Abstract
Purpose
Germline mutations in the aryl-hydrocarbon receptor interacting protein (AIP) have been identified often in the setting of familial isolated pituitary adenoma (FIPA). To date there is no strong evidence linking germline AIP mutations to other neoplasms apart from the pituitary. Our primary objective was to investigate the prevalence of AIP gene mutations and mutations in genes that have been associated with neuroendocrine tumors in series of tumors from patients presenting with both pituitary adenomas and differentiated thyroid carcinomas (DTCs).
Methods
Pathology samples were retrieved from all pituitary adenomas in patients with concomitant DTCs, including one with a known germline AIP variant. Subsequently, two additional patients with known germline AIP variants were included, of which one presented only with a follicular thyroid carcinoma (FTC).
Results
In total, 17 patients (14 DTCs and 15 pituitary adenomas) were investigated by targeted next generation sequencing (NGS). The pituitary tumor samples revealed no mutations, while among the thyroid tumor samples BRAF (6/14, 42.9%) was the most frequently mutated gene, followed by NRAS (3/11, 27.3%). In one AIP-mutated FIPA kindred, the AIP-variant c.853C>T; p.Q285* was confirmed in the FTC specimen, including evidence of loss of heterozygosity (LOH) at the AIP locus in the tumor DNA.
Conclusion
Although most observed variants in pituitary adenomas and DTCs were similar to those of sporadic DTCs, we confirmed in one AIP mutation-positive case the AIP-variant and LOH at this locus in an FTC specimen, which raises the potential role of the AIP mutation as a rare initiating event.
Keywords: Pituitary tumors, Thyroid carcinoma, AIP, Acromegaly
Introduction
Pituitary adenomas are mostly benign monoclonal neoplasms that arise from any of the five hormone-secreting cell types of the anterior lobe of the pituitary gland, and cause disease due to hormonal hypersecretion and tumor mass effects. Most pituitary adenomas occur sporadically (95%). Although in the majority of these sporadic cases the exact molecular pathogenesis remains unknown, in a significant proportion of somatotropinomas (30%) and corticotropinomas (60%) activating somatic mutations have been found in the GNAS and USP8 genes, respectively [1, 2]. In addition, germline mutations may predispose to pituitary tumorigenesis, which together represent about 5% of patients with pituitary adenomas [3].
Germline mutations have been described in the aryl-hydrocarbon receptor interacting protein (AIP) gene in the setting of either familial isolated pituitary adenoma (FIPA) or in simplex, young-onset pituitary adenomas, such as pituitary gigantism [4–6]. The AIP gene encodes a 330-amino-acid co-chaperone involved in subcellular trafficking, nuclear receptor stability, and transactivation potential [4, 7]. It is postulated that in AIP-mutated pituitary adenomas, AIP loses its activity as a tumor suppressor, which is supported by the association of loss-of-function mutations and the presence of loss of heterozygosity (LOH) at the AIP locus in the pituitary adenoma. To date there is no strong evidence linking germline AIP mutations to other neuroendocrine neoplasms apart from the pituitary.
The frequency of differentiated thyroid carcinomas (DTCs) is increased in patients with somatotropinomas, with papillary thyroid carcinoma (PTC) being the most frequently reported type (up to 25%) [8–13]. As thyroid follicular epithelial cells express insulin-like growth factor 1 (IGF-1) receptors and IGF-1 is an important factor for promoting replication and reducing apoptosis of these cells [14], IGF-1 could potentially be linked to the promotion of thyroid cancer in acromegalic patients. BRAF mutations have proved to be the most common genetic event (about 60% of cases) involved in the onset of PTC in the general population [15]; other frequently identified genetic events include point mutations of the RAS genes and RET/PTC and PAX8/PPARɣ chromosomal rearrangements [15, 16]. Based on earlier reports, LOH of chromosome 22 is particularly common in follicular thyroid carcinomas (FTCs), and it is associated with the widely invasive type [17–19].
Given the frequency of malignant thyroid tumors in somatotropinomas, the potential for a common mechanism behind both tumors remains valid. The role of an AIP mutation as an initiating event is open to question since the AIP protein may interact with the tyrosine kinase receptor, encoded by the RET protooncogene in the pituitary [20–24]. Coexistence of PTCs with somatotropinomas in AIP-mutated patients is very rare and has been described in three cases [25, 26]. Although only one case of LOH at the AIP locus (11q13) in FTCs is previously described [27], Daly et al. recently described an FTC in a teenaged AIP mutation-positive carrier in which decreased AIP staining was seen in the FTC tumor that was accompanied by LOH at the AIP locus in the tumor DNA [28]. Thus, the finding of DTCs and pituitary adenomas in the same individuals or kindreds could represent a rare association of germline AIP mutations.
To date, there has only been one study that reported in 12 patients with somatotropinomas and concomitant DTC that AIP was not overexpressed in the thyroid tumor tissue using immunohistochemistry [29]. Here we studied the presence of mutations in AIP in patients with DTCs and concomitant pituitary adenomas, including all five adenoma types. Subsequently, the available tumors from these patients were investigated using targeted next generation sequencing (NGS) for mutations in AIP and additional neuroendocrine tumor-related genes. Since these features are relative rare, a nationwide survey was performed in the Netherlands.
Materials and methods
Patients
From Pathologisch-Anatomisch Landelijk Geautomatiseerd Archief (PALGA), the nationwide Dutch network and registry of histo- and cytopathology, all patient records of individuals included 1993–2016 were retrieved matching the following search criteria: pituitary adenoma (i.e., prolactinomas, nonfunctioning pituitary adenomas (NFPAs), somatotropinomas, corticotropinomas, and thyrotropinomas) and DTC (i.e., FTC, follicular variant of papillary thyroid carcinoma (FVPTC), and PTC). The standardized records contain an encrypted patient identification number (allowing for identification of multiple samples of one patient), data on age at diagnosis and sex, date of arrival of the histological tissue, presence of metastasis, and the diagnosis of the pathology report.
