Abstract
A dichotomic distinction between “organic” and “functional” hypogonadism is emerging. The former is an irreversible condition due to congenital or “acquired” “organic” damage of the brain centers or of the testis. Conversely, the latter is a potentially reversible form, characterized by borderline low testosterone (T) levels mainly secondary to age-related comorbidities and metabolic derangements, including metabolic syndrome (MetS). Life-style modifications, - here reviewed and, when possible, meta-analyzed -, have documented that weight-loss and physical exercise are able to improve obesity-associated functional hypogonadism and its related sexual symptoms. A rabbit experimental model, of MetS originally obtained in our lab, showed that endurance training (PhyEx) completely reverted MetS-induced hypogonadotropic hypogonadism by reducing hypothalamus inflammation and testis fibrosis eventually allowing for a better corpora cavernosa relaxation and response to sildenafil. Physicians should strongly adapt all the reasonable strategies to remove/mitigate the known conditions underlying functional hypogonadism, including MetS and obesity. Physical limitations, including reduced muscle mass and increased fat mass, along with low self-confidence, also due to the sexual problems, might limit a subject's propensity to increase physical activity and dieting. A short term T treatment trial, by improving muscle mass and sexual function, might help hypogonadal obese patients to overcome the overfed, inactive state and to become physically and psychologically ready for changing their lifestyle.
Keywords: Male hypogonadism, Obesity, Physical exercise, Testosterone, Weight loss
INTRODUCTION
1. Male hypogonadism definition and classifications
Historically, male hypogonadism (HG) - and in particular testosterone (T) deficiency (TD) - has been classified according to the site of origin of the putative damage to the hypothalamus-pituitary-testis (HPT) axis (i.e., primary and secondary) or according to the gonadotropin levels (i.e., hypo- or hyper-gonadotropic). Primary HG is hypergonadotropic and secondary HG is hypogonadotropic. In addition, an impaired T bioactivity - due to alterations in the receptor status or in steroid free hormones - might also result in a hypogonadal-like syndrome [1]. This classification has the advantage of giving insights to the clinician on the possible therapeutic approaches, e.g., gonadotropins or T in secondary forms and only T in the primary forms [2,3]. However, the clinical scenario and subject phenotype of the HG patient is dramatically dependent upon timing of appearance of the TD [2]. When the deficit is orchestrating its effect very early on during fetal life, the masculine phenotype is severely compromised, whereas, when TD is manifested during adulthood, the phenotype is only minimally affected and symptoms and signs are often vague and unspecific. The most characteristic symptoms of this latter form (often called late onset hypogonadism or LOH) are sexual symptoms, including reduced sexual desire and impaired spontaneous and sexual-related erections [4,5]. In adult men older than 40 years of the European general population, a TD is a frequent finding (i.e., 12% and 2%, for secondary and primary HG, respectively). However, the large majority of these men do not present the aforementioned cluster of sexual symptoms, which are present in only 2% of them. According to the majority of Andrology Societies [6,7,8,9], and to the Endocrine Society [3], only TD men presenting typical symptoms can be categorized and treated as a true HG.
LOH, also in its symptomatic form, is often associated with aging and its related comorbidities, including obesity, metabolic syndrome (MetS) and diabetes mellitus type 2 (T2DM). In particular, the latter metabolic conditions increase the risk of secondary HG, whereas the aging process per se increases the likelihood of having a primary HG [10,11,12]. Considering that a secondary HG is by far more prevalent than the primary one, understanding the molecular mechanism(s) leading to a comorbidity-dependent hypothalamic-pituitary failure is relevant. In a minority of cases, secondary HG could be associated with other potentially reversible conditions, such as use or abuse of opiates and anabolic androgens as well as hyperprolactinemia [3,13]. In all these cases of secondary HG, the Endocrine Society suggests that removing the underlying noxious condition may have a beneficial effect on HG, along with obvious additional health benefits [3]. This position partially endorses a previous US Food and Drug Administration [14] and Health Canada [15] Drug Safety Notification that recommended T therapy (TTh) only in those subjects with proven “organic” damages to the HPT axis (organic HG). Similarly, the Endocrine Society of Australia stated that TTh is not justified in older HG men with comorbidities and that obese men should be firstly encouraged to lose weight [3].
2. Organic and functional hypogonadism
A clear distinction between an “organic” and a “functional” HG was formalized few years ago in a perspective article by Grossmann and Matsumoto [16]. Organic HG is an essentially irreversible HG, characterized by specific symptoms and signs and very low T levels. In organic HG, TTh is supposed to result in expected net effects, along with a reasonable therapy risk, when considering benefits [16]. In contrast, “functional” HG is a reversible form, with borderline low T levels, characterized by sexual symptoms that are only partially ameliorated by TTh, and, more importantly, with an unknown therapy risk. For “functional” HG, change in lifestyle and removing the underlying condition leading to the TD is the recommended strategy to increase endogenous T. Although this position might have a more than reasonable background - i.e., removing the cause of the disease and not just buffering its consequences - changing lifestyle is not an easy task to reach in everyday clinical practice. In addition, it should be emphasized that the large majority of subjects consulting for sexual dysfunction and having low T can be categorized as functional HG (85%), with secondary HG the prevalent form, with a ratio of 5.5:1 vs. primary HG [17]. Only 50% and 10% of primary and secondary HG, respectively, show an organic origin for the TD. In particular, the large majority of secondary functional HG is associated with unhealthy metabolic conditions, such as MetS, diabetes, and obesity [17].
3. Obesity, metabolic syndrome and functional hypogonadism
Obesity is an expansion of adipose tissue, and, therefore, an expansion of the body's largest energy reservoir. According to this view and to the concept of “survival of the fittest”, obesity should represent an evolutionary advantage for the species. However, despite socioeconomic deprivation, we have nowadays more than sufficient food for most, and therefore little need to have an expanded energy reservoir. Hence, obesity is no longer considered a survival advantage but a source of obesity-associated comorbidities, which chronically orchestrate unhealthy consequences as a function of lifetime expanding [18,19]. There is no doubt that secondary, functional HG is one of these unhealthy consequences. The association among obesity, and in particular visceral obesity, and other comorbidities, such as dyslipidemia, glucose impairment, and hypertension, is clustered in the concept of MetS. MetS is essentially a diagnostic category, helping physicians to identify subjects at major risk for metabolic and cardiovascular complications. Actually, MetS is also deeply underpinned with secondary HG and erectile dysfunction (ED) [12,20].
AIM OF THE REVIEW
In this review, we will overview the effect of treatments of functional HG alternative to pharmacological substitution with T or gonadotropins and their effects on sexual symptoms, including ED. It is important to note that several pharmacological treatments of either HG or ED are available and all are very efficacious, however, they are not the main topic of this review, as it has recently been covered elsewhere [2,21]. We will pay particular attention to obesity- and MetS-associated HG, because of their prevalence, affecting more than 600 million adults worldwide. When possible, we used a meta-analytic analysis for scrutinizing results, because meta-analyses are considered as the highest level of evidence for evaluating interventions in healthcare. Meta-analyses have the advantage of extending the number of observations putting together the results of single trials, thus increasing the strength of the conclusions.
