Abstract
Brain GABAΑ receptors are ionotropic receptors belonging to the class of Cys-loop receptors and are important drug targets for the treatment of anxiety and sleep disorders. By screening a compound library (2,112 compounds) at recombinant human α4β1δ GABAΑ receptors heterologously expressed in a HEK cell line, we identified a scaffold of spirocyclic compounds with nanomolar antagonist activity at GABAΑ receptors. The initial screening hit 2027 (IC50 of 1.03 μM) was used for analogue search resulting in 018 (IC50 of 0.088 μM). 018 was most potent at α3,4,5-subunit containing receptors, thus showing preference for forebrain-expressed extrasynaptic receptors. Schild analysis of 018 at recombinant human α4β1δ receptors and displacement of [3H]muscimol binding in rat cortical homogenate independently confirmed a competitive profile. The antagonist profile of 018 was further validated by whole-cell patch-clamp electrophysiology, where kinetic studies revealed a slow dissociation rate and a shallow hill slope was observed. Membrane permeability studies showed that 2027 and 018 do not cross membranes, thus making the compounds less attractive for studying central GABAΑ receptors effects, but conversely more attractive as tool compounds in relation to emerging peripheral GABAΑ receptor-mediated effects of GABA e.g. in the immune system.
Subject terms: Receptor pharmacology, Ion channels in the nervous system, Drug screening
Introduction
GABAΑ receptors (GABAΑRs) are ligand-gated chloride channels belonging to the Cys-loop receptor family responsible for mediating the majority of inhibition in the CNS. The receptors are assembled from 19 different subunits (α1-6, β1-3, γ1-3, ρ1-3, ε, π and θ)1 with distinct combinations depending on regional brain distribution, cell type and subcellular localization2. The predominant GABAΑR subtype in the forebrain is α1β2γ2 which is found at synaptic locations mediating fast synaptic inhibition2. This is in contrast to receptors containing the δ-subunit which are located at extrasynaptic sites and mediate tonic inhibition3. In forebrain regions such as thalamus and hippocampus, the δ-subunit predominantly partners with the α4-subunit, whereas in cerebellum it exclusively partners with the α6-subunit4,5, in all cases forming receptors primarily found at extrasynaptic sites. α5βγ receptors are also found at extrasynaptic sites6 and, similar to the δ-containing receptors, they generally show a high affinity for GABA (activated by GABA spill-over from the synapse) and slow desensitization compared to synaptic receptors3,7.
GABAΑRs are well established drug targets, with a number of approved drugs including the anxiolytic and sleep-inducing benzodiazepines, e.g. diazepam, and anaesthetics, e.g. propofol. Over the last decades, extrasynaptic GABAΑRs have been the focus of many studies as they are proposed to be involved in a number of neurological disorders including epilepsy, sleep disorders, depression and stroke where changes in the tonic inhibition seem to be part of the pathology8–11. α4βδ receptors have also been found to be important for synaptic pruning as the expression of these receptors are increased in hippocampal CA1 neurons during puberty12. An increase in the expression of αβδ receptors, and hence higher levels of tonic inhibition in hippocampal CA1, has been linked to impaired synaptic plasticity, thus indicating that these receptors also play an important role under normal physiological conditions13. The α5βγ2 receptor subtype has also been found to be important for cognition and learning14. Altogether this posits compounds that selectively target extrasynaptic GABAΑRs as interesting tool compounds and potential drug candidates.
Nonetheless, only a limited number of extrasynaptic-preferring compounds have been identified. The most renowned compound is the orthosteric agonist gaboxadol/THIP, a super-agonist at δ-containing receptors15, which was in clinical trials for the treatment of primary insomnia, but failed16. Potentially, gaboxadol is having a revival as it is currently in clinical trials for the rare disorder Angelman syndrome (trial number NCT02996305) and Fragile X (trial number NCT03697161) (as Ov101)17, underscoring the continued interest in targeting δ-containing receptors and tonic inhibitory current levels. In addition to the δ-preferring agonist, a number of positive allosteric modulators (PAMs) have been reported. These include delta selective compound 2 (DS2)18, AA-2950419, the flavonoid 2’MeO6MF20,21, and neurosteroid analogues22,23, however none of these discriminate between α4βδ and α6βδ receptors. Interestingly, no truly selective antagonists for δ-containing receptors have been identified although the competitive antagonist DPP-4-PIOL was found to be approximately 20 times more potent in inhibiting tonic over phasic currents (IC50 of 0.87 nM) in rat slice recordings from dentate gyrus granule cells24. As a whole, inhibitors of extrasynaptic GABAΑRs would constitute highly useful pharmacological tool compounds, and potentially be of therapeutic relevance in e.g. functional recovery after stroke and certain types of absence epilepsies10,25.
Here we used our recently implemented cell-based assay, measuring changes in membrane potential by fluorescence26, to search for novel α4βδ receptor antagonists using α4β1δ GABAΑRs as a model receptor to screen an in-house assembled small-molecule compound library, and report the identification of a novel class of antagonists with a clear competitive profile and preference for α3,4,5-containing extrasynaptic GABAΑRs with low to mid nanomolar potency.
Results
Validation of FMP assay for compound screening
We have previously established a FLIPR membrane potential (FMP) assay to study recombinant δ-containing GABAΑRs entailing a HEK293 Flp-In cell line stably expressing the human δ-subunit which is suitable for co-transfection with any desired α/β subunits26. Initially, we validated the applicability of the FMP assay for efficient compound screening in 96-well microtiter plates using the human α4β1δ GABAΑR heterologously expressed in HEK cells. This receptor was chosen as we have previously shown this system to be reliable in expressing δ-containing receptors26. To this end, we determined the Z′-factor, which is a statistical factor used to measure the suitability of an assay for high throughput screening27. An assay is regarded an excellent assay when the value of the Z′-factor is in the range of 0.5 ≤ Z′ < 1, as this indicates a large separation between positive and negative controls27. The Z′-factor was determined to 0.68 (three independent experiments with 48 positive and negative control wells distributed evenly across the ligand plate), thus categorizing the FMP assay on α4β1δ receptors as an excellent assay for screening with high sensitivity and reproducibility.
Primary screening using the FMP assay
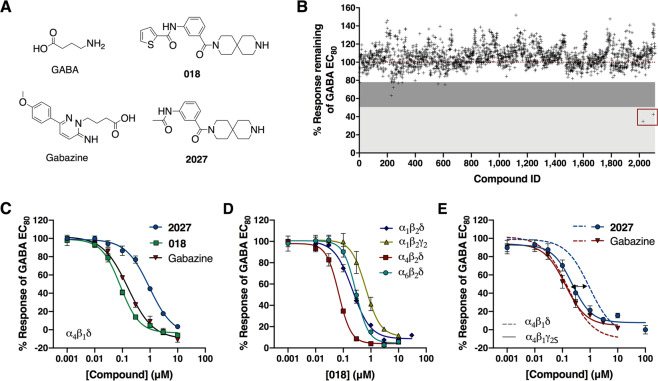
The compound library was screened for antagonist activity at α4β1δ receptors in the FMP assay in a single concentration of 10 μM in singlicates by applying the compounds together with a concentration of GABA corresponding to GABA EC80 (Table 1). In the primary screening, 10 compounds inhibited the GABA EC80 signal by more than 22%, but only two compounds, compound 2027 (N-(3-(3,9-diazaspiro[5.5]undecane-3-carbonyl)phenyl)acetamide) and 2100 (1-(furan-2-ylmethyl)-N-(2-methoxyphenyl)piperidine-4-carboxamide), were able to inhibit the signal by more than 50% (Fig. 1A,B). In the following hit validation, the same 10 compounds were tested in concentrations of 100, 50 and 10 μM in triplicates, in which only 2027 showed antagonistic effect, prominent already at a concentration of 10 μM (See Supplementary Fig. S1). Thus, we went on to further characterize 2027 by making a full concentration-inhibition curve. 2027 was able to completely inhibit the GABA EC80 -induced response in a concentration-dependent manner at α4β1δ receptors with an IC50 value of 1.03 μM (Fig. 1C, Table 1). Gabazine was included as reference compound having an IC50 value of 0.24 μM (6.61 ± 0.058), (n = 3) at α4β1δ receptors (Fig. 1A,C).
