Abstract
This study evaluated the growth and biochemical composition of farming Gracilaria gracilis (Stackhouse) M. Steentoft, L. M. Irvine & W. F. Farnham in the Bizerte Lagoon (BL) and Bizerte Bay (BB) in the North Coast of Tunisia, using lantern nets. Effects of site and depth on alga daily growth rate (DGR) and biochemical composition were investigated. The DGR was affected by culture site (1.42 ± 0.65% day−1 and 1.19 ± 0.34% day−1 for the BL and the BB respectively). Agar yield, was higher (p < 0.05) in the BB than the BL (23.31 ± 2.64% vs. 19.19 ± 2.32%) with a higher (p < 0.05) 3,6-anhydrogalactose (3,6-AG) contents (41.37 ± 3.68% vs 23.30 ± 5.40%) and a lower (p < 0.05) sulphate degree (6 ± 2.00% vs 8.80 ± 0.86%). The proteins contents were independent of the site and depth of culture (20.74 ± 7.22% and 22.02 ± 6.34% for the BL and the BB respectively). R-phycoerythrin (R-PE) contents were significantly higher (p < 0.05) in the BB (0.86 ± 0.31 mg g−1) than those obtained in the BL (0.33 ± 0.12 mg g−1). The salinity, transparency, nitrate and ammonium were monitored in both sites, and their influences were discussed. Our results suggest that G. gracilis cultured in Bizerte Bay can be used in a cascading biorefinery approach.
Subject terms: Biochemistry, Ocean sciences
Introduction
Global seaweed production, largely derived from aquaculture (96.5 percent by volume of the wild-collected and cultivated aquatic plants combined), has changed considerably since 20051. This production increased from 13 million in 2005 to reach 30 million tons (live weight) in 2016. Moreover, 99% of the world’s production comes from Asian countries, notably China (47.9%), Indonesia (38.7%), Philippines (4.7%), Republic of Korea (4.5%), Democratic People’s Republic of Korea (1.6%), Japan (1.3%) and Malaysia (0.7%). Furthermore, trade in aquatic plants increased from USD 60 million in 1976 to more than USD 1 billion in 2016, with Indonesia, Chile and the Republic of Korea the major exporters, and China, Japan and the United States of America the leading importers. Accordingly, red seaweed production accounts for 53% of the world production1. The most exploited red algae, expressed in a million tons year−1, are Euchema seaweeds nei and Eucheuma spp. (10.5), Gracilaria spp. (4.1) and Porphyra spp. (1.3). In contrast, the red alga Gracilaria spp. was barely farmed at all in 1990. However, the increase in Gracilaria spp. production, by aquaculture, is mainly due to the growing demand for agar. Consequently, Gracilaria spp. aquaculture has been initiated by many countries, in different regions of the world, such as Thailand, Chile, Vietnam, Portugal, Australia, Brazil and India2.
The red alga Gracilaria gracilis, which grow in Asian coasts, was introduced into the Mediterranean Sea3, and established in the lagoons4–7. Consequently, the alga was found all year round, but is the most component of the BL flora between April and June4. In Tunisia, the quantity of seaweeds harvested, was restricted along the Bizerte Lagoon and the Tunis Lake, which is inadequate to supply the raw material requirement of the industries. Hence, G. gracilis aquaculture was indicated as the main solution4. However, the BL was chosen to initiate many cultivation attempts of G. gracilis. As a result, a low biomass supply was obtained due to the interaction with environmental factors (Grazing, epiphitism, hydrodynamism, etc…) and limited surface suitable for the benthic culture methods. As a consequence, these culture methods were applied only in a depth less than 2 m, which represent a 10% of the BL surface8. Furthermore, agar obtained (gel strength less than 400 g cm−2) was of low quality compared to that extracted from Gelidium latifolium (gel strength higher than 800 g cm−2), which considered as the highest and used in many industrial applications9. Hence, no large-scale culture of G. gracilis is being developed outside the Asian region. That’s why suspended culture method should be an alternative to enhance biomass production in lagoon and sea8,9. In addition, when we move toward the lagoon depth and sea, we will look for the possibilities to improve the alga quality.
