Abstract
Purpose
Ginseng has been used as a tonic to improve overall health, restore balance in the body, help the body to heal itself, reduce stress, boost energy, and enhance the immune system. The aim of this review was to assess current evidence that ginseng improves sperm quality.
Materials and Methods
We searched twelve databases (PubMed, EMBASE, AMED, the Cochrane Library, five Korean medical databases, and three Chinese medical databases), using a cut-off date of 1st December 2019. We included clinical studies in which healthy men or men with fertility issues were treated with ginseng. We used Cochrane's risk of bias (ROB) tool to assess the ROB in the studies examined.
Results
From two-hundred and nineteen potentially eligible studies, just five relevant studies were selected (two randomized clinical trials [RCTs], one controlled clinical trial [CCT], and two observational studies). Of these studies, one RCT reported some positive results when using Korean red ginseng to improve sperm quality in infertile men. In addition, the other RCT reported that the effects of ginseng on sperm quality were equivalent to those found when valerian tablets are taken by healthy people. One CCT and two uncontrolled observational studies, however, did not demonstrate the clear effectiveness of ginseng in improving sperm quality in infertile men.
Conclusions
Currently, there are few trials investigating the efficacy of ginseng for improving sperm quality. The available studies demonstrate a high ROB. It can be suggested that overall, the evidence regarding ginseng improving sperm quality is limited.
Keywords: Ginseng, Infertility, Sperm, Spermatogenesis, Systematic review
INTRODUCTION
In recent reports, the prevalence of male infertility (which can be defined as infertility experienced for at least 12 months) varies from 9% to 15.8% of the general population [1]. Many conventional therapies are available to treat male infertility, but their efficacy is unclear [2,3]. People with infertility problems often seek complementary and alternative medicine [4]. Several studies have shown that many sufferers have used some form of herbal medicine, such as dang gui, maca, or ginseng, in order to try to improve their fertility, libido, and sexual performance [4,5,6,7,8,9,10,11]. Ginseng is the root of the Panax plant. It can be administered in various forms, including tablets, liquid extracts, tinctures, powdered roots, sliced roots and teas. Ginseng has been found to have several classes of chemical constituents, including ginsenosides, polysaccharides, alkaloids, glucosides, and phenolic acid [12,13]. Many studies have demonstrated the bioactivity of ginseng, namely its antioxidant, anti-inflammatory, anti-aging, anti-diabetic, anti-cancer, neuroregulation, lipid regulation and antithrombotic properties, as well as its pharmacokinetics [13,14]. As such, ginseng has been utilized as an ‘adaptogen for promoting resistance to external and internal stresses or stimuli and improving both physical and mental faculties’ [14].
Several preclinical studies have reported that ginseng promotes spermatogenesis, as well as improving testicular problems, sperm quality, and sperm mobility, due to be a cyclic adenosine monophosphate-responsive element modulator [15,16,17,18,19,20]. Recent reviews summarize that ginsenosides—the predominant active compounds of ginseng—may affect estrogen and androgen activity, and act as an aphrodisiac [21,22]. Ginseng polysaccharides may modulate estrogen receptors and the beneficial effects on estrogen-induced physiological states. In addition, they play a role in maintaining healthy levels of steroid hormone receptors, ensuring the proper functioning of androgen hormones in turn [22]. However, the exact mechanism through which ginseng functions as an enhancer of sperm quality has not been studied in sufficient detail to date; there has been no systematic review of the role of ginseng in improving semen quality parameters. The objective of this study, therefore, is to assess current evidence that ginseng can improve sperm quality.
MATERIALS AND METHODS
1. Study registration and published protocol
We registered the following protocol on PROSPERO: CRD42017078797 (http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017078797). The full protocol was published as recommended, and the methods described in this systematic review are the same as those published [23].
2. Ethical statement
No ethical approval was required for this manuscript as this study did not involve human subjects or laboratory animals.