Table 1.
Antagonist activity of 2027 and 018 plus GABA agonist potencies at selected GABAΑ receptor subtypes determined in the FMP assay.
| Receptor | 2027 (IC50 (μM), pIC50 ± SEM, n) | 018 (IC50 (μM), pIC50 ± SEM, n) | GABA (EC50 (μM), pEC50 ± SEM, n) | GABA EC80, μM | |||
|---|---|---|---|---|---|---|---|
| α1β2δ | 6.68 | (5.17 ± 0.10, 3) | 0.24 | (6.61 ± 0.050, 4) | 6.71 | (5.17 ± 0.087, 4)a | 10–14 |
| α1β2γ2 | 4.96 | (5.30 ± 0.17, 4) | 0.79 | (6.10 ± 0.11, 4) | 1.71 | (5.77 ± 0.022, 3) | 8.0 |
| α2β2γ2 | 2.96 | (5.53 ± 0.19, 3) | 0.32 | (6.49 ± 0.13, 3) | 1.47 | (5.83 ± 0.045, 3) | 10 |
| α3β2γ2 | 0.29 | (6.54 ± 0.17, 3) | 0.079 | (7.10 ± 0.18, 3) | 2.21 | (5.63 ± 0.11, 3) | 10 |
| α4β1δ | 1.03 | (5.99 ± 0.028, 3) | 0.088 | (7.06 ± 0.11, 4) | 0.17 | (6.77 ± 0.11, 3) | 0.27–1.0 |
| α4β1γ2 | 0.17 | (6.78 ± 0.081, 3) | 0.037 | (7.43 ± 0.094, 3) | 1.40 | (5.86 ± 0.014, 3) | 4.0–8.0 |
| α4β2δ | 0.36 | (6.44 ± 0.12, 3) | 0.068 | (7.17 ± 0.080, 3) | 0.25 | (6.61 ± 0.030, 3)a | 0.80–1.0 |
| α5β2γ2 | 0.59 | (6.23 ± 0.19, 4) | 0.051 | (7.29 ± 0.19, 4) | 0.47 | (6.33 ± 0.20, 3) | 3.0 |
| α6β2δ | 4.13 | (5.38 ± 0.050, 3) | 0.33 | (6.48 ± 0.082, 3) | 0.21 | (6.68 ± 0.13, 3)a | 3.0 |
The antagonist activity was determined using the stated GABA EC80 concentration calculated from the determined GABA potencies.
IC50/EC50 values are determined from concentration response curves and given as means. aData from L’Estrade et al.49.
Figure 1.
The identified small-molecule ligands 2027 and 018 are antagonists at GABAΑRs. (A) Chemical structures of 018, 2027, GABA and Gabazine. (B) Results of primary screening of the compound library at α4β1δ receptors in antagonist mode in the FMP assay (96-well format). Data shown are normalized to GABA EC80 of compounds tested in 10 μM in singlicates. (C) Concentration-response curves of 2027 and 018 at α4β1δ receptors, with gabazine shown as reference. (D) Concentration-response curves of 018 at selected GABAΑ receptor subtypes showing α-subunit dependent inhibition of the GABA EC80 signal. (E) Concentration-response curves of 2027 and gabazine at α4β1δ and α4β1γ2 showing a subtype-dependent right shift of the curve only for 2027. Data are shown as representative curves obtained in the FMP assay with three technical replicates (means ± SD). Collected IC50 values ± SEM and GABA EC80 values are given in Table 1.
Identification of the hit analogue 018 displaying increased potency
Next, we identified 52 structural analogues of compound 2027 by substructure searches in a database of commercially available compounds, which were tested for antagonist activity in the FMP assay in concentrations of 5 μM and 0.5 μM (For structures and data see Supplementary Figs. S2 and S3). Of the 52 analogues, 20 showed antagonist activity at 5 μM, and of these, 14 were found to be equipotent or more potent than compound 2027. Of the 20 active analogues, 018 (N-(3-(3,9-diazaspiro[5.5]undecane-3-carbonyl)phenyl)thiophene-2-carboxamide) was identified as the most potent compound with an IC50 value of 0.09 μM at α4β1δ, thus being approximately 10 times more potent than 2027 (Fig. 1C, Table 1). Interestingly, all identified active compounds were found to have a common 3,9-diazaspiro[5.5]undecane moiety (Fig. 1A). Thus, based on this common structural motif, additional 44 analogues were purchased, but none of these showed to be more potent than 018 (Data not shown). We thus decided to use 018 alongside 2027 as model compounds to look further into the pharmacological properties of this new class of GABAΑR antagonists.
Compound 018 shows preference for extrasynaptic GABAΑ receptor subtypes
To assess if the primary hit 2027 and the more potent analogue, 018 (Fig. 1A), displayed any subtype selectivity we wanted to determine the potency at receptor subtypes in which either α, β or γ/δ subunits were interchanged. We chose subtype combinations that were both informative and physiologically relevant. Thus α1−3, 5 were expressed together with the γ2 subunit and α4,6 with the δ-subunit1. Additionally, α1β2δ and α4β1γ2 receptors were included, as they are relevant for comparison and found to be expressed in hippocampus28,29. Further, we chose to use the β2-subunit for most receptors as it is convenient to express and because expression of β3 leads to homomers in our cell system26 (Table 1). First, we determined the GABA EC50 values at all the selected receptors in order to calculate the concentrations corresponding to GABA EC80. These values are stated in Table 1, and are overall similar to values determined by others at human subtypes expressed in HEK cells30, with GABA being more potent at the extrasynaptic and δ-containing receptors compared to γ-containing synaptic receptors. Next, we determined and compared the potencies of 2027 and 018 at the selected receptors. From these comparisons, we found that the observed potency depends most strongly on the specific α-subunit (Fig. 1D, Table 1). 018 was most potent at α3−5 containing receptors, with potencies in the mid nanomolar range (37–88 nM) whereas the potency at α1,2,6 receptors were in the high nanomolar range (240–790 nM). From this it follows that at a concentration of 10–100 nM, 018 would result in more than 50% inhibition of the α3–5 containing receptors but less than 20% inhibition at the α1,2,6 containing receptors. Comparing the potency at α4β2δ receptors (IC50 of 68 nM) and α1β2γ2 receptors (IC50 of 790 nM) showed 12 times increased potency of 018 at the α4- compared to the α1-containing receptors. The potency ranking based on α-subunit, α4 = α5 = α3 > α2 > α6 = α1, indicates a preference for the forebrain extrasynaptic GABAΑRs, often carrying α4 (Fig. 1D, Table 1). Overall, a similar trend in potency order was seen for 2027 albeit right-shifted 5–10 times, except for α1β2δ, where the potency was 28 times reduced for 2027 compared to 018 (Table 1). Finally, to assure that the observed subtype differences were not coincidental, we compared with data from the non-subtype selective antagonist, gabazine, tested at α4β1γ2 cf. α4b1δ subtypes. As shown in Fig. 1E, gabazine has a similar IC50 value at both subtypes (IC50 of 0.11 μM (6.94 ± 0.021), n = 4 and 0.24 μM, respectively), whereas 2027, by comparison, has a clear preference for α4β1δ.