Around the world Gracilaria biomass was used usually for agar extraction. In contrast, it contains a wide variety of valuable compounds, such as proteins and pigments10. The cascading biorefinery, as an alternative to single product extraction approach, aim to extract all components present in the algae biomass11,12. According to13,14, it is financially attractive to firstly extract R-PE, and then agar will be extracted from the by-product obtained. Furthermore, the agar by-product can be treated as waste to produce biofuels. Consequently, it is necessary to study the ecophysiology responses of G. gracilis to the various factors, which affect their growth and chemical composition, such as light intensity, nutrients, salinity and temperature variations. In Tunisia, no attempt has been done to understand G. gracilis behavior in deeper lagoon and sea. Therefore, the purpose of this study was to provide new insights on G. gracilis growth capacities and biochemical composition (agar, proteins and R-PE) in the BL and the BB. In addition, it may also provide knowledge on algal biomass uses.
Materials and methods
Preparation of experimental material
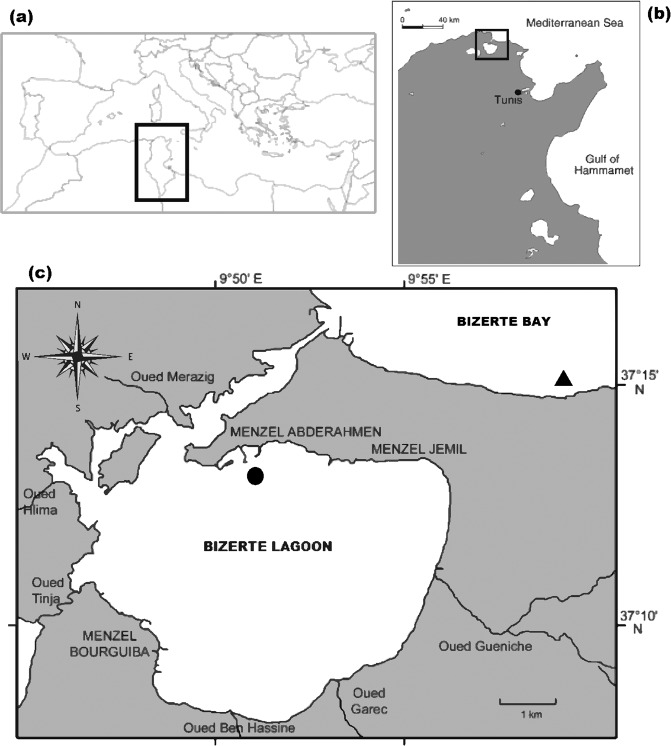
Gracilaria gracilis was collected between 0.5 and one meter depth from the Bizerte Lagoon (Fig. 1), North Tunisia (37°13′N; 9°55′E) at the end of Februray 2016. The collected thalli were transported to the laboratory in a cool environment to reduce stress. Collected samples were subjected to a series of washing steps using filtered sea water to eliminate diatoms contamination, epiphytes and other competing organisms. Then, the cleaned thalli, were placed in a tank with physicochemical parameters (dissolved oxygen, temperature and salinity) similar to those of the lagoon. Homogenous thalli with bright-red color, similar lengths and branches were selected.
Figure 1.
(a) Situation on Tunisia map within the Mediterranean region. (b) Gracilaria gracilis culture area. (c) Culture site location in the Bizerte Lagoon and Bay.
Cultivation of gracilaria gracilis
The G. gracilis culture experiments were conducted from March to May 2016 (90 days), in the Bizerte Lagoon (BL; 37°13N, 9°51′W) and Bay (BB; 37°15N, 9°59 W) in North Tunisia (Fig. 1). The experimental sites were located within sites specifically devoted to aquaculture with average water depths of 8 and 20 m for the BL and the BB, respectively. The suspended culture in the two sites was carried out using lantern nets (Fig. 2). The lantern net is formed by solid steel rings and crossbars coated with anti-corrosive. The crossbars were used to fix the G. gracilis thalli. Each lantern net is 3.75 m in length and contained fifteen experimental baskets (40 cm of diameter and 0.25 m in height) enclosed by monofilament netting of 6 mm mesh size. Each compartment has an opening through which thalli can be inserted or removed. Lantern nets loaded with thalli were hung down into the water column from long lines with buoys, which were placed in 1.3 m intervals. Lantern nets followed the tide without changing their position relative to the water surface during tidal cycles; get a vertical suspension due to a heavy-duty chain length. A synthetic rope of 36 mm in thickness and threefold the water depth in length was attached to the chain and rose to the marker buoys. Prepared tufts (500 g) were placed in the basket, being attached to the crossbars by a braid wire. Every lantern net, as well as baskets, was marked in such a way that each tuft represents an experimental unit. Three lantern nets were used in each site. As a result, at each depth, there were three experimental samples for statistical analyses. Lantern nets were then immersed in the filtered sea water, whose salinity was gradually increased into that of the BB to prevent thallus loss until they were transferred to the culture site.
