Abstract
Synaptic neurotransmission with dopamine (DA), norepinephrine (NE), and serotonin (5-HT) is terminated primarily by reuptake into presynaptic terminals via the DA, NE, and 5-HT transporters (DAT/NET/SERT, respectively). Monoamine transporter inhibitors constitute one class of drugs used to treat both depression and pain, and therapeutic effects by these compounds often require repeated treatment for days or weeks. The present study compared antinociceptive effects produced by repeated treatment with monoamine transporter inhibitors in a preclinical assay of pain-related depression of positively reinforced operant responding. Adult Sprague-Dawley rats equipped with microelectrodes targeting a brain-reward area responded for pulses of electrical brain stimulation in an intracranial self-stimulation (ICSS) procedure. Intraperitoneal injection of dilute lactic acid served as a noxious stimulus that repeatedly depressed ICSS and also produced weight loss during 7 days of repeated acid administration. Acid-induced depression of both ICSS and body weight were completely blocked by repeated pretreatment with the nonsteroidal anti-inflammatory drug ketorolac. The DAT-selective inhibitor bupropion also fully blocked acid-induced ICSS depression and weight loss throughout all 7 days of treatment. The NET-selective inhibitor nortriptyline and the SERT-selective inhibitor citalopram were generally less effective, but both drugs blocked acid-induced ICSS depression by the end of the 7-day treatment. Acid-induced depression of ICSS and body weight were not blocked by the kappa opioid receptor (KOR) agonist U69593 or the KOR antagonist norbinaltorphimine. These results support effectiveness of bupropion to alleviate signs of pain-related behavioral depression in rats and further suggest that nortriptyline and citalopram produce significant but less reliable effects.
Keywords: pain-depressed behavior, intracranial self-stimulation, ketorolac, bupropion, nortriptyline, citalopram, norbinaltorphimine, U69593, rat, antidepressant
INTRODUCTION
Mu opioid receptor agonists (e.g. morphine) and cyclooxygenase inhibiting nonsteroidal anti-inflammatory drugs (NSAIDs, e.g. ketorolac) are among the most widely used analgesics for treatment of moderate to severe pain, but they are not always effective, and their use is often constrained by side effects (Litvak and McEvoy 1990; Matava 2018; Yaksh and Wallace 2018). Drugs that inhibit the norepinephrine transporter (NET), serotonin transporter (SERT), and/or dopamine transporter (DAT) represent another class of drugs that is sometimes used to treat pain (Obata 2017; Sutherland et al. 2018). Norepinephrine (NE), serotonin (5-HT), and dopamine (DA) are monoamine neurotransmitters involved in a wide range of physiological and behavioral processes (Jacob and Nienborg 2018; Nutt 2008). Monoamine transporters located on presynaptic terminals are the primary mechanism for neurotransmitter clearance from a synapse after monoamine release, and transporter inhibition reduces neurotransmitter clearance, increases synaptic neurotransmitter concentrations, and increases signaling via the associated monoamine receptors (Aggarwal and Mortensen 2017; Lin et al. 2011).
Monoamine transporter inhibitors are most widely used for the treatment of major depression (Cipriani et al. 2018; O’Donnell et al. 2018); however, pain is often associated with depression-like signs and symptoms, and at least some dimensions of pain may be mediated by changes in monoamine signaling similar to those that are also present in major depression (Boakye et al. 2016; Goesling et al. 2013). The effectiveness of monoamine transporter inhibitors for pain treatment was first established with so-called “tricyclic” antidepressants, and tricyclics such amitriptyline and its primary metabolite nortriptyline, which act primarily as NET inhibitors, continue to be used (Finnerup et al. 2015; Moore et al. 2015; Paoli et al. 1960). Several more recently developed drugs display greater selectivity for monoamine transporters vs. non-transporter targets and may act either selectively at a single transporter (e.g. the moderately DAT-selective inhibitor bupropion or the highly SERT-selective inhibitor citalopram) or simultaneously at multiple transporters (e.g. the NET/SERT inhibitor duloxetine) (Bymaster et al. 2005; Hyttel et al. 1992; O’Donnell et al. 2018; Stahl et al. 2004). Analgesic effectiveness is best established for NET/SERT inhibitors (Attal 2019; Wang et al. 2015), but DAT-selective inhibitors (Pud et al. 2017; Shah and Moradimehr 2010) and SERT-selective inhibitors (Barakat et al. 2018; Lunn et al. 2015) may also be effective under at least some conditions.
Monoamine transporter inhibitors have been reported previously to produce antinociception in preclinical laboratory-animal procedures that rely on “pain-stimulated behaviors,” which can be defined as behaviors that increase in rate, frequency, or intensity after delivery of a putative pain stimulus (e.g. paw or tail withdrawal from thermal or mechanical stimuli) (Gatch et al. 1998; Hall et al. 2011; Pedersen et al. 2005; Ventafridda et al. 1990). However, pain states can also be associated with decreases in behavior, and pain-related behavioral depression is both a common criterion of pain diagnosis and a target of pain treatment in both human and veterinary medicine (Brown et al. 2008; Dworkin et al. 2005). Accordingly, we and others have developed preclinical assays of pain-depressed behaviors, which can be defined as behaviors that decrease in rate, frequency, or intensity after delivery of a putative pain stimulus (Negus 2019; Tappe-Theodor et al. 2019). As one example, we reported previously that intraperitoneal delivery of dilute lactic acid (IP acid) could serve as a noxious stimulus to decrease positively reinforced operant responding maintained by delivery of rewarding electrical brain stimulation in an intracranial self-stimulation (ICSS) procedure (Negus 2013). IP acid-induced depression of ICSS can be alleviated by acute treatment with clinically effective opioid and NSAID analgesics, but not by clinically ineffective classes of analgesics (e.g. centrally active kappa opioid receptor (KOR) agonists such as U69593) (Altarifi et al. 2015; Negus et al. 2010; Pereira Do Carmo et al. 2009). Among monoamine transporter inhibitors, acute treatment with bupropion was also effective to relieve IP acid-induced ICSS depression, but NET- and SERT-selective inhibitors (including nortriptyline and citalopram) were not (Rosenberg et al. 2013).
A distinguishing characteristic of monoamine transporter inhibitors for pain treatment is that they typically require repeated treatment for days to weeks before analgesic effects emerge (Sutherland et al. 2018). Accordingly, the main goals of the present study were to determine (a) if acute antinociception by bupropion would be sustained during repeated treatment, and (b) if effects of nortriptyline or citalopram would emerge with repeated treatment. The effects of these monoamine transporter inhibitors were compared to effects of ketorolac and U69593 as a positive and negative controls, respectively. The KOR antagonist norbinaltorphimine (norBNI) was also tested because of preclinical evidence to suggest that KOR antagonists may also produce antidepressant-like and analgesic-like effects by blocking stress- or injury-induced dynorphin signaling mediated by KORs (Jacobson et al. 2020; Knoll and Carlezon 2010; Liu et al. 2019). All drugs were evaluated in a procedure in which rats received daily treatment with IP acid for 7 days, and we have shown previously that repeated IP acid produces repeatable ICSS depression that can be blocked by daily co-administration of morphine (Miller et al. 2015). We hypothesized that ketorolac and all three monoamine transporter inhibitors would alleviate IP acid-induced ICSS depression after repeated treatment while U69593 would not. With regard to norBNI, we have shown previously that, like nortriptyline and citalopram, norBNI fails to alleviate acid-induced ICSS depression acutely (Leitl et al. 2014a; Negus et al. 2010), and here we evaluated the degree to which antinociceptive effects might emerge over time.