Compound 018 is a competitive antagonist
To determine the type of antagonism displayed by 018, we performed a Gaddum/Schild analysis at α4β1δ receptors in the FMP assay. Thus, GABA concentration-response curves were generated in the absence or presence of increasing concentrations of 018 ranging from 0.03 μM to 3 μM. This resulted in a decrease in the potency of GABA with increasing concentrations of 018 (right-shift of curves) but without any changes in the slope and/or the efficacy of GABA at any of the tested concentrations of 018 (Fig. 2A). The Schild slope for 018 was determined to 1.03 [0.96–1.06], (n = 5) and was not significantly different from unity (P = 0.668), indicating that 018 is a competitive antagonist at α4β1δ receptors (Fig. 2B). Additionally, the KΒ for 018 was determined to 19 nM [0.0138; 0.0278] from the Schild analysis. To further support the finding that 018 is a competitive antagonist, its ability to inhibit radioligand binding of [3H]muscimol to rat brain cortical homogenate was tested by performing competition binding studies. These experiments showed that 018 and 2027 were able to fully inhibit the binding of [3H]muscimol with Ki values of 20 nM (7.71 ± 0.06), (n = 4) and 0.56 μM (6.25 ± 0.06), (n = 4), respectively (Fig. 2C). The determined KΒ and Ki values correspond very well supporting a competitive profile of the compounds.
Figure 2.
Compound 018 is a competitive antagonist at α4β1δ receptors and displaces [3H]muscimol binding. (A) Schild plot of 018 at α4β1δ receptors determined in the FMP assay showing a right shift of the GABA concentration-response curve with increasing concentrations of 018 without affecting efficacy. Shown are pooled data from five independent experiments with three technical replicates given as means ± SEM. (B) Schild plot of data displayed in (A) showing a Schild slope of 1.03 indicative of competitive antagonism. (C) Concentration-dependent inhibition of [3H]muscimol binding by 018 and 2027 in rat cortical homogenate, with GABA as reference, showing a competitive profile of the compounds. Data are given as means ± SEM from four independent experiments performed in technical triplicates. The Ki of GABA is 0.049 μM, data from Krall et al.48.
Validation using whole-cell patch-clamp electrophysiology
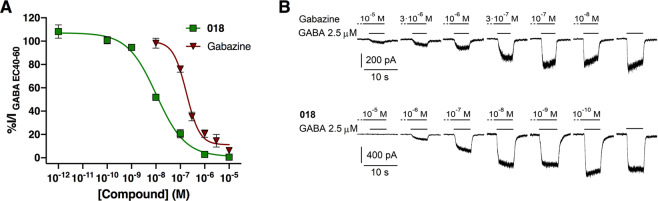
To validate the results from the FMP assay, compound 018 was tested in whole-cell patch-clamp electrophysiology at α4β1δ receptors conveniently expressed in the same δ-HEK cell line as used for the FMP assay. Again, 018 was found to be a full antagonist and the IC50 value was determined to 10.8 nM [6.27; 14.7], (n = 7), which is 9 times more potent than determined in the FMP assay. The Hill slope for 018 was found to be −0.74 [−0.95; −0.54] (Fig. 3), which is more shallow than what would be expected for a competitive GABAΑR antagonist31. To test if the shallow Hill slope was an effect of the compound or the assay, we tested the classical competitive antagonist gabazine using the same setup. The IC50 of gabazine was determined to 0.18 μM [0.11; 0.29], (n = 5) with a Hill slope of −1.54 [−2.2; −0.84] (Fig. 3). This is significantly steeper than that of 018 (**P = 0.0099, unpaired t-test), thus indicating that the shallow Hill slope of 018 is not a technical issue but an effect of the compound.
Figure 3.
Whole-cell patch-clamp electrophysiology studies of α4β1δ receptors support the antagonism determined in the FMP assay. (A) 018 concentration-dependently inhibits the current induced by 2.5 μM GABA (corresponding to GABA EC30-40) at α4β1δ receptors with an IC50 of 10.8 nM and a Hill slope of −0.74. Gabazine was included as reference with an IC50 of 0.18 μM and a Hill slope of −1.53. (B) Representative current traces for 018 and gabazine. The dotted line represents 30 sec pre-application of the antagonist. Data are shown as pooled data from 5–7 cells given as means ± SEM and normalized to the GABA 2.5 μM control.
Compound 018 displays slow dissociation kinetics
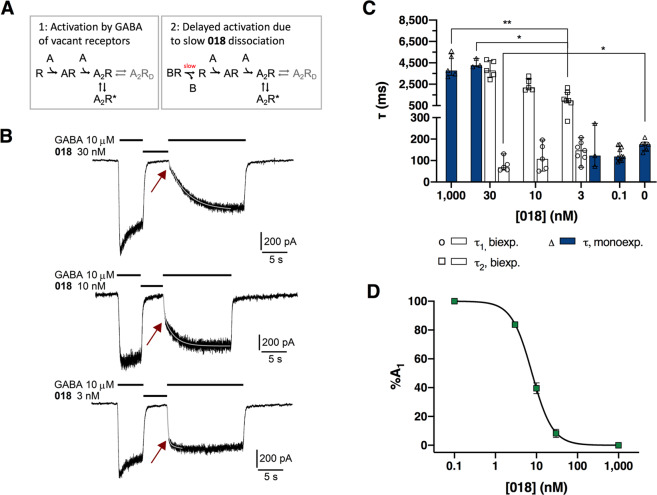
From the patch-clamp current traces of the concentration-response curve of 018 it was apparent that the presence of 018 concentration-dependently delayed the activation phase of GABA-activated currents (Fig. 3B). For concentrations of 018 close to its IC50 value, it was further apparent that the activation phase followed a biphasic time course. In order to obtain detailed information on the dissociation kinetics of 018 we decided to further investigate the time-course of GABA activation in the presence of 018. To this end, we tested concentrations of 018 ranging from 0.1 to 1000 nM pre-applied before application of 2.5 μM GABA, and determined the number of exponential components with their time constants and amplitudes that best fitted the time course of the activation phase (Fig. 4B+C, Table 2). The results show that the time course of the activation phase of the GABA currents could be described well using mono- or biexponential functions. Thus, it confirmed our initial observations, as the activation phase of the GABA current after preapplication of 3, 10 and 30 nM 018 could be fitted using biexponential functions, except for 6 of the tested cells that were fitted by mono-exponential functions (Fig. 4C, Table 2). At a concentration of 30 nM, four out of five tested cells could be fitted to both a mono- and a biexponential function, but statistical analysis showed that all were fitted best by the biexponential function (F-test, P < 0.001). The GABA activation phases with 0.1 nM and 1000 nM 018 could only be fitted using mono-exponential functions, as the slow and fast component, respectively, were virtually absent at these concentrations. The determined activation time constants are given in Table 2 and Fig. 4C. The time constant for the fast component, τ1, for 0.1–10 nM 018 was not significantly different from the single time constant, τ, for 2.5 μM GABA alone, but for 30 nM 018, the determined τ1 of 68.0 ms was significantly lower compared to GABA alone (P = 0.0068, Kruskal-Wallis ANOVA followed by Dunn’s multiple comparison). Comparing the time constants of the slow component, τ2, determined with the different concentrations of 018 showed that the time constant τ2 for 3 nM 018 was significantly lower than for 1000 nM and 30 nM (P = 0.0066 and P = 0.024 respectively, Kruskal-Wallis ANOVA followed by Dunn's multiple comparison). Additional experiments using 100 μΜ GABA showed that the determined slow time constants, τ2, were not significantly different from those using 2.5 μM GABA (Multiple t-test) (See Supplementary Fig. S4 and Table S1).