3. Inclusion and exclusion criteria
1) Types of study
We included all prospective randomized clinical trials (RCTs), controlled clinical trials (CCTs), and uncontrolled observational studies (UOS) available across the twelve databases selected. We excluded case studies, case series, and retrospective clinical studies.
2) Types of participant
We included healthy men, or men with subfertility or infertility, regardless of age.
3) Types of intervention
We included studies involving any type of ginseng (used alone or as a co-intervention with conventional medicines), regardless of its origin, age, or processing status, or the dose used. The inclusion criteria for the controls were no treatment, placebos, or conventional medicines.
4) Types of outcome measure
(1) Primary outcomes: sperm motility and sperm concentration
(2) Secondary outcomes: sperm morphology, sperm count, semen volume, and adverse events (AEs)
4. Search methods
1) Searched electronic databases
We searched twelve databases, without placing limits on the language of publication or on publication status, from the databases' inception to 1st December, 2019. The databases included were PubMed, the Cochrane Library, EMBASE, AMED, five Korean medical databases (the Research Information Service System [RISS], the Korean Studies Information Service System [KISS], KoreaMed, DBpia, OASIS), and three Chinese medical databases (China National Knowledge Infrastructure [CNKI], the Wanfang Database, and the Chinese Scientific Journals Database [VIP]).
With a view to including ongoing studies, we searched the following databases: World Health Organization International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/); Chinese Clinical Trial Registry (www.chictr.org); ISRCTN (www.controlledtrials.com/isrctn/); National Institutes of Health Clinical Trials Database (www.clinicaltrials.gov); Clinical Research Information Service (CRiS) of the Republic of Korea (https://cris.nih.go.kr/cris/en/).
2) Other sources
We also searched ‘The Journal of Ginseng Research’ and ‘Proceedings of the Ginseng Society Conference’ manually. Then, we reviewed all the studies included again in order to select those potentially suitable for inclusion in our study.
3) Search strategy
The search terms we utilized were ‘Panax ginseng OR ginseng’ and ‘semen OR sperm OR hyposperm OR subfertility’. The search strategy involved using a mixture of free text and thesaurus terms in English, Korean, and Chinese.
5. Selection of studies and data extraction
Two reviewers (HWL, KJK) selected potentially eligible articles by reading through titles and abstracts. These reviewers examined hard copies of the publications in order to determine their suitability. Any disagreements were resolved through discussion, and where necessary, in consultation with a third reviewer (MSL).
Two reviewers (HWL, KJK) extracted data from the studies included. Information that was excluded was as follows: the characteristics of subjects, the types of ginseng therapy used, the outcomes, and the results. We resolved disagreements through discussion and in consultation with the third author (MSL), who acted as an arbiter.
6. Assessment of risk of bias
Two reviewers (HWL, MSL) independently evaluated the risk of bias (ROB) in the studies included by following the guidelines given in the “Cochrane Handbook of Systematic Reviews of Interventions (i.e., sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting)” [24]. Although ROB tools generally assess the ROBs in RCTs, we also used this method to examine CCTs and UOSs for bias.
7. Data synthesis
We presented dichotomous data in terms of risk ratios, with 95% confidence intervals (CIs). We used the mean difference with 95% CIs for continuous data, and the standard mean difference for outcome variables with different scales. We were unable to conduct a meta-analysis due to the small number of studies included.