The PALGA search identified 15 patients with a history of thyroid carcinoma and pituitary adenoma with no known genetic background (i.e., sporadic), except for one with a known germline AIP variant from the Erasmus University Medical Center that was part of the PALGA search data range as well. Two additional patients from this center with known germline AIP variants were included in the study, of which one who presented only with an FTC. The latter has a familial history of pituitary adenomas (i.e., father was AIP mutation carrier and diagnosed with acromegaly), however, the pituitary gland was not affected in this patient. Therefore, this patient was not identified in the PALGA search data range. The second patient with a somatotropinoma and classical-variant PTC was successfully treated by total thyroidectomy in 1975, and therefore not part of the PALGA search date range, while the tumor sample showed well-preserved histomorphology. In total, we included 17 patients.
Approval from the Medical Ethical Committee of the Erasmus University Medical Center and informed consent to use the tumor tissues for research purposes were obtained. Tumor tissues from all Dutch medical centers were used according to the code of conduct, Proper Secondary Use of Human Tissue, established by the Dutch Federation of Medical Scientific Societies [30].
Data collection
Anonymized data were collected on age at diagnosis, sex, year of diagnosis, presence of metastasis, immunohistochemical staining results (adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), GH, luteinizing hormone (LH), PRL) and type of DTC (FTC, FVPTC, or classical-variant PTC).
Genetic analysis of germline AIP mutation
As mentioned above, three patients from the Erasmus University Medical Center included in the study were previously investigated for the presence of AIP mutations. This was performed using leukocyte DNA extracted from peripheral blood as described by Vierimaa et al. [5]; multiplex ligation-dependent probe amplification studies were performed as described previously [31, 32]. Normal population genetic databases were assessed for the presence of AIP variant frequencies; AIP variant pathogenicity was assessed using Alamut (Interactive Biosoftware). In addition, classification of variants was also performed according reported guidelines [33]. All patients provided informed written consent for genetic testing.
Tumor DNA samples
We excluded low quality tissue of pituitary adenoma (n = 2) or thyroid carcinoma (n = 2) from the study. As mentioned before, patient no. 17 presented only with an FTC. In total 29 tumor DNA samples from 17 index patients were studied; DNA obtained exclusively from thyroid tumor was available for 14 (82.4%) of the cases, and only pituitary tumor DNA for 15 (88.2%) of the cases.
DNA was isolated from representative tumor areas by microdissection, from ~10 hematoxylin stained sections from formalin-fixed, paraffin-embedded (FFPE) tumor tissue, using proteinase-K and 5% Chelex 100 resin. Selection of representative tumor areas was performed on a paraffin slide stained with hematoxylin and eosin by a pathologist (L.O. and F.G.). In addition, DNA was quantified with the Quant-iT PicoGreen dsDNA Assay Kit (Thermo Fisher Scientific, Waltham, MA). All tumor DNAs that were used for mutation screening contained ≥60% of tumor cells.
Targeted NGS and data analysis
A custom-made targeted gene panel (TGP) was designed using the TruSeq Custom Amplicon 1.5 kit system (Illumina, San Diego, CA) and the Ion AmpliSeq designer software (https://ampliseq.com/; Thermo Fisher Scientific, Breda, the Netherlands), to study DNA from FFPE tumor tissues (Table 1). The panel was designed specifically for FFPE-DNA use (amplicon range 125–175 bp). Targeting contained the entire coding sequences of AIP (coverage based on design: 92.11%), CDKN1B (96.84%), GNAS (82.93%), GPR101 (97.46%), HRAS (63.08%), KRAS (82.28%), MEN1 (84.73%), NRAS (100.00%), PIK3CA (96.55%), PRKACB (91.46%), PRKAR1A (100.00%), RET (86.34%), SDHA (93.48%), SDHAF2 (100.00%), SDHB (98.65%), SDHC (91.93%), SDHD (77.95%), and the hotspot region BRAF (p.V600E). In addition, single-nucleotide polymorphisms (SNPs) were selected on chromosome 11 and 22 to enable copy number variation (CNV) detection (Table 1). Mutation detection was performed using the S5-XL system (Ion Torrent) with manufacturer’s materials and protocols (Thermo Fisher Scientific). Library preparations and sequencing was performed as described earlier [34]. Data analysis was performed using SeqPilot version 4.2.2. (JSI medical systems). CNV detection was evaluated using SNPitty, which visualizes B-allele frequencies from NGS sequencing data [35]. The American College of Medical Genetics and Genomics standards and guidelines were used for interpretation of sequence variants of unknown significance (VUS) [33]. When classifying and reporting a variant we used the online software prediction program Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/) and Align GVGD (http://agvgd.hci.utah.edu/agvgd_input.php) as well as the gnomAD database (https://gnomad.broadinstitute.org), cBioportal database (https://www.cbioportal.org), and the Cosmic database (https://cancer.sanger.ac.uk/cosmic).
Table 1.
Characteristics of the custom-made targeted gene panel
| Characteristics | Panel I | |
|---|---|---|
| Type of sample | FFPE-DNA | |
| Amplicon length, bp | 125–175 | |
| Amplicons designed | 399 (X 2) | |
| Common genes included (pituitary adenoma and DTC) | 1. AIP (NM_003977): exon 1–6; | |
| 2. BRAF (NM_004333): exon 15; | ||
| 3. CDKN1B (NM_ 004064): exon 1–2 | ||
| 4. GNAS (NM_016592): exon 1–13 | ||
| 5. GPR101 (NM_054021): exon 1; | ||
| 6. HRAS (NM_005343): exon 2–6; | ||
| 7. KRAS (NM_004985): exon 2–5; | ||
| 8. MEN1 (NM_000244): exon 1–10; | ||
| 9. NRAS (NM_002524): exon 3; | ||
| 10. PIK3CA (NM_006218): exon 2–21; | ||
| 11. PRKACB (NM_207578): exon 1–10; | ||
| 12. PRKAR1A (NM_212471): exon 2–11; | ||
| 13. RET (NM_020975): exon 2–20; | ||
| 14. SDHA (NM_004168): exon 2–15; | ||
| 15. SDHAF2 (NM_017841): exon 1– 4; | ||
| 16. SDHB (NM_003000): exon 1–8; | ||
| 17. SDHC (NM_003001): exon 1–6; | ||
| 18. SDHD (NM_003002): exon 1–4 | ||
| SNPs target region chromosome 11 | – rs2513613 | – rs10838307 |
| – rs34593780 | – rs4267090 | |
| – rs2631403 | – rs7939803 | |
| – rs330253 | – rs2887046 | |
| – rs73455029 | – rs11233227 | |
| – rs7949600 | – rs6483324 | |
| – rs1247726 | – rs2851171 | |
| – rs4943948 | – rs736287 | |
| – rs681017 | – rs2510718 | |
| – rs10750552 | – rs1638585 | |
| – rs1620333 | – rs7110021 | |
| – rs630172 | – rs35787427 | |
| – rs481303 | – rs611697 | |
| – rs1455113 | ||
| SNPs target region chromosome 22 | – rs1970640 | – rs2017869 |
| – rs3747031 | – rs1894252 | |
| – rs5996639 | – rs956548 | |
| – rs2285206 | – rs2038010 | |
| – rs2294206 | – rs2143695 | |
| – rs62636244 | – rs5769583 | |
| – rs17003592 | – rs1296750 | |
| – rs3884944 | – rs6010046 | |
NM and ENST are both available at http://www.ensembl.org.