EFFECT OF DIET-INDUCED WEIGHT LOSS ON FUNCTIONAL HYPOGONADISM
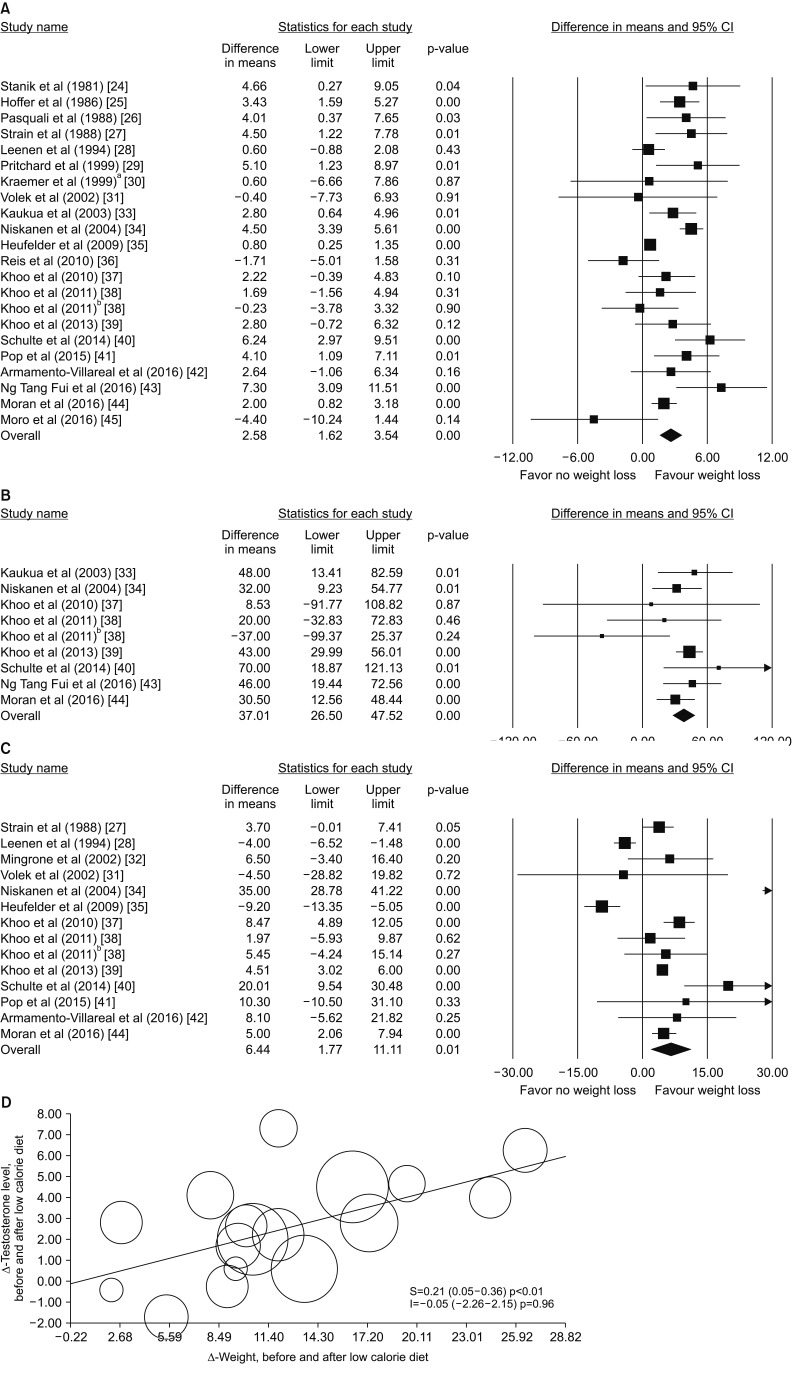
The clinical practice guidelines for medical care of patients with obesity from the American Association of Clinical Endocrinologists and the American College of Endocrinology [22] indicate that reducing total caloric intake should be the main component of any weight-loss intervention, irrespective to the macronutrient composition. We previously meta-analyzed the effect of any low-calorie diet in 13 studies published until mid-2012 and addressing the effect of caloric restriction on HPT [23]. We found that an average diet-induced weight loss of 9.8% was associated with a significant increase in total T (TT) of 2.8 nmol/L (1.68–4.07 nmol/L) and 2.05 (0.93–3.16 nmol/L), when paired and non-paired t-test models were applied. At that time, not enough studies were available to evaluate in a sub-analysis the effect of diet-induced weight reduction on gonadotropin levels. However, when data from diet were combined with those from bariatric surgery (see below), we reported a significant weight loss-induced increase in luteinizing hormone (LH) (1.31 mU/L [0.80–1.82 mU/L]) and follicle stimulating hormone (1.79 mU/L [1.28–2.30 mU/L]) [23]. Since that time, several new studies have been published. Nowadays, 22 studies evaluating the effect of low calorie diet on T levels and its metabolites are available [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. These trials enrolled 567 patients with a mean age of 44.9 years, a mean body mass index (BMI) of 36.0 kg/m2, and a mean follow-up of 23 weeks. These trials differ in basal TT levels and type of diet applied (Table 1). For a more conservative approach, endpoint values of each parameter were evaluated in a non-paired fashion (non-paired analysis). Information on T levels at endpoint were available for 21 studies. Combining the results of those trials, low calorie diet results in a significant increase in TT levels at endpoint (Fig. 1A). We observed similar results when hormone binding globulin (SHBG) and calculated free T were considered (Fig. 1B, 1C, respectively). Meta-regression analysis showed that a higher Δ-weight is associated with a higher T increase, meaning that each 5 kg of weight reduction results in one nmol/L increases (Fig. 1D). The latter finding was confirmed in a multivariate regression analysis, after the adjustment for age and trial duration (adjusted r =−1.21; p<0.0001).
Table 1. Moderators and outcome variables in individual studies included in the meta-analysis.
| Study | Location | Type of study | FU (wk) | Age (y) | BMI (kg/m2) | DM (%) | TT (nmol/L) |
|---|---|---|---|---|---|---|---|
| Stanik et al (1981) [24] | Sepulveda, CA, USA | BA | 8 | - | - | 4.2 | 13.9±3.4 |
| Hoffer et al (1986) [25] | Boston, MA, USA | BA | 4 | 34.0±7.0 | 33.1 | 0 | 13.0±1.0 |
| Pasquali et al (1988) [26] | Bologna, Italy | BA | 10 | 35.2±5.6 | 43.4±6.3 | 22.2 | 11.9 |
| Strain et al (1988) [27] | New York, USA | BA | 68 | 34.0±11.0 | - | 0 | 8.3±4.0 |
| Leenen et al (1994) [28] | Utrecht, The Netherlands | BA | 13 | 40.0±6.0 | 30.7±2.2 | 0 | 12.7±3.2 |
| Pritchard et al (1999) [29] | Laval, Canada | BA | 13 | 21.0±0.8 | 26.2±5.5 | 0 | 12.3±4.1 |
| Kraemer et al (1999)a [30] | Pennsylvania, USA | RCT | 12 | 39.7±6.6 | 33.1 | 0 | 15.9±7.7 |
| Volek et al (2002) [31] | Storrs, CT, USA | BA | 6 | 36.0±11.9 | - | 0 | 21.5±7.6 |
| Mingrone et al (2002) [32] | Rome, Italy | RCT | 54 | - | 47.8±8.8 | 0 | - |
| Kaukua et al (2003) [33] | Helsinki, Finland | RCT | 10 | 45.9±9.0 | 39.3±3.3 | 5.3 | 11.1±3.4 |
| Niskanen et al (2004) [34] | Kuopio, Finland | BA | 9 | 46.3±7.5 | 36.1±3.8 | 24.0 | 11.2±3.9 |
| Heufelder et al (2009) [35] | Berlin, Germany | RCT | 52 | 55.9±6.0 | 32.5±2.4 | 100 | 10.4±0.8 |
| Reis et al (2010) [36] | Saõ Paulo, Brazil | RCT | 104 | 42.2±10.5 | 54.0±6.7 | 0 | 11.7±5.1 |
| Khoo et al (2010) [37] | Adelaide, Australia | CBA | 8 | 49.6±11.0 | 34.4±4.0 | 38.8 | 20.2±6.9 |
| Khoo et al (2011) [38] | Adelaide, Australia | RCT | 52 | 62.3±5.9 | 35.6±4.8 | 100 | 13.9±3.3 |
| Khoo et al (2011)b [38] | Adelaide, Australia | RCT | 52 | 58.1±11.4 | 35.1±4.3 | 100 | 11.7±3.6 |
| Khoo et al (2013) [39] | Singapore, Singapore | BA | 90 | 40.8 | 32.7 | - | 12.2±5.7 |
| Schulte et al (2014) [40] | Kiel, Germany | BA | 13 | 44.0 | 42.7 | 0 | 6.9 |
| Pop et al (2015) [41] | New Brunswick, USA | RCT | 38 | 58±6 | 31.9±4.4 | 0 | 11.2±3.8 |
| Armamento-Villareal et al (2016) [42] | Houston, USA | RCT | 9 | 68.3±3.9 | 39.1±4.5 | - | 7.2±4.8 |
| Ng Tang Fui et al (2016) [43] | Melbourne, Australia | RCT | 49 | 54.3 | 37.5 | 20.4 | 6.8 |
| Moran et al (2016) [44] | Adelaide, Australia | RCT | 118 | 49.6 | 33.2 | 0 | 13.8 |
| Moro et al (2016) [45] | Padua, Italy | RCT | 34 | 29.9 | 26.6 | - | 21.3±16.9 |
Values are presented as mean only or mean±standard deviation.