RESULTS
1. Study description
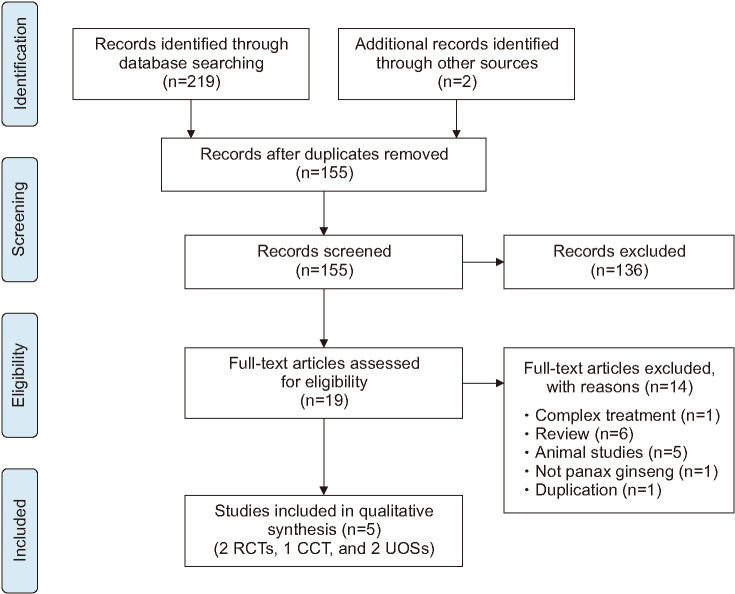
Of 219 eligible hits, 136 studies were excluded following screening the abstracts and titles. After assessing 19 full-text articles, 14 studies were excluded for several reasons (Fig. 1). Finally, five studies met our inclusion criteria [25,26,27,28,29]. We summarize the key data from these studies in Table 1. Two of the studies were RCTs [25,26], one was a CCT [27], and two were UOSs [28,29]. Of the five studies, one trial was performed in the USA [26], three were performed in Korea [25,27,28], and one was conducted in Italy [29]. All the studies used oral ginseng, and the daily dosages were 1.5–4 g of ginseng for 10 days to 12 weeks. One study included healthy men [26], two were undertaken with infertile men [25,29], and the other two studies included both infertile and healthy men [27,28].
Fig. 1. Flow chart for the selection of included trials. RCT: randomised clinical trial, CCT: controlled clinical trial, UOS: uncontrolled observational study.
Table 1. Summary of clinical trials focusing on the effect of ginseng on semen quality parameters.
| First author (year)/location | Sample size and condition | Intervention (regimen) | Control intervention (regimen) | Main outcome measures | Results | Adverse effects |
|---|---|---|---|---|---|---|
| Park (2016) [25]/Korea | RCT 80 infertile men 25–45 y |
(A) KRG (500 mg/capsule, 3 capsules/d, total 1.5 g/d for 12 wk, n=20) (B) KRG (500 mg/capsule, 3 capsules/d, total 1.5 g/d for 12 wk, n=20), plus varicocelectomy |
(C) Placebo (n=20) (D) Placebo (n=20), plus varicocelectomy |
(1) Sperm concentration (2) Sperm morphology (3) Semen viability (4) Sperm motility |
1) A vs. C: p=0.009, 0.86 (0.21–1.51); B vs. D: NS, 0.15 (-0.49–0.80) (2) A vs. C: p<0.001, 5.7 (4.24–7.15); B vs. D: p<0.001, 2.15 (1.32–2.98) (3) A vs. C: p<0.001, 3.23 (2.26–4.20); B vs. D: p=0.002, 1.08 (0.39–1.78) (4) A vs. C: p<0.001, 2.25 (1.45–3.06); B vs. D: NS, 0.59 (-0.07–1.26) |
n.r. KGC supported (ginseng) |
| Mkrtchyan (2005) [26]/Armenia | RCT 14 healthy men 21–31 y |
(A) Ginseng mixture (ginsenoside, 85.7 m/d=3 g of dry ginseng/ginseng for 10 d, n=5) | (B) Valerian tablet (1.28 g/d for 10 d, n=5) (C) Herbal mixtures (company commercial product, 60 mg/d, for 10 d, n=5)a (D) Herbal mixtures (company commercial product, 120 mg/d, for 10 days, n=5)a (E) Herbal mixtures (company commercial product, 180 mg/d, for 10 d, n=6)a |
(1) Semen volume (2) Sperm concentration (3) Motile sperm count (4) Total sperm count |
(1) NS, -0.13 (-1.37–1.11) (2) NS, -0.52 (-1.66–0.62) (3) NS, -0.08 (-1.32–1.16) (4) NS, -0.11 (-1.35–1.13) |
None |
| Lee (2006) [27]/Korea | CCT 43 participants (33 infertile men; 10 healthy men) n.r. |
(A) Mountain ginseng (n.r. in details, n=21) | (B) Placebo (n=12) (C) Health control (n=12) |
(1) Semen volume (2) Sperm concentration (3) Sperm morphology (4) Sperm motility |
(1)–(3) p<0.05 for A but NS for B and C (intergroup analysis) (4) NS for all groups (intergroup analysis) |
None Abstract only |
| Kim (2002) [28]/Korea | UOS (case control) Total 37 participants (25 infertile men; 12 healthy men) 34.3 y |
(A) KRG (300 mg/capsule, 8 capsules/d, total 2.4 g for 8 wk, n=37) | N/A | (1) Semen volume (2) Sperm concentration (3) Sperm morphology (4) Sperm motility |
