Abstract
Higher serum 6-bromotryptophan has been associated with lower risk of chronic kidney disease (CKD) progression, implicating mechanisms beyond renal clearance. We studied genetic determinants of urine 6-bromotryptophan and its association with CKD risk factors and incident end-stage kidney disease (ESKD) in 4,843 participants of the German Chronic Kidney Disease (GCKD) study. 6-bromotryptophan was measured from urine samples using mass spectrometry. Patients with higher levels of urine 6-bromotryptophan had higher baseline estimated glomerular filtration rate (eGFR, p < 0.001). A genome-wide association study of urine 6-bromotryptophan identified two significant loci possibly related to its tubular reabsorption, SLC6A19, and its production, ERO1A, which was also associated with serum 6-bromotryptophan in an independent study. The association between urine 6-bromotryptophan and time to ESKD was assessed using Cox regression. There were 216 ESKD events after four years of follow-up. Compared with patients with undetectable levels, higher 6-bromotryptophan levels were associated with lower risk of ESKD in models unadjusted and adjusted for ESKD risk factors other than eGFR (<median level: cause-specific hazard ratio [HR] 0.70, 95% confidence interval [CI] 0.51 to 0.97; ≥median level: HR 0.50, 95% CI 0.34 to 0.74). Upon adjustment for baseline eGFR, this association became attenuated, suggesting that urine 6-bromotryptophan may represent a correlated marker of kidney health.
Subject terms: Prognostic markers, Kidney diseases, Biomarkers, Epidemiology, Genetics research, Outcomes research, Kidney diseases, Risk factors
Introduction
Chronic kidney disease (CKD) is an important global health burden accounting for 1.2 million deaths worldwide in 20151,2. The course of CKD progression is highly variable. Novel biomarkers of CKD progression may lead to improvements in our understanding of the pathophysiology of CKD progression and its prediction3. Recently, higher serum 6-bromotryptophan has been associated with lower risk for CKD progression in multiple studies4. This molecule is a metabolite formed from the bromination of tryptophan, an essential amino acid, but little is known about its generation, metabolism, or excretion.
Previous studies have shown that higher levels of many blood metabolites are associated with unfavorable renal outcomes and all-cause mortality5–8 and thought to reflect reduced renal clearance. It is therefore remarkable that higher serum levels of 6-bromotryptophan were associated with lower risk of CKD progression, implicating mechanisms beyond renal clearance. To learn more about the metabolism of 6-bromotryptophan and its potential handling by the kidney, we carried out a hypothesis-generating genome-wide association study (GWAS) to gain insights into the biological mechanisms influencing 6-bromotryptophan levels in urine in the German Chronic Kidney Disease (GCKD) study, a large contemporary nationwide cohort of CKD patients. Significant loci were validated for association with serum 6-bromotryptophan in an independent cohort of European descent. Among GCKD participants, we also studied the association of urine 6-bromotryptophan with risk factors of CKD and CKD progression.
Results
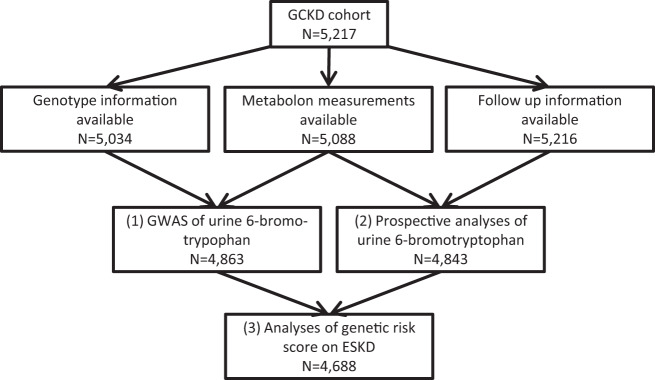
The majority of analyses used data from the GCKD study, with the workflow depicted in Fig. 1. Of the 5,217 GCKD participants, 4,843 participants with urine 6-bromotryptophan and incident ESKD information were included in the prospective analysis, and 4,863 participants (including 97% of those in the prospective analysis, n = 4,688) with urine 6-bromotryptophan and genetic data were included in the genome-wide association study.
Figure 1.
Workflow of analyses in the GCKD study.
Baseline characteristics of GCKD participants
Overall, the mean age of the 4,843 GCKD participants in the prospective analysis was 60 years, and 60% were male (Table 1). Prevalence of diabetes, hypertension, and coronary heart disease was 35%, 96%, and 20%, respectively. In contrast to tryptophan detected in urine of all participants, 6-bromotryptophan was only detectable in 57.3%. The Spearman correlation between tryptophan and 6-bromotryptophan in urine was moderate (r = 0.45).
Table 1.
Baseline characteristics of the study population (N = 4,843).