Figure 4.
Compound 018 displays slow dissociation kinetics. (A) Simple kinetic models for the activation by GABA of vacant receptors (Model 1) and receptors initially blocked by 018 (Model 2), respectively. When present, dissociation of 018 is the slowest (rate-limiting) step and delays the overall process of activation by GABA. Openings are assumed to occur only with two agonist molecules bound (A2R* state) and desensitization (A2RD, in grey) to be of minor importance during the activation phase. (B) Examples of current traces for 2.5 μM GABA before and after 5 sec application of 10 nM 018 showing slow dissociation kinetics of 018. A biexponential function (grey line) was fitted to the activation phase of the current traces, with the fast component interpreted as reflecting GABA activation of vacant receptors (Model 1), and the slow component reflecting receptors, where activation had to await dissociation of 018 (Model 2). The arrow indicates the break point where the fast component is essentially completed. (C) Time constants, τ, for currents induced by 2.5 μM with or without pre-application of 018. Constants determined by fitting to a monoexponential function are shown as blue bars (Δ) and biexponential fittings in white bars (☐,○). Data are shown as median ± interquartile range for 5–10 cells. Statistical analysis was performed using Kruskal-Wallis ANOVA follow by Dunn’s multiple comparison. τ1 is compared to 0 (2.5 μM GABA without pre-application of 018) and for τ2 all values are compared. (D) The contribution from the fast-rising amplitude %A1 concentration dependently decrease for increasing concentrations of 018 using 2.5 μM GABA, giving a functional KB of 7.80 nM. %A1 was determined from the fitting a to biexponential function using data displayed in (C). The fractional amplitude of the fast component (%A1) is a measure of the fraction of receptors that are vacant when GABA is applied. For values see Table 2. Additional data for similar experiment using 100 μM GABA is given in supplementary Fig. S4 and Table S1.
Table 2.
Summary of the time constant determined for 2.5 μM GABA with and without preincubation with 018 and calculated percentage contribution of the fast-rising current (%A1) relative to the total induced current.
| 018 (nM) | τ1 (ms) | τ2 (ms) | %A1 | n |
|---|---|---|---|---|
| 0 | 174 [148;186]a | — | — | 6 |
| 0.1 | 119 [101;167]a | — | — | 10 |
| 3 |
147 [115;183] 123 [73.3;273]a |
1090 [960;2180] — |
83.8 — |
7 3 |
| 10 | 107 [57.5;180] | 2170 [1970;3010] | 39.6 | 5 |
| 30 |
68.0 [56.3;106] — |
3780 [3160;4710] 4270 [4140;4920]a |
8.3 — |
5 3 |
| 1000 | — | 3780 [3330;5350]a | — | 5 |
If not otherwise stated, current traces were best fitted using a biexponential function. Data are given as medians followed by 25–75% quartiles in squared brackets. n denotes the number of tested cells. Statistics are shown and described in Fig. 4B. aCurrent traces were best fitted using a monoexponential function.
For those activation phases of the GABA current best fitted using a biexponential function, we determined the fraction of the total current amplitude contributed by the fast component (%A1) (Fig. 4D, Table 2). For 1000 nM and 0.1 nM, which could only be fitted using mono-exponential functions (slow and fast, respectively), the %A1 were set to 0% and 100%, respectively. This revealed a decrease in the relative contribution from the fast component with increasing antagonist concentrations (Fig. 4D). We interpret these observations based on the models presented in Fig. 4A, which are simple kinetic models for activation of receptors by GABA, and are based on models by Mortensen et al. and Keramidas & Harrison, for α4β3δ and α4β2δ receptors32,33, respectively, assuming that α4β1δ receptors display similar kinetics. Model 1 describes the activation of vacant receptors by GABA and Model 2 describes the situation when receptors are initially blocked by binding of 018. When receptors are bound to 018, the number of vacant receptors that GABA can activate will be reduced depending on the concentration and affinity of 018, thus delaying the overall process of activation, with the dissociation of 018 being the rate-limiting step. Assuming that the fast component of activation is due to GABA activating the population of initially vacant receptors, it follows that the decrease in the relative contribution of the fast component (%A1) with increasing antagonist concentrations (Fig. 4D) must reflect a decrease of the fraction of vacant receptors and a corresponding increase in fraction of receptors that are occupied by antagonist at the end of the pre-application.
Specifically, a %A1 of 50% should correspond to a receptor occupation by 018 of 50%. The corresponding concentration of 018 is then a measure of affinity, a “functional KΒ“, provided that binding equilibrium is obtained during pre-application. In this way, the functional KΒ for 018 at α4β1δ receptors was determined to 6.90 nM [5.99; 7.91] for 2.5 μM GABA. The same experiment was performed using 100 μM GABA giving a similar result with a functional KΒ of 7.80 nM [6.93; 8.73] (See Supplementary Fig. S4 and Table S1). These values concur both with the determined KΒ from the Schild analysis and Ki from the binding studies.
018 and 2027 display very low membrane permeability
To investigate the potential of both 018 and 2027 for in vivo studies, the compounds were tested in an in vitro permeability model using MDCK-MDR1 cells, to assess the bidirectional permeability. Results from these experiments indicate very low permeability of both 018 and 2027. The apical to the basal permeability was 0.00 cm/s for both of the compounds whereas the basal to apical permeability was 0.79 and 0.67 10−6 cm/s for 018 and 2027, respectively. Additionally, based on a high recovery both before and after (above 85%), sticking of compounds to the cells is not an issue (See Supplementary Table S2)”. This indicates that the compounds most likely will not pass cell membranes and thus not get into the brain.
Discussion
We have identified a new class of competitive GABAΑR antagonists with preference for α3,4,5-containing extrasynaptic receptors typical in forebrain regions such as cortex, thalamus and hippocampus. Having identified 2027 as a low-micromolar GABAΑR antagonist, we were able to increase the potency approximately 10 times by testing of analogues with the identification of 018. This confirmed the predictions of the library, which was designed to contain compounds that had at least 50 commercially available structural analogues, with the purpose of increasing potency of the primary hit and narrowing down the searchable chemical space.