(1)–(4) NS for both infertility and healthy participants | Heat (8), headache (1), indigestion (1) 26 participants were analyzed (18 infertility and 8 healthy men) |
| Salvati (1996) [29]/Italy | UOS (case control) Total 66 participants (30 oligoasthenospermia; 16 oligoasthenospermia with idiopathic varicocele; 20 healthy men) n.r. |
(A) Panax ginseng (extract, 4 g/d for 3 mo, n=66) | N/A | (1) Sperm counts (2) Sperm motility |
(1), (2) Increased compared with baseline but n.r. in statistically significant levels | n.r. |
RCT: randomized clinical trial, CCT: controlled clinical trial, UOS: uncontrolled observational study, n.r.: not reported, KRG: Korean red ginseng, NA: not available, NS: not significant, KGC: Korean Ginseng Corporation.
aThese parts were not considered in the analysis.
2. Risk of bias
Only one RCT [25] noted the use of random sequence generation methods; the other RCT [26] failed to do so (Fig. 2). In the former RCT, both the patients and practitioner were blinded [25], while the latter was an open-labeled RCT [26]. The CCT was published as an abstract only, and it noted the use of a double-blinded multi-center trial [27]. The other two studies were UOSs, with high ROBs [28,29].
Fig. 2. (A) Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies; (B) Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
3. Outcomes
1) Infertile men
One RCT tested the efficacy of Korean red ginseng (KRG) alone and KRG plus varicocelectomy in improving sperm quality parameters, when compared with the use of a placebo or a placebo plus varicocelectomy in 80 infertile men [25]. This trial's results indicated the beneficial effects of using KRG alone, as sperm concentration, morphology, viability, and motility were found to be improved with KRG, when the results were compared with those for the placebo group. Additionally, the use of KRG plus varicocelectomy was shown to have positive effects on sperm morphology and viability, when compared with the use of a placebo plus varicocelectomy. The CCT showed that mountain ginseng has significant positive effects on semen volume, sperm concentration, and morphology but not on sperm motility, when compared with pre-treatment scores [27]. On the other hand, the two UOSs failed to show that ginseng has any significant impact on semen parameters in infertile men [27,28].
2) Healthy volunteers
One of the RCTs included investigated the efficacy of a ginseng mixture in improving semen quality, when compared with the results for valerian tablets in healthy men. The study showed that the ginseng mixture had positive effects on semen volume, concentration, motile sperm count, and total sperm count, all of which were equivalent to the results obtained by utilizing valerian tablets [26]. The CCT, however, did not find that there was a significant improvement in semen quality parameters after taking ginseng, when compared with the use of a placebo [27]. Additionally, the two UOSs indicated that ginseng does not have significant effects on semen quality, when compared with baseline values [28,29].
3) Adverse events
Although all of the five studies investigated AEs, two did not report on them. Two of the studies found that there were no AEs [26,27], while another stated that elevated temperature, headache, and indigestion were reported [28].