Figure 2.

Lantern nets hang down into the water column in Bizerte Bay.
Weights of tufts were recorded at the beginning and at the end of the experiments to quantify algae growth. DGR was calculated by the formula in15:
| 1 |
where Wf is the final fresh weight after the t days of culture, and W0 is the initial fresh weight.
At the end of the culture period, a percentage tufts loss (PTL) was calculated using the following formula:
| 2 |
where Nf and N0 were the final and initial number of tufts.
Environmental measures
Three water samples were collected every month (March, April and May) from the BL and the BB, in acid-washed 1 L plastic bottles from a depth of 0.5 to 1 m using a Van Dorn water sampler three liters. For each sample taken, three replicates were analysed. Water samples fixed with 0.5 mL of H2SO4 4N. In the laboratory, triplicate samples (3 L) were filtered using Whatman® Grade GF/C Glass Microfiber filters to determine NO3−, NH4+ and PO4−. Water samples were analysed for Ν and Ρ according to16,17 methods. During the culture period Secchi depth, temperature, salinity and dissolved oxygen were measured. Three measures of Secchi depth per month were done according to the recommendations of18, by the same person at the same time of the day. Water temperature, oxygen and salinity were measured in situ using Hack multi-parameter (HQ40D), at the same time in the day, according to the recommendations of19.
Agar extraction and quality determination
At the end of the culture period, dry-cleaned seaweed samples were first washed with tap water to remove salts. Then, they were placed in 400 mL of a 5% H2SO4 solution for 1 h at room temperature and further rinsed thoroughly using tap water. Agar extraction was performed in acid-washed 500 mL glass bottles at 100 °C for 90 min using a 2.5 (% w/v) of dried alga and distilled water. The heated solution was then filtrated using a bûchner filter and a vacuum pump. The filtrate obtained was then transferred to a flat steeled recipient until it was cooled at room temperature for 20 min and then frozen overnight at −18 °C. Next day, the filtrate was thawed at room temperature until a thin agar film was formed. The agar yield was calculated as follows:
| 3 |
where Wa is the dry agar weight and Ws is the dry seaweed weight (g).
Sulfaphate content in polysaccharides was determined turbidimetrically using Barium chloride after acid hydrolysis as described by20. First, a barium chloride-gelatin solution was prepared by dissolving 600 mg of gelatin in 200 mL heated osmosis water (60–70 °C) and placed for 1 to 2 h at room temperature after cooling to 4 °C during 16 h. Then, 2 g of Barium chloride was dissolved in the resulting gelatin solution and left to stand for 2–3 h. Next, a hydrochloric acid solution (HCL 0.5N) was prepared. Thereafter, a sulfate standard curve was generated from a series of K2SO4 solutions (3 mg mL−1) containing between 0.02 and 0.20 mg mL−1. Lastly, agar (10 mg) was weighed and dissolved in 0.5 mL of hydrochloric acid for two hours at 100 °C. The hydrolyzate was compensated to 10 mL and centrifuged at 5000 G for 10 minutes. Then, 1 mL was placed in a test tube with the presence of 1 mL of HCl (0.5N) and 0.5 mL of barium chloride-gelatin solution, then compensated by 9 mL of osmosis water and mixed by vortex. After 15 min at room temperature, the absorbance was determined at λ = 360 nm with UV-visible spectrophotometer (Jenway 6405 type) and the absorbance value was obtained. Hydrochloric acid solution was used as a blank.