FU: follow-up, BMI: body mass index, DM: diabetes mellitus, TT: total testosterone, BA: controlled cohort before-and-after comparisons in the same group of patients, RCT: randomized controlled trials, CBA: controlled before-and-after study between two or more groups of participants receiving different interventions.
aLow calorie diet only group. bLow energy diet group.
Fig. 1. Weighted differences (with 95% confidence interval [CI]) of mean total testosterone (TT; A), calculated free testosterone (B), sex hormone binding globulin (C) and before and after weight loss. aLow calorie diet only group. bLow energy diet group. (D) Influence of Δ-weight loss on TT weighted mean differences before and after low calorie diet as derived from meta-regression analysis.
EFFECT OF BARIATRIC SURGERY-INDUCED WEIGHT LOSS ON FUNCTIONAL HYPOGONADISM
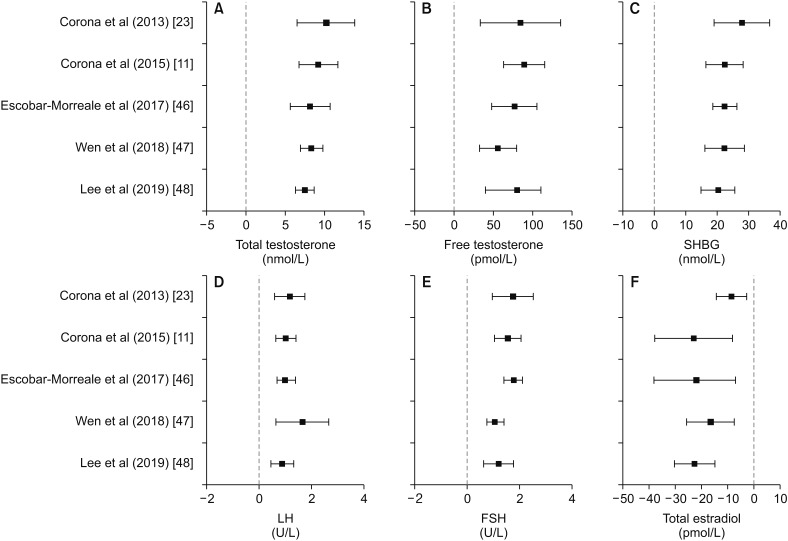
So far, five systematic meta-analyses [11,23,46,47,48] have been published concerning changes in sex hormone levels upon bariatric surgery. Despite differences in the number of studies analyzed and in the inclusion criteria for the study selection (Table 2), all the meta-analyses found a significant, sustained increase in TT and free T after bariatric surgery, as compared with the pre-surgical values (Fig. 2A). The mean increase in T was comparable in all the available meta-analyses ranging from 8 to 10 nmol/L for TT and from 75 to 90 pmol/L for free T (Fig. 2B). A significant increase in SHBG and gonadotropins was also consistently found in all these meta-analyses (Fig. 2C–2E). Total estradiol was significantly decreased after bariatric surgery, with a mean reduction of about 20 pmol/L, as documented by most meta-analyses, except for the earliest one [11] which found a decline of around a half (Fig. 2F).
Table 2. Available meta-analyses evaluating the effects of bariatric surgery on testosterone levels.
| Study | No. of trial | No. of patient | Main inclusion criteria |
|---|---|---|---|
| Corona et al (2013) [23] | 11 | 262 | Studies comparing testosterone levels before and after bariatric surgery |
| Corona et al (2015) [11] | 15 | 398 | Studies comparing testosterone levels before and after bariatric surgery |
| Escobar-Morreale et al (2017) [46] | 7 | 382 | Studies showing data on prevalence of obesity-associated secondary hypogonadism among patients undergoing to bariatric surgery |
| Wen et al (2018) [47] | 23 | 654 | Studies reporting sexual function and at least one sex hormone level with a follow-up time of at least 6 months |
| Lee et al (2019) [48] | 28 | 1,022 | Studies evaluating the effect of bariatric surgery on sex hormones or sperm parameters |
Fig. 2. Summary of the results obtained by available the meta-analyses, which evaluated the effects of bariatric surgery on sex hormones, gonadotropins, and SHBG. SHBG: sex hormone binding globulin, LH: luteinizing hormone, FSH: follicle stimulating hormone.
EFFECT OF DRUG-INDUCED WEIGHT LOSS ON FUNCTIONAL HYPOGONADISM
Only one study investigated the effect of an anti-obesity drug, i.e., liraglutide, on HPT in obese hypogonadal men with sexual symptoms and poor responses to lifestyle measures [49]. Liraglutide is a glucagon-like peptide-1 receptor agonist first approved for treatment of T2DM at doses up to 1.8 mg/d and later on approved for weight loss at a higher dosage (3.0 mg/d). In this trial [49], effects of liraglutide on TT levels were compared to those of TTh (1% T gel) in a 16-week study with 15 subjects enrolled per arm. Liraglutide, but not TTh, induced a 6% reduction in weight. Both treatments ameliorated sexual symptoms and increased significantly TT, although the increase was more evident with T gel (5.9 vs. 2.6 nmol/L with liraglutide). As expected, TTh further reduced LH levels, whereas liraglutide marginally improved it (0.7 mU/L).