DISCUSSION
Our results show that a small body of research exists on the effects of ginseng on semen quality parameters. One RCT that we investigated demonstrated the efficacy of KRG in improving semen quality parameters in infertile men [25], although another RCT that we examined failed to find any improvement in these parameters in healthy men [26]. The remaining three studies, all of which had high ROBs, also failed to show that KRG, mountain ginseng, and white ginseng respectively have positive effects on these parameters in either infertile or healthy men [27,28,29]. It should be acknowledged that the collective evidence was limited; the total number of studies and the total sample size were both too small for definite conclusions to be drawn.
One of the studies included was published as an abstract only and had not been through a rigorous peer review process, meaning that the study could not be evaluated to any great extent [27]. Two of the studies were UOSs with a high risk of potential bias; for example, the treatment effects were overstated in places [28,29]. Furthermore, all of the studies included had small sample sizes (ranging between 14 and 80), and were prone to type II errors.
One common concern surrounding the use of ginseng it is that it may bring about more AEs than conventional therapies. Two trials found that there were no AEs [26,27], while one study reported that were no severe AEs [28]. As such, the potential for ginseng use to lead to AEs needs to be investigated further in future studies.
The doses of ginseng reported in the studies included in our analysis were 3 g/d for 10 days and 4 g/d for 12 weeks. No detailed information was provided for mountain ginseng. The dose of KRG was 1.5 g/d for 12 weeks and 2.4 g/d for 8 weeks. Hence, all the RCTs in our review reported various doses of ginseng. It is clear that further study of clinical trials comparing the dosage, duration, and type of ginseng are required.
Assuming that ginseng is beneficial for improving sperm quality, its possible mechanisms of action may be of interest. As in the case of all herbal extracts, ginseng preparations are complex mixtures of multiple pharmacologically active ingredients. The most important and best researched of the active ingredients in ginseng are the ginsenosides, a diverse group of saponins that are structurally similar to steroid hormones. Thus, it was speculated that the effects of ginsenosides on sperm formation are the result of activation of steroid hormone receptors [22,30]. It was also suggested that the effects of ginseng may extend to other biochemical variables, including hormones, oxidative biomarkers, reactive oxygen species, and apoptosis [20,30]. In this review, three of the included studies assessed the biochemical parameters, including hormones [25,28,29]. However, the results were not consistent, and several limitations prevented their confirmation. Therefore, the mechanisms of action of ginseng are diverse, complex and often somewhat unclear. Further basic research is needed to fully understand these mechanisms.
This review also has several limitations. Although we conducted a comprehensive search, we cannot completely rule out the possibility that we may have overlooked other relevant articles. In addition, many studies on ginseng have received support from manufacturers of ginseng products, which could cause potential conflicts of interest and intrinsic biases. For instance, one of the studies included in this review received ginseng from an associated company. Moreover, the quality of the reporting in the studies examined was poor, lacking detail. It is very important to follow rigorous methods and CONSORT reporting guidelines for improving the evidence levels. Further limitations include a lack of methodological rigor in the primary data examined and the fact that most of the studies failed to minimize bias.
CONCLUSIONS
Our results suggest that there is limited evidence that ginseng can improve semen quality parameters. However, given the overall ROB, the small number of studies included, and the total sample size, we cannot draw firm conclusions. This study contends, therefore, that more academically rigorous studies are required in order to examine this subject further.
ACKNOWLEDGEMENTS
Hye Won Lee and Myeong Soo Lee were supported by Korea Institute of Oriental Medicine (K18043 and KSN2013210), Korea.
Footnotes
Conflict of Interest: Dr. Myeong Soo Lee is one of editorial board of Journal of Ginseng Research, but it made no influence on this work in relation with topic. Other authors have nothing to disclose.
- Conceptualization: HWL, MSL.
- Data curation: HWL, MSL.
- Formal analysis: HWL, MSL.
- Funding acquisition: HWL, MSL.
- Investigation: HWL, MSL.
- Methodology: HWL, MSL.
- Project administration: HWL, MSL.
- Resources: HWL, MSL.
- Software: HWL, MSL.
- Supervision: HWL, MSL.
- Validation: KJK, MSL.
- Visualization: HWL, MSL.
