Abstract
Torsion of the spermatic cord is a urological emergency that must be treated with acute surgery. Possible long-term effects of torsion on testicular function are controversial. This review aims to address the impact of testicular torsion (TT) on the endocrine- and exocrine-function of the testis, including possible negative effects of torsion on the function of the contralateral testis. Testis tissue survival after TT is dependent on the degree and duration of TT. TT has been demonstrated to cause long-term decrease in sperm motility and reduce overall sperm counts. Reduced semen quality might be caused by ischemic damage and reperfusion injury. In contrast, most studies find endocrine parameters to be unaffected after torsion, although few report minor alterations in levels of gonadotropins and testosterone. Contralateral damage after unilateral TT has been suggested by histological abnormalities in the contralateral testis after orchiectomy of the torsed testis. The evidence is, however, limited as most human studies are small case-series. Theories as to what causes contralateral damage mainly derive from animal studies making it difficult to interpret the results in a human context. Large long-term follow-up studies are needed to clearly uncover changes in testicular function after TT and to determine the clinical impact of such changes.
Keywords: Fertility; Infertility, male; Spermatic cord torsion; Testicular torsion; Testis; Testosterone
INTRODUCTION
Testicular torsion (TT) is an acute urological emergency affecting 1 in 4,000 males aged <25 years and occurs due to a rotation of the spermatic cord [1]. This can include rotation of the tunica vaginalis (extravaginal torsion) or solely be the spermatic cord and testis that rotate within the tunica vaginalis (intravaginal torsion). Extravaginal torsion happens during descent of the testis into the scrotum and therefore happens almost exclusively in the perinatal period (<6 weeks of age). The majority of TT cases are the result of intravaginal torsion occurring mainly in pubertal boys [2]. This is made possible by a congenital malformation in which the tunica vaginalis does not attach to the epididymis and the posterior surface of the testis, but instead envelops the testis, making it possible for the testis to rotate around a longitudinal axis. The anatomical variant making the intravaginal rotation possible is called ‘bell clapper’ deformity.
The rotation and subsequent arterial constriction results in ischemia causing damage to the testis tissue, which is why the condition must be treated with acute surgery. The affected testis can be completely removed by performing an orchiectomy or it can be manually untwisted and fixated in the scrotum by doing an orchiopexy.
This narrative review investigates the impact of TT on testicular function and discusses possible damage to the contralateral testis.
TESTICULAR FUNCTION AFTER TESTICULAR TORSION
The duration and the degree of spermatic cord torsion are the main determinants of testis tissue survival after hypoxia induced by arterial constriction [1,3]. A systematic review compiled 2,116 cases from 30 papers and investigated the correlation between testis survivability and the duration of torsion. When operated within 0 to 6 hours of torsion 97.2% of testes survived and after 25 to 48 hours of torsion only 24.4% of testes survived [4]. Testicular survival after prolonged torsion might reflect that the testicular blood flow was not fully constricted, or that intermittent torsion occurred, highlighting the importance of the degree of torsion.
The choice of surgical procedure is determined by intraoperative appearance of the torsed testis. Completely black and necrotic testes are generally removed by performing an orchiectomy. If the testis seems salvageable an orchiopexy is performed, where the testis is detorsed and fixated in the scrotum. Salvageability of the torsed testis has been found to correlate with the duration of torsion and thereby overall time of ischemia [5,6]. This was demonstrated in a study by Yang and colleagues [3] who found that patients undergoing orchiopexy experienced a mean of 360 degrees torsion and 12 hours of pain symptoms and that the orchiectomy patients experienced a mean of 540 degrees torsion and 90 hours of pain symptoms. The European Association of Urology guidelines do not state when to perform an orchiectomy or orchiopexy of the ipsilateral testis but state that this depends on the degree and duration of torsion [7]. It is further recommended that a contralateral orchiopexy is performed, since an estimated 60% to 80% of boys born with bell clapper deformity will have the deformity on the contralateral testis putting them at risk for a new episode of TT on the contralateral side [8].
Despite detorsion of the affected testis and subsequent orchiopexy, atrophy of the ipsilateral testis on clinical examination has been reported in several studies [9,10,11]. Long-term damage to the ipsilateral testis after TT is thought to be caused by the period of ischemia during torsion and by oxidative stress after detorsion [12]. When the testis is detorsed oxygen rich blood flows back in to the testis subsequently causing the formation of reactive oxygen species (ROS). ROS damages the testicular DNA, causing germinal cell apoptosis [13].
A study by Lian and colleagues [10] found that among 53 TT patients whose testis were salvageable, half of them had developed clinical evidence of atrophy (>50% difference in testicular volume compared with the contralateral testis) 14 months after the torsion event. Table 1 [3,5,9,11,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] summarizes the available retrospective case-series and case-control studies published on testicular function after TT. Follow-up time in the presented studies vary from a couple of months to 23 years and are dominated by small case series with a limited population of generally 20 to 30 patients.
Table 1. Studies investigating testicular function after TT.