METHODS
Subjects
Adult male and female Sprague-Dawley rats (Envigo, Somerset, NJ) with initial weights of 376 – 508 g (males) and 266 – 328 g (females) were housed individually and maintained on a 12-h light/dark cycle with lights on from 6:00AM to 6:00PM in an AAALAC International-accredited housing facility. Food and water were available ad libitum in the home cage. Animal-use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee and were in accordance with the National Academy of Science’s Guide for the Care and Use of Laboratory Animals (National_Research_Council 2003).
Intracranial self-stimulation (ICSS)
Surgery.
Male and female rats (N=52 rats, 27 male and 25 female) were anesthetized with isoflurane (2.5–3% in oxygen; Webster Veterinary, Phoenix, Arizona, USA) and implanted with electrodes (Plastics One, Roanoke, Virginia, USA) in the left medial forebrain bundle at the level of the lateral hypothalamus using previously published procedures and coordinates (Males: 2.8 mm posterior to bregma, 1.7 mm lateral to the midsagittal suture, 8.8 mm below skull surface; Females: 3.8 mm posterior to bregma, 1.6 mm lateral to the midsaggital suture, 8.7 mm below skull surface (Leitl et al. 2014b; Miller et al. 2015). The electrode was secured to the skull with orthodontic resin and skull screws. Ketoprofen (Spectrum Chemical, New Brunswick, NJ, 5 mg/kg) was administered immediately and 24 hours after surgery as a postoperative analgesic, and rats recovered for 7 days prior to initiation of ICSS training.
Apparatus.
Studies were conducted in sound-attenuating boxes containing modular acrylic and metal test chambers (29.2 × 30.5 × 24.1 cm; Med Associates, St Albans, VT, USA). Each chamber contained a response lever (4.5 cm wide, 2.0 cm deep, 3.0 cm above the floor), three stimulus lights (red, yellow, and green) centered 7.6 cm above the lever, a 2-W house light, and an ICSS stimulator. Electrodes were connected to the stimulator via bipolar cables routed through a swivel commutator (Model SL2C, Plastics One, Roanoke, VA, USA). Computers and interface equipment operated by custom software controlled all operant sessions and data collection (Med Associates).
Training.
Rats were trained to respond for brain stimulation using procedures identical to those previously described (Leitl et al. 2014b; Miller et al. 2015). Briefly, a white house light was illuminated during behavioral sessions, and responding under a fixed-ratio (FR) 1 schedule produced a 500-ms train of 0.1-ms square-wave cathodal pulses together with 500-ms illumination of stimulus lights over the response lever. The terminal schedule consisted of sequential 10-min components. Each component consisted of 10 1-min trials, and the available brain-stimulation frequency decreased in 0.05 log Hz increments from one trial to the next (158–56 Hz). Each frequency trial consisted of a 10-s timeout, during which five noncontingent stimulations were delivered at the frequency available during that trial, followed by a 50-s “response” period, during which responding resulted in electrical stimulation. Training continued with presentation of three sequential components per day until the following two criteria for stable responding were met for three consecutive days: (1) ≤5% variability in the maximum rate of reinforcement in any trial, and (2) ≤10% variability in the total number of stimulations per component.
Testing.
Experiments were conducted using an 8-day protocol. On Day 0, a three-component ICSS session was conducted to establish pre-drug baseline parameters for the number of total stimulations per component and the maximal control rate (MCR) (see Data Analysis). On Days 1 – 7, rats were weighed, and daily experimental sessions consisted of three daily baseline ICSS components followed first by a timeout period when treatments were administered and then by two ICSS test components. Fourteen groups of rats were used to evaluate effects of fourteen different experimental conditions. Specifically, six groups received daily IP injections of vehicle or one of five test drugs followed by treatment with IP 1.8% lactic acid. Six other groups received the same daily injections of vehicle or test drug followed by treatment with acid vehicle. The test drugs and doses were as follows: 10 mg/kg ketorolac, 3.2 mg/kg bupropion, 3.2 mg/kg nortriptyline, 10 mg/kg citalopram, and 0.18 mg/kg U69593. The interval between administration of test drug or drug vehicle and subsequent administration of IP acid or acid vehicle was 30 min for vehicle, bupropion, nortriptyline, citalopram, 15 min for ketorolac, and 10 min for U69–593, and test components were initiated immediately after IP injection of acid or its vehicle. These doses and pretreatment times were based on previously published studies that examined acute effects of these drugs on ICSS performance in the absence or presence of the IP acid noxious stimulus (Leitl et al. 2014a; Moerke et al. 2019; Rosenberg et al. 2013). Two additional groups were used to test the KOR antagonist norBNI. In view of its potency and very long duration of action (Craft and McNiel 2003; Friese et al. 1997; Holtzman 2000; Negus et al. 2010), norBNI (20 mg/kg SC) was administered as a single injection on Day 0, but IP 1.8% lactic acid or its vehicle was still administered daily immediately before test components on the subsequent days.
Each treatment was tested in 6–7 rats. Most rats (37) were included in two different groups, receiving no more than one of the monoamine reuptake inhibitors (bupropion, nortriptyline, or citalopram) and no more than one of the other test drugs (ketorolac, norBNI, or U69593). Rats tested twice received repeated IP acid with only one treatment and repeated IP acid vehicle with the other treatment. Evaluation of sex differences in treatment effects was not a goal of the present study, but to comply with National Institute of Health guidance for inclusion of both sexes in preclinical research, each group consisted of 3–4 male rats and 3–4 female rats.
Data analysis.
ICSS data were analyzed as previously described (Leitl et al. 2014b; Miller et al. 2015). The primary dependent measure was the total number of reinforcements per component (i.e. the total number of stimulations delivered across all brain-stimulation frequencies during each 10-min component). For each rat on each day, data from the second and third baseline components were averaged to provide the baseline data for that day, and data from the two test components were averaged to yield the test data for that day. The baseline number of stimulations per component on Day 0, before the first treatment, served as the Pre-Drug Baseline value, and all subsequent baseline and test data across days were expressed as a percentage of the Pre-Drug Baseline using the equation: % Pre-Drug Baseline Reinforcements per Component = (Daily-Baseline or Daily-Test Reinforcements per Component on a Test Day ÷ Pre-Drug Baseline Reinforcements per Component) × 100. These data were averaged across rats on each test day and compared across groups using two-way ANOVAs, with treatment day as a within-subjects factor and treatment group as a between-subjects factor. A significant two-way ANOVA was followed by the Holm-Sidak post-hoc test. In general, the criterion for significance was p<0.05. In cases where effects of multiple test groups were compared to the same control group(s), the criterion for significance was adjusted for multiple comparisons using the Bonferroni correction (0.05 ÷ number of comparisons) (McDonald, 2014).
A secondary and more granular measure of ICSS performance was used for within-subject analysis of “frequency-rate curves” that related brain-stimulation frequency to reinforcement rate in stimulations per frequency trial on Day 7, the final day of treatment in each group. Raw reinforcement rates for each rat from each trial were converted to “Percent Maximum Control Rate” (%MCR), with MCR defined as the mean of the maximal rates observed during any trial of the second and third components of the pre-drug baseline session (Day 0). Thus, %MCR values for each daily-baseline and test trial on Day 7 were calculated as: %MCR = (reinforcement rate during a Day 7 baseline or test frequency trial ÷ MCR) × 100. %MCR values were then averaged across rats, and within-group baseline vs. test data were compared by repeated-measures two-way ANOVA, with ICSS frequency as one factor and Day 7 baseline vs. test performance as the second factor. A significant two-way ANOVA was followed by the Holm-Sidak post-hoc test, and the criterion for significance was p<0.05.