| Characteristic | Overall | Category of urine 6-bromotryoptophana | |||
|---|---|---|---|---|---|
| Undetectable | Low | High | P-valueb | ||
| N | 4843 | 2068 | 1392 | 1383 | |
| 6-bromotryptophan, mean (SD) | 0.96 (0.56) | NA | 0.55 (0.17) | 1.37 (0.51) | |
| Leading CKD cause acc. to treating nephrologist, % (n)c | 0.001 | ||||
| Vascular nephropathy | 23 (1107) | 21 (443) | 23 (321) | 25 (343) | |
| Primary glomerulopathy | 19 (912) | 18 (381) | 19 (268) | 19 (263) | |
| Diabetic nephropathy | 15 (704) | 16 (340) | 16 (217) | 11 (147) | |
| Systemic disease | 8 (387) | 8 (175) | 7 (102) | 8 (110) | |
| Male sex, % (n) | 60 (2917) | 54 (1108) | 61 (856) | 69 (953) | <0.001 |
| Age, years, mean (SD) | 60.1 (12) | 60.6 (11.42) | 60.5 (11.89) | 58.8 (12.85) | 0.001 |
| Smoking, % (n) | <0.001 | ||||
| Non-smoker | 41 (1967) | 39 (805) | 40 (558) | 44 (604) | |
| Former smoker | 43 (2102) | 44 (900) | 43 (597) | 44 (605) | |
| Current smoker | 16 (774) | 18 (363) | 17 (237) | 13 (174) | |
| Education, % (n) | 0.020 | ||||
| Higher | 17 (806) | 15 (314) | 17 (240) | 18 (252) | |
| Middle | 28 (1343) | 27 (551) | 28 (396) | 29 (396) | |
| Lower | 54 (2595) | 56 (1168) | 52 (726) | 51 (701) | |
| Other | 2 (99) | 2 (35) | 2 (30) | 2 (34) | |
| Body mass index, kg/m², mean (SD)d | 29.8 (5.9) | 29.7 (6.03) | 29.7 (5.8) | 29.9 (5.82) | 0.718 |
| Waist-to-hip ratio, median (IQR) | 0.94 (0.88–1) | 0.93 (0.87–0.99) | 0.94 (0.89–1) | 0.95 (0.89–1.01) | <0.001 |
| Blood pressure, systolic, mmHg, mean (SD) | 139.4 (20.3) | 138.6 (20.91) | 139.1 (20.02) | 140.9 (19.59) | 0.006 |
| Blood pressure, diastolic, mmHg, mean (SD) | 79.3 (11.74) | 77.8 (11.76) | 79.1 (11.49) | 81.7 (11.59) | <0.001 |
| Diabetes mellitus, % (n) | 35 (1705) | 40 (826) | 36 (505) | 27 (374) | <0.001 |
| Hypertension, % (n) | 96 (4664) | 97 (2012) | 97 (1353) | 94 (1299) | <0.001 |
| Coronary heart disease, % (n) | 20 (967) | 23 (469) | 19 (258) | 17 (240) | <0.001 |
| Serum albumin, g/dL, mean (SD)d | 3.84 (0.44) | 3.83 (0.44) | 3.81 (0.49) | 3.87 (0.40) | 0.009 |
| Serum C-reactive protein, mg/L, median (IQR) | 2.3 (1.01–4.92) | 2.2 (1–4.81) | 2.3 (0.98–4.94) | 2.3 (1.06–5.1) | 0.398 |
| HDL-C, mg/dL, mean (SD) | 52 (18.14) | 53.4 (19.64) | 51.6 (17.07) | 50.2 (16.63) | <0.001 |
| LDL-C, mg/dL, mean (SD) | 118.3 (43.51) | 116.2 (43.47) | 119.8 (45.69) | 119.7 (41.18) | <0.001 |
| Serum triglycerides, mg/dL, median (IQR)d | 167.9 (117.54–238.76) | 167.6 (116.02–250.66) | 170.7 (119.04–235.96) | 164.2 (117.64–230.51) | 0.226 |
| eGFR, mL/min/1.73 m², mean (SD) | 49.4 (18.24) | 45 (16.53) | 48.7 (16.83) | 56.7 (19.71) | <0.001 |
| UACR, mg/g, median (IQR) | 50.5 (9.55–384.17) | 58.1 (12.26–498.97) | 53.3 (8.71–405.42) | 37.2 (6.6–265.69) | <0.001 |
| Blood pressure medication: by renin-angiotensin system, % (n) | 81 (3924) | 82 (1701) | 83 (1152) | 77 (1071) | <0.001 |
| * ACE inhibitors | 47 (2292) | 48 (983) | 49 (680) | 45 (629) | |
| * ARB | 42 (2021) | 44 (919) | 42 (582) | 38 (520) | |
| * ACE and ARB | 8 (389) | 10 (201) | 8 (110) | 6 (78) | |
| * ACE or ARB | 73 (3535) | 73 (1500) | 75 (1042) | 72 (993) | |
| Diuretic use, % (n) | 60 (2926) | 71 (1466) | 58 (813) | 47 (647) | <0.001 |
| * Potassium-sparing | 10 (477) | 11 (228) | 10 (134) | 8 (115) | |
| * Thiazide | 26 (1281) | 26 (533) | 29 (407) | 25 (341) | |
| * Aldosterone antagonist | 8 (383) | 9 (194) | 8 (106) | 6 (83) | |
| * Loop diuretics | 38 (1838) | 54 (1122) | 30 (421) | 21 (295) | |
| Death event, % (n)e | 7 (325) | 8 (167) | 6 (78) | 6 (80) | |
| Major causes of death, % (n)f | |||||
| Due to forgoing of dialysis | 0 (9) | 0 (4) | 0 (2) | 0 (3) | |
|
Cardiovascular disease (e.g. myocardial infarction) |
2 (108) | 3 (60) | 2 (25) | 2 (23) | |
|
Cerebrovascular disease (e.g. stroke) |
0 (14) | 0 (6) | 0 (4) | 0 (4) | |
| Infection | 1 (64) | 2 (39) | 1 (11) | 1 (14) | |
| ESKD event, % (n)e | 4 (216) | 6 (127) | 4(55) | 2 (34) | |
Abbreviations. HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; UACR, urine albumin-to-creatinine ratio; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; ESKD, end-stage kidney disease; IQR, interquartile range (25th and 75th percentiles); * Subcategories; aSubcategories according to levels of urine 6-bromotryptophan: undetectable levels; low, range: 0.135 to < 0.84 (median); high, range: 0.84 (median) to 5.31; bStatistical comparison of groups for categorical variables (Pearson’s Chi-squared test) and for continuous variables (Kruskal-Wallis rank sum test); cMajor leading causes of CKD as reported by the treating nephrologist (N > 300); dVariables with missing values: body mass index N = 41, serum albumin N = 1, serum triglycerides N = 2 (overall < 1%); eFollow-up time was restricted to the period from study entry to 1,500 days (4.11 years); fCauses of death with at least 50 events, except for death due to forgoing of dialysis.
Across the three groups of 6-bromotryptophan levels (undetectable, low, and high), there were significant differences in kidney function: eGFR (mean levels per group: undetectable 45.0, low 48.7, high 56.7 ml/min/1.73 m2, p < 0.001) and UACR (median levels per group: undetectable 58.1, low 53.3, high 37.2 mg/g, p < 0.001). Higher urine 6-bromotryptophan was also significantly associated with lower proportions of prevalent diabetes, hypertension, coronary heart disease, diuretic use, and current smoking status (Table 1).