| Study (year) | Study characteristics | Main findings and major limitations |
|---|---|---|
| Bartsch et al (1980) [11] | - Retrospective case series | - Semen samples were pathological (sperm counts: <20 millon/mL, motility: <40%, volume: <1.5 mL) in 12/30. |
| - 42 TT patients | - Patients with abnormal semen analyses had elevated FSH (13.6±6.7 mU/mL) and LH (16.3±13.7 mU/mL). | |
| - Blood samples and semen samples (30 sampels were delivered) | ||
| Danner et al (1982) [14] | - Retrospective case series | - T and LH were normal in all patients. 50% of patients had FSH in the upper normal range or slightly elevated, which correlated with abnormal semen samples. |
| - 20 TT patients, evaluated a mean of 22 months (5–60 months) after torsion | - Semen analyses (12 TT patients) found decreased motility (<50%) in 8 samples. | |
| - Testis biopsies, semen samples and blood samples | - Histology was abnormal (spermatogenic arrest, lack of spermatozoa, infiltration of red blood cells and leucocytes) in all patients with a torsion of more than 6 | |
| Mastrogiacomo et al (1982) [15] | - Retrospective case-control study | - The presences of ASA in TT patients showed a significant association with low sperm count (<30 million/mL) (p>0.001). |
| - 25 TT patients (6 months–7 years after TT) and a control group | - ASA were present in 20% of the TT patients. | |
| - Semen samples | - Control group were healthy sperm donors. | |
| Thomas et al (1984) [9] | - Retrospective case series | - LH, FSH, and testosterone were within normal range for all patients. |
| - 67 TT patients, evaluated a mean of 4 years (3 months–12 years) after TT | - 39% had sperm counts <20 millon/mL. Low sperm counts correlated with the duration of torsion (p<0.001). | |
| - Blood samples and semen samples | ||
| Goldwasser et al (1984) [16] | - Retrospective case series | - 11/16 were operated within 12 hours of first pain symptoms and 4/11 (36.4%) had normal semen analysis (sperm count between 32–164 millon/mL, sperm motility between 39%–78% and sperm morphology between 45%–81%). 1/5 operated after 12 hours of pain symptoms had a normal semen analysis. |
| - 16 TT patients, evaluated 4 months–5 years after torsion | - T, FSH, and LH were normal in 14/16 of TT patients. | |
| - Semen samples and blood samples | ||
| Fraser et al (1985) [17] | - Retrospective case series | - 13/32 semen samples (40.6%) had low sperm density (21 millon/mL, p<0.005) and motility (25%, p<0.005). |
| - 47 TT patients, evaluated 2–10 years after TT | - 8 had fathered children. | |
| - Blood samples (44 samples was obtained) and semen samples (32 patients delivered semen samples) | - Teststerone was normal for all patients. | |
| - Mean FSH was elevated (8.9±4 u/L, p<0.005) in 19/44 patients (43.2%). | ||
| - ASA was not found in any of the patients. | ||
| Puri et al (1985) [18] | - Retrospective case series | - 10 semen samples were normal. 2 had low sperm concentration (mean 10 million/mL) and 1 had abnormal semen volume (0.7 mL), sperm concentration (10 million/mL), and motility (15%). |
| - 18 TT patients, evaluated 7–23 years after TT | - MAR test didn't show ASA in any patient. | |
| - Interview and semen samples (13 patients delivered semen samples) | ||
| Anderson and Williamson (1986) [19] | - Retrospective case series | - 20/35 biopsies showed histological evidence of pre-existing partial maturation arrest. 19 of the patients with partial maturation arrest attended postoperative review 3–6 months after TT. 15/19 with this abnormality had oligozoospermia (p<0.002). |
| - 56 TT patients, evaluated 3–6 months after TT | - Preoperatively no patients' serum showed ASA, postoperatively ASA was found in 3/35. | |
| - Testis biopsies contralateral testis biopsies taken at the time of surgery, blood samples and semen samples (from 35 patients) | ||
| Fisch et al (1988) [20] | - Retrospective case series | - TT patients had a greater FSH response than control group, the response was greatest in patients treated with orchioctomy (as was the LH response). |
| - 14 TT patients, evaluated a mean of 33 months after TT. 5 normal men was used as controls. | - Small population | |
| - IV bolus GRH test | ||
| Laor et al (1990) [21] | - Retrospective case series | - 12/20 contralateral biopsies showed abnormalites (maturation arrest, germ cell degeneration, tubular hyalinisation, immature tubules, focal thickening of the basement membrane). |
| - 20 TT patients | ||
| - Contralateral testis biopsies taken at the time of surgery | ||
| Jones (1991) [22] | - Retrospective case series | - FSH, LH, and T was normal in all patients. |
| - 43 patients with recurrent subacute torsion, evaluated preoperative and 3 months postoperative | - 3 patients had abnormal semen analyses (sperm counts: 22 millon/mL, 43% abnormal forms, motility: 35%) the same 3 had abnormal testicular biopsies (Johnson scores: mean 5.7 [4.9–6.8]). | |
| - Bilateral testis biopsies taken at the time of surgery, blood samples and semen samples | ||
| Hagen et al (1992) [23] | - Retrospective case series | - 7 with normal semen analyses 2–8 years after torsion, 19 had OAT syndrome, 10 had asthenozoospermia, and 19 had teratozoospermia |
| - 55 TT patients, evaluated 2–8 years after TT | - Testis biospies were abnormal (desquamination of the germinative epithelium, atrophy of leydig cells, and malformation of spermatids) in 30/34. | |
| - Contralateral testis biopsies taken at the time of surgery, semen samples and blood samples (at the time of surgery, and at follow-up) | - ASA was preoperatively found in 2/36, and at follow-up in 2/36. | |
| Anderson et al (1992) [24] | - Retrospective case control study | - Orchiectomy patients (n=7) had a significant decrease in semen quality (sperm density was average 29 million/mL) compared with controls (p=0.001). |
| - 16 TT patients and 10 controls | - Semen quality was not significantly different in patients treated with orchiopexy compared to control (p=0.25). | |
| - Blood samples, semen samples and GRH stimulation test, presence/ absence of ASA | - Control group: fertile sperm donors | |
| - Small population | ||
| Brasso et al (1993) [25] | - Retrospective case series | - Duration of torsion correlated with orchioctomy. |
| - 35 TT patients, evaluated 6–11 years after torsion | - Duration of torsion correlated with reduced sperm counts. | |
| - Blood samples and semen samples | - FSH and LH were normal. | |