Through the testing of analogues of 2027, we identified the 3,9-diazaspiro[5.5]undecane moiety as an important structural element for antagonist activity. Interestingly, this structure does not contain the carboxylic acid/GABA moiety like typical orthosteric GABAΑR antagonists like gabazine34 and DPP-4-PIOL24. It was thus interesting that the Schild analysis and binding studies of the compounds convincingly showed a competitive profile. However, there are other examples of orthosteric GABAΑR antagonists lacking the carboxylic acid, including bicuculline and the acid-sensing ion channel type 3 agonist 2-guanidine-4-methylquinazoline (GMQ)35, underlining that the presence of a “GABA”-like moiety is not necessary for binding to the orthosteric site. Additionally, we found that further substitution on the secondary amine of the 3-substituted 3,9-diazaspiro[5.5]undecane structure abolished the activity of the compounds (Supplementary Figs. S2 and S3), indicating that this part of the structure is important. By comparing the structures of 018 and 2027 it is clear that there is space for rather large substituents in the 3-position of the 3,9-diazaspiro[5.5]undecane. The studies of 018 and 2027 at selected GABAΑ receptor subtypes showed that the α-subunit dictates potency. Comparing the IC50 values we found that 018 is more potent at α3,4,5-subunit containing receptors than the α1-containing receptors, regardless of the presence of either the δ or γ subunit and the type of β-subunit. This conclusion could be strengthened with determination affinity-based KB values for all subtypes. A study by Ebert et al. looked into the α-subunit selectivity of the known competitive antagonist gabazine, bicuculline and Thio-4-PIOL at αβγ2 receptors, but did not find any differences between the receptor subtypes when studying functional responses36. It is therefore interesting that this new class of GABAΑR antagonists seemingly discriminate between the different α-subunits. It is however known that the GABA sensitivity is dependent on the type of α-subunit37 which we also show here. Interestingly, there is no clear correlation between the GABA potency and those of 2027 and 018 at the different subtypes, thus underlining that the compounds might also interact with residues outside the GABA binding site that are important for the potency, e.g. via hydrophobic interactions of the distal amide and aromatic moieties assuming that the protonated 9-amino group has the same interactions as GABA and thus is located under the c-loop.
The competitive profile of 018 was corroborated by similar affinity constants obtained from the Schild analysis, radioligand binding studies, and electrophysiology, although the specific receptor subtypes present in the native membranes is not equivocal. It has previously been shown that there is good correlation between affinity and potency for GABAΑR antagonists36, as shown here for both 018 and 2027. However, as the data can be fitted to a one-site binding model, this indicates binding to either a uniform receptor population or only high-affinity δ-containing receptors because the assay protocol employed only a low nanomolar [3H]muscimol concentration38. Immunoprecipitation studies from cortex have shown that the δ-containing receptors constitute around 10% of the total GABAΑRs39, thus only making up a small part of the available binding sites. Thus, at this point we cannot rule out that the differences observed for the α-subunits are only functional.
Patch-clamp electrophysiology studies of 018 corroborated the antagonistic profile from the FMP assay, however with a 9 times increased potency, likely relating to assay differences. Whole-cell patch-clamp is more sensitive and gives more details compared to the FMP assay, and has the advantage of measuring the response of a single cell in contrast to an average of cells in a specific well as in the FMP assay. It has previously been reported that the results between the two assays differ18,40. Additionally, we have also previously reported 5–8 times in potency differences of agonists between the two assays26. This difference however does not seem to be consistent, as the potency determined for gabazine in this study was similar in the two assays, with IC50 values of 0.18 μM and 0.24 μM in whole-cell patch-clamp and FMP, respectively. In the FMP assay there is the potential of compounds interfering with the fluorescence which may skew responses. However, both 2027 and gabazine do not interfere with the fluorescence signals in concentrations below 10 μM, (Supplementary Fig. S5) ruling out this as a cause of the discrepancy. Additionally, we used a GABA EC80 concentration in the FMP assay compared to GABA EC40 in patch-clamp to avoid desensitization of the response.
From the patch-clamp experiments we observed a surprisingly shallow Hill slope for 018 compared to the classical orthosteric antagonist gabazine when using the same cells and setup. Therefore, it is more likely that the shallow Hill slope is a compound effect, which is interesting as both the Schild analysis and binding studies confirmed that the compound is competitive and thus must share or at least have an overlap with the GABA binding site. Further, it does not seem likely that the slow dissociation kinetics of the compound is the cause, as Vestergaard et al.41 did not observes differences in Hill slopes for a series of analogues (competitive antagonists) of the partial GABAΑR agonist 4-PIOL, which included both fast and slowly dissociating antagonists. Thus, we have not been able to find an explanation for the shallow Hill slope other than being a characteristic of the compound.
The kinetics of GABA currents in the presence of 018 were studied in order to obtain additional information on the interaction of 018 with the α4β1δ receptor. The observation, that the activation phase of GABA currents in the presence of 018 consisted of two exponential components, was interpreted as GABA interacting with two “populations” of receptors: those that are initially vacant and those that are initially occupied with antagonist, giving rise to the fast and slow, respectively, component of the activation phase (Fig. 4A,B). This is a simplification because, due to the reversibility and branching of the underlying processes (binding, gating, desensitization), the two populations do not remain distinct in time. Nevertheless, we found indications that the interpretation of the parameters obtained from biexponential fitting of the activation time course is indeed meaningful: First, the time constant of the fast component of activation, τ1 (interpreted as GABA activation of vacant receptors), is similar to the time constant for activation by GABA alone, both with the high and low GABA concentration used. Second, the time constant of the slow component of activation, τ2 (interpreted as reflecting the rate limiting antagonist dissociation), is independent of the GABA concentration and largely independent of the antagonist concentration. A trend towards faster τ2 with lower concentrations of 018 was observed for both 100 and 2.5 μM GABA. However, this can be explained by the fact that at the lower concentrations of antagonist, the fractional amplitude of the slow component (%A2) is small, thus giving less reliable estimates of the τ2. Third, the fractional amplitude of the fast component, %A1, interpreted as the fraction of receptors that are vacant and immediately available for GABA to bind to and activate, decreases with the antagonist concentration, thus reflecting the concentration-dependent binding of 018 before application of GABA. The functional KB obtained from this relationship is similar with both 2.5 μM and 100 μM GABA and in agreement with the Ki determined from receptor binding and the KΒ from functional experiments (radioligand binding and FMP with Schild analysis). Also, the potential influence of drug application needs to be considered, since the solution exchange rate sets a limit to how fast processes can be resolved. With saturating GABA concentration, time constants as short as 35 ms were obtained, and therefore we conclude that solution exchange does not influence the slower τ values obtained in the kinetic experiments and analysis. This allows us to conclude that the time constant of the slow component of the GABA activation time course (τ2) obtained in the presence of 018 is an estimate of the dissociation time constant of 018. Thus, 018 is characterized as slowly dissociating with a time constant of the same order of magnitude as the most slowly dissociating GABAΑ antagonists described41.
To address future in vivo usage of the compounds, the cellular permeability of 2027 and 018 were assessed and found to be very low, indicating that neither of these compounds will likely pass the blood-brain barrier, thus limiting their use for in vivo CNS studies. Nonetheless, as several studies have shown that GABAΑR subunits are expressed outside CNS, on for example several types of peripheral immune cells42 and that GABAΑRs can be potential targets for cancer therapy, this is not discouraging as such. For these purposes it would be an advantage to have compounds that do not get into the brain, as this will limit unwanted neurological side-effects. Thus, a compound such as 018 would be highly interesting to examine for such peripheral GABAΑR-mediated inhibitory effects and may have a favourable pharmacokinetic profile because of the slow dissociation kinetics. Furthermore, it would be of interest to probe the binding site further, e.g. by the development of a radiolabelled analogue, and/or computational modelling to gain more information on the binding site residues and, ultimately, the α-subunit dependence. Lastly, the α3,4,5-extrasynaptic receptor preference displayed by 018 presents an alternative, and highly desired, way to study (in vitro) tonic inhibition in the forebrain, e.g. by slice electrophysiology. Interestingly, the functional selectivity of the identified compounds for extrasynaptic over synaptic receptors make them promising tools, especially in the absence of δ-selective antagonist.