DTC differentiated thyroid carcinoma, FFPE formalin-fixed, paraffin-embedded, SNPs single-nucleotide polymorphisms
In patient no. 17, we also examined the most common FTC driver gene alterations [36] by a targeted NGS designed to study PTEN and the TERT promoter. The panel included the entire coding sequences of CDKN2A, KEAP1, PTEN, STK11, and TP53, as well as hotspots: AKT1 (exon 3), AKT2 (3), AKT3 (2), ALK (20, 22–25), APC (16), ARAF (7), BRAF (11, 12, 14, 15), CDK4 (2, 4, 7, 8), CTNNB1 (3, 7, 8), DDR2 (14–19), EGFR (12, 18–21), EIF1AX (1, 2), ERBB2 (HER2) (8, 17–21), ERBB3 (3, 6–10, 21, 23), ESR1 (4, 5, 7, 8), EZH2 (16), FBWX7 (9, 10), FGFR1 (4, 7, 12–14), FGFR2 (7, 9, 12), FGFR3 (7, 9, 14, 15), FOXL2 (1), GNA11 (4, 5), GNAQ (4, 5), GNAS (8, 9), HRAS (2–4), IDH1 (4), IDH2 (4), JAK2 (14), JAK3 (4, 16), KIT (8, 9, 11, 13–18), KNSTRN (1), KRAS (2–4), MAP2K1 (1–6), MET (2, 14, 19, 20), MTOR (30, 39, 40, 43, 47, 53, 56, 57), MYD88 (5), NFE2L2 (2), NOTCH1 (26, 27), NRAS (2–4), OXA1L (1), PDGFRA (12, 14, 18), PIK3CA (2, 5, 8, 10, 14, 21), POLD1 (6, 8, 12, 15–17, 24), POLE (9–14, 21, 25), RAC1 (2), RAF1 (7), RET (11, 16), RHOA (2), RIT1 (4, 5), RNF43 (2–10), ROS1 (36–41), SF3B1 (14, 15), and SMAD4 (3, 9, 12). In addition, it also covers the known C228T, 242_243delinsTT, and the C250T of the TERT promoter. To investigate the presence of driver fusions, the FTC tumor of patient no. 17 was investigated using Archer technology. RNA was isolated according to manufactures instructions using the RNeasy kit (Qiagen). Subsequently, Archer was performed with the Archer FusionPlex CTL panel (Illumina) according to manufacturer’s instructions and analysed using the S5-XL system. Sequencing data were uploaded and analyzed using the Archer Analysis software (https://analysis.archerdx.com). If all quality criteria were met as indicated by the Archer’s instructions, data were considered valid. Details are available on request.
Statistical analysis
We calculated proportions and rates for categorical variables, means ± standard deviations, or medians and ranges for parametric or nonparametric variables. For statistical analysis, the Statistical Package for the Social Sciences (SPSS) version 23.0.0 (IBM Corp, Armonk, NY, USA) was used. The significance level was set at p < 0.05 for all tests.
Results
Cohort characteristics
In total, seventeen patients were included for pathology NGS analysis. Clinical characteristics are summarized in Table 2. In most patients, the onset of thyroid carcinoma was detected later than the onset of the pituitary adenoma (median 51.5 years (IQR 48.3–66.3) versus 57.0 years (44.0–69.0)). Thyroid carcinoma was diagnosed before the pituitary adenoma in five cases, from 1 to 18 years before their pituitary adenoma had been diagnosed. Classical-variant PTC was reported in most patients (n = 9), following by FTC (n = 5) and FVPTC (n = 3). Thyroid carcinoma metastasis was found in five patients (29.4%); three had locoregional lymph node metastases, one had skeletal metastases, and the other had lung metastases.
Table 2.
Clinical characteristics of patients included in the study
| Characteristics | Value | |
|---|---|---|
| Patients from PALGA search | n = 14 | |
| Type of sample available | Pituitary tumor DNA, n = 13 (92.9%) | |
| Thyroid tumor DNA, n = 11 (78.6%) | ||
| Patients with known AIP germline variants | n = 3 | |
| Type of sample available | Pituitary tumor DNA, n = 2 (66.7%) | |
| Thyroid tumor DNA, n = 3 (100.0%) | ||
| Sex | Female/male: n = 15 (88.2%)/2 (11.8%) | |
| Age at onset pituitary adenoma (yrs) | Median, 51.5 (IQR 48.3–66.3) | |
| Age at onset thyroid carcinoma (yrs) | Median, 57.0 (IQR 44.0–69.0) | |
| No. and type of pituitary adenoma from available samples | Single, n = 12 (80.0%) | Multiple, n = 3 (20.0%) |
| Nonfunctioning, n = 5 (33.3%) | FSH + LH, n = 1 (6.7%) | |
| ACTH, n = 2 (15.0%) | FSH + TSH, n = 1 (6.7%) | |
| GH, n = 2 (15.0%) | GH + PRL, n = 1 (6.7%) | |
| LH, n = 1 (6.7%) | ||
| PRL, n = 1 (6.7%) | ||
| Unknown, n = 1 (6.7%) | ||
| No. and type of thyroid carcinoma from available samples | Single, n = 14 (100.0%) | |
| PTC, n = 7 (50.0%) | ||
| FTC, n = 4 (28.6%) | ||
| FVPTC, n = 3 (21.4%) | ||
| Metastasis | n = 5 (29.4%) | |
ACTH adrenocorticotropic hormone, FSH follicle-stimulating hormone, FTC follicular thyroid cancer, FVPTC follicular variant of papillary thyroid carcinoma, GH growth hormone, IQR interquartile range, LH luteinizing hormone, SS Sanger sequencing, TSH thyroid-stimulating hormone, PA pituitary adenoma, PRL prolactin, PTC papillary thyroid carcinoma, TC thyroid carcinoma, yrs years
Regarding the pituitary adenomas, no pituitary hormonal staining was reported in most patients (i.e., NFPAs; n = 5), while others stained positively for ACTH (n = 2), GH (n = 2), LH (n = 1), and PRL (n = 1). Combined expression was reported in three patients: GH and PRL, and FSH with either LH, or TSH. The staining data were not reported in two patients.