Body Weight
Rats were weighed daily before treatment injections on Days 1–7, and body weight data for each rat on each day were expressed as a percent of the initial Pre-Drug weight on Day 1 using the equation % Pre-Drug Body Weight = (Weight on a Test Day ÷ Weight on Day 1) × 100. Data across groups from Days 2–7 were then analyzed by two-way ANOVA with treatment day as a within-subjects factor and treatment group as a between-subjects factor. As above, the criterion for significance was adjusted for multiple comparisons to common control groups using the Bonferroni correction (0.05 ÷ number of comparisons), and a significant ANOVA was followed by the Holm-Sidak post-hoc test.
Drugs
Lactic acid, ketorolac HCl, nortriptyline HCl, and bupropion HCl were purchased from Sigma Chemical Company (St. Louis, MO). Citalopram HBr was purchased from Spectrum Chemical Manufacturing Corporation (Gardena, CA). Norbinaltorphimine 2 HCl was kindly provided by Drs. K. Cheng and K. Rice (National Institute on Drug Abuse, Bethesda, MD). U69593 was obtained from the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). All drugs were dissolved in sterile water for IP or SC injection.
RESULTS
Drug effects on ICSS and body weight in the absence of IP acid.
For all rats in ICSS studies, the mean ± SEM pre-drug baseline number of reinforcements per component was 164.3 ± 17.4, and the mean ± SEM pre-drug baseline maximum control rate was 59.6 ± 6.4 reinforcements per trial. Relative to these pre-drug baselines, daily baseline ICSS performance did not change across days in any of the 14 treatment groups, and daily test performance in rats that received repeated treatment with drug vehicle + acid vehicle did not differ from daily baseline performance in that group (data not shown). Thus, daily baseline ICSS performance was stable across time in all groups and unaffected by repeated daily treatment with drug vehicle + acid vehicle. Figure 1 compares effects produced by repeated treatment with each drug in combination with acid vehicle on both ICSS and body weight, and statistical results for this and all other figures are shown in the figure legends. No drug produced effects on ICSS performance that differed from effects of vehicle treatment. Similarly, within-subject analysis of ICSS frequency-rate curves on Day 7 found that no treatment produced effects different from the Day 7 daily baseline (data not shown). Body weights were also similar over time in rats treated with vehicle, ketorolac, bupropion, or citalopram. However, significant weight decreases were observed in rats treated with nortriptyline (across all 7 Days), norBNI (Days 3 and 4), and U69593 (Day 7).
Figure 1: Drug effects on ICSS and body weight in the absence of IP acid.
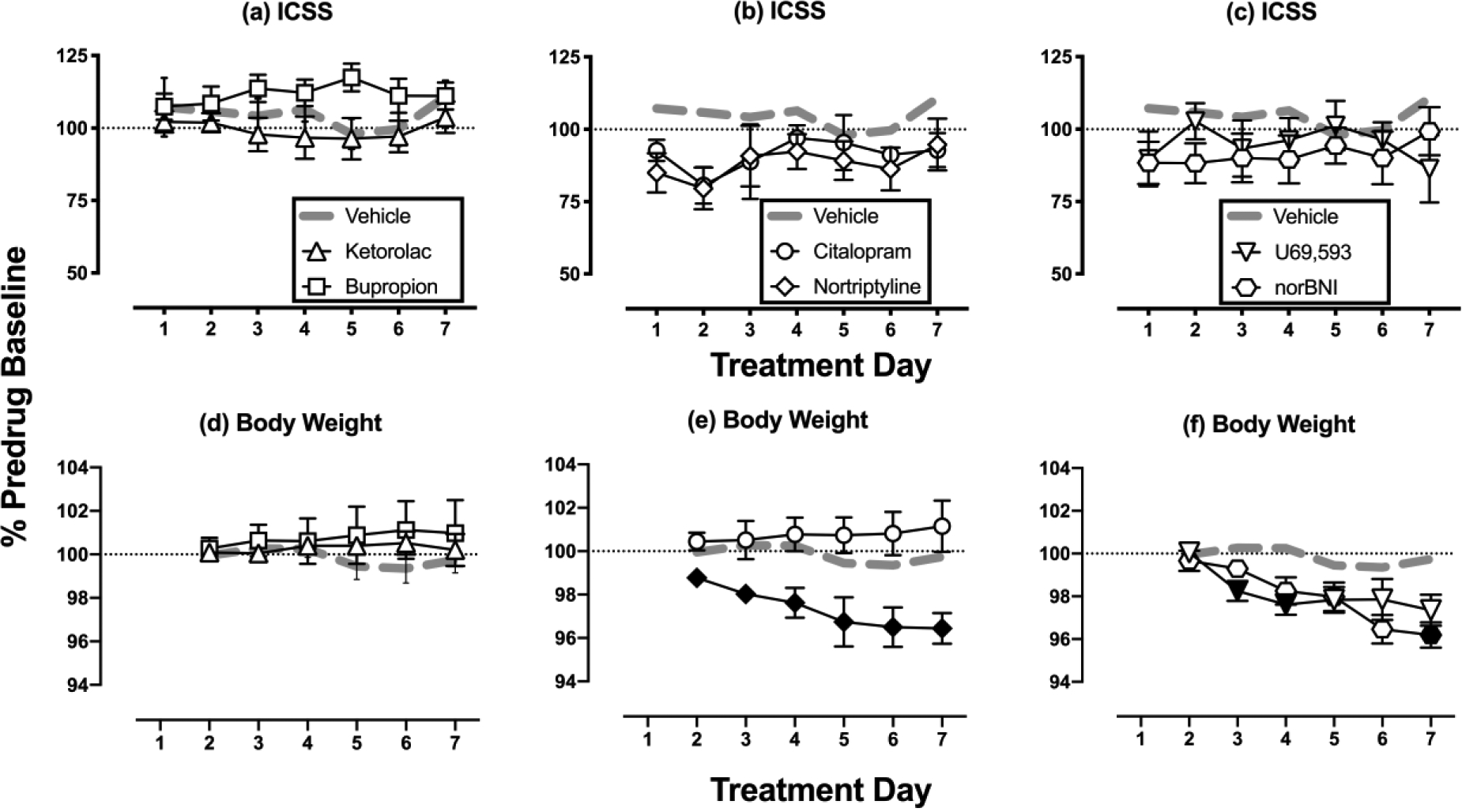
Horizontal axes: Time in days of treatment. Vertical axes: (Panels a-c) ICSS performance expressed as the % baseline number of pre-drug reinforcements earned per 10-min component; (Panels d-f) Body weight expressed as the % baseline weight. All points show mean±SEM for N=6 rats (3 male and 3 female) except for the vehicle, ketorolac, and nortriptyline groups (N=7 rats, 4 male and 3 female). Filled symbols in the bottom panels indicate points significantly different from “Vehicle” on a given treatment day as indicated by a significant Treatment × Day interaction or a main effect of Treatment in the two-way ANOVA followed by a Holm-Sidak post-hoc test. Because effects of each drug were compared to vehicle in separate two-way ANOVAs, the criterion for significance was adjusted for multiple comparisons to the vehicle group (p<0.0083 = 0.05 ÷ 6 different test drugs). Significant statistical results are as follows: For nortriptyline (Panel e), there was a main effect of Treatment [F(1,12)=11.570, p=0.005], for norBNI (Panel f), there was a significant Treatment × Day interaction [F(5,55)=3.834, p=0.005], for U69593 (Panel f), there was a significant Treatment × Day interaction [F(5,55)=5.670, p<0.001].