The 3,6-AG content was determined by the colorimetric method of21, using the resorcinol–acetal reagent and fructose as standard. The acetal solution was prepared by diluting 1 mL of acetal in 100 mL of deionized water. The resorcinol solution was prepared by diluting 150 mg of resorcinol in 100 mL of deionized water. Then, 9 mL of resorcinol solution was added to 1 mL of acetal solution diluted to 1/25. Next, 2 mL of agar solution (0.02 mg mL−1) was transferred into a test tube containing 10 mL of cold resorcinol-acetal reagent, mixed thoroughly and allowed to cool in ice bath for 3 min. Then, the tube was placed into water bath (80 °C) for 10 min. After cooling (15 mn) at room temperature, the absorbance was determined at λ = 555 nm with UV-visible spectrophotometer (Jenway 6405 type). A standard curve was prepared using D-fructose solution concentration, ranging from 0.018 to 0.09 mM. The 3,6-AG content was calculated and expressed as the percentage (dry weight basis). Experiments were performed in triplicate.
Determination of crude protein and R-PE
Crude protein was determined according to the22 method. Fresh alga (1 g) was placed in 20 mL of deionized water. A seaweed extract sample (1 mL) was putted in a hemolytic tube to which was added 2 mL of Coomassie Blue reagent and then homogenized. Absorbance was determined at λ = 595 nm after 5 min, using an UV-visible spectrophotometer (Jenway 6405 type). Curve calibration was performed using a Bovine Serum Albumin (BSA) solution between 0 and 2.0 mg mL−1. Protein content Q (mg) in seaweed samples was calculated as follows:
| 4 |
where C is the protein concentration (mg g−1 fresh weight), obtained using the calibration curve; V = initial sampling volume (mL). Results are presented as percentage of dry weight (15%). The R-PE content was determined as described by13 and the absorbance was measured at 565 nm, which is the maximum of R-PE absorbance. Beer-Lambert law established the absorbance at 565 nm as follows:
| 5 |
where C2 was calculated by the following equation:
| 6 |
A: absorbance at 565 nm,
ε: R-PE extinction coefficient (2.106 M−1 cm−1),
L: optic length (=1 cm),
C1: molar concentration of R-PE (M),
C2: Concentration of R-PE (mg mL−1),
MW: molecular weight of R-PE (260000 Da).
Statistical analyses
All results were expressed as mean ± Standard deviation. The Daily growth rate (% day−1), Agar yields (%), sulphate (%), 3,6-AG (%), protein (%) and R-PE (mg g−1 fw) obtained in different depths and sites were examined using the statistical package Statistica, version 5.123, as shown in Table 1. After verification of the homogeneity of the variances and the normality of the data, the results, were subjected to two-way ANOVA analysis to assess the impacts of sites and depths according to the GLM procedure. When ANOVA proved to be significant, the Duncan’s test was used to compare averages; the significance level of 5% was retained.
Table 1.
Physico-chemical parameters at the Bizerte Lagoon and Bay water. Values are means ± SD; n = 9 for physical parameters and n = 18 for chemical parameters. Means in same row followed by same superscript letter are not significantly different according to the Duncn’s test (P > 0.05).
| BL | BB | |
|---|---|---|
| Chemical parametres | ||
| Nitrate (μ mol L−1) | 21.87a ± 2.17 | 1.64b ± 0.59 |
| Ammonium (μ mol L−1) | 13.32a ± 2.80 | 11.73a ± 1.91 |
| Orthophosphate (μ mol L−1) | 4.53a ± 0.49 | 3.54a ± 0.71 |
| Physical parametres | ||
| Secchi depth (m) | 2.37a ± 0.25 | 4.50b ± 0.50 |
| Salinity (psu) | 35.86a ± 0.04 | 36.90b ± 0.36 |
| Temperature (°C) | 17.45a ± 3.57 | 19.43a ± 1.25 |
| Dissolved oxygen (mgL−1) | 7.98a ± 0.02 | 8.28a ± 0.71 |
| pH | 8.18a ± 0.07 | 8.36a ± 0.16 |
BL: the Bizerte lagoon; BB: the Bizerte bay; Means in same row followed by same superscript letter are not significantly different (P > 0.05).
Ethical statement
This article doesn’t contain any studies with animals performed by any of the authors.