Genetic characterization
Detection of variants in sporadic patients
NGS analysis of the 14 patients from the PALGA search revealed no known mutations in targeted genes in pituitary tumor DNA and eight mutations in thyroid tumor DNA. Table 3 summarizes the identified mutations and CNVs (i.e., LOH) of chromosome 11 and 22. The 13 pituitary tumor samples showed no gene mutations. Among the 11 thyroid tumor samples, BRAF (5/11, 45.5%) was the gene most frequently mutated, followed by NRAS (3/11, 27.3%). These classical BRAF (p.V600E) point mutation were found in 57.1% (n = 4) of classical-variant PTC specimen and once (33.3%) in FVPTC specimen (Fig. 1a). NRAS codon 61 point mutation is the most common among RAS mutations and this was only observed in FTC specimen: p.Q61R twice (50.0%) (Fig. 1b) and p.Q61K once (25.0%).
Table 3.
Cluster of mutations and CNVs
Cases are categorized by pituitary adenoma and differentiated thyroid carcinoma
ACTH adrenocorticotropic hormone, FSH follicle-stimulating hormone, FTC follicular thyroid cancer, FVPTC follicular variant of papillary thyroid carcinoma, GH growth hormone, LH luteinizing hormone, LOH loss of heterozygosity, Non nonfunctioning pituitary adenomas, TSH thyroid-stimulating hormone, PA pituitary adenoma, PRL prolactin, PTC classical-variant papillary thyroid carcinoma, TC thyroid carcinoma, UK unknown, VUS variant of unknown significance, yrs years
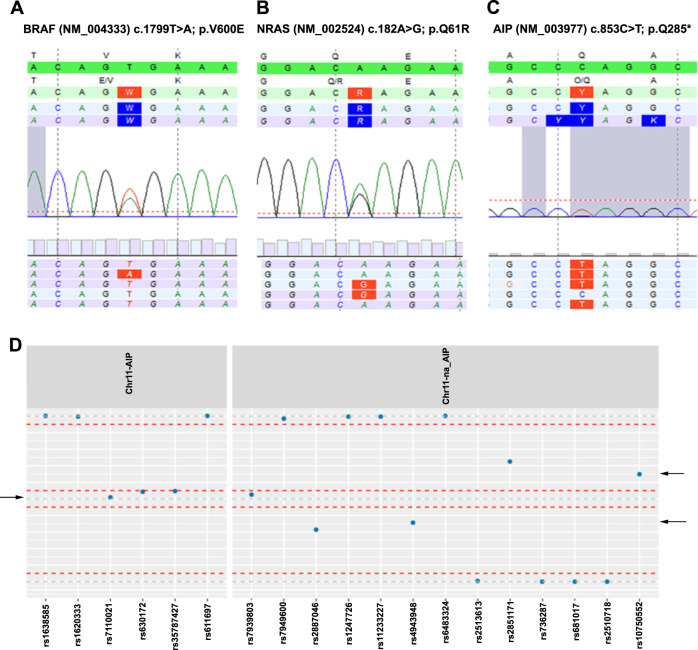
Fig. 1.
Direct sequencing of PCR antisense products in thyroid tumor samples obtained from 14 patients revealing the presence of (a corresponding with patient no. 2) the BRAF p.V600E variant in six patients, (b corresponding with patient no. 1) the NRAS p.Q61R variant in two patients, and (c corresponding with patient no. 17) the AIP p.Q285 variant in one patient. LOH of chromosome 11 was identified in two of the 15 pituitary tumor samples; one was a partial chromosome 11 LOH deletion. In thyroid tumor samples, in 2 of the 14 samples chromosome 11 was identified; one was a partial chromosome 11 LOH deletion. Chromosome 22 was identified in two of the 14 thyroid tumor samples. (d arrow: corresponding with patient no. 12) Demonstrates a representative example of LOH. LOH loss of heterozygosity
In addition, two VUSs were found in pituitary tumor DNA. These VUSs involved AIP-variant c.433C>T; p.145S (n = 1) and HRAS-variant c.505C>T; p.R169W (n = 1) (Table 3). Prediction software to determine pathogenicity predicted the AIP p.145S variant as benign (Align GVGD Class C0) to probably damaging (Polyphen-2 score of 0.978 (sensitivity: 0.76; specificity: 0.96)). The variant was never detected in the healthy population (gnomAD), nor is it found in large series of different tumor types from the cBioportal (n = 10,967 tumor samples) and Cosmic (n = 92,857 tumor samples) databases. Therefore, we considered AIP p.145S as a VUS. The prediction software predicted HRAS p.R169W as probably damaging (GVGD Class C15 and a Polyphen-2 score of 0.988 (sensitivity: 0.73; specificity: 0.96)). However, the variant also appeared in the European and American population with an allele frequency of 0.01% (rs151229168; gnomAD). In addition, a TCGA PanCancer Atlas Studies search using the cBioportal database did not report the HRAS p.R169W variant in the 10,967 tumor samples. Furthermore, the variant is also not reported by the Cosmic database in all tumor types, including thyroid tumors (cBioportal 500 and Cosmic 9985 thyroid samples). So, although the prediction software indicates the HRAS variant as probably damaging, we consider HRAS p.R169W as a VUS.