Drug effects on ICSS in the presence of IP acid.
Figure 2 shows ICSS performance in rats during daily treatment with test drugs in combination with IP acid. IP acid alone (drug vehicle + IP acid) produced a sustained and repeatable decrease in ICSS relative to responding in rats treated with drug vehicle + acid vehicle (panel a), and a major goal of the study was to evaluate the effectiveness of test drugs to block this sustained and repeatable IP acid-induced ICSS depression. The NSAID k etorolac (10 mg/kg/day) produced sustained antinociception as indicated by a complete blockade of IP acid-induced ICSS depression across all seven days of treatment (panel b). Like ketorolac, bupropion (3.2 mg/kg/day) also completely blocked IP acid-induced ICSS depression across all seven days of treatment (panel c). Nortriptyline and citalopram had more nuanced effects. With nortriptyline (3.2 mg/kg/day), there was a main effect of treatment and a trend for increasing antinociception over days; however, the treatment × day interaction did not reach the criterion for statistical significance. When data were collapsed across days, effects of nortriptyline + acid were different from both drug vehicle + acid and drug vehicle + acid vehicle, indicating a partial antinociceptive effect (panel d). Citalopram (10 mg/kg/day) also produced a main effect of treatment, a trend toward increasing antinociception over days, but no treatment × day interaction, again indicating a partial antinociceptive effect (panel e). Both the KOR antagonist norBNI (20 mg/kg single injection, panel f) and the KOR agonist U69593 (0.18 mg/kg/day, Supplemental figure 1a) failed to alleviate acid-induced ICSS depression.
Figure 2: Drug effects on ICSS in the presence of IP acid.

Horizontal axes: Time in days of treatment. Vertical axes: ICSS performance expressed as the % baseline number of pre-drug reinforcements earned per 10-min component. All points show mean±SEM for N=6 rats (3 male and 3 female) except IP Vehicle and IP Acid in Panel a (4 males, 3 females) and Nortriptyline + Acid in Panel e (3 males, 4 females). Gray dotted and solid lines in Panels b-f replicate data from Panel a for Drug Vehicle + IP Acid Vehicle (IP vehicle) or Drug Vehicle + IP Acid (IP Acid), respectively, for statistical comparison to effects of Test Drug + IP Acid. Filled symbols indicate significantly different from IP Vehicle but not from IP Acid (i.e. no antinociception), open symbols indicate significantly different from IP Acid but not from IP Vehicle (i.e. antinociception), and half-filled symbols indicate significantly different from both IP Vehicle and IP Acid (i.e. partial antinociception) as indicated by significant main effect of Treatment without a significant Treatment × Day interaction in the two-way ANOVA followed by a Holm-Sidak post-hoc test. As in Figure 1, the criterion for significance was adjusted for multiple comparisons to the control groups (p<0.0083 = 0.05 ÷ 6 different test drugs). Significant statistical results for the main effect of treatment in each panel are as follows. Panel a: [F(1,12)=91.14, p<0.001], Panel b: [F(2,17)=42.16, p<0.001], Panel c: [F(2,17)=17.56, p<0.001], Panel d: [F(2,18)=35.82, p<0.001], Panel e: [F(2,17)=28.36, p<0.001], and Panel f: [F(2,17)=31.49, p<0.001].
Figure 3 shows a within-subjects comparison of full ICSS frequency-rate curves before and after each treatment in combination with IP acid on Day 7. Relative to the daily baseline ICSS frequency-rate curve on Day 7, drug vehicle + IP acid depressed ICSS by shifting the frequency-rate curve to the right. The ICSS frequency-rate curve was also shifted to the right in rats treated with norBNI + IP acid (panel f) and with U69593 + IP acid (Supplemental Figure 1b). However, in the other test groups, there was no difference between baseline and test frequency-rate curves on Day 7. The failure of IP acid to depress ICSS in these groups on Day 7 provides additional evidence for antinociception by ketorolac (panel b), bupropion (panel c), nortriptyline (panel d), and citalopram (panel e).
Figure 3: Drug + Acid effects on Day 7 ICSS frequency-rate curves.
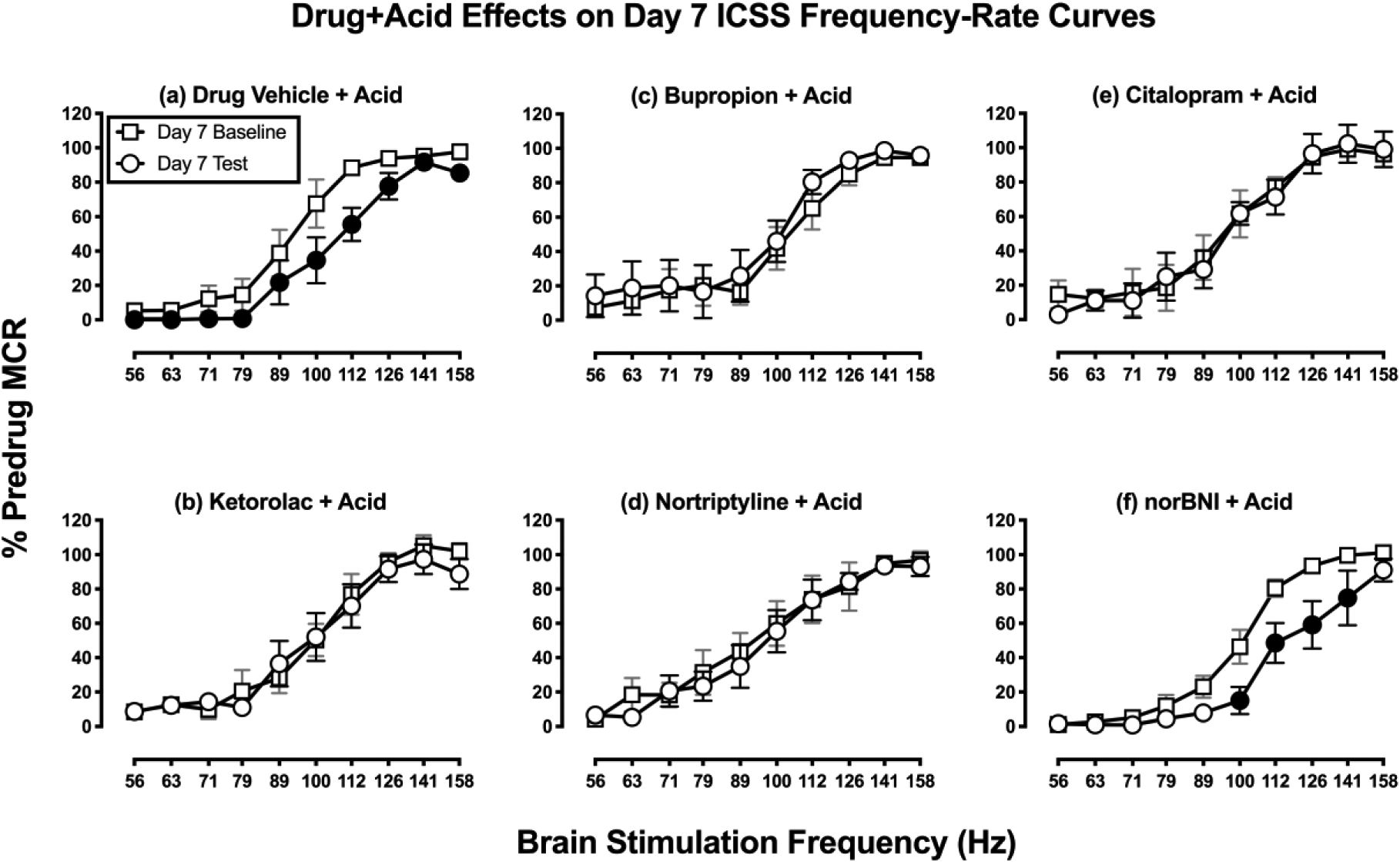
Horizontal axes: frequency of brain stimulation in hertz (Hz; log scale). Vertical axes: ICSS performance expressed as the % pre-drug maximal control rate (MCR). For all panels, squares indicate Daily Baseline data collected before the designated treatment, and circles indicate Daily Test data collected after the designated treatment. Filled circles indicate significantly different from the Day 7 Baseline as indicated by a significant two-way ANOVA main effect of Test (Panel a) or by both a main effect of treatment and a significant interaction of Test and Frequency (Panel f) followed by the Holm-Sidak post-hoc test, p<0.05. All points show mean±SEM for N=6 rats (3 male and 3 female) except IP Acid in Panel a (4 males, 3 females) and Nortriptyline + Acid in Panel e (3 males, 4 females). There was a significant main effect of frequency for all panels, and results are not shown. Other significant statistical results are as follows. Panel a: Significant main effect of Baseline vs. Test [F(1,6)=16.75, P=0.006], Panel f: Significant main effect of Baseline vs. Test [F(1,5)=14.80, p=0.012] and a significant interaction of Test and Frequency [F(9,45)=2.76, p=0.012].