| Tryfonas et al (1994) [26] | - Retrospective case series | - Duration and degree of torsion with orchioctomy. |
| - 25 TT patient, evaluted after 1–12 years after TT | - 4/4 semen samples were abnormal. | |
| - Ultrasound of testis, semen samples (only in 4 patients) | ||
| Daehlin et al (1996) [27] | - Retrospective case series | - Oligoazoospermia was found in 2/13 semen samples. |
| - 52 TT patients, evaluted 4–10 years after TT | - Testosterone level was normal. | |
| - Blood samples and semen samples (n=13) | ||
| Hadziselimovic et al (1998) [28] | - Retrospective case control study | - All TT patients' contralateral testis biopsies showed atrophic Leydig cells, malformed late spermatids, often binuclear spermatids, apoptosis of spermatocytes and pathological changes in the cytoplasm of Sertoli cells. |
| - 17 TT patients and 3 controls | ||
| - Bilateral testis biopsies | ||
| Arap et al (2007) [29] | - Retrospective case control study | - Median FSH was statistically higher in patients treated with orchiectomy (n=15) compared with orchiopexy (n=9): median 7.6 UI/L vs. 5,6 UI/L. |
| - 24 TT patients, evaluated a mean of 6 years (5–7 years) and 10 years (5–12 years) after torsion for patients treated with orchiopexy and orchioctomy, repsectively | - Median T were significantly higher in TT patients compared with controls: 701 ng/ dL (p<0.001) and 641 ng/dL (p=0.017) for patients treated with orchiectomy and orchiopexy, respectively vs. 440 ng/dL in controls. | |
| - 20 voluntary men requesting vasectomy as the control group | - Sperm motility was better in patients treated with orchiectomy compared with orchiopexy: 77% vs. 54% (p=0.028). | |
| - Blood samples and semen samples | - ASA was abnormal for TT patients (21% and 20%) compared with control group (14.5%), but had no siginificant correlation with sperm concentration (p=0.51), sperm motility (p=0.87), or testosteron level (p=0.75). | |
| - Control group was proven fertile men. | ||
| Romeo et al (2010) [5] | - Retrospective case control study | - FSH, LH, and T were within normal range. Mean inhibin B was significantly reduced in TT patients compared with controls: 34.5±5.2 vs. 63.9±12.8 pg/mL, p=0.02. This significantly correlated with T levels (p=0.02) and testis volume (p=0.03). |
| - 20 TT patient and 15 age-matched controls, evaluated a mean of 5 years after TT | - Subfertility (negative WHO fertility index) was found in 6/7 semen samples. | |
| - Blood samples, semen samples (7 samples were delivered), testes ultrasound | ||
| Yang et al (2011) [3] | - Retrospective case control study | - Duration and degree of torsion correlates with testicular salvageability (p=0.008 and p=0.011, respectively.) |
| - 86 TT patients, evaluated a mean of 7 years after surgery (3 months–16.5 years) and 60 controls | - FSH, LH, and T were within normal range when compared to age matched controls regardless of surcigal management. | |
| - Ultrasound of the testis, urine samples and blood samples | ||
| Gielchinsky et al (2016) [30] | - Retrospective case series | - Pregnancy rates in TT patients were 90.2% and 90.9% for orchiectomy and orchioepexy patients, respectively, vs. the accepted pregnancy rate in the general population of 82% to 92%. |
| - 63 TT patients, married more than 1 year, with proven female fertility | - 6/63 of the TT patients (9.5%) had been diagnosed infertile. | |
| - Questionnaire |
TT: testicular torsion, FSH: follicle-stimulating hormone, LH: luteinizing hormone, T: testosterone, ASA: anti-sperm antibodies, MAR: mixed antiglobulin reaction, IV: intravenous, GRH: gonadotropin realizing hormone, OAT: oligoasthenoteratozoospermia, WHO: World Health Organization.
1. Endocrine function of the testis after torsion
The primary endocrine function of the testes is production of testosterone. This is regulated by luteinizing hormone (LH) as part of the hypothalamic-pituitary-gonadal axis. LH from the pituitary gland stimulates the production of testosterone from Leydig cells. A decline in testicular endocrine function causes a compensatory increase in LH levels and, depending on the remaining testosterone secretory capacity of the testes, a decrease in serum testosterone may be observed.
The postoperative testicular endocrine function after TT has been investigated in several studies (Table 1). Generally, follicle-stimulating hormone (FSH) is included in these studies although the level of FSH is a marker of spermatogenesis and not a part of the testicular endocrine function per se. To avoid unnecessary repetitive summaries of relevant studies, FSH levels are included in this section.
A study by Yang et al [3] evaluated 86 TT patients' endocrine function at a mean of 7 years following surgery and found that their endocrine parameters were not significantly different when compared to 60 age-matched controls. However, a major limitation of the study is that the patients had mean age of 9.3 years and the findings is therefore not indicative of the mature male endocrine function. Thomas et al [9] investigated 67 TT patients' endocrine parameters and found that LH, FSH and testosterone were within normal levels. A study by Goldwasser et al [16] investigated the testicular endocrine function of 16 TT patients 4 months to five years after TT. A total of 14 of the 16 patients had normal FSH, LH and testosterone at follow-up. The two remaining patients had insignificant elevations of FSH, LH and a decrease in testosterone level. Romeo et al [5] evaluated blood samples of 20 TT patients, 8 treated with orchiectomy and 12 with detorsion, at a mean follow-up of 22 months after torsion and found that the patients had a normal FSH (6.2±1.2 mIU/mL and 2.9±0.6 mIU/mL, respectively; p=0.04), LH (5.6±1.4 mIU/mL and 2.2±0.56 mIU/mL, respectively; p=0.06), and testosterone (505±129 and 417±107 ng/dL, respectively; p=0.43) with a small statistically significant difference in FSH levels between those who had orchiectomy and those who had detorsion. Surprisingly, a study by Arap et al [29] found statistically significantly higher median testosterone levels in TT patients compared with controls (701 ng/dL, p<0.001 and 641 ng/dL, p=0.017 for patients treated with orchiectomy and orchiopexy, respectively compared with a control group [440 ng/dL]) though the values were all within the normal range of testosterone (264–916 ng/dL [31]). Although this finding is contraintuitive it supports that testosterone levels are not affected after TT.