LOH of chromosome 11 was identified in two of 13 pituitary tumor samples (15.4%), both in 11q13; one had a partial chromosome 11 LOH deletion (Table 3). A representative example of LOH is demonstrated in Fig. 1d. No pituitary tumor samples showed LOH of chromosome 22. Out of the 11 patients with thyroid carcinomas, two patients had LOH of chromosome 22. No LOH of chromosome 11 was identified in the thyroid carcinomas.
Detection of variants in patients with known germline AIP variants
Genetic screening of germline DNA from patients 15, 16, and 17 revealed several AIP variants. Patient no. 15 had two AIP-variants: c.787 + 25 G>A; p.? and *60 G>C; p.?. Variant prediction software noted *60 G>C as probably benign, whereas c.787 + 25 G>A was noted in 2/4 prediction models to lead to a new splice acceptor site at c.787 + 27. In the second patient (patient no. 16), two AIP-variants were detected: c.682 C>A; p.Q288K, which is a known benign polymorphism, and c.920 A>G; p.Q307R; considered a benign variant. In patient no. 17, a pathological AIP-variant c.853 C>T; p.Q285* was identified.
NGS analysis of the three patients with known germline AIP variants revealed no known mutations in the pituitary tumor DNA, however, two mutations were identified in the thyroid tumor DNA. In patient no. 17, the AIP-variant c.853 C>T; p.Q285* was confirmed in FTC specimen (allele frequency 83%), while no mutations in other genes or translocations were observed (Fig. 1c). The BRAF (p.V600E) point mutation was found in patient no. 16. No pituitary tumor samples showed LOH of chromosome 11. LOH of chromosome 11 was identified in two (patient no. 16 and 17) of the three thyroid carcinomas (66.7%); one was a partial chromosome 11 deletion. No LOH of chromosome 22 was identified in both pituitary adenomas and thyroid carcinomas.
Discussion
To our knowledge, this is the first study to analyze the prevalence of AIP gene mutations and mutations in genes that have been associated with neuroendocrine tumors in series of tumors from patients presenting with both pituitary adenomas and DTCs. We showed that genetic alterations were observed in 71.4% (10/14) of DTCs and in 13.3% (2/15) of pituitary adenomas tissues, while there was no overlap between genetic alterations within tissues from the same patient. Among patients with pathological germline AIP variants, one AIP variant c.853 C>T; p.Q285* was confirmed in the FTC specimen (patient no. 17), including evidence of loss of the AIP wild-type allele, based on the relatively high allele frequency (83%) of the germline mutation in the tumor DNA. Unfortunately, we were unable to confirm this LOH based on the SNPs analysis, due to low quality of the FTC tissue. This patient came from an AIP-mutated FIPA kindred, however, her pituitary gland was unaffected. This supports that the finding of DTCs and pituitary adenomas are not totally fortuitous coexistence in an AIP mutation-positive FIPA kindred, thereby echoing a recent finding of FTC in an AIP mutation carrier by Daly et al. [28]. In a second patient with a somatotropinoma with two benign AIP-variants (p.Q288K and p.Q307R), a somatic BRAF (p.V600E) mutation was detected in PTC specimen in combination with a partial chromosome 11 LOH deletion. Although the partial chromosome 11 LOH deletion could indicate a second hit in the thyroid tissue, the observed LOH concerns SNPs located downstream (3′) of the AIP gene, while the SNPs located in the AIP gene did not indicate LOH.
It is noteworthy that although the most common mechanism to lose the wild-type copy of a tumor suppressor gene (e.g., AIP) in DTC specimen is a large deletion affecting the wild-type allele, other mechanisms could also play a role, such as another somatic mutation in other parts of the gene, or silencing of the wild-type copy with epigenetic mechanism-promoter methylation or microRNAs which are not covered by NGS. Moreover, we should emphasize that DTCs are more progressed in transformation since they are malignant when compared with pituitary adenomas. Therefore, it might be interesting to investigate the role of AIP mutation in thyroid adenomas (i.e., earlier in the transformation) in further studies.
In the total cohort, the most common oncotype in pituitary adenoma-related DTC was classical-variant PTC (9 out of 14 cases; see Table 2) with a high frequency (42.9%, 6/14) of BRAF (p.V600E) mutations, whereas none of these cases harbored NRAS mutations. These results confirm and build upon previous studies stating that among PTC, virtually all tumors that harbor a RAS mutation grow forming neoplastic follicles and no papillary structures and are, therefore, diagnosed as the FVPTC, while BRAF is the most frequent genetic alteration in classical-variant PTC [15, 16]. In line with this, the NRAS codon 61 point mutations were only observed in FTC specimen in three cases (21.4%) [37], which was the second most frequently mutated gene among the thyroid tumor samples. Although the limited number of DTCs in the present series prevents us from drawing any final conclusions on the prevalence of BRAF and NRAS mutations in DTCs in patients with versus those without pituitary adenomas, BRAF and NRAS seems the main genetic drivers of thyroid follicular epithelial cell transformation in our cases.
Our results are not in accordance with previous data, which suggested that BRAF mutation may not play a dominant role in development of DTC in patients with acromegaly [11, 38]. In these studies only one (9.1%) [11] or two (14.3%) [38] patients with concomitant PTC had the BRAF mutation, which in both studies was more frequently present in PTC patients without acromegaly. This discrepancy might be explained by [1] the inclusion of relative more FVPTC patients in the study from Aydin et al. [38] which is different to our cohort, or [2] our distinct study population, as included patients had not only of GH-producing adenomas but all five hormone-secreting cell types. In fact, previous studies [11, 29, 38] were carried out exclusively in acromegaly patients, while the patients we studied included only one patient with a GH-producing tumor. Therefore, direct comparison between our cohort and the acromegaly cohorts is limited.
In line with our findings, studying 12 DTC patients with acromegaly, Mian et al. reported that 70% of PTC patients with acromegaly were BRAF positive [29]. Moreover, AIP expression was similar between neoplastic and normal tissue, while the aryl-hydrocarbon receptor (AHR) was expressed more in PTCs carrying BRAF mutations than in normal tissue, irrespective of acromegaly status [29]. These data suggest that BRAF mutations and AHR overexpression may be associated with DTC risk in acromegaly, at least in patients with concomitant PTC.