Drug effects on body weights in the presence of IP acid.
Figure 4 shows body weights in rats during daily treatment with test drugs in combination with IP acid. IP acid alone (drug vehicle + IP acid) produced a significant loss of body weight relative to rats treated with drug vehicle + acid vehicle (panel a). This loss of body weight was blocked across all days by ketorolac (panel b), bupropion (panel c), and nortriptyline (panel d) as indicated by a main effect of treatment. Citalopram (panel e) and U69593 (Supplemental Figure 1c) did not significantly alleviate acid-induced weight loss, although rats treated with citalopram+acid or U69593+acid also did not differ significantly from rats treated acid vehicle. NorBNI (panel f) also generally failed to alleviate acid-induced weight loss.
Figure 4: Drug effects on body weights in the presence of IP acid.

Horizontal axes: Time in days of treatment. Vertical axes: Body weight expressed as the % pre-drug baseline body weight. All points show mean±SEM for N=6 rats (3 male and 3 female) except IP Vehicle and IP Acid in Panel a (4 males, 3 females) and Nortriptyline + Acid in Panel e (3 males, 4 females). Gray dotted and solid lines in Panels b-f replicate data from Panel a for Drug Vehicle + IP Acid Vehicle (IP vehicle) or Drug Vehicle + IP Acid (IP Acid), respectively, for statistical comparison to effects of Test Drug + IP Acid. Filled symbols indicate points significantly different from IP Vehicle but not from IP Acid and open symbols indicate significantly different from IP Acid but not from IP Vehicle, and gray symbols indicate no difference from either IP Vehicle or IP Acid as indicated by either a significant main effect of Treatment or a Treatment × Day interaction in the two-way ANOVA followed by a Holm-Sidak post-hoc test. As in Figure 1, the criterion for significance was adjusted for multiple comparisons to the control groups (p<0.0083 = 0.05 ÷ 6 different test drugs). Significant Treatment × Day interaction results are as follows. Panel a: [F(5,60)=4.246, p=0.002], Panel b: [F(10,85)=3.742, p<0.001], Panel c: [F(10,85)=4.995, p<0.001], and Panel f: [F(10,85)=3.576, p<0.001]. For Nortriptyline + Acid in Panel d, there was a main effect of treatment [F(2,18)=9.365, p=0.002] but no significant interaction. For Citalopram + Acid in Panel e, there was no main effect of treatment and no significant interaction.
DISCUSSION
This study evaluated effects of monoamine-transporter-inhibitor antidepressants on pain-related behavioral depression produced in male and female rats by IP acid as a visceral noxious stimulus. Repeated daily administration of IP acid for seven consecutive days decreased both body weight and positively reinforced operant responding in an ICSS procedure. This acid-induced depression of ICSS and body weight provided an opportunity to compare antinociceptive effectiveness of repeated test-drug administration. There were three main findings. First, consistent with the clinical effectiveness of NSAIDs for treatment of inflammatory pain, ketorolac blocked acid-induced depression of both ICSS and body weight. Second, the monoamine-transporter-inhibitor antidepressants bupropion, nortriptyline, and citalopram also alleviated acid effects, though to different degrees. Of these, bupropion produced the most robust antinociception, whereas nortriptyline and citalopram produced weaker and less reliable effects. Lastly, the KOR agonist U69593 and KOR antagonist norBNI failed to produce antinociception in this study. The poor effectiveness of U69593 is consistent with evidence for poor clinical effectiveness of centrally acting KOR agonists as analgesics in humans. The poor effectiveness of norBNI extends the range of conditions across which KOR antagonists have failed to produce antinociception in preclinical studies of pain-depressed behavior and does not support the utility of these compounds for pain treatment.
Effects of repeated IP acid.
ICSS is one type of positively reinforced operant behavior that can be reduced by some noxious stimuli that are thought to produce pain states in rodents (Negus 2013). We and others have shown that acute IP acid can serve as a visceral noxious stimulus sufficient to produce ICSS depression as a model of pain-related behavioral depression in rats (Brust et al. 2016; Pereira Do Carmo et al. 2009). The present results confirm and extend our previous finding that repeated daily IP acid produces repeatable ICSS depression in male rats and extends this finding to females (Miller et al. 2015). Although IP acid effects on food consumption were not evaluated directly in this study, prior studies have shown that IP acid can decrease feeding in both rats and mice (Kwilasz and Negus 2012; Stevenson et al. 2006), and consistent with these findings, the present study found that repeated IP acid produced weight loss. This effectiveness of repeated IP acid to produce ICSS depression and weight loss provides an opportunity to evaluate effects of repeated treatment with candidate analgesics. We found previously that the mu opioid receptor agonist morphine retained its antinociceptive effectiveness to alleviate IP acid-induced ICSS depression during 7 days of repeated morphine treatment (Miller et al. 2015). The present study used this same procedure to evaluate effects of repeated treatment with monoamine-transporter-inhibitor antidepressants in comparison to the NSAID ketorolac as a positive control, the KOR agonist U69593 as a negative control, and the KOR antagonist norBNI as a candidate type of antidepressant with a different mechanism of action than monoamine uptake inhibitors.
Effects of ketorolac and U69593.
Ketorolac effects are consistent with the analgesic effectiveness of ketorolac in human and veterinary medicine (Litvak and McEvoy 1990; Matava 2018; Mathews et al. 1996) and also agree with our previous report that acute ketorolac treatment blocked acute IP acid-induced ICSS depression (Moerke et al. 2019). Ketorolac and other NSAIDs can produce gastric ulceration upon repeated exposure, and this in turn has the potential to reduce body weight (Bjarnason 2013; Moore and Scheiman 2018); however, the dosing regimen used here in the absence or presence of repeated IP acid was not sufficient to alter body weight. Rather, ketorolac alleviated weight loss induced by repeated IP acid.