Current evidence does not suggest that testicular endocrine function is affected by TT. However, it is important to acknowledge that the available studies have focused on endocrine function compared to a normal range and do not explore if LH levels increase, or testosterone levels decrease as compared with baseline levels before TT. Furthermore, long-term follow-up is lacking, and it is unknown if the ageing man after TT is at a higher risk of late onset hypogonadism as the testosterone secretory capacity is potentially already challenged early on. Still, it is questionable if the occasional reported minor alterations in endocrine parameters would have any meaningful clinical impact. No studies have investigated possible symptoms of testosterone deficiency in men with prior TT.
2. Fertility after torsion
Although endocrine function seems unhindered by TT, there is evidence to suggest that TT may negatively impact a man's fertility measured by semen parameter changes. Thomas and colleagues [9] assessed semen quality at a mean follow-up of 4 years after torsion in 67 TT patients. They found that 64% of patients had abnormally low sperm motility or morphology and 39% were classified as subfertile (sperm counts<20 million/mL). Only 14% had semen parameters within the normal range. Furthermore, they found that low total motile sperm count correlated with the duration of torsion (p<0.001). Fifty-five of the 67 patients included in the study underwent orchiopexy demonstrating that having a salvagable testis does not prevent future reduced semen quality. Anderson et al [24] evaluated 16 TT patients, 9 treated with orchiopexy and 7 with orchiectomy, and found a significant decrease in sperm density (average sperm density 29 million/mL) after orchiectomy (p=0.001) but semen parameters did not differ significantly after orchiopexy (p=0.25), when compared to a control group consisting of proven fertile sperm donors. Goldwasser and collegues [16] evaluated 16 TT patients and found that only 20% of semen samples were normal (sperm count between 32–164 millon/mL, sperm motility between 39%–78% and sperm morphology between 45%–81% [not strict morphology]) after more than 12 hours of TT symptoms, when compared to a control group consisting of proven fertile donors. In the previously mentioned study conducted by Arap et al [29] no overall differences in semen quality were found when comparing 24 TT patients with 20 healthy proven fertile men. However, when assessed individually 25% of TT patients presented with oligospermia compared to 0% of men in the control group. Mean sperm counts were 38.3 million/mL for patients treated with orchiectomy and 47 million/mL for patients treated with orchiopexy vs. 99.3 million/mL in the control group (p=0.46 and p=0.10, respectively).
Overall, it seems that at long-term follow-up after TT some patients experience decreased sperm motility and reduced overall sperm counts possibly rendering them subfertile. However, high quality studies are lacking and available studies are limited by selection bias, as they often include men with proven fertility as controls instead of unselected healthy controls with unknown fertility status. Furthermore, studies lack evaluating the clinical consequences of reduced semen quality after TT. For these reasons a definitive conclusion on the long-term effects of TT on fertility cannot be made.
Only one study investigated pregnancy rates among 63 couples where the men had been treated for TT. They found no decrease in paternity rates among TT men when compared to the general population [30].
To more clearly determine the endocrine and exocrine function after TT there is a need for larger prospective long-term follow-up studies using unselected control groups and reporting on future paternity rates.
THE IMPACT OF TESTICULAR TORSION ON THE CONTRALATERAL TESTIS
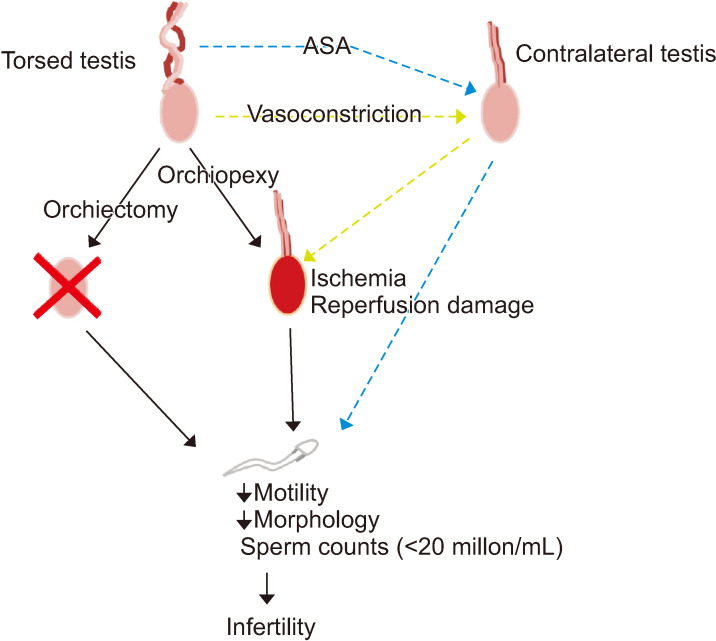
Evidence is contradictory regarding how the torsed testis should be surgically managed. Removing it or leaving it in the scrotum after detorsion both seem to have a negative effect on testicular function, perhaps indicating that torsion causes bilateral damage or that the contralateral testis does not have sufficient capacity to increase spermatogenesis and testosterone production. Possible contralateral damage after TT is debated and three main hypotheses exist as to what would cause the abnormality (Fig. 1). 1) Ipsilateral reperfusion injury causes contralateral reflectory sympathetic mediated vasoconstriction [32] leading to hypoxia. 2) The torsion of the ipsilateral spermatic cord breaks down the blood-testis barrier. This initiates an immunological process where immunoglobulins have antibody activity against sperm antigens [33]. These immunoglobulins, also called anti-sperm antibodies (ASA), will in turn reduce sperm motility and sperm concentration [34]. 3) The contralateral testicular function is compromised before TT due to pre-existing congenital testicular dysgenesis [19].
Fig. 1. Testicular torsion is managed by either orchiectomy or orchiopexy. Detorsion during orchiopexy may cause reperfusion injury and combined with ischemic damage due to arterial constriction spermatogenesis might be altered resulting in reduced sperm concentration, reduced motility and reduced morphologically normal sperm. Studies have also demonstrated a negative impact of testicular torsion on the contralateral testis. This has been hypothesized to be caused by the formation of anti-sperm antibodies (ASA) and contralateral vasoconstriction resulting in hypoxia and subsequent reperfusion damage.