EFFECT OF PHYSICAL EXERCISE ON FUNCTIONAL HYPOGONADISM
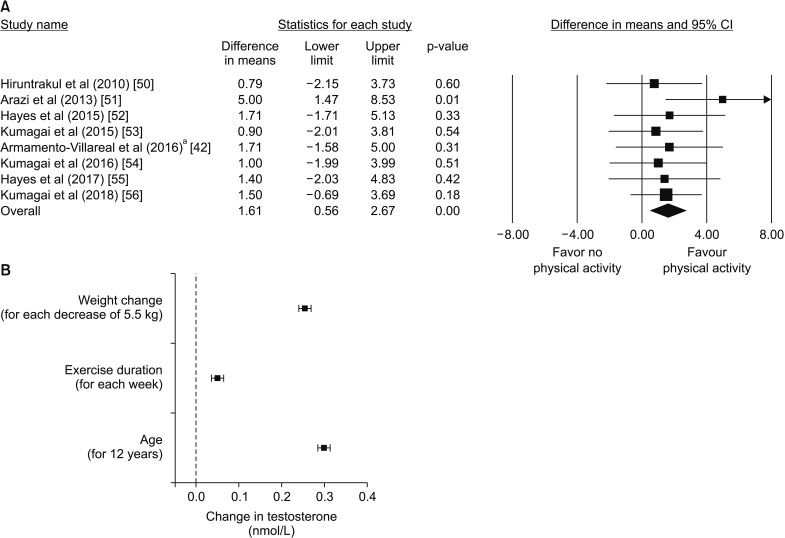
Overall, 8 studies evaluated the effect of physical exercise on T levels [42,50,51,52,53,54,55,56]. These trials enrolled 202 patients with a mean age 51.8 years, mean BMI of 28.5 kg/m2, and mean follow-up of 15 weeks. Trials differ in basal TT levels and physical exercise protocol (Table 3). For a more conservative approach, endpoint TT values of each parameter were evaluated in a non-paired fashion (non-paired analysis). Combining the results of those trials, physical exercise resulted in a significant increase in TT levels at endpoint (Fig. 3A). In order to evaluate the major determinants of the increase of TT after physical exercise (Δ-TT), a multivariate regression analysis was performed using (Δ-TT) as the dependent variable and age, trial duration as well as percent reduction of weight (Δ-weight) after physical exercise as putative predictors. The standardized coefficients are shown in Fig. 3B and suggest that a greater weight loss and a longer trial duration are significantly associated with an increase in TT levels, independently of age. Interestingly, the analysis also shows that for equal weight change, a greater improvement in TT levels is obtained in older men.
Table 3. Moderators and outcome variables in individual studies included in the meta-analysis.
| Study | Location | No. of subject | Type of physical exercize | Type of study | FU (wk) | Age (y) | BMI (kg/m2) | TT (nmol/L) |
|---|---|---|---|---|---|---|---|---|
| Hiruntrakul et al (2010) [50] | KhonKaen, Thailand | 19 | D: 50′ | RCT | 12 | 21.00±2.00 | 20.99±3.35 | 18.46±5.62 |
| F: 1/wk | ||||||||
| T: bicycle ergometry | ||||||||
| I: 60% of maximal effort | ||||||||
| Arazi et al (2013) [51] | Rasht, Iran | 8 | D: 60′ | CBA-S | 8 | 49.7±2.1 | 26.3 | 11.76±0.96 |
| F: 3/wk | ||||||||
| T: anaerobic floor exercises (resistance training) | ||||||||
| Hayes et al (2015) [52] | Hamilton, UK | 28 | I: moderate | CBA | 28 | 63±5 | 29.7 | 13.25±6.15 |
| D: 150′ | ||||||||
| F: minimum 2/wk | ||||||||
| T: aerobic exercise | ||||||||
| I: moderate and vigorous | ||||||||
| Kumagai et al (2015) [53] | Tsukuba, Japan | 44 | D: 40–60′ | BA | 12 | 51.0±13.3 | 29±6.6 | 12.3±6.0 |
| F: 3/wk | ||||||||
| T: aerobic (walking and jogging) | ||||||||
| I: gradually from light to hard | ||||||||
| Armamento-Villareal et al (2016)a [42] | St. Louis, USA | 12 | D: 90′ | RCT | 52 | 68.7±1.8 | 35.1±5.2 | 9.28±4.34 |
| F: 3/wk | ||||||||
| T: aerobic exercises | ||||||||
| I: moderate | ||||||||
| Kumagai et al (2016) [54] | Tsukuba, Japan | 41 | D: up to 90′ | CBA | 12 | 49.2±6.0 | 29.0±3.0 | 12.2±6.04 |
| F: 4–7/wk | ||||||||
| T: aerobic (walking and/or jogging) | ||||||||
| I: moderate and vigorous | ||||||||
| Hayes et al (2017) [55] | Hamilton, UK | 22 | D: 150′ | CBA | 6 | - | - | - |
| F: every 5 days | ||||||||
| T: bicycle ergometry | ||||||||
| I: 40% of maximal effort | ||||||||
| Kumagai et al (2018) [56] | Tsukuba, Japan | 28 | D+F: at least 150′/wk | CBA-S | 12 | 50.0±6.4 | 27.4±2.1 | 15.4±7.4 |
| T: aerobic (walking and/ or light jogging+aerobic exercises at home) | ||||||||
| I: moderate to vigorous |
Values are presented as mean only or mean±standard deviation.
FU: follow-up, BMI: body mass index, TT: total testosterone, D: duration of each session of physical activity, F: frequency of physical activity, T: type of physical activity, I: intensity of physical activity, RCT: randomized controlled trials, CBA: controlled before-and-after study between two or more groups of participants receiving different interventions, BA: controlled cohort before-and-after comparisons in the same group of patients.
aExercise only group.
Fig. 3. (A) Weighted differences (with 95% confidence interval [CI]) of mean total testosterone (TT), after physical activity. aExercise only group. (B) Change in TT (dependent variable) as a function of weight change, exercise duration and age (independent variables) as derived from a multivariate linear regression weighted for trial participants. Data derive from the meta-analysis of the available trials on the effect of physical exercise on testosterone levels. The independent variables were transformed as z-values (standardized parameters). The value corresponding to 1 standard deviation is reported below each independent variable.
EFFECT OF LIFESTYLE CHANGE ON SEXUAL SYMPTOMS
Considering that sexual complaints are the main symptoms related to LOH - and most probably to functional HG - it is important to examine the effect of lifestyle modifications on them, and, in particular, on ED. However, this is not the main topic of the present review, even because this issue was extensively covered by two recent meta-analysis [57,58]. One of them examined the effect of different lifestyle factors in population-based research. Cigarette smoking, high alcohol intake, and lack of physical activity all have deleterious effects on erectile function [58]. However, the most impressive results were observed for regular physical activity, which reduced the odds ratio for ED by 50% [58]. In addition, this effect was apparent in almost all the individual studies scrutinized [58]. Another meta-analysis investigates the effect of intervention with any physical activity on ED patients in randomized controlled trails, having, as an end-point, variation in ED scoring, as evaluated through the International Index of Erectile Function erectile function domain (IIEF-EFD). They found that on average physical activity increased IIEF-EFD by almost 4 points, an effect that is not very far from the 5 point increase - as evaluated in the meta-analysis - by the most recent phosphodiesterase type 5 inhibitor (PDE5i) on the market, avanafil [59].
MECHANISM OF ACTION OF PHYSICAL ACTIVITY ON FUNCTIONAL HYPOGONADISM AND ITS RELATED ERECTILE DYSFUNCTION
Human data derived from intervention trials aimed at investigating the effect of changing lifestyle on functional HG indicate that reducing caloric intake (diet or bariatric surgery) or increasing caloric expenditure (physical activity) improve TD. Overall, the data summarized above suggest that they are efficacious in restoring an altered HPT axis, as often observed in several metabolic conditions, including obesity and MetS. In particular, a recent meta-analysis indicates that consistent aerobic endurance exercise is the most effective strategy for preventing ED [58], beside increasing T levels. However, human data does not offer insights on how changing lifestyle acts in ameliorating TD and its associated symptoms.