Results
The physicochemical characteristics of water in the BL and the BB, during the culture period, are shown in Table 1. Consequently, the water transparency (m), salinity (psu) and nitrate concentration (µmol L−1) varied significantly between the BL and the BB. In contrast, the water temperature (°C), dissolved oxygen (mgL−1), pH, ammonium (µmol L−1) and orthophosphate concentration (µmol L−1) were similar. The BL water was less transparent compared to the BB (2.37 ± 0.25 m vs 4.50 ± 0.50 m). The average salinity were different between the BL and the BB (35.86 ± 0.04 psu vs 36.90 ± 0.36 psu). The nitrate showed the higher concentrations in the BL compared to the BB (21.87 ± 2.17 μmol L−1 vs 1.64 ± 0.59 μmol L−1).
Results of DGR (%), agar yields (%),3,6-AG (%), sulphate (%), proteins (%) and R-PE (mg g−1 fresh wt) were shown in Tables 2, 3, 4 and Supplementary Fig. S1. Hence, G. gracilis grows differently (P < 0.05) between the site and the culture depth (Table 2). Accordingly, the DGR recorded in the BL was higher than that of the BB (1.42 ± 0.65% day−1 vs 1.19 ± 0.34% day−1). In the BL, we recorded a decrease of DGR from the surface to 3.75 m. Consequently, a highest DGR were recorded between the surface and 1 m. As a result, DGR recorded at 1 m is the highest (2.33 ± 0.03% day−1). Whereas, a lowest DGR ( <1%) was recorded over 3 m. In addition, the DGR at 3.5 m does not exceed 0.5% day−1 and showed null values at 3.75 m, by far the lowest, due to thallus degeneration. In contrast, DGR recorded in the BB were homogenous in the studying depths (p > 0.05) and varied from 0.86 ± 0.32% day−1 to 1.57 ± 0.42% day−1 (Table 3). In the BL, the thalli loss was insignificant from the water surface to 1.5 m. Consequently, the PTL ranges between 1% and 2%. In contrast, in the BB, up to 1.5 m, we recorded the higher PTL, especially near the water surface (10%).
Table 2.
Daily Growth Rate (DGR %), Agar yield (%), Sulfate (%), 3,6-Anhydrogalactose (3,6-AG %), Proteins (%) and R-phycoerythrin (R-PE mg g−1fw) obtained from Gracilaria gracilis samples cultured at different depths in two sites (Bizerte Lagoon and Bizerte Bay, Northeast Tunisia) analyzed by ANOVA at 95% confidence level.
| Factors | SS | df | MS | F | p |
|---|---|---|---|---|---|
| DGR (%) | |||||
| Intercept | 154.07 | 1 | 154.07 | 1557.88 | <0.05 |
| Site | 1.22 | 1 | 1.22 | 12.36 | <0.05 |
| Depth | 7.85 | 14 | 0.56 | 5.67 | <0.05 |
| Site*Depth | 10.24 | 14 | 0.73 | 7.39 | <0.05 |
| Error | 5.93 | 60 | 0.10 | ||
| Agar (%) | |||||
| Intercept | 40636.38 | 1 | 40636.38 | 6314.463 | <0.05 |
| Site | 382.75 | 1 | 382.75 | 59.475 | <0.05 |
| Depth | 110.93 | 14 | 7.92 | 1.231 | >0.05 |
| Site*Depth | 58.34 | 14 | 4.17 | 0.648 | >0.05 |
| Error | 386.13 | 60 | 6.44 | ||
| Sulfate (%) | |||||
| Intercept | 4932.622 | 1 | 4932.622 | 3507.418 | <0.05 |
| Site | 176.084 | 1 | 176.084 | 125.207 | <0.05 |
| Depth | 54.125 | 14 | 3.866 | 2.749 | <0.05 |
| Site*Depth | 74.098 | 14 | 5.293 | 3.763 | <0.05 |
| Error | 84.380 | 60 | 1.406 | ||
| 3,6-AG(%) | |||||
| Intercept | 97159.40 | 1 | 97159.40 | 36228.44 | <0.05 |
| Site | 6518.91 | 1 | 6518.91 | 2430.75 | <0.05 |
| Depth | 853.10 | 14 | 60.94 | 22.72 | <0.05 |
| Site*Depth | 1196.31 | 14 | 85.45 | 31.86 | <0.05 |
| Error | 160.91 | 60 | 2.68 | ||
| Proteins(%) | |||||
| Intercept | 41150.41 | 1 | 41150.41 | 979.3834 | <0.05 |
| Site | 36.62 | 1 | 36.62 | 0.8715 | >0.05 |
| Depth | 976.25 | 14 | 69.73 | 1.6596 | >0.05 |
| Site*Depth | 661.06 | 14 | 47.22 | 1.1238 | >0.05 |
| Error | 2521.00 | 60 | 42.02 | ||
| R-PE(mgg−1fw) | |||||
| Intercept | 31.92 | 1 | 31.92 | 535.76 | <0.05 |
| Site | 6.30 | 1 | 6.30 | 105.83 | <0.05 |
| Depth | 1.15 | 14 | 0.08 | 1.38 | >0.05 |
| Site*Depth | 0.23 | 14 | 0.02 | 0.27 | >0.05 |
| Error | 3.57 | 60 | 0.06 | ||
Table 3.