1. Animal studies
Evaluating the impact on the contralateral testis in humans can be difficult and is often unethical in the acute phase. Performing extra analyses might prolong the time of torsion potentially further damaging the torsed testis. Animal studies have been valuable in understanding the various hypotheses as to what causes potential contralateral testicular damage after TT.
The effect of ipsilateral reperfusion injury after detorsion is thought to manifest itself bilaterally and simultaneously result in a contralateral reflectory sympathetic mediated vasoconstriction of the testicular blood vessels. This reduces the contralateral blood flow causing a period of potential ischemia and subsequent reperfusion damage when blood flow increases again. The hypothesis of contralateral vasoconstriction after TT mainly derives from animal studies which have shown that contralateral microvascular changes occur after TT [35]. In rabbits and piglets it has been shown that torsion causes a simultaneous decrease in contralateral testicular blood flow and upon detorsion blood flow returns to normal [36,37]. Likewise in rats a 43% decrease in contralateral blood flow has been found during torsion [38]. These vascular changes are hypothesized to cause hypoxia subsequently damaging the testis tissue. Certain biomarkers such as the level of lactic acid and hypoxanthine are indicative of whether hypoxia occurs. A study conducted by Akgür and colleagues [39] found that values of lactic acid, hypoxanthine and thiobarbituric acid reactive substances were significantly elevated (p<0.05) in the ipsilateral and contralateral testis after unilateral torsion when compared to a control group consisting of 10 rats having undergone sham surgery. This was hypothesized to be caused by altered blood flow bilaterally during unilateral TT.
Few studies have been conducted on the relevance of ASA in relation to fertility problems after TT. Pakyz et al [40] demonstrated that the contralateral testis damage is reduced after performing an orchiectomy in rats compared to a group where a detorsion was performed. Futhermore, they found that immunosupression (cyclosporin and prednisone) given to the rats undergoing detorsion prevented further damage. This treatment showed a siginifcant (p<0.05) increase in pregnant female rats after torsion and contralateral testis weight was significantly higher (p<0.05) compared with rats who underwent detorsion with no immunosuppresion. Another study investigated groups of 10 rats who underwent torsion for 3, 6, 12, and 24 hours and was then compared to a control group of 10 untreated rats. The presence of ASA was only detected in the groups who had torsion for 12 and 24 hours (5/8 rats and 6/7 rats, respectively) [41]. A study by Nagler and White [42] investigated contralateral testis histology after unilateral torsion in rats. The histological assessment of the contralateral testis consisted of mean seminiferous tubular diameter and presence or absence of spermatozoa. If spermatozoa were not present the spermatogenesis was regarded as not existing. They found that 12/20 of rats (60.0%) with untreated torsion had no spermatogenesis and a mean seminiferous tubule diameter of 196.66±7.65 microns in the contralateral testis which was significant when compared to the mean seminiferous tubule diameter in the sham group of 252.88±4.43 microns (p<0.001). Furthermore, all rats in the sham group had contralateral spermatogenesis. When orchiectomy of the torsed testis was performed only 6/20 of rats (30.0%) had no spermatogenesis and had a mean seminiferous tubules diameter of 244±7.41 microns in the contralateral testis. The mean seminiferous tubules diameter in this group was significantly different when compared to the untreated torsion group (p<0.001). Interestingly, they found that rats submitted to torsion and treated with both immunosuppression and splenectomy maintained the contralateral seminiferous tubular diameter compared with rats undergoing untreated torsion (p<0.01). This could indicate that TT is followed by a possible unwanted immune response affecting the contralateral testis.
Animal studies have generally demonstrated TT's negative contralateral effect in that the contralateral testis seems to be affected by reperfusion injury, potentially causing long-term damage. Furthermore, the presence of an active immune system after TT has been demonstrated displaying a possible role of ASA.
2. Human trials: anti-sperm antibodies
The formation of ASA has been shown after trauma to the testes and after vasectomies [43]. During torsion of the spermatic cord it is hypothesized that breakdown of the blood-testis barrier exposes sperm to the bloodstream, subsequently activating the immune system which results in the formation of ASA [33]. The effect of ASA on fertility is an area of controversy. A systematic review by Cui and colleagues [34] concluded based on 8 studies that ASA had a negative effect on sperm concentration and sperm motility, though it had no significant effect on semen volume, sperm morphology, sperm progressive motility and sperm viability (Fig. 1). The presence of ASA have been found in around 13% of semen samples from infertile men and in 1% to 2% of fertile men's semen [34,44,45,46].
To detect the presence of ASA in semen or blood a mixed antiglobulin reaction test, immunobead test or ELISA test can be used.
From Table 1 it is clear that only few studies have conducted ASA tests on human semen or blood samples from TT patients making conclusions on their relevance hard to draw. Zanchetta et al [47] found that 13% (n=68) of TT patients had antibody activity against testicular- and spermatozoa antigens compared to 0% in the control group, consisting of 21 healthy men and 13 healthy women. In another study, ASAs were found in 20% (n=25) of TT patients' semen samples when evaluated 6 months to 7 years after TT. Three of the 25 patients were treated with orchiectomy and the rest by detorsion. The presences of ASA in the TT patients showed a significant association with low sperm count (<30 million/mL) (p>0.001) [15]. This was not found in patients until 2 years after torsion and the authors therefore suggested that the immunological response have a slow onset. Arap et al [29] found no differences in ASA between patients treated with orchiectomy (21%, p=0.073) and orchiopexy (20%, p=0.17) when compared to the control group (14.5%), and ASA showed an insignificant correlation to sperm concentration (p=0.51), motility (p=0.87), LH levels (p=0.33), and testosterone levels (p=0.75). While ASA had no significant effect on semen quality and endocrine parameters the presence of ASA after torsion was still detected. The authors concluded that ASA could be due to an irreversible autoimmune response triggered by the torsion itself, thereby implying that ASA is not affected by the method of treatment.