Genetic determinants of 6-bromotryptophan levels
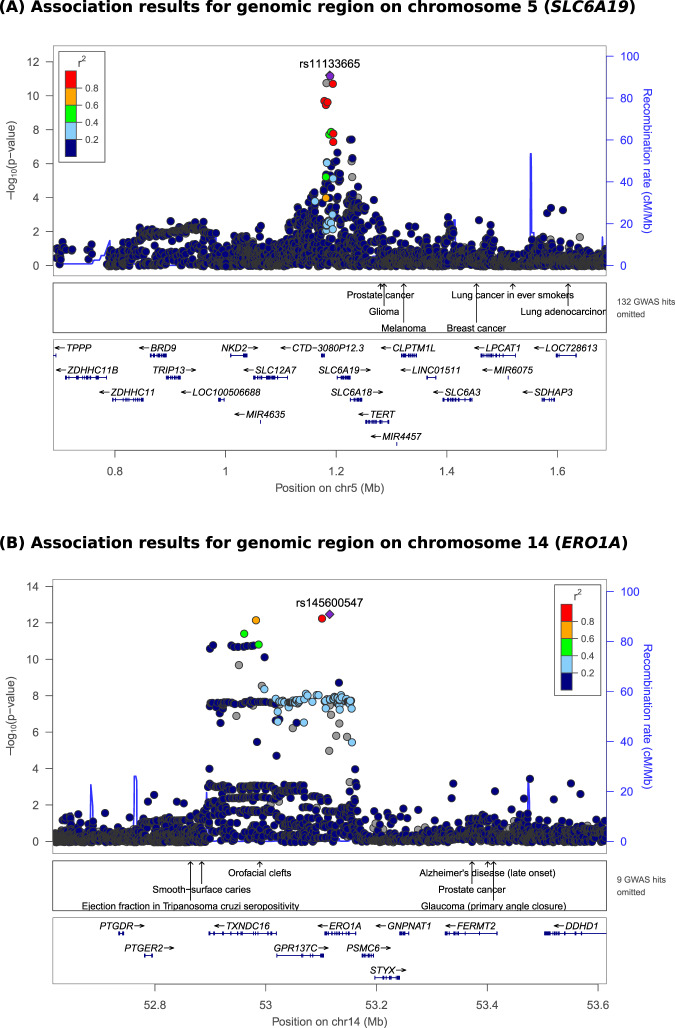
In the GCKD study, we detected two genome-wide significant loci of urine 6-bromotryptophan. The index SNP on chromosome 5 was rs11133665 (Fig. 2A). This SNP is located upstream of SLC6A19, which encodes a renal transport protein known to mediate the reuptake of amino acids such as tryptophan from the urine. It therefore is a plausible candidate for the reuptake of 6-bromotryptophan (Fig. 3). Each copy of the A allele was associated with 11.1% lower urine 6-bromotryptophan levels (p = 6.2 × 10−12, Table 2). The other index SNP was rs145600547, an intronic insertion deletion polymorphism in the ERO1A (Endoplasmic Reticulum Oxidoreductase 1 Alpha) gene on chromosome 14 (Fig. 2B). Each copy of a CT deletion was associated with 27% lower urine 6-bromotryptophan levels (p = 3.4 × 10−13, Table 2). In support, the deletion at rs145600547 was significantly associated with lower ERO1A expression in whole blood in the GTEx Project V8 data (p=4.3e-8; https://gtexportal.org/). These two index SNPs together explained 2% of the variance of urine 6-bromotryptophan in GCKD.
Figure 2.
Regional association plots for genome-wide significant loci of urine 6-bromotryptophan in GCKD. Regional association plots for the genome-wide significant loci at chromosome 5 (SLC6A19, A) and at chromosome 14 (ERO1A, B). Y-axis: -log10(p-value), X-axis: chromosomal location. Marker color corresponds to correlation (r2) with the indicated index SNP.
Figure 3.
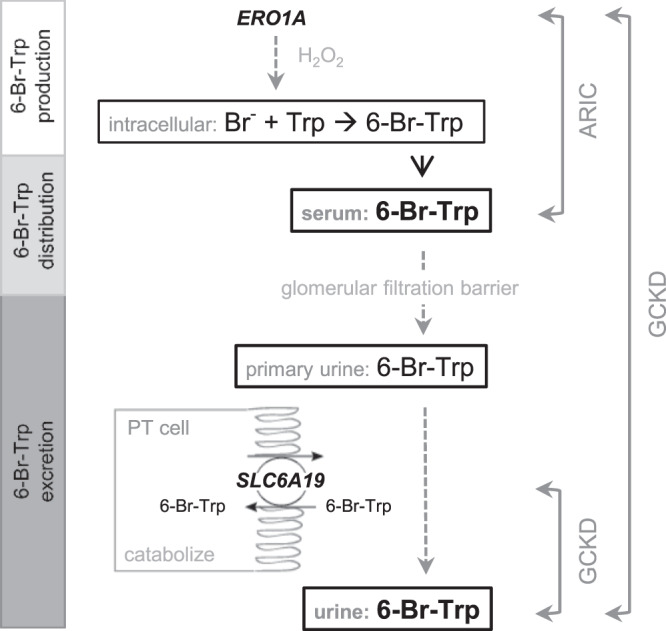
Proposed model of 6-bromotryptophan metabolism based on the findings from this study. Dotted lines and gray font represent hypothesized mechanisms. The genetic association of the ERO1A variant with serum (ARIC) and urine 6-bromotryptophan (GCKD) suggests that ERO1A may be involved in the production of 6-bromotryptophan. The genetic variant near SLC6A19 was associated with urine but not serum 6-bromotryptophan, suggesting a mechanism related to reabsorption of 6-bromotryptophan followed by catabolization. SLC6A19 is a plausible biological candidate to mediate this reabsorption. Abbreviations: Br-: bromine; Trp: tryptophan; 6-Br-Trp: 6-bromotryptophan; GCKD: German Chronic Kidney Disease study; ARIC: Atherosclerosis Risk in Communities (ARIC) study.
Table 2.