- Writing — original draft: HWL, KJK.
- Writing — review & editing: HWL, MSL.
References
- 1.Barratt CLR, Björndahl L, De Jonge CJ, Lamb DJ, Osorio Martini F, McLachlan R, et al. The diagnosis of male infertility: an analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum Reprod Update. 2017;23:660–680. doi: 10.1093/humupd/dmx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabaja AA, Hammoud AO. Male factor infertility. BMJ Best Practice 2017. London: BMJ; 2017. [Google Scholar]
- 3.Jungwirth A, Diemer T, Kopa Z, Krausz C, Minhas S, Tournaye H. EAU guidelines on male infertility [Internet] Arnhem: European Association of Urology; [cited 2019 Nov 30]. Available from: http://uroweb.org/guideline/male-infertility/ [Google Scholar]
- 4.Clark NA, Will M, Moravek MB, Fisseha S. A systematic review of the evidence for complementary and alternative medicine in infertility. Int J Gynaecol Obstet. 2013;122:202–206. doi: 10.1016/j.ijgo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Coulson C, Jenkins J. Complementary and alternative medicine utilisation in NHS and private clinic settings: a United Kingdom survey of 400 infertility patients. J Exp Clin Assist Reprod. 2005;2:5. doi: 10.1186/1743-1050-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu M, Zhang Y, Ma H, Ng EH, Wu XK. Eastern medicine approaches to male infertility. Semin Reprod Med. 2013;31:301–310. doi: 10.1055/s-0033-1345589. [DOI] [PubMed] [Google Scholar]
- 7.Lee MS, Lee HW, You S, Ha KT. The use of maca (Lepidium meyenii) to improve semen quality: a systematic review. Maturitas. 2016;92:64–69. doi: 10.1016/j.maturitas.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Smith CA, Armour M, Ee C. Complementary therapies and medicines and reproductive medicine. Semin Reprod Med. 2016;34:67–73. doi: 10.1055/s-0035-1571194. [DOI] [PubMed] [Google Scholar]
- 9.Smith JF, Eisenberg ML, Millstein SG, Nachtigall RD, Shindel AW, Wing H, et al. Infertility Outcomes Program Project Group. The use of complementary and alternative fertility treatment in couples seeking fertility care: data from a prospective cohort in the United States. Fertil Steril. 2010;93:2169–2174. doi: 10.1016/j.fertnstert.2010.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stankiewicz M, Smith C, Alvino H, Norman R. The use of complementary medicine and therapies by patients attending a reproductive medicine unit in South Australia: a prospective survey. Aust N Z J Obstet Gynaecol. 2007;47:145–149. doi: 10.1111/j.1479-828X.2007.00702.x. [DOI] [PubMed] [Google Scholar]
- 11.Zini A, Fischer MA, Nam RK, Jarvi K. Use of alternative and hormonal therapies in male infertility. Urology. 2004;63:141–143. doi: 10.1016/j.urology.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Angelova N, Kong HW, van der Heijden R, Yang SY, Choi YH, Kim HK, et al. Recent methodology in the phytochemical analysis of ginseng. Phytochem Anal. 2008;19:2–16. doi: 10.1002/pca.1049. [DOI] [PubMed] [Google Scholar]
- 13.Ru W, Wang D, Xu Y, He X, Sun YE, Qian L, et al. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.) Drug Discov Ther. 2015;9:23–32. doi: 10.5582/ddt.2015.01004. [DOI] [PubMed] [Google Scholar]