Other studies have revealed no elevated levels of ASA. Hagen et al [23] studied 55 TT patients 2 to 8 years after TT and found that 7 out of 55 had normal semen analyses according to World Health Organization (WHO) reference values used at that time. ASA tests were done in 36 of the semen samples and ASA was only present in 2 out of 36. Similar results have been demonstrated by Fraser and colleagues [17] who evaluated 47 TT patients; 11 of these men were treated with orchiectomy and the rest was detorsed. Thirty-two patients delivered a semen sample between 2 to 10 years after TT and it was found that 41% (n=32) of semen samples were classified as subnormal since they failed one or more of the WHO standards. The group classifed as subnormal had low sperm density (21 millon/mL) and motility (25%) when compared to the normal group of TT patients who had normal sperm density (mean 184 millon/mL, p<0.005) and motility (mean 55%, p<0.005) according to WHO standards at that time, though ASA was not found in any patient. Puri and colleagues [18] investigated 18 TT patients; 14 of them had undergone orchiectomy and the rest of the patients were detorsed. Thirteen of the men delivered semen samples. ASA was not present in any of the samples. Anderson and Williamson [19] demonstrated similar results when evaluating 32 TT patients. Preoperatively no patients' serum showed ASA, postoperatively ASA was found in only 3 of the 32 patients.
In summary, studies investigating the effect of ASA on fertility in patients with prior TT are contradictory. Some studies demonstrate the presences of ASA after TT, though a majority of studies does not support an elevated level of ASA after TT.
3. Human trials: pre-existing congenital testicular dysgenesis
Testicular dysgenesis syndrome (TDS) describes a maldevelopment of the male uro-genital tract as a common cause for long-term consequences in adult men, such as infertility, hypogonadism and an increased risk of testis cancer [48]. TDS might be caused by an underlying genetic predisposition, intrauterine growth disorders, lifestyle factors including lifestyle factors of the mother and exposure to endocrine disruptors. It is hypothesized that the anatomical variant causing torsion is part of the TDS which could mean that semen quality is affected before torsion. This theory is supported by biopsies taken from both testes during surgery for TT showing abnormal contralateral histology. Laor et al [21] reviewed 20 TT patients contralateral testis biopsies taken at the time of surgery and found that 12 out of 20 contralateral biopsies had abnormalites (maturation arrest, germ cell degeneration, tubular hyalinisation, immature tubules, focal thickening of the basement membrane). Anderson and Williamson [19] found that 20 out of 35 testis biopsies from TT patients showed histological evidence of preexisting partial maturation arrest. Nineteen of the patients with partial maturation arrest had their semen analyzed 3 to 6 months after surgery and 79% were diagnosed with oligozoospermia. This finding is further backed by Hagen et al [23] who investigated 34 TT patients' contralateral testis biopsies and found that 88% were abnormal (desquamination of the germinative epithelium, atrophy of Leydig cells and malformation of spermatids).
In conclusion, it is possible that the above mentioned hypotheses of contralateral damage after TT are overlapping and that contralateral testis damage is a result of a multifactorial process. All of the mentioned hypotheses are primarily based animal studies and low-quality human trials. The observed negative effects of TT might simply be a consequence of damage to the torsed testis with the contralateral testis being unable to compensate sufficiently with increased spermatogenesis. In fact, a study investigating semen samples from patients who had one testis removed due to TT or other reasons such as testis cancer, cryptorchidism or after trauma found that orchiectomy in general decreased semen quality (sperm counts <20 million/mL was found in 53% of men with cryptorchidism, 57% of the men diagnosed with TT, 50% of the men diagnosed with testicular cancer and 56% of the men who had lost a testis after trauma) in all patients regardless of their diagnosis [49].
CONCLUSION
The torsed testis' survivability depends on the degree and duration of TT. Long-term testicular damage occurs due to ischemia and oxidative stress following detorsion. The endocrine function of the testes seems undisturbed while semen quality is more frequently affected after TT. However, impact on fertility evaluated by paternity rates after TT remains unclear. Contralateral testis damage after TT has been demonstrated by histological examination but the cause remains unclear. Large long-term prospective follow-up studies are needed to clearly assess changes in testicular function after TT and the possible clinical implications of these.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Writing—original draft: FMJ.
- Writing—review & editing: all authors.
