses to painful stimuli: a functional near-infrared spectroscopy study. Sci Rep. 2015;5:9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sorkpor S, Ahn H, Pollonini L, Do J. Cortical hemodynamic response to contact thermal stimuli in older adults with knee osteoarthritis: a functional near infrared spectroscopy pilot study. J Pain. 2019;20:S33. [Google Scholar]
- [27].Bevilaqua-Grossi D, Zanin M, Benedetti C, Florencio L, Oliveira A. Thermal and mechanical pain sensitization in patients with osteoarthritis of the knee. Physiother Theory Pract. 2019;35:139–47. [DOI] [PubMed] [Google Scholar]
- [28].Gillick BT, Feyma T, Menk J, Usset M, Vaith A, Wood TJ, et al. Safety and feasibility of transcranial direct current stimulation in pediatric hemiparesis: randomized controlled preliminary study. Phys Ther. 2015;95:337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cha YH, Urbano D, Pariseau N. Randomized Single Blind Sham Controlled Trial of Adjunctive Home-Based tDCS after rTMS for Mal De Debarquement Syndrome: Safety, Efficacy, and Participant Satisfaction Assessment. Brain Stimul. 2016;9:537–44. [DOI] [PubMed] [Google Scholar]
- [30].Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann. 1979;2:197–207. [DOI] [PubMed] [Google Scholar]
- [31].Nguyen TD, Attkisson CC, Stegner BL. Assessment of patient satisfaction: development and refinement of a service evaluation questionnaire. Eval Program Plann. 1983;6:299–313. [DOI] [PubMed] [Google Scholar]
- [32].Ahn H, Woods AJ, Kunik ME, Bhattacharjee A, Chen Z, Choi E, et al. Efficacy of transcranial direct current stimulation over primary motor cortex (anode) and contralateral supraorbital area (cathode) on clinical pain severity and mobility performance in persons with knee osteoarthritis: An experimenter- and participant-blinded, randomized, sham-controlled pilot clinical study. Brain Stimul. 2017;10:902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cope M, Delpy DT. System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Med Biol Eng Comput. 1988;26:289–94. [DOI] [PubMed] [Google Scholar]
- [34].Delpy DT, Cope M, van der Zee P, Arridge S, Wray S, Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol. 1988;33:1433–42. [DOI] [PubMed] [Google Scholar]
- [35].Barker JW, Aarabi A, Huppert TJ. Autoregressive model based algorithm for correcting motion and serially correlated errors in fNIRS. Biomed Opt Express. 2013;4:1366–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Santosa H, Zhai X, Fishburn F, Huppert T. The NIRS brain AnalyzIR toolbox. Algorithms. 2018;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Taylor AG, Anderson JG, Riedel SL, Lewis JE, Kinser PA, Bourguignon C. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Manag Nurs. 2013;14:327–35. [DOI] [PubMed] [Google Scholar]
- [38].Tan G, Rintala DH, Jensen MP, Richards JS, Holmes SA, Parachuri R, et al. Efficacy of cranial electrotherapy stimulation for neuropathic pain following spinal cord injury: a multi-site randomized controlled trial with a secondary 6-month open-label phase. J Spinal Cord Med. 2011;34:285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yennurajalingam S, Kang DH, Hwu WJ, Padhye NS, Masino C, Dibaj SS, et al. Cranial Electrotherapy Stimulation for the Management of Depression, Anxiety, Sleep Disturbance, and Pain in Patients With Advanced Cancer: A Preliminary Study. J Pain Symptom Manage. 2018;55:198–206. [DOI] [PubMed] [Google Scholar]
- [40].Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36:169–76. [DOI] [PubMed] [Google Scholar]