Index SNPs at genome-wide significant loci of urine 6-bromotryptophan in GCKD and their association with serum 6-bromotryptophan in ARIC participants of European ancestry. Abbreviations: GCKD: German Chronic Kidney Disease study; ARIC: Atherosclerosis Risk in Communities (ARIC) study.
| Chromosome | 5 | 14 | ||||
| Gene | SLC6A19 | ERO1A | ||||
| Function | upstream | intronic | ||||
| Index SNP | rs11133665 | rs145600547 | ||||
| Coded allele/noncoded allele | A/G | A/ACT | ||||
| Coded allele frequence | GCKD: 0.28/ARIC: 0.28 | GCKD: 0.03/ARIC: 0.03 | ||||
| Association with 6-bromo-tryptophan in … | Effect in %* | Beta* (SE) | P-value | Effect in %* | Beta* (SE) | P-value |
| urine (GCKD, N = 4863) | −11.1 | −0.17 (0.02) | 6.2E-12 | −27.0 | −0.45 (0.06) | 3.4E-13 |
| serum (ARIC, N = 1433) | 1.2 | 0.02 (0.01) | 7.5E-02 | −11.1 | −0.17 (0.03) | 1.9E-09 |
*beta on the log2 scale and effect in % = (2beta -1)*100.
Imputation quality: rs11133665, GCKD 1.0, ARIC 0.99; rs145600654, GCKD 0.93, ARIC 0.76 (info from impute2 output).
rs11133665 function based on UCSC, downstream gene is CTD-3080P12.3.
We next tested whether these two index SNPs were associated with serum 6-bromotryptophan levels among participants of European descent in the Atherosclerosis Risk in Communities (ARIC) study, a population-based cohort with a mean age of 55 and mean eGFR of 98 mL/min/1.73 m2 (Supplementary Table 1). Whereas rs11133665, upstream of SLC6A19, did not show a significant association with serum 6-bromotryptophan levels (p = 0.07), rs145600547 at ERO1A was significantly associated in a direction consistent with its effect on urine 6-bromotryptophan (each CT deletion was associated with 11.1% lower serum levels, p = 1.9 × 10−9, Table 2) and had the strongest association in the entire region (Supplementary Fig. 1). The association at the SLC6A19 locus with urine but not serum 6-bromotryptophan is consistent with the reuptake of filtered 6-bromotryptophan by the SLC6A19 transporter at the apical membrane of tubular epithelial cells that subsequently does not exit the cell on the basolateral side into the blood, for example, due to intracellular catabolism (Fig. 3). In contrast, the association with 6-bromotryptophan at ERO1A was observed in blood and may reflect mechanisms related to the production of 6-bromotryptophan rather than its reuptake (Fig. 3).
Association between urine 6-bromotrytophan and ESKD
Among participants in the GCKD study, there were 216 ESKD events after four years of follow-up (median [range] of follow-up in years: 4.04 [0.06–4.11]). Compared with those having undetectable urine 6-bromotryptophan levels, those with low and high levels had lower risk of ESKD in unadjusted analysis, age- and sex-adjusted analyses, and with additional adjustment for baseline non-kidney risk factors (Fig. 4, Table 3). For example, compared to those with undetectable levels, those with high urine 6-bromotryphan levels had a cause-specific hazard ratio (HR) of 0.39 (95% CI: 0.26 to 0.58) after adjustment for age, sex, and non-kidney risk factors (Model 3, see Methods for selection of risk factors). With the addition of baseline UACR as a covariate, the cause-specific hazard ratios were somewhat attenuated but remained significant (Model 4). A sensitivity analysis that categorized urine 6-bromotryptophan levels into five groups yielded very similar results (Supplementary Table 2), with no indication of departure from linearity. With the addition of baseline eGFR, the association between urine 6-bromotryptophan and ESKD was no longer significant (Model 5, low group HR: 0.98, 95% CI: 0.71 to 1.36; high group HR: 1.25, 95% CI: 0.83 to 1.89, Table 3). In the competing risk analysis using death not due to forgoing dialysis and without ESKD previously as the competing event, urine 6-bromotryptophan was also not significantly associated with ESKD in Model 5 (Supplementary Table 3).
Figure 4.
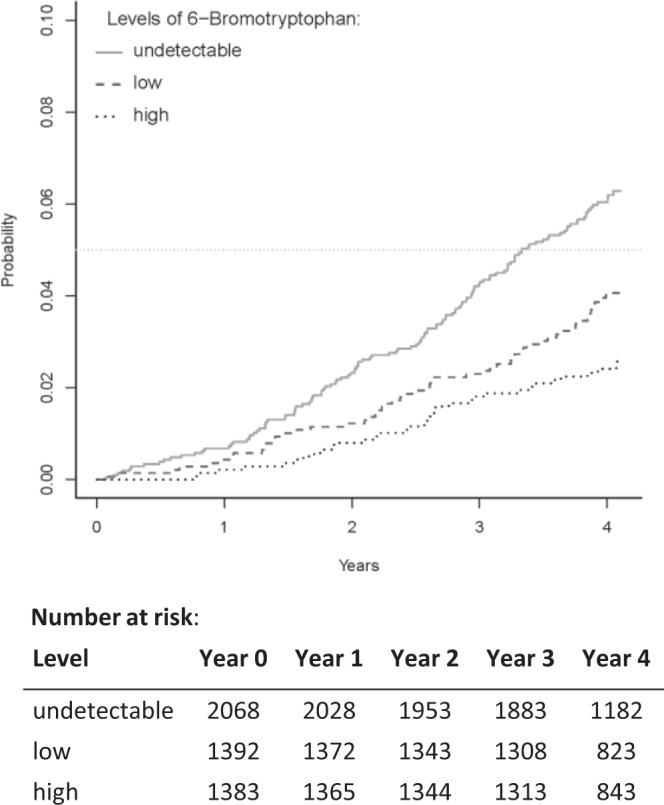
Cumulative incidence function of ESKD events by the three categories of urine 6-bromotrytophan levels. Categories of urine 6-bromotryptophan levels: (1) undetectable: patients with undetectable levels; (2) low: patients with levels < median detected level (0.84); (3) high: patients with levels ≥0.84.
Table 3.
Association between urine 6-bromotryptophan and incident ESKD.
| Categorya: | Cause-specific hazard ratio (95% confidence interval) | ||
|---|---|---|---|
| Undetectable | Low | High | |
| Model 1 | 1.00 | 0.63 (0.46, 0.86) | 0.39 (0.27, 0.57) |
| Model 2 | 1.00 | 0.59 (0.43, 0.81) | 0.33 (0.23, 0.49) |
| Model 3 | 1.00 | 0.64 (0.46, 0.88) | 0.39 (0.26, 0.58) |
| Model 4 | 1.00 | 0.70 (0.51, 0.97) | 0.50 (0.34, 0.74) |
| Model 5 | 1.00 | 0.98 (0.71, 1.36) | 1.25 (0.83, 1.89) |
N = 4,843, Nevents = 216.
aSubcategories according to levels of urine 6-bromotryptophan: undetectable levels; low: range: 0.135 to <0.84 (median); high: range: 0.84 (median) to 5.31.