1. Establishment of a rabbit model of metabolic syndrome-associated hypogonadotropic hypogonadism
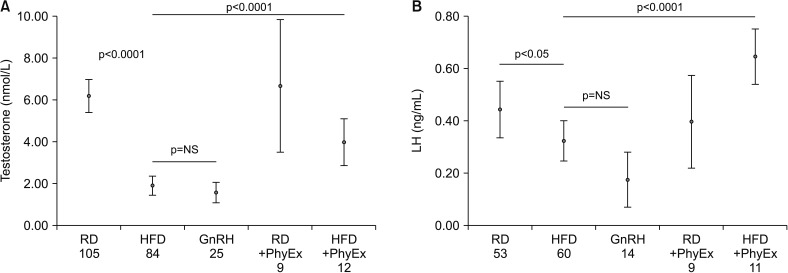
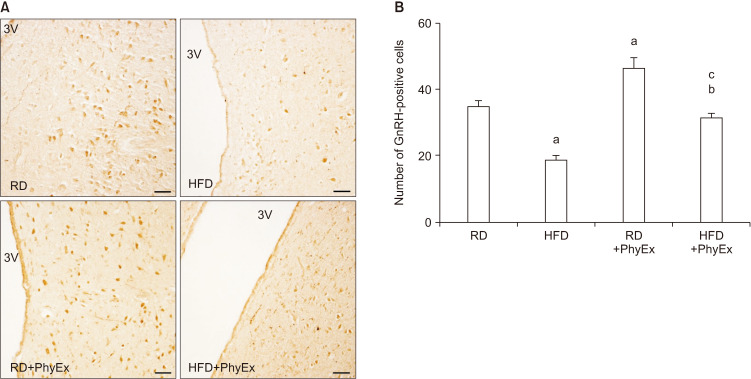
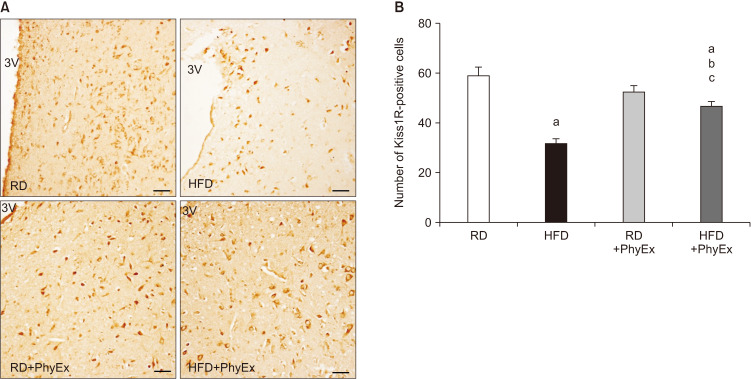
In the last ten years, we have developed an animal (rabbit) model of MetS by feeding rabbits a high fat diet (HFD) [60]. This animal model shares with the human phenotype several basic characteristics of MetS, i.e., hypertension, glucose intolerance, dyslipidemia, and increased visceral fat [60]. In addition, these rabbits show a MetS factor-dependent hypogonadotropic HP and ED [60]. Fig. 4A, shows the HFD-dependent decline in circulating T in a large number of rabbits. The same Fig. 4B shows that in MetS rabbits not only T levels were reduced but also LH levels are, at levels not statistically different from those induced by impairing LH secretion through gonadotropin-releasing hormone (GnRH) analog administration. Hence, HFD induced a genuine hypogonadotropic HP. We have previously demonstrated that the MetS-induced hypogonadotropic HP was associated with a peculiar inflammation of the medial preoptic area (MPO) of the hypothalamus, where GnRH neurons are located, characterized by an increased expression of cyclooxygenase-2 and macrophage markers [61,62]. Interestingly, in MPO of MetS rabbits, immunopositivity for GnRH was decreased, along with an altered expression of the main neurotransmitters regulating GnRH secretion, as Kiss1 and its receptor (Kiss1R) [60,61,62]. Fig. 5 and 6 show immunostaining for GnRH and Kiss1R in hypothalamic sections from rabbits fed a regular diet (RD; Fig. 5A, 6A) and in MetS-animals fed a HFD (Fig. 5B, 6B). We previously reported that, upon HFD, genes coding inhibitory factors for GnRH were increased, as well as neuropetide Y and prodynorphin, along with their cognate receptors [62]. In addition, we also reported an increased expression in MPO of estrogen receptor (ERβ and GPR30), which - in the male hypothalamus - mediates a negative feedback on GnRH [61,62]. Finally, in MPO of MetS rabbits several genes related to glucose transporter (GLUT) #1–4 and metabolism (insulin receptor substrate 1) were up-regulated [61,62]. MetS-associated HG was also associated with symptoms and signs of TD, as decreased prostate weight and ED [61,62]. In MetS animals, ED was characterized by impaired responsiveness to acetylcholine (Ach) and PDE5i, due to an impairment in nitric oxide formation and action [60,62]. Interestingly, we previously reported that T administration was able to restore sensitivity to Ach and PDE5i [60].
Fig. 4. Effects of physical exercise on hormonal circulating levels in regular diet (RD) and high fat diet (HFD) rabbits. Testosterone (A) and luteinizing hormone (LH; B) plasma levels were measured in rabbits fed a RD or a HFD with and without physical exercise (PhyEx), according to a previous protocol [62]. Results obtained in rabbits treated with the gonadotropin-releasing hormone (GnRH) analog triptorelin [60] were also shown, for comparison. Numbers of experimental observations are reported below each group. Statistical analysis between groups was performed with Kruskal-Wallis and post-hoc Mann-Whitney non-parametric tests. p-values are reported in each panel. NS: non-significant.
Fig. 5. Gonadotropin-releasing hormone (GnRH) immunohistochemical analysis in experimental rabbits. (A) Representative images of coronal hypothalamic sections, including the preoptic region lining the 3rd ventricle (3V). (B) Quantification of GnRH-positive cells, as calculated by counting at least ten fields of three independent experiments (mean±standard error; n=3 for each group; ap<0.001 vs. RD, bp<0.001 vs. HFD, cp<0.001 vs. RD+PhyEx). Scale bar 50 µm. RD: regular diet, HFD: high fat diet, PhyEx: physical exercise.
Fig. 6. Immunohistochemical analysis of Kiss1 receptor (Kiss1R) in rabbit hypothalamus. (A) Representative images of coronal hypothalamic sections, including the preoptic region lining the 3rd ventricle (3V). (B) Quantification of Kiss1R-positive cells, as calculated by counting at least ten fields of three independent experiments (mean±standard error; n=3 for each group; ap<0.001 vs. RD, bp<0.001 vs. HFD, cp<0.05 vs. RD+PhyEx). Scale bar 50 µm. RD: regular diet, HFD: high fat diet, PhyEx: physical exercise.