- 14.Jia L, Zhao Y, Liang XJ. Current evaluation of the millennium phytomedicine- ginseng (II): collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr Med Chem. 2009;16:2924–2942. doi: 10.2174/092986709788803204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eskandari M, Jani S, Kazemi M, Zeighami H, Yazdinezhad A, Mazloomi S, et al. Ameliorating effect of ginseng on epididymo-orchitis inducing alterations in sperm quality and spermatogenic cells apoptosis following infection by uropathogenic escherichia coli in rats. Cell J. 2016;18:446–457. doi: 10.22074/cellj.2016.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopalli SR, Cha KM, Jeong MS, Lee SH, Sung JH, Seo SK, et al. Pectinase-treated Panax ginseng ameliorates hydrogen peroxide-induced oxidative stress in GC-2 sperm cells and modulates testicular gene expression in aged rats. J Ginseng Res. 2016;40:185–195. doi: 10.1016/j.jgr.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopalli SR, Cha KM, Lee SH, Ryu JH, Hwang SY, Jeong MS, et al. Pectinase-treated Panax ginseng protects against chronic intermittent heat stress-induced testicular damage by modulating hormonal and spermatogenesis-related molecular expression in rats. J Ginseng Res. 2017;41:578–588. doi: 10.1016/j.jgr.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopalli SR, Cha KM, Ryu JH, Lee SH, Jeong MS, Hwang SY, et al. Korean red ginseng improves testicular ineffectiveness in aging rats by modulating spermatogenesis-related molecules. Exp Gerontol. 2017;90:26–33. doi: 10.1016/j.exger.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Kopalli SR, Hwang SY, Won YJ, Kim SW, Cha KM, Han CK, et al. Korean red ginseng extract rejuvenates testicular ineffectiveness and sperm maturation process in aged rats by regulating redox proteins and oxidative defense mechanisms. Exp Gerontol. 2015;69:94–102. doi: 10.1016/j.exger.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Park WS, Shin DY, Kim DR, Yang WM, Chang MS, Park SK. Korean ginseng induces spermatogenesis in rats through the activation of cAMP-responsive element modulator (CREM) Fertil Steril. 2007;88:1000–1002. doi: 10.1016/j.fertnstert.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 22.Park J, Song H, Kim SK, Lee MS, Rhee DK, Lee Y. Effects of ginseng on two main sex steroid hormone receptors: estrogen and androgen receptors. J Ginseng Res. 2017;41:215–221. doi: 10.1016/j.jgr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HW, Kil KJ, Lee Y, Lee MS. Ginseng for improving semen quality parameters: a protocol of systematic review. Medicine (Baltimore) 2018;97:e9732. doi: 10.1097/MD.0000000000009732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Altman DG . Sterne JAC on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. London: The Cochrane Collaboration; 2011. pp. 187–241. [Google Scholar]
- 25.Park HJ, Choe S, Park NC. Effects of Korean red ginseng on semen parameters in male infertility patients: a randomized, placebo-controlled, double-blind clinical study. Chin J Integr Med. 2016;22:490–495. doi: 10.1007/s11655-015-2139-9. [DOI] [PubMed] [Google Scholar]
- 26.Mkrtchyan A, Panosyan V, Panossian A, Wikman G, Wagner H. A phase I clinical study of Andrographis paniculata fixed combination Kan Jang versus ginseng and valerian on the semen quality of healthy male subjects. Phytomedicine. 2005;12:403–409. doi: 10.1016/j.phymed.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Lee H, Woo S, Kim H, Park H, Kim T, Youn N, et al. Doubleblind, case-control, multi-center study for therapeutic effects of panax ginseng C. A. Meyer extract in male unfertility with low sperm quality. Urol. 2006;68(Suppl 5A):57. [Google Scholar]
- 28.Kim YT, Lee HL, Lee SC, Shin KH, Han KH, Lee SC, et al. Effect of Panax ginseng water extract for treatment of male infertility. Korean J Androl. 2002;20:94–99. [Google Scholar]
- 29.Salvati G, Genovesi G, Marcellini L, Paolini P, De Nuccio I, Pepe M, et al. Effects of Panax ginseng C.A. Meyer saponins on male fertility. Panminerva Med. 1996;38:249–254. [PubMed] [Google Scholar]
- 30.Leung KW, Wong AS. Ginseng and male reproductive function. Spermatogenesis. 2013;3:e26391. doi: 10.4161/spmg.26391. [DOI] [PMC free article] [PubMed] [Google Scholar]