Covariate adjustment:
Model 1: unadjusted.
Model 2: age + sex.
Model 3: Model 2 + smoking + waist-to-hip ratio + diastolic blood pressure + prevalent diabetes + coronary heart disease + high density lipoprotein cholesterol + low density lipoprotein cholesterol + diuretic use.
Model 4: Model 3 + urine albumin-to-creatinine ratio.
Model 5: Model 4 + eGFR.
The genetic score summarizing a genetic effect on higher urine 6-bromotryptophan levels showed cause-specific hazard ratios in the same direction, but was not significantly associated with ESKD (N = 4,688, 209 events, HR: 0.73, 95% CI: 0.32, 1.69, p = 0.46).
Lastly, we evaluated the addition of urine 6-bromotryptophan to the Tangri 4-variable kidney failure risk model, a widely-validated model9,10. This addition led to a negligible increase in the C-statistic (0.8526 vs 0.8531), and a likelihood ratio test comparing both models did not reveal any significant difference (p = 0.74). These results indicate that urine 6-bromotryptophan does not contribute to risk prediction beyond the variables included in the Tangri model.
Discussion
We detected two genetic loci of urine 6-bromotryptophan levels providing insights into its metabolism: while SLC6A19 encodes a transporter and likely responsible for its tubular reuptake, the signals at the ERO1A locus may reflect its generation. Among GCKD participants, 6-bromotryptophan was detected in urine in just over half of the population. Compared to those with undetectable levels, those with detectable levels of urine 6-bromotryptophan had higher kidney function and lower prevalence of risk factors of ESKD. In prospective analyses, higher urine 6-bromotryptophan levels were significantly associated with lower risk of ESKD in models that were not adjusted for eGFR and did not have significant association after adjusting for eGFR.
Previously, higher serum 6-bromotryptophan levels were reported to be associated with lower risk of CKD progression in three cohorts: the African American Study of Kidney Disease and Hypertension (AASK), BioMe, and the Modification of Diet in Renal Disease (MDRD) study4. Associations were robust after adjustment for eGFR and albuminuria as well as other kidney disease risk factors. In the present study, the direction of association between urine 6-bromotryptophan and ESKD was consistent with the reported association of higher serum 6-bromotryptophan with higher kidney function and lower risk of CKD progression, but the results were not independent of eGFR. This suggests that the mechanisms related to its tubular reuptake may be correlated with eGFR.
Whereas urine 6-bromotryptophan was detected in 57% of the GCKD participants, serum 6-bromotryptophan was detected in the overwhelming majority of the participants in other studies, including the ARIC study4. This is consistent with free filtration of this metabolite, followed by its tubular reabsorption, for which we detected SLC6A19 as a candidate transporter in an unbiased genome-wide screen. This finding is biologically plausible: rs11133665, the index SNP at SLC6A19, has been identified in a previous GWAS of urinary metabolites, with the A allele associated with lower levels of tyrosine and histidine11, directionally consistent with its association with lower urine 6-bromotryptophan and reflecting more efficient reuptake of amino acids. Moreover the associations of SLC6A19 variants with urine tyrosine and histidine levels were not observed in blood11, consistent with the lack of significant association at the SLC6A19 locus for serum 6-bromotryptophan. SLC6A19 encodes a transporter of amino acids at the apical membrane of tubular epithelial cells12. Rare mutations in SLC6A19 cause autosomal-recessive Hartnup disorder13, a disease featuring the loss of amino acids in urine, including tryptophan14. The mouse model of Hartnup disorder shows elevated concentrations of many neutral amino acids in urine, while plasma concentrations were normal15, which is consistent with our observation of genetic associations with urine but not serum 6-bromotryptophan levels.
Little is known about the generation of 6-bromotryptophan; it is a metabolite formed from the bromination of tryptophan but the localization of this reaction is unknown. Bromine, a trace element16, was shown to be an essential co-factor for the formation of sulfilimine, a crosslink protein in the assembly of collagen IV scaffolds17. This reaction involves peroxidasin, a heme peroxidase, hydrogen peroxide, and bromide, the ionic form of bromine, to generate hypobromous acid17,18. Here, the other genetic locus detected in the GWAS of 6-bromotryptophan levels may provide novel insights: the identified insertion deletion polymorphism rs145600547 maps into an intron of ERO1A and had the strongest association with urine 6-bromotryptophan in GCKD and with serum 6-bromotryptophan in ARIC. The oxidoreductase encoded by ERO1A plays a role in disulfide bond formation, with reactive oxygen species such as hydrogen peroxide generated as part of this pathway. It is therefore tempting to speculate that ERO1A is involved in the generation of hydrogen peroxide required for the bromination of tryptophan19, and this hypothesis would also be consistent with the observed association of the ERO1A locus on both urine and blood 6-bromotryptophan levels.
We observed that adjustment for eGFR led to an attenuation of the association between urine 6-bromotryptophan levels with ESKD, whereas this was not the case with serum 6-bromotryptophan in previous studies of CKD patients. A potential explanation is that urine levels of 6-bromotryptophan reflect processes that are correlated with eGFR more strongly than those of serum 6-bromotryptophan. If urine 6-bromotryptophan and eGFR levels show stronger correlations than serum 6-bromotryptophan and eGFR, for example because tubular reabsorptive capacity is correlated with both eGFR and urine 6-bromotryptophan levels, this could explain the attenuation of the association of urine 6-bromotryptophan and ESKD risk upon adjustment for eGFR. In agreement, our genetic studies support that tubular re-uptake of the metabolite does not influence its blood levels, making this a plausible explanation for differences to previous studies of serum 6-bromotryptophan levels. Alternatively or in addition, a more sensitive assay for very low levels of 6-bromotrypotophan in urine (e.g., a targeted MS assay) might allow for better discrimination of ESKD risk. Lastly, other differences between the GCKD study and previous studies, such as the proportion of individuals with diabetes, may also contribute to the observed differences. In any case, our study suggests that urine 6-bromotryptophan represents an interesting novel biomarker of ESKD risk, even if it does not improve ESKD prediction in CKD patients beyond eGFR. In contrast to established markers of kidney function such as creatinine and urea, where higher levels reflect poor kidney function, higher levels of both serum and urine 6-bromotryptophan levels were associated with better eGFR and lower levels of markers of kidney damage. These results support the notion that 6-bromotryptophan levels may mark an essential protective process in the kidney.