Daily growth rate and proximate biochemical composition of Gracilaria gracilis cultivated in Bizerte lagoon (BL) and bay (BB) at different depth (from 0.25 m to 3.75 m). Values are means ± SD; n = 9. Means with the same superscripts are not significantly different (p = 0.05).
| Depth (m) | DGR (%) | Agar (%) | Sulfate (%) | 3,6-AG(%) | Proteins (%) | R-PE (mgg−1fw) | |
|---|---|---|---|---|---|---|---|
| BL | 0.25 | 2.33a ± 0.03 | 18.40a ± 1.44 | 7.97e ± 0.54 | 32.41a ± 1.39 | 22.06a ± 10.72 | 0.25a ± 0.05 |
| BL | 0.50 | 1.96ab ± 0.46 | 18.07a ± 2.25 | 8.70bcde ± 0.83 | 32.79a ± 1.81 | 17.10a ± 6.96 | 0.24a ± 0.04 |
| BL | 0.75 | 2.06ab ± 0.32 | 20.70a ± 0.26 | 8.43cde ± 0.54 | 32.98a ± 2.30 | 20.13a ± 7.76 | 0.25a ± 0.03 |
| BL | 1.00 | 1.96ab ± 0.19 | 19.43a ± 2.63 | 8.13e ± 0.40 | 40.68a ± 3.30 | 16.36a ± 6.58 | 0.31a ± 0.09 |
| BL | 1.25 | 1.75abc ± 0.27 | 18.90a ± 1.65 | 8.28de ± 0.29 | 29.98a ± 0.57 | 18.50a ± 7.42 | 0.28a ± 0.03 |
| BL | 1.50 | 1.58bc ± 0.13 | 21.23a ± 3.96 | 8.39cde ± 0.17 | 31.47a ± 1.34 | 16.56a ± 6.30 | 0.31a ± 0.06 |
| BL | 1.75 | 1.34bc ± 0.39 | 17.43a ± 2.67 | 9.42abcd ± 0.24 | 32.64a ± 1.15 | 21.86a ± 9.56 | 0.36a ± 0.16 |
| BL | 2.00 | 1.49bc ± 0.27 | 19.27a ± 1.36 | 9.58abc ± 0.17 | 31.93a ± 1.86 | 21.77a ± 8.46 | 0.37a ± 0.19 |
| BL | 2.25 | 1.42bc ± 0.11 | 21.57a ± 3.71 | 9.42abcd ± 0.29 | 30.42a ± 1.10 | 22.64a ± 10.59 | 0.37a ± 0.17 |
| BL | 2.50 | 1.53bc ± 0.14 | 18.50a ± 2.11 | 7.52e ± 0.52 | 35.97a ± 5.95 | 23.97a ± 8.64 | 0.41a ± 0.19 |
| BL | 2.75 | 1.43bc ± 0.16 | 19.17a ± 2.10 | 9.69ab ± 0.46 | 36.43a ± 0.63 | 29.63a ± 8.23 | 0.43a ± 0.21 |
| BL | 3.00 | 1.05c ± 0.15 | 18.00a ± 2.55 | 8.62bcde ± 0.40 | 30.73a ± 0.46 | 27.18a ± 6.52 | 0.45a ± 0.19 |
| BL | 3.25 | 1.05cd ± 0.07 | 18.80a ± 4.16 | 9.96a ± 0.50 | 35.04a ± 1.84 | 26.05a ± 2.79 | 0.30a ± 0.03 |
| BL | 3.50 | 0.57d ± 0.25 | 19.07a ± 0.95 | 7.97e ± 0.37 | 27.26a ± 0.53 | 22.30a ± 3.09 | 0.32a ± 0.05 |