Although there is no gender preponderance in pituitary adenoma patients, the vast majority of those with concomitant DTCs were female (15 out of 17) and is in accordance with previous literature, probably reflecting a trend seen in the general population. When comparing differences between patients with and without concomitant DTC, ist seems the former were relatively older. The mean age at onset and diagnosis of pituitary adenoma was mean 55 years [SD 12] in the cohort vs. mean 44 years [SD 17] in the general population, with the mean age at diagnosis in female patients being younger; 34 years [39]. The onset and diagnosis of DTC was median 57 years [IQR 44–69] in the cohort vs. 46 years [IQR 10–85] in the general population [40], with the median age at diagnosis in female patients being younger; 45 years [40]. In addition, in the two previously reported cases of acromegaly and concomitant PTC, and harboring a germline AIP variant, both patients were female and diagnosed with acromegaly at age 67 and 74, respectively.
This is in contrast to the clinical characteristics of patients bearing germline AIP mutations; the disease usually manifests in the second decade of life, almost all cases are diagnosed before the age of 30 years [28, 41–44] and they are predominantly males [45]. With this in mind, it should be stressed that after progress is made in the treatment of pituitary adenomas and its complications, these patients may live long enough to reach the age of increased cancer risk.
Strength of our study lies in the relatively large number of patients in which the pituitary adenoma and concomitant DTC tumor tissue were systematically investigated by targeted NGS. The main limitations of our study lie in the retrospective collection of tumor samples, and we had to exclude several tissues due to low quality. Another limitation is the lack of clinical data from the patients, including follow-up and family history data. Therefore, it should be stressed that we cannot rule out if patients from the PALGA search had additional risk factors for DTCs (e.g., received radiotherapy). At last, we should be borne in mind that the increased number of the diagnoseis of thyroid cancer in these patients could be due to the fact that they are examined more accurately and more frequently than before (i.e., surveillance bias).
In conclusion, the absence of somatic AIP mutations observed in patients with pituitary adenomas and concomitant DTCs suggest that their contribution to tumoral pathogenesis is probably limited and seems unlikely the genetic cause predisposing to the higher DTC risk observed in these patients. Though the finding of DTCs and pituitary adenomas could represent a new variant of MEN syndrome with a de novo germline mutation in a not yet identified gene, we suggest that this may be a fortuitous coexistence based on our observed variants that were similar to those of sporadic DTCs. In view of this and in line with the clinical practice guidelines from Katznelson et al. [46], we recommend including regularly thyroid examination and thyroid ultrasound only if there is a palpable thyroid nodularity. While the finding of the AIP-variant and LOH at this locus in FTC specimen in one AIP mutation-positive case, opens up a potential role for AIP mutation as an initiating event, further studies of AIP genetic status among DTCs in FIPA kindred cohorts are warranted to answer this question.
Supplementary information
Acknowledgements
We are indebted to the many pathologists, scientists, and other collaborators who have contributed to developing and maintaining PALGA. In addition, we acknowledge the neurosurgeons (Alof H.G. Dallenga and Ian K. Haitsma) and L. Oudijk (L.O.) and F.H. Groenendijk (F.G.) who performed histological evaluation of the tissues. All contributed to the study.
Funding
Fonds d’Investissement pour la Recherche Scientifique of the CHU de Liège (to A.B.) This work did not receive any other specific grant from any funding agency in the public, commercial, or non-profit sector.
Author contributions
Design of the study, and acquisition, analysis, and interpretation of data for the work: E.C.C. and E.K. Targeted NGS and data analysis: M.H and E.K. Drafting the work: E.C.C. and E.K. Critical review of drafts for important intellectual content: A.M., A.F.D., W.W.H, F.J.K., A.B., A.J.L., S.J.C.M.M.N.
Compliance with ethical standards
Conflict of interest
E.C.C, F.J.K., M.H., and E.K. have nothing to disclose. A.M. has received a speaker fee from Novartis. A.F.D. has received grants and/or speakers fees from Pfizer and Ipsen. W.W.H. has received travel or speaker fees from Novartis and Ipsen, and research funds from Ipsen. A.J.L. is a consultant for Pfizer, and has received speaker fees from Novartis, Ipsen, and Pfizer. A.B. is a consultant for Ipsen Pharma and received research funding from Ipsen Pharma, Novartis, and Pfizer. S.J.C.M.M.N. received research and speakers’ fee grants from Ipsen Pharma International, Novartis Pharma, Pfizer International and consulting fee from Ipsen Pharma International.
Ethical approval
Approval from the Medical Ethical Committee of the Erasmus University Medical Center and informed consent to use the tumor tissues for research purposes were obtained. Tumor tissues from all Dutch medical centers were used according to the code of conduct, Proper Secondary Use of Human Tissue, established by the Dutch Federation of Medical Scientific Societies (30).
Informed consent
All patients provided informed written consent for genetic testing.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: E. Korpershoek, S. J. C. M. M. Neggers
Supplementary information
The online version of this article (10.1007/s12020-020-02303-7) contains supplementary material, which is available to authorized users.