Our study has several strengths. It was conducted in a large contemporary cohort of patients with CKD with rich phenotypic characterization and over 4 years of follow-up in whom urine 6-bromotryptophan was measured and adjusted for urine dilution based on a probabilistic quotient derived from a large number of metabolites. The two genome-wide significant loci of 6-bromotryptophan provide additional insight on the metabolism of 6-bromotryptophan with direct relevance in humans. However, the modest effects of the genetic variants on urine 6-bromotryptophan levels limited statistical power to use a genetic score to investigate a potential causal relationship between 6-bromotryptophan and ESKD. Other limitations include levels of urine 6-bromotryptophan were measured from spot urine with relative quantification, and information on dietary factors that may influence 6-bromotryptophan levels were not available. The GCKD study does not have measures of serum 6-bromotryptophan for direct comparison with urine 6-bromotryptophan for risk association and the estimation of fractional excretion. In addition, information on eGFR slopes over time is not yet available for an analysis of changes in kidney function, as creatinine collection at follow-up visits is still ongoing in the GCKD study.
In conclusion, this study extends previous work on the association between higher serum 6-bromotryptophan and lower risk of CKD progression by investigating the association between urine 6-bromotryptophan and ESKD in a large cohort of CKD patients. We show that urine 6-bromotryptophan is detectable in approximately half of the patients and has strong associations with markers of baseline kidney function and damage. The strong association with ESKD is not independent of eGFR. Our study shows how integration of genetic information with the evaluation of novel biomarkers of kidney disease yields novel insights into their metabolism, and supports the notion that higher 6-bromotryptophan may mark a protective process with respect to kidney function that is different from filtration and worth further investigation.
Materials and Methods
German Chronic Kidney Disease (GCKD) study
The GCKD study is a prospective cohort study in Germany that enrolled 5,217 adult patients of European descent under regular care of nephrologists20. Main eligibility criteria were eGFR of 30–60 mL/min/1.73 m2 or eGFR > 60 mL/min/1.73 m2 with urinary albumin-to-creatinine ratio (UACR) > 300 mg/g. All participants provided written informed consent21. The GCKD study was approved by local ethics committees of each of the nine participating German institutions reported in the Supplementary Note and is registered in the German national registry for clinical studies (DRKS 00003971). The study is conducted according to national laws and relevant guidelines.
All patients underwent standardized, questionnaire-based interviews and physical examinations by trained personnel. Biosamples collected were immediately processed and shipped on dry ice to a central biobank where they were stored at −80 °C for future usage22. Measurement and definitions of baseline characteristics used in this project are described in the Supplementary Methods section. These variables were selected a priori to include study-specific characteristics, diagnostic measures of CKD as well as known CKD risk factors23. End-stage kidney disease (ESKD) and death including the cause of death, were obtained from dialysis protocols, hospital discharge records, and death certificates collected at yearly follow-up visits, and systematically abstracted by physicians on a continuous basis.
Measurement of urine 6-bromotryptophan
6-bromotryptophan was measured from spot urine samples collected at the GCKD enrollment visit. 6-bromotryptophan was measured by Metabolon, Inc (Morrisville, NC)4. Details of the methods for metabolite profiling are reported in the Supplementary Methods section. Briefly, 6-bromotryptophan was quantified using reverse phase ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MSn) methods and negative ion mode electrospray ionization24 and identified by matching detected features to an in-house spectral and chromatographic library of authentic reference standards containing the retention time/index (RI), mass to charge ratio (m/z), and MS/MS fragmentation data. The levels were quantified using area-under-the-curve of the MS peaks following inter-day normalization. After quality control, measurements for 5,088 GCKD patients were available for analyses. 6-bromotryptophan was detected in 57% (n = 2,909) of the participants, with missingness likely due to values being below the limit of detection of the untargeted metabolite profiling approach25. We evaluated potential reasons for missingness but did not find a link, for instance to enrollment year (Supplementary Fig. 2A,B). To account for urine concentration, we applied the probabilistic quotient normalization method, which uses the median values of all metabolites with complete measurements as reference and was found to have better performance in CKD patients than other methods that use a single substance, such as urine creatinine26,27.
Imputation and genome-wide association analysis of urine 6-bromotryptophan
Detailed information on genotyping and imputation based on the 1000 Genomes Project ALL haplotypes – Phase 328 integrated variant set using SHAPEIT v229 and IMPUTE2 (v2.3.1)30 in the GCKD study was reported previously27. Filtering for imputation quality (info) value of >0.8 and minor allele frequency (MAF) ≥ 1% yielded 9,281,895 high-quality imputed markers.
Given the limit of detection is a major contributing factor to missing values in the untargeted metabolite approach25, missing values of urine 6-bromotryptophan were imputed to the minimum observed measurement before correction for urine dilution using the probabilistic quotient normalization method for the GWAS in order to increase statistical power. Measurements were log2-transformed resulting in a near normal distribution (Supplementary Fig. 2C). Linear regression was conducted using SNPtest v2.5.231 adjusting for age, sex, the first three genetic principal components, log-transformed eGFR and UACR. The GWAS included 4,863 participants with imputed genotype dosage, urine 6-bromotryptophan and covariate information (Fig. 1). The significance threshold for the GWAS was set at 5*10−8. The quantile-quantile plot and Manhattan plot of the results are presented in Supplementary Fig. 3, and indicated no major sources of unmodeled stratification. Regional association plots were generated by LocusZoom (v1.3)32 using genotypes from the GCKD study to calculate linkage disequilibrium.
Association analysis of baseline characteristics and ESKD with urine 6-bromotryptophan
Given the limit of detection is a major contributing factor to missing values in the untargeted metabolite approach25, we categorized 6-bromotryptophan levels into three groups: undetectable (43%), low (<median among those with detectable levels) and high (≥median). Separating the detectable levels at the median allows for some inspection of linearity of effects.
Baseline characteristics of the participants were analyzed by these three groups of 6-bromotryptophan levels using chi-square-test for categorical variables and Kruskal-Wallis rank sum test for continuous variables.