In contrast to ketorolac, the KOR agonist U69593 failed to block IP acid-induced ICSS depression or weight loss. These results are consistent with other evidence from preclinical animal studies that acute treatment with U69593 and other centrally acting KOR agonists fails to alleviate pain-related behavioral depression (Bagdas et al. 2016; Brust et al. 2016; Lazenka et al. 2018; Negus et al. 2010; Negus et al. 2015; Negus et al. 2012). We have interpreted these results to suggest that apparent antinociception by KOR agonists in many conventional assays of pain-stimulated behavior likely reflects false-positive effects due to motor impairment rather than analgesia (Negus, 2019). This conclusion is also consistent with clinical evidence to suggest that centrally acting KOR agonists not effective analgesics in humans up to doses that produce unacceptable side effects (Lazenka et al. 2018; Pande et al. 1996).
Effects of bupropion.
Like ketorolac, bupropion blocked IP acid-induced ICSS depression and weight loss throughout the 7 days of treatment. The effects of bupropion on Day 1 replicate our earlier report that acute bupropion treatment alleviates acute IP acid-induced ICSS depression in male rats (Rosenberg et al. 2013), and the sustained effectiveness of bupropion in the present assay of repeated IP acid-induced ICSS depression agrees with the sustained effectiveness of repeated 7-day bupropion to alleviate formalin-induced ICSS depression (Leitl and Negus 2016). Taken together, these findings provide preclinical evidence for analgesic effectiveness of bupropion and suggest that it may be especially effective to alleviate behavioral depressant signs of pain. Additionally, these preclinical findings agree with evidence for clinical analgesic effectiveness of bupropion and the mechanistically similar mixed DAT/NET inhibitor methylphenidate under at least some conditions (Pud et al. 2017; Shah and Moradimehr 2010).
When bupropion was administered alone in the absence of the IP acid noxious stimulus, it did not alter baseline ICSS. This is consistent with our previous findings with this relatively low dose of 3.2 mg/kg bupropion (Leitl and Negus 2016; Rosenberg et al. 2013); however, higher bupropion doses produce robust and significant ICSS facilitation similar to that produced by abused monoamine transporter inhibitors such as cocaine, methylphenidate, and methylenedioxypyrovalerone (MDPV) (Bonano et al. 2014; Kornetsky and Esposito 1979; Lazenka and Negus 2017). ICSS facilitation is suggestive of abuse potential (Carlezon and Chartoff 2007; Negus and Miller 2014; Wise 1996), and although bupropion is not currently scheduled by the Drug Enforcement Administration in the United States, it does produce abuse-related effects in other preclinical procedures in laboratory animals (e.g. drug self-administration (Lamb and Griffiths 1990; Nicholson et al. 2009)), and abuse has been observed clinically in the United States and elsewhere (Dagan and Yager 2018; Stall et al. 2014; Stassinos and Klein-Schwartz 2016). Thus, while results of the present study are consistent with other evidence to suggest that bupropion has potential as a non-opioid analgesic, its potential for abuse should also be considered.
Effects of nortriptyline and citalopram.
In contrast to bupropion, our primary between-subjects analysis of nortriptyline and citalopram effect indicated only a partial blockade of acid-induced ICSS depression with a trend toward increasing antinociceptive effectiveness over days of treatment. This trend for emerging antinociception is further supported by within-subject analysis of full ICSS frequency-rate curves from our previous study (Rosenberg et al. 2013) and the present study. Specifically, our previous study found that acute treatment with nortriptyline, citalopram, and the related compounds nisoxetine (a NET-selective inhibitor) and clomipramine (a SERT-selective inhibitor) failed to block acute IP acid-induced ICSS depression (Rosenberg et al. 2013), and similarly, nortriptyline and citalopram failed to block acid-induced rightward shifts in ICSS frequency-rate curves on Day 1 of the present study (data not shown). However, after 7 days of repeated treatment, both compounds significantly blocked IP acid-induced rightward shifts in ICSS frequency-rate curves (see Figure 3). This antinociceptive effectiveness after repeated treatment is consistent with clinical evidence that repeated treatment with NET- and/or SERT-inhibitor antidepressants is usually required before analgesic effectiveness is apparent (Sutherland et al. 2018). Moreover, the presence of antinociceptive effectiveness during repeated treatment with nortriptyline or citalopram + IP acid was not accompanied by ICSS facilitation in rats treated with nortriptyline or citalopram + acid vehicle. The failure of these compounds to facilitate ICSS even after repeated treatment is consistent with their low abuse liability (Negus and Miller 2014), and also suggests that blockade of IP acid-induced ICSS depression cannot be attributed to a nonselective increase in ICSS responding. Rather, these results suggest that repeated treatment with nortriptyline and citalopram engaged processes that selectively attenuated pain-related ICSS depression.
The effectiveness of nortriptyline to block acid-induced weight loss was unexpected given that nortriptyline significantly decreased body weight when it was administered alone. However, in contrast to its effects on acid-induced ICSS depression, nortriptyline effects on acid-induced weight loss tended to diminish rather than increase over time. Citalopram did not significantly alleviate acid-induced weight loss. High variability in this group resulted in low power, so it cannot be concluded that an effect was absent; however, as with nortriptyline, any antinociceptive blockade of acid-induced weight loss tended to decrease over time. Taken together, nortriptyline and citalopram effects on acid-induced weight loss are not consistent with emerging antinociception during repeated treatment.
Effects of norbinaltorphimine.
Accumulating evidence has been interpreted to suggest that stress and/or injury may produce prodepressant effects by activating KOR-mediated dynorphin signaling in brain regions such as nucleus accumbens, and that KOR antagonists might have therapeutic effectiveness to treat depression or some affective dimensions of pain by blocking KOR-mediated dynorphin signaling (Jacobson et al. 2020; Knoll and Carlezon 2010; Liu et al. 2019; Massaly et al. 2019). However, results of the present study extend the range of conditions across which KOR antagonists have failed to alleviate pain-related behavioral depression in rodents. For example, we have shown previously that KOR antagonists did not alleviate acute IP acid-induced depression of either ICSS or mesolimbic dopamine release in rats (Leitl et al. 2014a; Negus et al. 2010), more sustained ICSS depression produced by intraplantar formalin as a model of sustained neuropathic pain in rats (Leitl et al. 2014b), acute IP acid-induced depression of nesting in mice (Negus et al. 2015), or IP acid-induced conditioned place avoidance in mice (Bagdas et al. 2016). The failure of norBNI to block ICSS depression by repeated IP acid in the present study cannot be attributed to inadequate dosing, because lower doses of 5–10 mg/kg norBNI have been reported to produce long-lasting antagonism of exogenous KOR agonist effects in rats (Craft and McNiel 2003; Friese et al. 1997; Holtzman 2000; Negus et al. 2010). Rather, these results suggest that, while KOR antagonists may alleviate some depression-like effects of stress or injury, they are less effective than clinically available monoamine-transporter-inhibitor antidepressants to alleviate pain-related behavioral depression.
Limitations.