References
- 1.Yecies T, Bandari J, Schneck F, Cannon G. Direction of rotation in testicular torsion and identification of predictors of testicular salvage. Urology. 2018;114:163–166. doi: 10.1016/j.urology.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 2.Vasdev N, Chadwick D, Thomas D. The acute pediatric scrotum: presentation, differential diagnosis and management. Curr Urol. 2012;6:57–61. doi: 10.1159/000343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang C, Song B, Tan J, Liu X, Wei GH. Testicular torsion in children: a 20-year retrospective study in a single institution. ScientificWorldJournal. 2011;11:362–368. doi: 10.1100/tsw.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellick LB, Sinex JE, Gibson RW, Mears K. A systematic review of testicle survival time after a torsion event. Pediatr Emerg Care. 2017 doi: 10.1097/PEC.0000000000001287. [Epub] [DOI] [PubMed] [Google Scholar]
- 5.Romeo C, Impellizzeri P, Arrigo T, Antonuccio P, Valenzise M, Mirabelli S, et al. Late hormonal function after testicular torsion. J Pediatr Surg. 2010;45:411–413. doi: 10.1016/j.jpedsurg.2009.10.086. [DOI] [PubMed] [Google Scholar]
- 6.Castañeda-Sánchez I, Tully B, Shipman M, Hoeft A, Hamby T, Palmer BW. Testicular torsion: a retrospective investigation of predictors of surgical outcomes and of remaining controversies. J Pediatr Urol. 2017;13:516.e1–516.e4. doi: 10.1016/j.jpurol.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Tekgül S, Dogan HS, Erdem E, Hoebeke P, Kocvara R, Nijman JM, et al. Guidelines on pediatric urology. Arnhem: European Association of Urology; 2015. [Google Scholar]
- 8.Osumah TS, Jimbo M, Granberg CF, Gargollo PC. Frontiers in pediatric testicular torsion: an integrated review of prevailing trends and management outcomes. J Pediatr Urol. 2018;14:394–401. doi: 10.1016/j.jpurol.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Thomas WE, Cooper MJ, Crane GA, Lee G, Williamson RC. Testicular exocrine malfunction after torsion. Lancet. 1984;2:1357–1360. doi: 10.1016/s0140-6736(84)92056-7. [DOI] [PubMed] [Google Scholar]
- 10.Lian BS, Ong CC, Chiang LW, Rai R, Nah SA. Factors predicting testicular atrophy after testicular salvage following torsion. Eur J Pediatr Surg. 2016;26:17–21. doi: 10.1055/s-0035-1566096. [DOI] [PubMed] [Google Scholar]
- 11.Bartsch G, Frank S, Marberger H, Mikuz G. Testicular torsion: late results with special regard to fertility and endocrine function. J Urol. 1980;124:375–378. doi: 10.1016/s0022-5347(17)55456-7. [DOI] [PubMed] [Google Scholar]
- 12.Turner TT, Brown KJ. Spermatic cord torsion: loss of spermatogenesis despite return of blood flow. Biol Reprod. 1993;49:401–407. doi: 10.1095/biolreprod49.2.401. [DOI] [PubMed] [Google Scholar]
- 13.Turner TT, Bang HJ, Lysiak JL. The molecular pathology of experimental testicular torsion suggests adjunct therapy to surgical repair. J Urol. 2004;172:2574–2578. doi: 10.1097/01.ju.0000144203.30718.19. [DOI] [PubMed] [Google Scholar]
- 14.Danner C, Frick J, Rovan E. Testicular function after torsion. Int J Androl. 1982;5:276–281. doi: 10.1111/j.1365-2605.1982.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 15.Mastrogiacomo I, Zanchetta R, Graziotti P, Betterle C, Scrufari P, Lembo A. Immunological and clinical study of patients after spermatic cord torsion. Andrologia. 1982;14:25–30. doi: 10.1111/j.1439-0272.1982.tb03091.x. [DOI] [PubMed] [Google Scholar]
- 16.Goldwasser B, Weissenberg R, Lunenfeld B, Nativ O, Many M. Semen quality and hormonal status of patients following testicular torsion. Andrologia. 1984;16:239–243. doi: 10.1111/j.1439-0272.1984.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 17.Fraser I, Slater N, Tate C, Smart JG. Testicular torsion does not cause autoimmunization in man. Br J Surg. 1985;72:237–238. doi: 10.1002/bjs.1800720332. [DOI] [PubMed] [Google Scholar]
- 18.Puri P, Barton D, O'Donnell B. Prepubertal testicular torsion: subsequent fertility. J Pediatr Surg. 1985;20:598–601. doi: 10.1016/s0022-3468(85)80006-3. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JB, Williamson RC. The fate of the human testes following unilateral torsion of the spermatic cord. Br J Urol. 1986;58:698–704. doi: 10.1111/j.1464-410x.1986.tb05916.x. [DOI] [PubMed] [Google Scholar]
- 20.Fisch H, Laor E, Reid RE, Tolia BM, Freed SZ. Gonadal dysfunction after testicular torsion: luteinizing hormone and follicle-stimulating hormone response to gonadotropin releasing hormone. J Urol. 1988;139:961–964. doi: 10.1016/s0022-5347(17)42731-5. [DOI] [PubMed] [Google Scholar]
- 21.Laor E, Fisch H, Tennenbaum S, Sesterhenn I, Mostofi K, Reid RE. Unilateral testicular torsion: abnormal histological findings in the contralateral testis—cause or effect? Br J Urol. 1990;65:520–523. doi: 10.1111/j.1464-410x.1990.tb14800.x. [DOI] [PubMed] [Google Scholar]
- 22.Jones DJ. Recurrent subacute torsion: prospective study of effects on testicular morphology and function. J Urol. 1991;145:297–299. doi: 10.1016/s0022-5347(17)38319-2. [DOI] [PubMed] [Google Scholar]
- 23.Hagen P, Buchholz MM, Eigenmann J, Bandhauer K. Testicular dysplasia causing disturbance of spermiogenesis in patients with unilateral torsion of the testis. Urol Int. 1992;49:154–157. doi: 10.1159/000282415. [DOI] [PubMed] [Google Scholar]
- 24.Anderson MJ, Dunn JK, Lipshultz LI, Coburn M. Semen quality and endocrine parameters after acute testicular torsion. J Urol. 1992;147:1545–1550. doi: 10.1016/s0022-5347(17)37622-x. [DOI] [PubMed] [Google Scholar]
- 25.Brasso K, Andersen L, Kay L, Wille-Jørgensen P, Linnet L, Egense J. Testicular torsion: a follow-up study. Scand J Urol Nephrol. 1993;27:1–6. doi: 10.3109/00365599309180406. [DOI] [PubMed] [Google Scholar]