2. Effect of physical exercise in a rabbit with metabolic syndrome-associated hypogonadotropic hypogonadism
To evaluate the effect of physical activity on MetS-induced HG and ED, RD and MetS rabbits were exercise-trained to run on a treadmill for 12 weeks (RD+PhyEx and HFD+PhyEx). As mentioned before, HFD rabbits showed typical metabolic and cardiovascular features of MetS along with hypogonadotropic HG. Within the testis, the MetS condition down regulated, in a stepwise fashion, all the steroidogenic enzymes leading to T synthesis, along with an increase in fibronectin expression. PhyEx completely restored T and LH plasma levels, prostate weight and GnRH immunostaining (Fig. 5), doubling its gene expression in the preoptic area [62]. All the aforementioned HFD-induced increase in genes related to inflammation, estrogen signaling, and glucose metabolism in the hypothalamus were significantly reduced in HFD+PhyEx. In particular, in the hypothalamus, PhyEx increased Kiss 1 and decreased orexigenic and GnRH-inhibiting factors (dynorphin and its receptors OPRD1 and OPRK1), whereas it increased anorexigenic ones (proopiomelanocortin). PhyEx restored Kiss1 receptor immunostaining, which resulted decreased by HFD (Fig. 6B). Within the testis, PhyEx normalized fibronectin expression and increased genes related to T formation (17βHSD3) and metabolism (5α-reductase 1) [62]. Accordingly, PhyEx increased the ratio of androstenedione to T concentration within the testis, which resulted downregulated by HFD [62]. Corpora cavernosa (CC) strips from HFD rabbits showed a hypo-responsiveness to Ach and electrical field (EF) stimulation. In addition, sildenafil action on EF- or sodium nitroprusside-induced relaxation was also impaired in HFD CC. PhyEx reverted all these alterations. In CC extracts, several genes related to NO formation (DDAH1) and signaling (GCSa1, GCSb1, PDE5, PKG) were up-regulated by PhyEx, along with those involved in smooth muscle differentiation (SM22, αSMA) and androgen action (AR, STAMP2).
In conclusion, in this experimental model, endurance training (PhyEx) completely reverted MetS-induced hypogonadotropic HP and ED, having beneficial effects on the HPT axis and on the penis. In the hypothalamus, PhyEx reduced HFD-induced inflammation, in the testis, it reduced fibrosis and, in the penis, it allows a better relaxation and response to sildenafil. Hence, according to preclinical data aerobic exercise training can be considered an interesting strategy to combat MetS-associated HP and ED.
CONCLUSIONS
Functional HG is by far the most common condition determining, in the adult male, a TD that can be also symptomatic, having sexual complains - and in particular ED - as the most characteristic symptoms. The distinction between a functional and an organic form of HG is, in some ways, captious because, as demonstrated in our preclinical model, even diet-induced hypogonadotropic HG is associated with distinct, organic alterations within the hypothalamus, the testis and the penis [60,61,62]. However, the main advantage of considering a functional form of HG is that this is essentially a reversible form of HG. Therefore, physicians should strongly encourage subjects meeting the criteria of functional HG to adapt all the reasonable strategies to remove/mitigate the known conditions underlying functional HP, including MetS and obesity. Considering that combating obesity is not an easy task, the first step is to create a supportive environment to promote healthy living behaviors that prevent its appearance. However, when obesity is already present, change in lifestyle is imperative. Therapeutic diets and behavioral modifications are reasonable (but not often successful) clinical strategies, even for protecting against obesity-associated HG, as demonstrated by the meta-analyses also reported here. Physical activity, integrated within daily life, is another important option not only to induce a weight change but also for achieving a long-term weight management and overall health enhancement. In particular, according to recommendations (https://health.gov/paguidelines/second-edition/10things/), adults need at least 150 to 300 minutes of moderate-intensity aerobic activity each week, although any amount of physical activity has some health benefits. We reported here, by meta-analyzing available studies, that physical exercise is able to increase T levels, in particular in older individuals, with a benefit that is proportional to the amount and duration of the activity. Hence, we should encourage obese individuals in developing the skills necessary for physical activity and exercise. An open question is whether obese, hypogonadal individuals have the skills to progress safely and effectively along the continuum of changing their lifestyle. Physical limitations, including reduced muscle mass and increased fat mass, along with low self-confidence, also due to the sexual problems, might limit the propensity to increase physical activity and dieting. It is therefore conceivable that a short-term TRT trial, by improving muscle mass [63,64,65,66] and sexual problems [66,67], will help obese patients with HG to overcome the overfed, inactive state to become physically and psychologically ready for changing their lifestyle. In our opinion, this strategy could be more successful.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: MM, GC.
- Data curation: MM, GC, GR, ES, SC, AM.
- Formal analysis: GC, GR.
- Investigation: MM, GC.
- Methodology: MM, GC.
- Project administration: MM.
- Resources: MM, GC, GR, AM.
- Software: GC, GR, AM.
- Supervision: GC, LV, MM.
- Validation: MM, GC.
- Visualization: GC, MM.
- Writing—original draft: GC, GR, ES, AM, MM.
- Writing—review & editing: MM, LV, GC.
References
- 1.Rastrelli G, Corona G, Cipriani S, Mannucci E, Maggi M. Sex hormone-binding globulin is associated with androgen deficiency features independently of total testosterone. Clin Endocrinol (Oxf) 2018;88:556–564. doi: 10.1111/cen.13530. [DOI] [PubMed] [Google Scholar]
- 2.Rastrelli G, Maggi M, Corona G. Pharmacological management of late-onset hypogonadism. Expert Rev Clin Pharmacol. 2018;11:439–458. doi: 10.1080/17512433.2018.1445969. [DOI] [PubMed] [Google Scholar]
- 3.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715–1744. doi: 10.1210/jc.2018-00229. [DOI] [PubMed] [Google Scholar]
- 4.Rastrelli G, Corona G, Tarocchi M, Mannucci E, Maggi M. How to define hypogonadism? Results from a population of men consulting for sexual dysfunction. J Endocrinol Invest. 2016;39:473–484. doi: 10.1007/s40618-015-0425-1. [DOI] [PubMed] [Google Scholar]
- 5.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 6.Khera M, Adaikan G, Buvat J, Carrier S, El-Meliegy A, Hatzimouratidis K, et al. Diagnosis and treatment of testosterone deficiency: recommendations from the fourth International Consultation for Sexual Medicine (ICSM 2015) J Sex Med. 2016;13:1787–1804. doi: 10.1016/j.jsxm.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Morales A, Bebb RA, Manjoo P, Assimakopoulos P, Axler J, Collier C, et al. Canadian Men's Health Foundation Multidisciplinary Guidelines Task Force on Testosterone Deficiency. Diagnosis and management of testosterone deficiency syndrome in men: clinical practice guideline. CMAJ. 2015;187:1369–1377. doi: 10.1503/cmaj.150033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lunenfeld B, Mskhalaya G, Zitzmann M, Arver S, Kalinchenko S, Tishova Y, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18:5–15. doi: 10.3109/13685538.2015.1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeap BB, Grossmann M, McLachlan RI, Handelsman DJ, Wittert GA, Conway AJ, et al. Endocrine Society of Australia position statement on male hypogonadism (part 1): assessment and indications for testosterone therapy. Med J Aust. 2016;205:173–178. doi: 10.5694/mja16.00393. [DOI] [PubMed] [Google Scholar]