Methods
Materials
Dulbecco’s modified Eagle medium (DMEM) containing GlutaMAX-I, foetal bovine serum (FBS), Dulbecco’s phosphate-buffered saline (DPBS), penicillin-streptomycin, hygromycin B, trypsin-EDTA, and Hank’s balanced salt solution (HBSS) were all purchased from Life Technologies (Paisley, UK). Polyfect transfection reagent was purchased from Qiagen (West Sussex, UK). FLIPR membrane potential Blue dye was purchased from Molecular Devices (Crawley, UK). Buffer components, poly-D-lysine (PDL) and 4-(2-hydroxyethyl)-piperazine-1-ethanesulfonic acid (HEPES) were purchased from Sigma-Aldrich (St. Louis, MO, USA). GABA and gabazine were from Tocris Bioscience (Bristol, UK). Compounds in the screening library and analogues were purchased from either Enamine, Vita-M or ChemBridge as stated in the supplementary information. [3H]muscimol (36.9 Ci/mmol) was purchased from PerkinElmer (Waltham, MA, USA). Compounds 2027 and 018 can be purchased from Enamine and have ID numbers Z1839922409 and Z1839935473, respectively.
Compound design and analogue search
Starting from Enamine’s > 2 million in-stock compounds, a diverse library of 2,112 compounds was designed. This library was selected for structural diversity (MolPrint2D fingerprint and Tanimoto coefficient <0.5), small drug-/lead-like size (MW 300–350), solubility (LogS > −4 and LogP < =3.5), flexibility (2–7 rotatable bonds), polarity (hydrogen bond donors + hydrogen bond acceptors + charged atoms = 3–10) and lack of reactive groups. The physicochemical properties were calculated by employing LigPrep, Epik, QikProp from Schrödinger Maestro and cxcalc form ChemAxon. To enable primary assay hits to be optimised by an ‘analogue-by-catalogue’ approach, we selected library compounds with at least 50 available drug-like analogues (Tanimoto coefficient > 0.55). A diversity search was conducted in the pool of screening compounds by Schrödinger Canvas (molPrint2D fingerprint and Tanimoto Coefficient = <0.5). The library was ordered ready for assaying as freeze-dried compounds in 384-well plates.
Substructure queries were constructed in MarvinSketch 17.3.27.0, 2017, ChemAxon (http://www.chemaxon.com) based on compound 2027 allowing for variations in different parts of the molecule. The actual analogue searches were performed in Instant JChem 17.2.27.0, 2017, ChemAxon (http://www.chemaxon.com) in a database of commercially available compounds combined from different vendors.
Cell culturing and transient transfection
HEK293 cells were maintained in DMEM containing GlutaMAX-I, supplemented with 10% FBS and 1% penicillin-streptomycin and incubated at 37 °C and a humidity of 5% CO2. For expression of δ-containing GABAΑ receptors, a HEK293 Flp-InΤΜ cell line stably expressing the human GABAΑ δ-subunits (referred to as δ-HEK) was used and the γ-containing subtypes was expressed in a background HEK293 Flp-InΤΜ cell line stably expressing the G-protein coupled peptide receptor NPBWR2 (referred to as HEK background)26, both positively selected for using 200 μg/mL hygromycin B. α1β2γ2 receptors were stably expressed in a HEK293 cell line maintained as above but with no selection (gift from Marianne L. Jensen, Neurosearch).
To express recombinant GABAΑ receptors human α1, α2, α3, α5, α6, β2, γ2 (pcDNA3.1/Zeo) and α4, β1 (pUNIV)26 subunit cDNA were transiently transfected into either δ-HEK or HEK background cells. δ-HEK cells were transfected with α- and β-subunits in a 1:1 ration whereas HEK background cells were transfected with α-, β- and γ-subunits in a 1:1:2 ratio. Human BGT1 (pcDNA5/FRT)43 was transiently transfected into the δ-HEK cell line. δ-HEK cells transfected for whole-cell patch-clamp recordings were co-transfected with green fluorescent protein (GFP) as well as α- and β-subunits in a 0.5:1:1 ratio (0.8 μg + 1.6 μg + 1.6 μg in a 6 cm dish) in order to identify transfected cells. The transfection was carried out using Polyfect as transfection reagent according to the manufactures protocol expect for using half the recommended volume of Polyfect.
FLIPR membrane potential (FMP) assay
The FMP assay was performed as previously described with a few modifications26. Briefly, HEK cells were transfected as described26 and 16–20 hours later plated (50,000 cells/well in DMEM medium) into clear-bottomed black 96-well plates coated with poly-D-lysine and the assay performed 20–28 hours later. The assay protocol including the FMP blue dye incorporation into membranes was exactly as described in Falk-Petersen et al.26. Ligand solutions were prepared in 4x in assay buffer (HBSS supplemented with 20 mM HEPES, pH 7.4, on the day of assay supplemented with 2 mM CaCl2 and 0.5 mM MgCl2, which for all compounds tested for antagonism contained a concentration of GABA corresponding to approximately GABA EC80 (given in Table 1). The ligand solutions were added to a ligand plates and incubated for 10–15 min in the FLEXstation3 plate reader (Molecular Devices, Crawley, UK) pre-heated to 37 °C. In a few cases a similarly operating machine, NOVOStarΤΜ (BMG LABTECH GmbH, Offenburg, Germany) was used instead, giving similar results. Data was obtained as changes in fluorescence units (∆RFU) by measuring emission at 560 nm caused by excitation at 530 nm. Unless otherwise mentioned in the text or figure legends experiments were performed in at least three independent experiments each using three technical replicates. Due to an observed edge effect for the FMP assay, outer wells were not used except for the screening. For screening results with GABA EC80 controls giving signals of less than 60 ∆RFU in the outer wells, the compounds were retested.
Data obtained in the FMP assay are given as relative changes in fluorescence units (ΔRFU) by subtracting the average of the baseline fluorescence signal from the peak fluorescence signal after compound addition. All raw traces were inspected manually and peak signals resulting from compound/buffer addition together with signal artefacts were omitted from the analysis.
The concentration-response curves obtained in the FMP assay for both agonists (determining EC50 values) and antagonists (determining IC50 values) were fitted using the four-parameter concentration-response model:
EC/IC50 values describe the concentration resulting in the half-maximal response (halfway between top and bottom), and nΗ is the Hill coefficient. In order to pool the data from the individual experiments for the Schild analysis, ΔRFU were normalized to the buffer level set as 0% response and average response of the highest tested concentration of GABA was set to 100% in order to compensate for variation in the assay window between experiments. The presence of 018 did not result in a decreased plateau of the curves, as the maximum response was similar to the GABA max (100 μM) controls. Gaddum Schild analysis was performed using GraphPad Prism version 8.2.1 (GraphPad Software Inc., San Diego, CA, USA). EC50/IC50 values obtained in the FMP assay are given as means ± SEM from at least three independent experiments with three technical replicates, as specified in the individual figure legends.
Z′-factor determination
The Z′-factor for the FMP assay at α4β1δ receptors was calculated using the equation:
with σc- and σc+ denoting the standard deviation of the negative and positive controls, respectively, and μc+ and μc- the means of the positive and negative controls, respectively. GABA EC80 was the positive control and the buffer response the negative control.