ESKD events were defined as a composite endpoint of incident dialysis or kidney transplantation (N = 210) and death due to forgoing of dialysis (N = 6). To evaluate the association between urine 6-bromotryptophan categories and ESKD, we used cause-specific Cox regression models. Patients without an event were censored at the time of last visit or the time of death for reasons not attributable to kidney disease. We evaluated the association between urine 6-bromotryptophan categories and ESKD first using an unadjusted model (Model 1) and successively adding risk factors of CKD. Given that baseline UACR and eGFR are the two most important predictors of CKD progression9, these two variables were added last. Model 2 adjusted for age and sex. Model 3 added baseline non-kidney risk factors: smoking, waist-to-hip ratio, diastolic blood pressure, diabetes, coronary heart disease, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), and diuretic use. These factors were selected based on significantly association with urine 6-bromotryptophan categories in univariate analysis (Table 1, p < 0.001) and excluding categorical variables with >90% of the participants in one of its categories. Model 4 added log(UACR), and Model 5 added log(eGFR). We examined Schoenfeld residuals to assess the proportional hazards assumption, and found no violations (data not shown). The competing event, death from non-ESKD causes (N = 273), was investigated similarly. Reported analyses were restricted to complete cases (N = 4,843, Fig. 1).
In addition, a genetic score was calculated as the sum of the alleles of the index SNPs associated with higher urine 6-bromotryptophan in the GWAS of urine 6-bromotryptophan, weighted by respective effect estimates. We assessed the association between the genetic score and ESKD using cause-specific Cox regression controlling for age and sex among 4,688 GCKD participants with both genetic and prospective data (Fig. 1).
Sensitivity analysis
We conducted sensitivity analyses to investigate other factors that might influence the association between urine 6-bromotryptophan and kidney function: potential non-linear relations between urine 6-bromotryptophan and baseline kidney function. We inspected scatter plots of urine 6-bromotryptophan levels against baseline eGFR and UACR (Supplementary Fig. 4). For the prospective analysis of ESKD, we categorized urine 6-bromotryptophan levels into five groups: undetectable, and four groups by quartiles of the detectable values and reassessed the association between urine 6-bromotryptophan and ESKD using the Cox regression models described above. A trend test that coded the five groups as a numeric variable was used as a test for linearity.
To evaluate the extent to which urine 6-bromotryptophan might improve ESKD prediction, we compared goodness of fit measures of the Tangri 4-variable kidney failure risk model, a widely-validated model9,10, with the same model adding urine 6-bromotryptophan as a 3-category predictor in the GCKD study.
ARIC study
To determine whether genetic loci significantly associated with urine 6-bromotryptophan levels in GCKD were also associated with serum 6-bromotryptophan, we tested the association of the index SNPs with serum 6-bromotryptophan among participants of European descent in the ARIC study (N = 1,433, Supplementary Table 1). This study was approved by Institutional Review Board of the Johns Hopkins University. Participants included in this study have provided informed consent for genetic studies. The ARIC study is conducted according to national laws and relevant guidelines.
Details of genotyping and imputation in the ARIC study are reported in the Supplementary Methods. Details of the metabolite profiling in the ARIC study were reported previously33. Briefly, metabolite profiling of 2,153 participants (1,553 European descent and 600 African Americans) was performed using fasting serum samples that had been stored at −80 °C since collection in 1987–1989. In total, 1,160 known and unknown metabolites were quantified by Metabolon Inc. (Durham, USA) using untargeted, gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry (GC-MS and LC-MS)34. Serum 6-bromotryptophan was detected in 97.4% of the samples (1,510 European ancestry and 585 African Americans).
In the analysis, we performed linear regression using natural log-transformed serum 6-bromotryphan as the outcome adjusting for age, sex, study-center, and ten genetic principal components. The effect estimates were converted to log2 scale for reporting to match the transformation of urine 6-bromotryptophan in GCKD. The significance threshold was set at 0.05 divided by the number of tested index SNPs.
Supplementary information
Acknowledgements
The work of AK was supported by grants KO 3598/5-1, KO 3598/4-2, and CRC 1140 project number 246781735, all German Research Foundation (DFG). The work of UTS was supported by the CRC 1140 project number 246781735, by the Else Kroener Fresenius Foundation (NAKSYS) and by the German Federal Ministry of Education and Research (BMBF) within the framework of the e:Med research and funding concept (grant 01ZX1912B). The work of AT is supported by NIDDK grant R21DK112087. The GCKD study was/is funded by grants from the Federal Ministry of Education and Research (BMBF, grant number 01ER0804), the KfH Foundation for Preventive Medicine, and several industry partners. We thank Dr. Yong Li for his support in the generation of locuszoom plots. We are grateful for the willingness of the patients to participate in the GCKD study. The enormous effort of the study personnel of the various regional centers is highly appreciated. We thank the large number of nephrologists who provide routine care for the patients and collaborate with the GCKD study. The GCKD Investigators are listed in the Supplementary Note. Genotyping and metabolomics measurements in GCKD were supported by Bayer Pharma AG. The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services (contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I and HHSN268201700005I), R01HL087641, R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. The authors thank the staff and participants of the ARIC study for their important contributions. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. The article processing charge was funded by the Baden-Wuerttemberg Ministry of Science, Research and Art and the University of Freiburg in the funding programme Open Access Publishing.
Author contributions
Design of this study: A.T., P.S. and A.K. Recruitment and management of study: G.C.K.D. – U.T.S., I.S., S.B.A., K.U.E., A.K.; A.R.I.C. – E.B., J.C. Metabolite quantification: R.P.M. Bioinformatics and statistical analysis: P.S. and A.T. Interpretation of results: A.T., B.Y., S.L., R.P.M., P.S., M.G. and A.K. Draft of manuscript: P.S., A.T., M.G. and A.K. Critically read and approved the manuscript: all authors.
Data availability
With respect to GCKD, public posting of individual level participant data is not covered by the informed patient consent. As stated in the patient consent form and approved by the Ethics Committees, pseudonymized datasets can be obtained by collaborating scientists upon approval of a scientific project proposal by the steering committee of the GCKD study: www.gckd.org.
The ARIC study genetic data is available at dbGaP (accession: phs000090). The metabolite data is available upon approval of a manuscript proposal by the ARIC Publication Committee as described in https://sites.cscc.unc.edu/aric/distribution-agreements.