- 10.Tajar A, Forti G, O'Neill TW, Lee DM, Silman AJ, Finn JD, et al. EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–1818. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- 11.Corona G, Vignozzi L, Sforza A, Mannucci E, Maggi M. Obesity and late-onset hypogonadism. Mol Cell Endocrinol. 2015;418 Pt 2:120–133. doi: 10.1016/j.mce.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Corona G, Rastrelli G, Filippi S, Vignozzi L, Mannucci E, Maggi M. Erectile dysfunction and central obesity: an Italian perspective. Asian J Androl. 2014;16:581–591. doi: 10.4103/1008-682X.126386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coluzzi F, Billeci D, Maggi M, Corona G. Testosterone deficiency in non-cancer opioid-treated patients. J Endocrinol Invest. 2018;41:1377–1388. doi: 10.1007/s40618-018-0964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use [Internet] Silver Spring: U.S. Food and Drug Administration; c2015. [cited 2019 Feb 11]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-cautions-about-using-testosterone-products-low-testosterone-due. [Google Scholar]
- 15.Government of Canada. Summary safety review - testosterone replacement products - cardiovascular risk [Internet] Ottawa: Government of Canada; c2014. [cited 2019 Feb 11]. Available from: https://hpr-rps.hres.ca/reg-content/summary-safety-review-detail.php?linkID=SSR00058. [Google Scholar]
- 16.Grossmann M, Matsumoto AM. A perspective on middle-aged and older men with functional hypogonadism: focus on holistic management. J Clin Endocrinol Metab. 2017;102:1067–1075. doi: 10.1210/jc.2016-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corona G, Maggi M. Perspective: regulatory agencies' changes to testosterone product labeling. J Sex Med. 2015;12:1690–1693. doi: 10.1111/jsm.12951. [DOI] [PubMed] [Google Scholar]
- 18.Kjellberg J, Tange Larsen A, Ibsen R, Højgaard B. The socioeconomic burden of obesity. Obes Facts. 2017;10:493–502. doi: 10.1159/000480404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corona G, Monami M, Boddi V, Balzi D, Melani C, Federico N, et al. Is obesity a further cardiovascular risk factor in patients with erectile dysfunction? J Sex Med. 2010;7:2538–2546. doi: 10.1111/j.1743-6109.2010.01839.x. [DOI] [PubMed] [Google Scholar]
- 20.Rastrelli G, Filippi S, Sforza A, Maggi M, Corona G. Metabolic syndrome in male hypogonadism. Front Horm Res. 2018;49:131–155. doi: 10.1159/000485999. [DOI] [PubMed] [Google Scholar]
- 21.Corona G, Rastrelli G, Maggi M. The pharmacotherapy of male hypogonadism besides androgens. Expert Opin Pharmacother. 2015;16:369–387. doi: 10.1517/14656566.2015.993607. [DOI] [PubMed] [Google Scholar]
- 22.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22 Suppl 3:1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 23.Corona G, Rastrelli G, Monami M, Saad F, Luconi M, Lucchese M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168:829–843. doi: 10.1530/EJE-12-0955. [DOI] [PubMed] [Google Scholar]
- 24.Stanik S, Dornfeld LP, Maxwell MH, Viosca SP, Korenman SG. The effect of weight loss on reproductive hormones in obese men. J Clin Endocrinol Metab. 1981;53:828–832. doi: 10.1210/jcem-53-4-828. [DOI] [PubMed] [Google Scholar]
- 25.Hoffer LJ, Beitins IZ, Kyung NH, Bistrian BR. Effects of severe dietary restriction on male reproductive hormones. J Clin Endocrinol Metab. 1986;62:288–292. doi: 10.1210/jcem-62-2-288. [DOI] [PubMed] [Google Scholar]
- 26.Pasquali R, Casimirri F, Melchionda N, Fabbri R, Capelli M, Platè L, et al. Weight loss and sex steroid metabolism in massively obese man. J Endocrinol Invest. 1988;11:205–210. doi: 10.1007/BF03350136. [DOI] [PubMed] [Google Scholar]
- 27.Strain GW, Zumoff B, Miller LK, Rosner W, Levit C, Kalin M, et al. Effect of massive weight loss on hypothalamic-pituitary-gonadal function in obese men. J Clin Endocrinol Metab. 1988;66:1019–1023. doi: 10.1210/jcem-66-5-1019. [DOI] [PubMed] [Google Scholar]
- 28.Leenen R, van der Kooy K, Seidell JC, Deurenberg P, Koppeschaar HP. Visceral fat accumulation in relation to sex hormones in obese men and women undergoing weight loss therapy. J Clin Endocrinol Metab. 1994;78:1515–1520. doi: 10.1210/jcem.78.6.8200956. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard J, Després JP, Gagnon J, Tchernof A, Nadeau A, Tremblay A, et al. Plasma adrenal, gonadal, and conjugated steroids following long-term exercise-induced negative energy balance in identical twins. Metabolism. 1999;48:1120–1127. doi: 10.1016/s0026-0495(99)90125-7. [DOI] [PubMed] [Google Scholar]
- 30.Kraemer WJ, Volek JS, Clark KL, Gordon SE, Puhl SM, Koziris LP, et al. Influence of exercise training on physiological and performance changes with weight loss in men. Med Sci Sports Exerc. 1999;31:1320–1329. doi: 10.1097/00005768-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Volek JS, Sharman MJ, Love DM, Avery NG, Gómez AL, Scheett TP, et al. Body composition and hormonal responses to a carbohydrate-restricted diet. Metabolism. 2002;51:864–870. doi: 10.1053/meta.2002.32037. [DOI] [PubMed] [Google Scholar]
- 32.Mingrone G, Greco AV, Giancaterini A, Scarfone A, Castagneto M, Pugeat M. Sex hormone-binding globulin levels and cardiovascular risk factors in morbidly obese subjects before and after weight reduction induced by diet or malabsorptive surgery. Atherosclerosis. 2002;161:455–462. doi: 10.1016/s0021-9150(01)00667-0. [DOI] [PubMed] [Google Scholar]
- 33.Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Sex hormones and sexual function in obese men losing weight. Obes Res. 2003;11:689–694. doi: 10.1038/oby.2003.98. [DOI] [PubMed] [Google Scholar]
- 34.Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab. 2004;6:208–215. doi: 10.1111/j.1462-8902.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- 35.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–733. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- 36.Reis LO, Favaro WJ, Barreiro GC, de Oliveira LC, Chaim EA, Fregonesi A, et al. Erectile dysfunction and hormonal imbalance in morbidly obese male is reversed after gastric bypass surgery: a prospective randomized controlled trial. Int J Androl. 2010;33:736–744. doi: 10.1111/j.1365-2605.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 37.Khoo J, Piantadosi C, Worthley S, Wittert GA. Effects of a low-energy diet on sexual function and lower urinary tract symptoms in obese men. Int J Obes (Lond) 2010;34:1396–1403. doi: 10.1038/ijo.2010.76. [DOI] [PubMed] [Google Scholar]
- 38.Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8:2868–2875. doi: 10.1111/j.1743-6109.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- 39.Khoo J, Tian HH, Tan B, Chew K, Ng CS, Leong D, et al. Comparing effects of low- and high-volume moderate-intensity exercise on sexual function and testosterone in obese men. J Sex Med. 2013;10:1823–1832. doi: 10.1111/jsm.12154. [DOI] [PubMed] [Google Scholar]