Whole-cell patch-clamp electrophysiology
δ-HEK cells transiently expressing human α4β1δ receptors were seeded in 35 mm Petri dishes the day before experiment and used for recordings in a similar was as previously reported26. On the day of experiment the dishes were transferred to the stage of an Axiovert 10 microscope (Zeiss, Germany), and the culture medium was exchanged for artificial balanced salt solution (ABBS) at room temperature (20−24 °C). The ABSS solution contained the following (in mM): NaCl 140, KCl 3.5, Na2HPO4 1.25, MgSO4 2, CaCl2 2, glucose 10, and HEPES 10; pH 7.35. The cells were viewed at 200× magnification, and cells containing green fluorescent protein were visualized with UV light from an HBO 50 lamp (Zeiss, Germany). The cells were approached with micropipettes of 1.2−3.3 MΩ resistance manufactured from 1.5 mm OD glass (World Precision Instruments, Sarasota, Florida) on a microelectrode puller, model PP-830 (Narishige, Tokyo, Japan). The intrapipette solution contained the following (in mM): KCl 140, MgCl2 1, CaCl2 1, EGTA 10, MgATP 2, and HEPES 10; pH 7.3. Standard patch-clamp techniques in voltage clamp mode44 were used to record from cells in the whole-cell configuration using an EPC-9 amplifier (HEKA, Lambrecht, Germany). A clamping potential of −60 mV was used, and series resistance was 80% compensated. Whole-cell currents were recorded and subsequently analyzed using Pulse and PulseFit software (HEKA, Lambrecht, Germany).
ABSS solution containing the compounds were applied using two VC3–8xP pressurized application systems feeding into a sixteen-barreled perfusion pipette (ALA Scientific Instruments Inc., Farmingdale New York, USA) ending approximately 100 μm from the recorded cell. Between the compound applications, compound-free ABSS was applied from one of the barrels in order to quickly remove the compounds from the cell. For the concentrations-response dependent experiments, antagonist was pre-applied for 30 sec before 5 sec co-application with GABA EC40 (2.5 μM), with 1 min intervals between the applications to let the cells fully recover. For the kinetic studies GABA was first applied for 5 sec, followed by 5 sec application of antagonist and last 5–15 sec application of GABA, depending on when a plateau was reached. The cells were left to recover for 1 min before the next application.
For the antagonist concentration-response relationship, the equation:
was fitted to the experimental data, where I is the membrane current, A is the logarithm of the antagonist concentration, Imax is the maximum current that the reference agonist can induce, IC50 is the antagonist concentration inhibiting 50% of Imax, and nΗ is the Hill coefficient. Data were described using means and 95% confidence intervals given en squared brackets.
Current relaxations were fitted to a single or biexponential equation using a Simplex optimization algorithm (PulseFit; HEKA, Lambrecht, Germany).
With I denoting the currents at a time t and τ1 and τ2 the time constants. All curve-fitting results were carefully inspected visually. In some cases, the current relaxations were fitted to both a mono- and a biexponential function and an F-test was applied to the residual sum of squares to determine the appropriateness of using a second component:
where SS and RMS are the sum of squares and root mean square deviation between fit and data, respectively. A and B refer to the single and biexponential fit, respectively. df represents the degree of freedom for each fit. Time constants were given as medians followed by the 25–75% percentile in square brackets. All statistical analyses performed on time constants were performed by using non-parametric tests (Kruskal-Wallis ANOVA followed by Dunn’s multiple comparison), as the data do not follow a normal distribution.
All fractional amplitudes (%A) were determined from the second GABA pulse, for those concentrations of antagonist where a biexponential time course could be superimposed.
Radioligand binding assay
The binding studies were performed using rat brain homogenate prepared from the cortex of adult Sprague-Dawley male rats (250–350 g) obtained from commercial breeders (Janvier Laboratories, Le Genest-Saint-Isle, France), housed and cared for according to the Danish legislation regulating animal experiments and the European Communities Council Directive (2010/63/EU). Homogenate preparation was performed as described earlier45. On the day of assay, membranes stored at −20 °C was quickly thawed followed by addition of 40 volumes of ice-cold binding buffer (50 mM TRIS-HCl buffer; pH 7.4) and homogenization using and UltraTurrax homogenizer before being centrifuged at 48,000 ×g for 10 minutes at 4 °C. This washing procedure was repeated 4 times before the final pellet was suspended in the binding buffer to final concentration of approximately 0.5 mg/mL protein.
The radioligand binding assay was performed using [3H]muscimol in a 96-well format. [3H]muscimol (5 nM) was incubated with 75 μg/well protein with or without test compound or control at 0 °C for 1 hour in a total volume of 200 μL binding buffer in triplicates. GABA (1 mM) was used to determine non-specific binding. After incubation, the binding reaction was terminated by rapid filtration through GF/C unifilters (PerkinElmer) with the use of a 96-well Packard FilterMate cell harvester (PerkinElmer), followed by 3 times washing in 250 μL ice-cold binding buffer. After drying at 65 °C, microscintillation fluid was added to the dried filters and the radioactivity bound to the filter was quantified in a Packard TopCounter microplate scintillation counter. The obtained binding data was converted into specific binding (% of control) for each data point and fitted to the one-site competition model by linear regression (GraphPad Prism, version 8.2.1). The fitted IC50 values were converted into Ki values by means of the Cheng-Prusoff equation46. Data are from three independent experiments, performed with three technical replicates and Ki values are given as pKi ± SEM.
Membrane permeability
Bidirectional permeability was tested for 018 and 2027 in the Madin-Darby canine kidney (MDCK) cell line expressing human multidrug resistance protein (MDR1, P-glycoprotein) (referred to as MDR1-MDCK cells) as described previously47. To calculate efflux ratio the permeability in the basal-to-apical direction was divided by the permeability in the apical-to-basal direction. The obtained data is from triplicate measurements.
Data analysis and statistics
Detailed descriptions of data and statistics are stated in the respective method sections. Data and statistical analysis were performed using GraphPad Prism version 8.2.1 (GraphPad software Inc., San Diego, CA, USA).
Supplementary information
Acknowledgements
The work was financially supported by the Lundbeck Foundation (grant R230-2016-2562 to C.B.F.-P. and R163-2013-16327 to D.E.G.), the A.P. Møller Foundation for the Advancement of Medical Sciences (C.B.F.-P.), the Drug Research Academy, and in part by ERC Starting Grant 639125 (D.E.G.) and Lundbeck Foundation fellowship R133-A12270 (P.W.).
Author contributions
C.B.F.-P. and P.W. conceived the study. T.M.T. designed the compound library under supervision from D.E.G. K.H. was responsible for selecting analogues of the screening hit. M.S.N. performed the library screening under supervision of C.B.F.-P. and P.W. C.B.F.-P. performed and analysed all pharmacological data (FMP, binding and electrophysiology studies). U.K. contributed to the design and analysis of the electrophysiology studies. B.F. provided chemistry input. C.B. performed the permeability studies. C.B.F.-P. and P.W. wrote the manuscript and all authors approved the final version.
Data availability
The data generated and analysed during the present study is available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-66821-0.