Three limitations to this study warrant mention. First, only one dose of each compound was tested. Although dose selection was based on prior studies of acute treatment with each drug in assays of IP acid-induced ICSS depression, it is possible that other doses may have had different magnitudes or time courses of effects. However, these results do show sustained antinociception by repeated bupropion and significant antinociception after repeated nortriptyline and citalopram. Second, although this study included both male and female subjects, it was not powered to detect sex differences. No sex differences were apparent with the sample sizes used, and Supplemental Table 1 shows post-hoc power analysis results (Diester et al. 2019) that could be used to guide sample-size selection in future studies that do aim to investigate sex differences in drug effects. Lastly, this study did not include mixed-action NET/SERT inhibitors like duloxetine and milnacipran, which are also used for chronic pain management (Urits et al. 2019). We found previously that, like the NET- and SERT-selective inhibitors, acute milnacipran failed to alleviate IP acid-induced ICSS depression (Rosenberg et al. 2013); however, the present results with NET- and SERT-selective inhibitors suggest that repeated dosing may also be effective for mixed NET/SERT inhibitors.
Conclusions.
These results support the utility of monoamine uptake inhibitor antidepressant drugs to treat pain-related behavioral depression and suggest that the moderately selective DAT inhibitor bupropion might have a faster onset and higher effectiveness than NET- or SERT-selective inhibitors. Mechanisms that underlie antinociception produced by repeated treatment with NET and SERT inhibitors requires further study.
Supplementary Material
Acknowledgements
This work was supported by grants R01NS070715 (SSN) and F30CA213956 (LPL) from the National Institutes of Health. The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- Aggarwal S, Mortensen OV (2017) Overview of Monoamine Transporters. Curr Protoc Pharmacol 79: 12 16 1–12 16 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS (2015) Effects of mu-opioid receptor agonists in assays of acute pain-stimulated and pain-depressed behavior in male rats: role of mu-agonist efficacy and noxious stimulus intensity. J Pharmacol Exp Ther 352: 208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal N (2019) Pharmacological treatments of neuropathic pain: The latest recommendations. Rev Neurol (Paris) 175: 46–50. [DOI] [PubMed] [Google Scholar]
- Bagdas D, Muldoon PP, AlSharari S, Carroll FI, Negus SS, Damaj MI (2016) Expression and pharmacological modulation of visceral pain-induced conditioned place aversion in mice. Neuropharmacology 102: 236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat A, Hamdy MM, Elbadr MM (2018) Uses of fluoxetine in nociceptive pain management: A literature overview. Eur J Pharmacol 829: 12–25. [DOI] [PubMed] [Google Scholar]
- Bjarnason I (2013) Gastrointestinal safety of NSAIDs and over-the-counter analgesics. Int J Clin Pract Suppl: 37–42. [DOI] [PubMed] [Google Scholar]
- Boakye PA, Olechowski C, Rashiq S, Verrier MJ, Kerr B, Witmans M, Baker G, Joyce A, Dick BD (2016) A Critical Review of Neurobiological Factors Involved in the Interactions Between Chronic Pain, Depression, and Sleep Disruption. Clin J Pain 32: 327–36. [DOI] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS (2014) Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl) 231: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DC, Boston RC, Coyne JC, Farrar JT (2008) Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc 233: 1278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, Aube J, Jones SR, Martin TJ, Bohn LM (2016) Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal 9: ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Lee TC, Knadler MP, Detke MJ, Iyengar S (2005) The dual transporter inhibitor duloxetine: a review of its preclinical pharmacology, pharmacokinetic profile, and clinical results in depression. Curr Pharm Des 11: 1475–93. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr., Chartoff EH (2007) Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2: 2987–95. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, Geddes JR (2018) Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391: 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, McNiel DM (2003) Agonist/antagonist properties of nalbuphine, butorphanol and (−)-pentazocine in male vs. female rats. Pharmacol Biochem Behav 75: 235–45. [DOI] [PubMed] [Google Scholar]
- Dagan Y, Yager J (2018) Severe bupropion XR abuse in a patient with long-standing bulimia nervosa and complex PTSD. Int J Eat Disord 51: 1207–1209. [DOI] [PubMed] [Google Scholar]
- Diester CM, Banks ML, Neigh GN, Negus SS (2019) Experimental design and analysis for consideration of sex as a biological variable. Neuropsychopharmacology 44: 2159–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J, Immpact (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113: 9–19. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14: 162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese N, Diop L, Lambert C, Riviere PJ, Dahl SG (1997) Antinociceptive effects of morphine and U-50,488H on vaginal distension in the anesthetized rat. Life Sci 61: 1559–70. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Negus SS, Mello NK (1998) Antinociceptive effects of monoamine reuptake inhibitors administered alone or in combination with mu opioid agonists in rhesus monkeys. Psychopharmacology (Berl) 135: 99–106. [DOI] [PubMed] [Google Scholar]
- Goesling J, Clauw DJ, Hassett AL (2013) Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psychiatry Rep 15: 421. [DOI] [PubMed] [Google Scholar]
- Hall FS, Schwarzbaum JM, Perona MT, Templin JS, Caron MG, Lesch KP, Murphy DL, Uhl GR (2011) A greater role for the norepinephrine transporter than the serotonin transporter in murine nociception. Neuroscience 175: 315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman SG (2000) Further characterization of the discriminative stimulus effects of spiradoline. Pharmacol Biochem Behav 66: 517–22. [DOI] [PubMed] [Google Scholar]
- Hyttel J, Bogeso KP, Perregaard J, Sanchez C (1992) The pharmacological effect of citalopram residues in the (S)-(+)-enantiomer. J Neural Transm Gen Sect 88: 157–60. [DOI] [PubMed] [Google Scholar]
- Jacob SN, Nienborg H (2018) Monoaminergic Neuromodulation of Sensory Processing. Front Neural Circuits 12: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson ML, Browne CA, Lucki I (2020) Kappa Opioid Receptor Antagonists as Potential Therapeutics for Stress-Related Disorders. Annu Rev Pharmacol Toxicol 60: 615–636. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA Jr. (2010) Dynorphin, stress, and depression. Brain Res 1314: 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU (1979) Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc 38: 2473–6. [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS (2012) Dissociable effects of the cannabinoid receptor agonists Delta9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther 343: 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Griffiths RR (1990) Self-administration in baboons and the discriminative stimulus effects in rats of bupropion, nomifensine, diclofensine and imipramine. Psychopharmacology (Berl) 102: 183–90. [DOI] [PubMed] [Google Scholar]
- Lazenka MF, Negus SS (2017) Oral modafinil facilitates intracranial self-stimulation in rats: comparison with methylphenidate. Behav Pharmacol 28: 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenka ML, Moerke MJ, Townsend EA, Freeman KB, Carroll FI, Negus SS (2018) Dissociable effects of the kappa opioid receptor agonist nalfurafine on pain/itch-stimulated and pain/itch-depressed behaviors in male rats. Psychopharmacology (Berl) 235: 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Negus SS (2016) Pharmacological modulation of neuropathic pain-related depression of behavior: effects of morphine, ketoprofen, bupropion and [INCREMENT]9-tetrahydrocannabinol on formalin-induced depression of intracranial self-stimulation in rats. Behav Pharmacol 27: 364–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA Jr., Banks ML, Negus SS (2014a) Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous kappa-opioids. Neuropsychopharmacology 39: 614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon WA Jr., Negus SS (2014b) Sustained pain-related depression of behavior: effects of intraplantar formalin and complete freund’s adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol Pain 10: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Canales JJ, Bjorgvinsson T, Thomsen M, Qu H, Liu QR, Torres GE, Caine SB (2011) Monoamine transporters: vulnerable and vital doorkeepers. Prog Mol Biol Transl Sci 98: 1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak KM, McEvoy GK (1990) Ketorolac, an injectable nonnarcotic analgesic. Clin Pharm 9: 921–35. [PubMed] [Google Scholar]