- 40.Schulte DM, Hahn M, Oberhäuser F, Malchau G, Schubert M, Heppner C, et al. Caloric restriction increases serum testosterone concentrations in obese male subjects by two distinct mechanisms. Horm Metab Res. 2014;46:283–286. doi: 10.1055/s-0033-1358678. [DOI] [PubMed] [Google Scholar]
- 41.Pop LC, Sukumar D, Tomaino K, Schlussel Y, Schneider SH, Gordon CL, et al. Moderate weight loss in obese and overweight men preserves bone quality. Am J Clin Nutr. 2015;101:659–667. doi: 10.3945/ajcn.114.088534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armamento-Villareal R, Aguirre LE, Qualls C, Villareal DT. Effect of lifestyle intervention on the hormonal profile of frail, obese older men. J Nutr Health Aging. 2016;20:334–340. doi: 10.1007/s12603-016-0698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng Tang Fui M, Prendergast LA, Dupuis P, Raval M, Strauss BJ, Zajac JD, et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med. 2016;14:153. doi: 10.1186/s12916-016-0700-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moran LJ, Brinkworth GD, Martin S, Wycherley TP, Stuckey B, Lutze J, et al. Long-term effects of a randomised controlled trial comparing high protein or high carbohydrate weight loss diets on testosterone, SHBG, erectile and urinary function in overweight and obese men. PLoS One. 2016;11:e0161297. doi: 10.1371/journal.pone.0161297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14:290. doi: 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update. 2017;23:390–408. doi: 10.1093/humupd/dmx012. [DOI] [PubMed] [Google Scholar]
- 47.Wen JP, Wen LY, Zhao YJ, Li Q, Lin W, Huang HB, et al. Effect of bariatric surgery on sexual function and sex hormone levels in obese patients: a meta-analysis. J Endocrine Soc. 2018;2:117–132. [Google Scholar]
- 48.Lee Y, Dang JT, Switzer N, Yu J, Tian C, Birch DW, et al. Impact of bariatric surgery on male sex hormones and sperm quality: a systematic review and meta-analysis. Obes Surg. 2019;29:334–346. doi: 10.1007/s11695-018-3557-5. [DOI] [PubMed] [Google Scholar]
- 49.Jensterle M, Podbregar A, Goricar K, Gregoric N, Janez A. Effects of liraglutide on obesity-associated functional hypogonadism in men. Endocr Connect. 2019;8:195–202. doi: 10.1530/EC-18-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiruntrakul A, Nanagara R, Emasithi A, Borer KT. Effect of endurance exercise on resting testosterone levels in sedentary subjects. Cent Eur J Public Health. 2010;18:169–172. doi: 10.21101/cejph.a3589. [DOI] [PubMed] [Google Scholar]
- 51.Arazi H, Ghiasi A, Afkhami M. Effects of different rest intervals between circuit resistance exercises on post-exercise blood pressure responses in normotensive young males. Asian J Sports Med. 2013;4:63–69. [PMC free article] [PubMed] [Google Scholar]
- 52.Hayes LD, Sculthorpe N, Herbert P, Baker JS, Spagna R, Grace FM. Six weeks of conditioning exercise increases total, but not free testosterone in lifelong sedentary aging men. Aging Male. 2015;18:195–200. doi: 10.3109/13685538.2015.1046123. [DOI] [PubMed] [Google Scholar]
- 53.Kumagai H, Zempo-Miyaki A, Yoshikawa T, Tsujimoto T, Tanaka K, Maeda S. Lifestyle modification increases serum testosterone level and decrease central blood pressure in overweight and obese men. Endocr J. 2015;62:423–430. doi: 10.1507/endocrj.EJ14-0555. [DOI] [PubMed] [Google Scholar]
- 54.Kumagai H, Zempo-Miyaki A, Yoshikawa T, Tsujimoto T, Tanaka K, Maeda S. Increased physical activity has a greater effect than reduced energy intake on lifestyle modification-induced increases in testosterone. J Clin Biochem Nutr. 2016;58:84–89. doi: 10.3164/jcbn.15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayes LD, Herbert P, Sculthorpe NF, Grace FM. Exercise training improves free testosterone in lifelong sedentary aging men. Endocr Connect. 2017;6:306–310. doi: 10.1530/EC-17-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumagai H, Yoshikawa T, Zempo-Miyaki A, Myoenzono K, Tsujimoto T, Tanaka K, et al. Vigorous physical activity is associated with regular aerobic exercise-induced increased serum testosterone levels in overweight/obese men. Horm Metab Res. 2018;50:73–79. doi: 10.1055/s-0043-117497. [DOI] [PubMed] [Google Scholar]
- 57.Silva AB, Sousa N, Azevedo LF, Martins C. Physical activity and exercise for erectile dysfunction: systematic review and meta-analysis. Br J Sports Med. 2017;51:1419–1424. doi: 10.1136/bjsports-2016-096418. [DOI] [PubMed] [Google Scholar]
- 58.Allen MS, Walter EE. Health-related lifestyle factors and sexual dysfunction: a meta-analysis of population-based research. J Sex Med. 2018;15:458–475. doi: 10.1016/j.jsxm.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 59.Corona G, Rastrelli G, Burri A, Jannini EA, Maggi M. The safety and efficacy of Avanafil, a new 2(nd) generation PDE5i: comprehensive review and meta-analysis. Expert Opin Drug Saf. 2016;15:237–247. doi: 10.1517/14740338.2016.1130126. [DOI] [PubMed] [Google Scholar]
- 60.Filippi S, Vignozzi L, Morelli A, Chavalmane AK, Sarchielli E, Fibbi B, et al. Testosterone partially ameliorates metabolic profile and erectile responsiveness to PDE5 inhibitors in an animal model of male metabolic syndrome. J Sex Med. 2009;6:3274–3288. doi: 10.1111/j.1743-6109.2009.01467.x. [DOI] [PubMed] [Google Scholar]
- 61.Morelli A, Sarchielli E, Comeglio P, Filippi S, Vignozzi L, Marini M, et al. Metabolic syndrome induces inflammation and impairs gonadotropin-releasing hormone neurons in the preoptic area of the hypothalamus in rabbits. Mol Cell Endocrinol. 2014;382:107–119. doi: 10.1016/j.mce.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 62.Morelli A, Filippi S, Comeglio P, Sarchielli E, Cellai I, Pallecchi M, et al. Physical activity counteracts metabolic syndrome-induced hypogonadotropic hypogonadism and erectile dysfunction in the rabbit. Am J Physiol Endocrinol Metab. 2019;316:E519–E535. doi: 10.1152/ajpendo.00377.2018. [DOI] [PubMed] [Google Scholar]
- 63.Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, et al. Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J Endocrinol Invest. 2016;39:967–981. doi: 10.1007/s40618-016-0480-2. [DOI] [PubMed] [Google Scholar]
- 64.Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, et al. Therapy of endocrine disease: testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016;174:R99–R116. doi: 10.1530/EJE-15-0262. [DOI] [PubMed] [Google Scholar]
- 65.Corona G, Maseroli E, Maggi M. Injectable testosterone undecanoate for the treatment of hypogonadism. Expert Opin Pharmacother. 2014;15:1903–1926. doi: 10.1517/14656566.2014.944896. [DOI] [PubMed] [Google Scholar]
- 66.Isidori AM, Balercia G, Calogero AE, Corona G, Ferlin A, Francavilla S, et al. Outcomes of androgen replacement therapy in adult male hypogonadism: recommendations from the Italian society of endocrinology. J Endocrinol Invest. 2015;38:103–112. doi: 10.1007/s40618-014-0155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corona G, Rastrelli G, Morgentaler A, Sforza A, Mannucci E, Maggi M. Meta-analysis of results of testosterone therapy on sexual function based on international index of erectile function scores. Eur Urol. 2017;72:1000–1011. doi: 10.1016/j.eururo.2017.03.032. [DOI] [PubMed] [Google Scholar]