Competing interests
Robert P. Mohney is an employee of Metabolon, Inc. and, as such, has affiliations with or financial involvement with Metabolon, Inc. The other authors have nothing to declare.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Peggy Sekula and Adrienne Tin.
Supplementary information
is available for this paper at 10.1038/s41598-020-66334-w.
References
- 1.Xie Y, et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney International. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Neuen BL, Chadban SJ, Demaio AR, Johnson DW, Perkovic V. Chronic kidney disease and the global NCDs agenda. BMJ Glob Health. 2017;2:e000380. doi: 10.1136/bmjgh-2017-000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMahon GM, Waikar SS. Biomarkers in nephrology: Core Curriculum 2013. Am J Kidney Dis. 2013;62:165–178. doi: 10.1053/j.ajkd.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tin A, et al. Serum 6-Bromotryptophan Levels Identified as a Risk Factor for CKD Progression. J Am Soc Nephrol. 2018;29:1939–1947. doi: 10.1681/ASN.2017101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goek ON, et al. Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol Dial Transplant. 2013;28:2131–2138. doi: 10.1093/ndt/gft217. [DOI] [PubMed] [Google Scholar]
- 6.Hu JR, et al. Serum metabolites are associated with all-cause mortality in chronic kidney disease. Kidney Int. 2018;94:381–389. doi: 10.1016/j.kint.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu B, Heiss G, Alexander D, Grams ME, Boerwinkle E. Associations Between the Serum Metabolome and All-Cause Mortality Among African Americans in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 2016;183:650–656. doi: 10.1093/aje/kwv213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee EP, et al. A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol. 2013;24:1330–1338. doi: 10.1681/ASN.2012101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangri N, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305:1553–1559. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 10.Tangri N, et al. Multinational Assessment of Accuracy of Equations for Predicting Risk of Kidney Failure: A Meta-analysis. JAMA. 2016;315:164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raffler J, et al. Genome-Wide Association Study with Targeted and Non-targeted NMR Metabolomics Identifies 15 Novel Loci of Urinary Human Metabolic Individuality. PLoS Genet. 2015;11:e1005487. doi: 10.1371/journal.pgen.1005487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Leary NA, et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016;44:D733–745. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleta R, et al. Mutations in SLC6A19, encoding B0AT1, cause Hartnup disorder. Nat Genet. 2004;36:999–1002. doi: 10.1038/ng1405. [DOI] [PubMed] [Google Scholar]
- 14.Baron DN, Dent CE, Harris H, Hart EW, Jepson JB. Hereditary pellagra-like skin rash with temporary cerebellar ataxia, constant renal amino-aciduria, and other bizarre biochemical features. Lancet. 1956;271:421–428. doi: 10.1016/s0140-6736(56)91914-6. [DOI] [PubMed] [Google Scholar]
- 15.Symula DJ, Shedlovsky A, Guillery EN, Dove WF. A candidate mouse model for Hartnup disorder deficient in neutral amino acid transport. Mamm Genome. 1997;8:102–107. doi: 10.1007/s003359900367. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen FH. Ultratrace elements in nutrition. Annu Rev Nutr. 1984;4:21–41. doi: 10.1146/annurev.nu.04.070184.000321. [DOI] [PubMed] [Google Scholar]
- 17.McCall AS, et al. Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell. 2014;157:1380–1392. doi: 10.1016/j.cell.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mani AR, Moreno JC, Visser TJ, Moore KP. The metabolism and de-bromination of bromotyrosine in vivo. Free radical biology & medicine. 2016;90:243–251. doi: 10.1016/j.freeradbiomed.2015.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senthilmohan R, Kettle AJ. Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride. Arch Biochem Biophys. 2006;445:235–244. doi: 10.1016/j.abb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Eckardt KU, et al. The German Chronic Kidney Disease (GCKD) study: design and methods. Nephrol Dial Transplant. 2012;27:1454–1460. doi: 10.1093/ndt/gfr456. [DOI] [PubMed] [Google Scholar]
- 21.Titze S, et al. Disease burden and risk profile in referred patients with moderate chronic kidney disease: composition of the German Chronic Kidney Disease (GCKD) cohort. Nephrol Dial Transplant. 2015;30:441–451. doi: 10.1093/ndt/gfu294. [DOI] [PubMed] [Google Scholar]
- 22.Prokosch HU, et al. Designing and implementing a biobanking IT framework for multiple research scenarios. Stud Health Technol Inform. 2012;180:559–563. [PubMed] [Google Scholar]
- 23.Levin A, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390:1888–1917. doi: 10.1016/S0140-6736(17)30788-2. [DOI] [PubMed] [Google Scholar]
- 24.Evans, A. M., B.R., B., Liu, Q., Mitchell, M. W. & Robinson, R. J. High Resolution Mass Spectrometry Improves Data Quantity and Quality as Compared to Unit Mass Resolution Mass Spectrometry in High-Throughput Profiling Metabolomics. Metabolomics4 (2014).
- 25.Do KT, et al. Characterization of missing values in untargeted MS-based metabolomics data and evaluation of missing data handling strategies. Metabolomics. 2018;14:128. doi: 10.1007/s11306-018-1420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dieterle F, Ross A, Schlotterbeck G, Senn H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal Chem. 2006;78:4281–4290. doi: 10.1021/ac051632c. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, et al. Genome-Wide Association Studies of Metabolites in Patients with CKD Identify Multiple Loci and Illuminate Tubular Transport Mechanisms. Journal of the American Society of Nephrology. 2018;29:1513. doi: 10.1681/ASN.2017101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genomes Project C, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nat Methods. 2011;9:179–181. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- 30.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. Plos Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 32.Pruim RJ, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu B, et al. Genetic determinants influencing human serum metabolome among African Americans. Plos Genet. 2014;10:e1004212. doi: 10.1371/journal.pgen.1004212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
With respect to GCKD, public posting of individual level participant data is not covered by the informed patient consent. As stated in the patient consent form and approved by the Ethics Committees, pseudonymized datasets can be obtained by collaborating scientists upon approval of a scientific project proposal by the steering committee of the GCKD study: www.gckd.org.
The ARIC study genetic data is available at dbGaP (accession: phs000090). The metabolite data is available upon approval of a manuscript proposal by the ARIC Publication Committee as described in https://sites.cscc.unc.edu/aric/distribution-agreements.