References
- 1.Caimari F, Korbonits M. Novel genetic causes of pituitary adenomas. Clin. Cancer Res. 2016;22(20):5030–42. doi: 10.1158/1078-0432.CCR-16-0452. [DOI] [PubMed] [Google Scholar]
- 2.Vandeva S, Daly AF, Petrossians P, Zacharieva S, Beckers A. Genetics in endocrinology: somatic and germline mutations in the pathogenesis of pituitary adenomas. Eur. J. Endocrinol. 2019;181(6):R235–R254. doi: 10.1530/EJE-19-0602. [DOI] [PubMed] [Google Scholar]
- 3.Aflorei ED, Korbonits M. Epidemiology and etiopathogenesis of pituitary adenomas. J. Neurooncol. 2014;117(3):379–94. doi: 10.1007/s11060-013-1354-5. [DOI] [PubMed] [Google Scholar]
- 4.Beckers A, Aaltonen LA, Daly AF, Karhu A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocr. Rev. 2013;34(2):239–77. doi: 10.1210/er.2012-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312(5777):1228–30. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]
- 6.Rostomyan L, Daly AF, Petrossians P, Nachev E, Lila AR, Lecoq AL, et al. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr. Relat. Cancer. 2015;22(5):745–57. doi: 10.1530/ERC-15-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trivellin G, Korbonits M. AIP and its interacting partners. J. Endocrinol. 2011;210(2):137–55. doi: 10.1530/JOE-11-0054. [DOI] [PubMed] [Google Scholar]
- 8.Gasperi M, Martino E, Manetti L, Arosio M, Porretti S, Faglia G, et al. Prevalence of thyroid diseases in patients with acromegaly: results of an Italian multi-center study. J. Endocrinol. Investig. 2002;25(3):240–5. doi: 10.1007/BF03343997. [DOI] [PubMed] [Google Scholar]
- 9.Gullu BE, Celik O, Gazioglu N, Kadioglu P. Thyroid cancer is the most common cancer associated with acromegaly. Pituitary. 2010;13(3):242–8. doi: 10.1007/s11102-010-0224-9. [DOI] [PubMed] [Google Scholar]
- 10.Dagdelen S, Cinar N, Erbas T. Increased thyroid cancer risk in acromegaly. Pituitary. 2014;17(4):299–306. doi: 10.1007/s11102-013-0501-5. [DOI] [PubMed] [Google Scholar]
- 11.Kim HK, Lee JS, Park MH, Cho JS, Yoon JH, Kim SJ, et al. Tumorigenesis of papillary thyroid cancer is not BRAF-dependent in patients with acromegaly. PLoS ONE. 2014;9(10):e110241-e. doi: 10.1371/journal.pone.0110241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dal J, Leisner MZ, Hermansen K, Farkas DK, Bengtsen M, Kistorp C, et al. Cancer incidence in patients with acromegaly: a cohort study and meta-analysis of the literature. J. Clin. Endocrinol. Metab. 2018;103(6):2182–8. doi: 10.1210/jc.2017-02457. [DOI] [PubMed] [Google Scholar]
- 13.Lai NB, Garg D, Heaney AP, Bergsneider M, Leung AM. No Benefit of Dedicated Thyroid Nodule Screening in Patients with Acromegaly. Endocr. Pract. 2020;26(1):16–21. doi: 10.4158/EP-2019-0254. [DOI] [PubMed] [Google Scholar]
- 14.Onoda N, Ohmura E, Tsushima T, Ohba Y, Emoto N, Isozaki O, et al. Autocrine role of insulin-like growth factor (IGF)-I in a human thyroid cancer cell line. Eur. J. Cancer. 1992;28A(11):1904–9. doi: 10.1016/0959-8049(92)90033-x. [DOI] [PubMed] [Google Scholar]
- 15.Fagin JA, Wells SA., Jr. Biologic and clinical perspectives on thyroid cancer. N. Engl. J. Med. 2016;375(11):1054–67. doi: 10.1056/NEJMra1501993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011;7(10):569–80. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 17.Roque L, Rodrigues R, Pinto A, Moura-Nunes V, Soares J. Chromosome imbalances in thyroid follicular neoplasms: a comparison between follicular adenomas and carcinomas. Genes Chromosomes Cancer. 2003;36(3):292–302. doi: 10.1002/gcc.10146. [DOI] [PubMed] [Google Scholar]
- 18.Hemmer S, Wasenius VM, Knuutila S, Franssila K, Joensuu H. DNA copy number changes in thyroid carcinoma. Am. J. Pathol. 1999;154(5):1539–47. doi: 10.1016/S0002-9440(10)65407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung S-H, Kim MS, Jung CK, Park H-C, Kim SY, Liu J, et al. Mutational burdens and evolutionary ages of thyroid follicular adenoma are comparable to those of follicular carcinoma. Oncotarget. 2016;7(43):69638–48. doi: 10.18632/oncotarget.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cañibano C, Rodriguez NL, Saez C, Tovar S, Garcia-Lavandeira M, Borrello MG, et al. The dependence receptor Ret induces apoptosis in somatotrophs through a Pit-1/p53 pathway, preventing tumor growth. EMBO J. 2007;26(8):2015–28. doi: 10.1038/sj.emboj.7601636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozfirat Z, Korbonits M. AIP gene and familial isolated pituitary adenomas. Mol. Cell. Endocrinol. 2010;326(1-2):71–9. doi: 10.1016/j.mce.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Vargiolu M, Fusco D, Kurelac I, Dirnberger D, Baumeister R, Morra I, et al. The tyrosine kinase receptor RET interacts in vivo with aryl hydrocarbon receptor-interacting protein to alter survivin availability. J. Clin. Endocrinol. Metab. 2009;94(7):2571–8. doi: 10.1210/jc.2008-1980. [DOI] [PubMed] [Google Scholar]
- 23.de Oliveira SK, Hoffmeister M, Gambaryan S, Muller-Esterl W, Guimaraes JA, Smolenski AP. Phosphodiesterase 2A forms a complex with the co-chaperone XAP2 and regulates nuclear translocation of the aryl hydrocarbon receptor. J. Biol. Chem. 2007;282(18):13656–63. doi: 10.1074/jbc.M610942200. [DOI] [PubMed] [Google Scholar]
- 24.Kang BH, Altieri DC. Regulation of survivin stability by the aryl hydrocarbon receptor-interacting protein. J. Biol. Chem. 2006;281(34):24721–7. doi: 10.1074/jbc.M603175200. [DOI] [PubMed] [Google Scholar]
- 25.Urbani C, Russo D, Raggi F, Lombardi M, Sardella C, Scattina I, et al. A novel germline mutation in the aryl hydrocarbon receptor-interacting protein (Aip) gene in an Italian family with gigantism. J. Endocrinol. Investig. 2014;37(10):949–55. doi: 10.1007/s40618-014-0123-4. [DOI] [PubMed] [Google Scholar]