References
- 1.Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr. Top. Med. Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 2.Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 4.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 5.Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the δ subunit. J. Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Caraiscos VB, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brickley SG, Mody I. Extrasynaptic GABAA receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lie MEK, et al. GAT3 selective substrate L-isoserine upregulates GAT3 expression and increases functional recovery after a focal ischemic stroke in mice. J. Cereb. Blood Flow Metab. 2019;39:74–88. doi: 10.1177/0271678X17744123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarkson AN, Huang BS, MacIsaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mesbah-Oskui L, Orser BA, Horner RL. Thalamic δ-subunit containing GABAA receptors promote electrocortical signatures of deep non-REM sleep but do not mediate the effects of etomidate at the thalamus in vivo. J. Neurosci. 2014;34:12253–12266. doi: 10.1523/JNEUROSCI.0618-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SS. α4βδ GABAA receptors and tonic inhibitory current during adolescence: effects on mood and synaptic plasticity. Front. Neural Circuits. 2013;7:1–16. doi: 10.3389/fncir.2013.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whissell PD, et al. Acutely increasing δGABAA receptor activity impairs memory and inhibits synaptic plasticity in the hippocampus. Front. Neural Circuits. 2013;7:1–12. doi: 10.3389/fncir.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collinson N, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J. Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storustovu SI, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J. Pharmacol. Exp. Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- 16.Wafford KA, Ebert B. Gaboxadol - a new awakening in sleep. Curr. Opin. Pharmacol. 2006;6:30–36. doi: 10.1016/j.coph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Cogram P, et al. Gaboxadol normalizes behavioral abnormalities in a mouse model of fragile X syndrome. Front. Behav. Neurosci. 2019;13:1–9. doi: 10.3389/fnbeh.2019.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wafford KA, et al. Novel compounds selectively enhance δ subunit containing GABAA receptors and increase tonic currents in thalamus. Neuropharmacology. 2009;56:182–189. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Vardya I, et al. Positive modulation of δ-subunit containing GABAA receptors in mouse neurons. Neuropharmacology. 2012;63:469–479. doi: 10.1016/j.neuropharm.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Karim N, et al. 3-Hydroxy-2’-methoxy-6-methylflavone: a potent anxiolytic with a unique selectivity profile at GABAA receptor subtypes. Biochem. Pharmacol. 2011;82:1971–1983. doi: 10.1016/j.bcp.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson AN, et al. The flavonoid, 2’-methoxy-6-methylflavone, affords neuroprotection following focal cerebral ischaemia. J. Cereb. Blood Flow Metab. 2019;39:1266–1282. doi: 10.1177/0271678X18755628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogenkamp DJ, et al. Enaminone amides as novel orally active GABAA receptor modulators. J. Med. Chem. 2007;50:3369–3379. doi: 10.1021/jm070083v. [DOI] [PubMed] [Google Scholar]
- 23.Johnstone TBC, et al. Positive allosteric modulators of nonbenzodiazepine γ-aminobutyric acidA receptor subtypes for the treatment of chronic pain. Pain. 2019;160:198–209. doi: 10.1097/j.pain.0000000000001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boddum K, Frølund B, Kristiansen U. The GABAA antagonist DPP-4-PIOL selectively antagonises tonic over phasic GABAergic currents in dentate gyrus granule cells. Neurochem. Res. 2014;39:2078–2084. doi: 10.1007/s11064-014-1397-9. [DOI] [PubMed] [Google Scholar]
- 25.Cope DW, et al. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat. Med. 2009;15:1392–1398. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falk-Petersen CB, et al. Development of a robust mammalian cell-based assay for studying recombinant α4β1/3δ GABAA receptor subtypes. Basic Clin. Pharmacol. Toxicol. 2017;121:119–129. doi: 10.1111/bcpt.12778. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C. & Oldenburg. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 28.Glykys J, et al. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat. Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- 29.Maric D, et al. GABAA receptor subunit composition and functional properties of Cl- channels with differential sensitivity to zolpidem in embryonic rat hippocampal cells. J. Neurosci. 1999;19:4921–4937. doi: 10.1523/JNEUROSCI.19-12-04921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortensen M, Patel B, Smart TG. GABA potency at GABAA receptors found in synaptic and extrasynaptic zones. Front. Cell. Neurosci. 2012;6:1. doi: 10.3389/fncel.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J. Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortensen M, Ebert B, Wafford K, Smart TG. Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J. Physiol. 2010;588:1251–1268. doi: 10.1113/jphysiol.2009.182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keramidas A, Harrison NL. Agonist-dependent single channel current and gating in α4β2δ and α1β2γ2S GABAA receptors. J. Gen. Physiol. 2008;131:163–181. doi: 10.1085/jgp.200709871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heaulme M, et al. Biochemical characterization of the interaction of three pyridazinyl-GABA derivatives with the GABAA receptor site. Brain Res. 1986;384:224–31. doi: 10.1016/0006-8993(86)91158-3. [DOI] [PubMed] [Google Scholar]
- 35.Xiao X, Zhu MX, Xu T. Le. 2-Guanidine-4-methylquinazoline acts as a novel competitive antagonist of A type γ-aminobutyric acid receptors. Neuropharmacology. 2013;75:126–137. doi: 10.1016/j.neuropharm.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Ebert B, et al. Differences in agonist/antagonist binding affinity and receptor transduction using recombinant human γ-aminobutyric acid type A receptors. Mol. Pharmacol. 1997;52:1150–6. [PubMed] [Google Scholar]
- 37.Ebert B, Wafford KA, Whiting PJ, Krogsgaard-Larsen P, Kemp JA. Molecular pharmacology of γ-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different α, β, and γ receptor subunit combinations. Mol. Pharmacol. 1994;46:957–963. [PubMed] [Google Scholar]
- 38.Chandra D, et al. Prototypic GABAA receptor agonist muscimol acts preferentially through forebrain high-affinity binding sites. Neuropsychopharmacology. 2010;35:999–1007. doi: 10.1038/npp.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quirk K, Whiting PJ, Ian Ragan C, McKernan RM. Characterisation of δ-subunit containing GABAA receptors from rat brain. Eur. J. Pharmacol. Mol. Pharmacol. 1995;290:175–181. doi: 10.1016/0922-4106(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 40.Pálvölgyi A, et al. Loop F of the GABAA receptor alpha subunit governs GABA potency. Neuropharmacology. 2018;128:408–415. doi: 10.1016/j.neuropharm.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 41.Vestergaard HT, Cannillo C, Frølund B, Kristiansen U. Differences in kinetics of structurally related competitive GABAA receptor antagonists. Neuropharmacology. 2007;52:873–882. doi: 10.1016/j.neuropharm.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Barragan A, Weidner JM, Jin Z, Korpi ER, Birnir B. GABAergic signalling in the immune system. Acta Physiol. 2015;213:819–827. doi: 10.1111/apha.12467. [DOI] [PubMed] [Google Scholar]
- 43.Al-Khawaja A, et al. Pharmacological identification of a guanidine-containing β-alanine analogue with low micromolar potency and selectivity for the betaine/GABA transporter 1 (BGT1) Neurochem. Res. 2014;39:1988–1996. doi: 10.1007/s11064-014-1336-9. [DOI] [PubMed] [Google Scholar]
- 44.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 45.Ransom RW, Stec NL. Cooperative modulation of [3H]MK‐801 binding to the N‐methyl‐D‐aspartate receptor‐ion channel complex by l‐glutamate, glycine, and polyamines. J. Neurochem. 1988;51:830–836. doi: 10.1111/j.1471-4159.1988.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 46.Yung-Chi C, Prusoff WH. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 47.Risgaard R, et al. Radiolabelling and PET brain imaging of the α1-adrenoceptor antagonist Lu AE43936. Nucl. Med. Biol. 2013;40:135–140. doi: 10.1016/j.nucmedbio.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Krall J, et al. Discovery of α‐substituted imidazole‐4‐acetic acid analogues as a novel class of ρ1 γ‐aminobutyric acid type A receptor antagonists with effect on retinal vascular tone. ChemMedChem. 2016;11:2299–2310. doi: 10.1002/cmdc.201600356. [DOI] [PubMed] [Google Scholar]
- 49.L’Estrade ET, et al. Synthesis and pharmacological evaluation of [11C]4-methoxy-N-[2-(thiophen-2-yl)imidazo[1,2- a]pyridin-3-yl]benzamide as a brain penetrant PET ligand selective for the δ-subunit-containing γ-aminobutyric acid type A receptors. ACS Omega. 2019;4:8846–8851. doi: 10.1021/acsomega.9b00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated and analysed during the present study is available from the corresponding author upon request.