- Liu SS, Pickens S, Burma NE, Ibarra-Lecue I, Yang H, Xue L, Cook C, Hakimian JK, Severino AL, Lueptow L, Komarek K, Taylor AMW, Olmstead MC, Carroll FI, Bass CE, Andrews AM, Walwyn W, Trang T, Evans CJ, Leslie FM, Cahill CM (2019) Kappa Opioid Receptors Drive a Tonic Aversive Component of Chronic Pain. J Neurosci 39: 4162–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn TH, Frokjaer VG, Hansen TB, Kristensen PW, Lind T, Kehlet H (2015) Analgesic effect of perioperative escitalopram in high pain catastrophizing patients after total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. Anesthesiology 122: 884–94. [DOI] [PubMed] [Google Scholar]
- Massaly N, Copits BA, Wilson-Poe AR, Hipolito L, Markovic T, Yoon HJ, Liu S, Walicki MC, Bhatti DL, Sirohi S, Klaas A, Walker BM, Neve R, Cahill CM, Shoghi KI, Gereau RWt, McCall JG, Al-Hasani R, Bruchas MR, Moron JA (2019) Pain-Induced Negative Affect Is Mediated via Recruitment of The Nucleus Accumbens Kappa Opioid System. Neuron 102: 564–573 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matava MJ (2018) Injectable Nonsteroidal Anti-Inflammatory Drugs in Sport. Clin J Sport Med 28: 443–450. [DOI] [PubMed] [Google Scholar]
- Mathews KA, Paley DM, Foster RA, Valliant AE, Young SS (1996) A comparison of ketorolac with flunixin, butorphanol, and oxymorphone in controlling postoperative pain in dogs. Can Vet J 37: 557–67. [PMC free article] [PubMed] [Google Scholar]
- McDonald JH (2014) Handbook of Biological Statistics (3rd ed.). Sparky House Publishing, Baltimore, Maryland. [Google Scholar]
- Miller LL, Altarifi AA, Negus SS (2015) Effects of repeated morphine on intracranial self-stimulation in male rats in the absence or presence of a noxious pain stimulus. Exp Clin Psychopharmacol 23: 405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerke MJ, Li G, Golani LK, Cook J, Negus SS (2019) Effects of the alpha2/alpha3-subtype-selective GABAA receptor positive allosteric modulator KRM-II-81 on pain-depressed behavior in rats: comparison with ketorolac and diazepam. Behav Pharmacol 30: 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N, Scheiman JM (2018) Gastrointestinal safety and tolerability of oral non-aspirin over-the-counter analgesics. Postgrad Med 130: 188–199. [DOI] [PubMed] [Google Scholar]
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ (2015) Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev: CD008242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National_Research_Council (2003) Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. The National Academies Press, Washington, D.C. [PubMed] [Google Scholar]
- Negus SS (2013) Expression and treatment of pain-related behavioral depression. Lab Anim (NY) 42: 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS (2019) Core Outcome Measures in Preclinical Assessment of Candidate Analgesics. Pharmacol Rev 71: 225–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL (2014) Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev 66: 869–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC (2010) Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 210: 149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL (2015) Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain 156: 1153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC (2012) Effects of peripherally restricted kappa opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther 340: 501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KL, Balster RL, Golembiowska K, Kowalska M, Tizzano JP, Skolnick P, Basile AS (2009) Preclinical evaluation of the abuse potential of the analgesic bicifadine. J Pharmacol Exp Ther 330: 236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ (2008) Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry 69 Suppl E1: 4–7. [PubMed] [Google Scholar]
- O’Donnell JM, Bies RR, Shelton RC (2018) Drug therapy of depression and anxiety disorders In: Brunton LL, Hilal-Dandan R, Knollmann BC (eds) Goodman and Gilman’s The Pharmacological Basis of Therapeutics. McGraw Hill, New York, pp 267–278 [Google Scholar]
- Obata H (2017) Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande AC, Pyke RE, Greiner M, Cooper SA, Benjamin R, Pierce MW (1996) Analgesic efficacy of the kappa-receptor agonist, enadoline, in dental surgery pain. Clin Neuropharmacol 19: 92–7. [DOI] [PubMed] [Google Scholar]
- Paoli F, Darcourt G, Cossa P (1960) [Preliminary note on the action of imipramine in painful states]. Rev Neurol (Paris) 102: 503–4. [PubMed] [Google Scholar]
- Pedersen LH, Nielsen AN, Blackburn-Munro G (2005) Anti-nociception is selectively enhanced by parallel inhibition of multiple subtypes of monoamine transporters in rat models of persistent and neuropathic pain. Psychopharmacology (Berl) 182: 551–61. [DOI] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS (2009) Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain 144: 170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pud D, Broitman E, Hameed O, Suzan E, Aviram J, Haddad M, Hadad S, Shemesh R, Eisenberg E (2017) Methylphenidate attenuates the response to cold pain but not to aversive auditory stimuli in healthy human: a double-blind randomized controlled study. Pain Rep 2: e593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg MB, Carroll FI, Negus SS (2013) Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. J Pain 14: 246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah TH, Moradimehr A (2010) Bupropion for the treatment of neuropathic pain. Am J Hosp Palliat Care 27: 333–6. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S (2004) A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. Prim Care Companion J Clin Psychiatry 6: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall N, Godwin J, Juurlink D (2014) Bupropion abuse and overdose. CMAJ 186: 1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassinos GL, Klein-Schwartz W (2016) Bupropion “Abuse” Reported to US Poison Centers. J Addict Med 10: 357–62. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Bilsky EJ, Negus SS (2006) Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J Pain 7: 408–16. [DOI] [PubMed] [Google Scholar]
- Sutherland AM, Nicholls J, Bao J, Clarke H (2018) Overlaps in pharmacology for the treatment of chronic pain and mental health disorders. Prog Neuropsychopharmacol Biol Psychiatry 87: 290–297. [DOI] [PubMed] [Google Scholar]
- Tappe-Theodor A, King T, Morgan MM (2019) Pros and Cons of Clinically Relevant Methods to Assess Pain in Rodents. Neurosci Biobehav Rev 100: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urits I, Peck J, Orhurhu MS, Wolf J, Patel R, Orhurhu V, Kaye AD, Viswanath O (2019) Off-label Antidepressant Use for Treatment and Management of Chronic Pain: Evolving Understanding and Comprehensive Review. Curr Pain Headache Rep 23: 66. [DOI] [PubMed] [Google Scholar]
- Ventafridda V, Bianchi M, Ripamonti C, Sacerdote P, De Conno F, Zecca E, Panerai AE (1990) Studies on the effects of antidepressant drugs on the antinociceptive action of morphine and on plasma morphine in rat and man. Pain 43: 155–62. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Shi SY, Li SJ, Chen F, Chen H, Lin HZ, Lin JM (2015) Efficacy and Safety of Duloxetine on Osteoarthritis Knee Pain: A Meta-Analysis of Randomized Controlled Trials. Pain Med 16: 1373–85. [DOI] [PubMed] [Google Scholar]
- Wise RA (1996) Addictive drugs and brain stimulation reward. Annu Rev Neurosci 19: 319–40. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Wallace M (2018) Opioids, analgesia, and pain management In: Brunton LL, Hilal-Dandan R, Knollmann BC (eds) Goodman & Gilman’s The Pharmacological Basis of Therapeutics. McGraw Hill, New York, pp 355–386 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