- 26.Occhi G, Trivellin G, Ceccato F, De Lazzari P, Giorgi G, Dematte S, et al. Prevalence of AIP mutations in a large series of sporadic Italian acromegalic patients and evaluation of CDKN1B status in acromegalic patients with multiple endocrine neoplasia. Eur. J. Endocrinol. 2010;163(3):369–76. doi: 10.1530/EJE-10-0327. [DOI] [PubMed] [Google Scholar]
- 27.Nord B, Larsson C, Wong FK, Wallin G, Teh BT, Zedenius J. Sporadic follicular thyroid tumors show loss of a 200-kb region in 11q13 without evidence for mutations in the MEN1 gene. Genes Chromosomes Cancer. 1999;26(1):35–9. doi: 10.1002/(sici)1098-2264(199909)26:1<35::aid-gcc5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28.Daly A, Rostomyan L, Betea D, Bonneville JF, Villa C, Pellegata NS, et al. AIP-mutated acromegaly resistant to first-generation somatostatin analogs: long-term control with pasireotide LAR in two patients. Endocr. Connect. 2019;8(4):367–77. doi: 10.1530/EC-19-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mian C, Ceccato F, Barollo S, Watutantrige-Fernando S, Albiger N, Regazzo D, et al. AHR over-expression in papillary thyroid carcinoma: clinical and molecular assessments in a series of Italian acromegalic patients with a long-term follow-up. PLoS ONE. 2014;9(7):e101560. doi: 10.1371/journal.pone.0101560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermeulen E, Geesink I, Schmidt MK, Steegers C, Verhue D, Brom FW, et al. Secondary use of human tissue: consent and better information required. Ned. Tijdschr. Geneeskd. 2009;153:A948. [PubMed] [Google Scholar]
- 31.Barlier A, Vanbellinghen JF, Daly AF, Silvy M, Jaffrain-Rea ML, Trouillas J, et al. Mutations in the aryl hydrocarbon receptor interacting protein gene are not highly prevalent among subjects with sporadic pituitary adenomas. J. Clin. Endocrinol. Metab. 2007;92(5):1952–5. doi: 10.1210/jc.2006-2702. [DOI] [PubMed] [Google Scholar]
- 32.Georgitsi M, Heliovaara E, Paschke R, Kumar AV, Tischkowitz M, Vierimaa O, et al. Large genomic deletions in AIP in pituitary adenoma predisposition. J. Clin. Endocrinol. Metab. 2008;93(10):4146–51. doi: 10.1210/jc.2008-1003. [DOI] [PubMed] [Google Scholar]
- 33.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.W.R. Geurts-Giele, E.H. Rosenberg, Av. Rens, M.Ev. Leerdam, W.N. Dinjens, F.E. Bleeker, Somatic mosaicism by a de novo MLH1 mutation as a cause of Lynch syndrome. Mol. Genet. Genom. Med. 7(7), e00699 (2019) [DOI] [PMC free article] [PubMed]
- 35.van Riet J, Krol NMG, Atmodimedjo PN, Brosens E, van IWFJ, Jansen M, et al. SNPitty: an intuitive web application for interactive B-allele frequency and copy number visualization of next-generation sequencing data. J. Mol. Diagn. 2018;20(2):166–76. doi: 10.1016/j.jmoldx.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Giordano TJ. Genomic hallmarks of thyroid neoplasia. Annu. Rev. Pathol. Mech. Dis. 2018;13(1):141–62. doi: 10.1146/annurev-pathol-121808-102139. [DOI] [PubMed] [Google Scholar]
- 37.Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW, II, Tallini G, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J. Clin. Endocrinol. Metab. 2003;88(5):2318–26. doi: 10.1210/jc.2002-021907. [DOI] [PubMed] [Google Scholar]
- 38.Aydin K, Aydin C, Dagdelen S, Tezel GG, Erbas T. Genetic alterations in differentiated thyroid cancer patients with acromegaly. Exp. Clin. Endocrinol. Diabetes. 2016;124(3):198–202. doi: 10.1055/s-0035-1565061. [DOI] [PubMed] [Google Scholar]
- 39.Day PF, Loto MG, Glerean M, Picasso MFR, Lovazzano S, Giunta DH. Incidence and prevalence of clinically relevant pituitary adenomas: retrospective cohort study in a Health Management Organization in Buenos Aires, Argentina. Arch. Endocrinol. Metab. 2016;60:554–61. doi: 10.1590/2359-3997000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sciuto R, Romano L, Rea S, Marandino F, Sperduti I, Maini CL. Natural history and clinical outcome of differentiated thyroid carcinoma: a retrospective analysis of 1503 patients treated at a single institution. Ann. Oncol. 2009;20(10):1728–35. doi: 10.1093/annonc/mdp050. [DOI] [PubMed] [Google Scholar]
- 41.Daly AF, Tichomirowa MA, Petrossians P, Heliovaara E, Jaffrain-Rea ML, Barlier A, et al. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J. Clin. Endocrinol. Metab. 2010;95(11):E373–83. doi: 10.1210/jc.2009-2556. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez-Ramirez LC, Gabrovska P, Denes J, Stals K, Trivellin G, Tilley D, et al. Landscape of familial isolated and young-onset pituitary adenomas: prospective diagnosis in AIP mutation carriers. J. Clin. Endocrinol. Metab. 2015;100(9):E1242–54. doi: 10.1210/jc.2015-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams F, Hunter S, Bradley L, Chahal HS, Storr HL, Akker SA, et al. Clinical experience in the screening and management of a large kindred with familial isolated pituitary adenoma due to an aryl hydrocarbon receptor interacting protein (AIP) mutation. J. Clin. Endocrinol. Metab. 2014;99(4):1122–31. doi: 10.1210/jc.2013-2868. [DOI] [PubMed] [Google Scholar]
- 44.Korbonits M, Storr H, Kumar AV. Familial pituitary adenomas—who should be tested for AIP mutations? Clin. Endocrinol. (Oxf.) 2012;77(3):351–6. doi: 10.1111/j.1365-2265.2012.04445.x. [DOI] [PubMed] [Google Scholar]
- 45.Cazabat L, Bouligand J, Salenave S, Bernier M, Gaillard S, Parker F, et al. Germline AIP mutations in apparently sporadic pituitary adenomas: prevalence in a prospective single-center cohort of 443. J. Clin. Endocrinol. Metab. 2012;97(4):E663–E70. doi: 10.1210/jc.2011-2291. [DOI] [PubMed] [Google Scholar]
- 46.Katznelson L, Laws ER, Melmed S, Molitch ME, Murad MH, Utz A, et al. Acromegaly: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocr. Metab. 2014;99(11):3933–51. doi: 10.1210/jc.2014-2700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.