- 26.Tryfonas G, Violaki A, Tsikopoulos G, Avtzoglou P, Zioutis J, Limas C, et al. Late postoperative results in males treated for testicular torsion during childhood. J Pediatr Surg. 1994;29:553–556. doi: 10.1016/0022-3468(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 27.Daehlin L, Ulstein M, Thorsen T, Høisaeter PA. Follow-up after torsion of the spermatic cord. Scand J Urol Nephrol Suppl. 1996;179:139–142. [PubMed] [Google Scholar]
- 28.Hadziselimovic F, Geneto R, Emmons LR. Increased apoptosis in the contralateral testes of patients with testicular torsion as a factor for infertility. J Urol. 1998;160:1158–1160. doi: 10.1097/00005392-199809020-00053. [DOI] [PubMed] [Google Scholar]
- 29.Arap MA, Vicentini FC, Cocuzza M, Hallak J, Athayde K, Lucon AM, et al. Late hormonal levels, semen parameters, and presence of antisperm antibodies in patients treated for testicular torsion. J Androl. 2007;28:528–532. doi: 10.2164/jandrol.106.002097. [DOI] [PubMed] [Google Scholar]
- 30.Gielchinsky I, Suraqui E, Hidas G, Zuaiter M, Landau EH, Simon A, et al. Pregnancy rates after testicular torsion. J Urol. 2016;196:852–855. doi: 10.1016/j.juro.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 31.Travison TG, Vesper HW, Orwoll E, Wu F, Kaufman JM, Wang Y, et al. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102:1161–1173. doi: 10.1210/jc.2016-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karagüzel G, Güngör F, Karagüzel G, Yildiz A, Melikoğlu M. Unilateral spermatic cord torsion without ipsilateral spermatogenetic material: effects on testicular blood flow and fertility potential. Urol Res. 2004;32:51–54. doi: 10.1007/s00240-003-0377-3. [DOI] [PubMed] [Google Scholar]
- 33.Arora P, Sudhan MD, Sharma RK. Incidence of anti-sperm antibodies in infertile male population. Med J Armed Forces India. 1999;55:206–208. doi: 10.1016/S0377-1237(17)30443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui D, Han G, Shang Y, Liu C, Xia L, Li L, et al. Antisperm antibodies in infertile men and their effect on semen parameters: a systematic review and meta-analysis. Clin Chim Acta. 2015;444:29–36. doi: 10.1016/j.cca.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 35.Salman AB, Kilinç K, Tanyel FC. Torsion of only spermatic cord in the absence of testis and/or epididymis results in contralateral testicular hypoxia. Urol Res. 1997;25:413–415. doi: 10.1007/BF01268858. [DOI] [PubMed] [Google Scholar]
- 36.Tanyel FC, Büyükpamukçu N, Hiçsönmez A. Contralateral testicular blood flow during unilateral testicular torsion. Br J Urol. 1989;63:522–524. doi: 10.1111/j.1464-410x.1989.tb05949.x. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen L, Lievano G, Ghosh L, Radhakrishnan J, Fornell L, John E. Effect of unilateral testicular torsion on blood flow and histology of contralateral testes. J Pediatr Surg. 1999;34:680–683. doi: 10.1016/s0022-3468(99)90355-x. [DOI] [PubMed] [Google Scholar]
- 38.Kolettis PN, Stowe NT, Inman SR, Thomas AJ., Jr Acute spermatic cord torsion alters the microcirculation of the contralateral testis. J Urol. 1996;155:350–354. [PubMed] [Google Scholar]
- 39.Akgür FM, Kilinç K, Tanyel FC, Büyükpamukçu N, Hiçsönmez A. Ipsilateral and contralateral testicular biochemical acute changes after unilateral testicular torsion and detorsion. Urology. 1994;44:413–418. doi: 10.1016/s0090-4295(94)80105-3. [DOI] [PubMed] [Google Scholar]
- 40.Pakyz RE, Heindel RM, Kallish M, Cosentino MJ. Spermatic cord torsion: effects of cyclosporine and prednisone on fertility and the contralateral testis in the rat. J Androl. 1990;11:401–408. [PubMed] [Google Scholar]
- 41.Koşar A, Küpeli B, Alçigir G, Ataoglu H, Sarica K, Küpeli S. Immunologic aspect of testicular torsion: detection of antisperm antibodies in contralateral testicle. Eur Urol. 1999;36:640–644. doi: 10.1159/000020060. [DOI] [PubMed] [Google Scholar]
- 42.Nagler HM, White RD. The effect of testicular torsion on the contralateral testis. J Urol. 1982;128:1343–1348. doi: 10.1016/s0022-5347(17)53504-1. [DOI] [PubMed] [Google Scholar]
- 43.Fisch H, Laor E, BarChama N, Witkin SS, Tolia BM, Reid RE. Detection of testicular endocrine abnormalities and their correlation with serum antisperm antibodies in men following vasectomy. J Urol. 1989;141:1129–1132. doi: 10.1016/s0022-5347(17)41190-6. [DOI] [PubMed] [Google Scholar]
- 44.Sinisi AA, Di Finizio B, Pasquali D, Scurini C, D'Apuzzo A, Bellastella A. Prevalence of antisperm antibodies by SpermMARtest in subjects undergoing a routine sperm analysis for infertility. Int J Androl. 1993;16:311–314. doi: 10.1111/j.1365-2605.1993.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 45.Collins JA, Burrows EA, Yeo J, YoungLai EV. Frequency and predictive value of antisperm antibodies among infertile couples. Hum Reprod. 1993;8:592–598. doi: 10.1093/oxfordjournals.humrep.a138102. [DOI] [PubMed] [Google Scholar]
- 46.Lu JC, Huang YF, Lu NQ. Antisperm immunity and infertility. Expert Rev Clin Immunol. 2008;4:113–126. doi: 10.1586/1744666X.4.1.113. [DOI] [PubMed] [Google Scholar]
- 47.Zanchetta R, Mastrogiacomo I, Graziotti P, Foresta C, Betterle C. Autoantibodies against Leydig cells in patients after spermatic cord torsion. Clin Exp Immunol. 1984;55:49–57. [PMC free article] [PubMed] [Google Scholar]
- 48.Skakkebaek NE. Testicular dysgenesis syndrome: new epidemiological evidence. Int J Androl. 2004;27:189–191. doi: 10.1111/j.1365-2605.2004.00488.x. [DOI] [PubMed] [Google Scholar]
- 49.Ferreira U, Netto Júnior NR, Esteves SC, Rivero MA, Schirren C. Comparative study of the fertility potential of men with only one testis. Scand J Urol Nephrol. 1991;25:255–259. doi: 10.3109/00365599109024555. [DOI] [PubMed] [Google Scholar]