| BL | 3.75 | −0.16e ± 0.61 | 19.27a ± 1.82 | 9.96a ± 0.70 | 32.12a ± 1.52 | 24.20a ± 4.05 | 0.32a ± 0.05 |
| BB | 0.25 | 0.86a ± 0.32 | 21.13a ± 1.44 | 7.74ab ± 2.60 | 45.82abc ± 1.27 | 15.38a ± 5.69 | 0.63a ± 0.13 |
| BB | 0.50 | 1.03a ± 0.48 | 24.03a ± 2.25 | 5.38ab ± 0.75 | 46.12ac ± 2.78 | 15.47a ± 3.85 | 0.64a ± 0.11 |
| BB | 0.75 | 0.99a ± 0.43 | 22.47a ± 0.26 | 7.40ab ± 2.38 | 40.78bcd ± 0.81 | 27.74a ± 0.47 | 0.65a ± 0.09 |
| BB | 1.00 | 0.91a ± 0.78 | 20.87a ± 2.63 | 5.91ab ± 0.46 | 41.31abcd ± 0.41 | 16.30a ± 4.70 | 0.73a ± 0.22 |
| BB | 1.25 | 1.57a ± 0.42 | 22.73a ± 1.65 | 5.42ab ± 1.37 | 39.94bd ± 1.51 | 15.45a ± 2.76 | 0.77a ± 0.09 |
| BB | 1.50 | 1.32a ± 0.39 | 23.40a ± 3.96 | 8.28a ± 1.85 | 40.05d ± 1.93 | 25.39a ± 6.42 | 0.81a ± 0.15 |
| BB | 1.75 | 1.27a ± 0.15 | 21.27a ± 2.67 | 7.90ab ± 1.79 | 46.96a ± 4.52 | 18.14a ± 3.04 | 0.82a ± 0.41 |
| BB | 2.00 | 1.50a ± 0.29 | 25.23a ± 1.36 | 4.58ab ± 0.71 | 39.83bcd ± 0.30 | 19.23a ± 6.00 | 0.82a ± 0.49 |
| BB | 2.25 | 1.38a ± 0.07 | 25.50a ± 3.71 | 5.84ab ± 2.89 | 37.96d ± 0.35 | 28.88a ± 10.19 | 0.82a ± 0.43 |
| BB | 2.50 | 1.23a ± 0.01 | 22.47a ± 2.11 | 5.30ab ± 0.86 | 42.19abcd ± 2.94 | 17.06a ± 1.09 | 0.93a ± 0.50 |
| BB | 2.75 | 1.18a ± 0.11 | 22.93a ± 2.10 | 8.39b ± 0.29 | 45.74ac ± 1.52 | 20.87a ± 5.09 | 0.96a ± 0.56 |
| BB | 3.00 | 1.17a ± 0.21 | 23.60a ± 2.55 | 4.84ab ± 1.32 | 36.74d ± 2.29 | 23.44a ± 4.41 | 0.97a ± 0.49 |
| BB | 3.25 | 1.18a ± 0.22 | 22.90a ± 4.16 | 3.40b ± 1.04 | 38.61d ± 5.02 | 18.61a ± 2.91 | 1.07a ± 0.07 |
| BB | 3.50 | 1.09a ± 0.23 | 24.90a ± 0.95 | 5.19ab ± 1.94 | 39.41d ± 0.37 | 20.86a ± 7.37 | 1.12a ± 0.13 |
| BB | 3.75 | 1.19a ± 0.24 | 26.23a ± 1.82 | 4.50ab ± 1.00 | 39.06d ± 1.44 | 28.38a ± 5.29 | 1.16a ± 0.12 |
BL: the Bizerte Lagoon; BB:the Bizerte Bay; DGR: Daily Growth Rate;3,6-AG(%):3,6-anhydrogalactose; R-PE: R-phycoerythrin; SD: Standard deviation.
Table 4.
Daily growth rate and proximate biochemical composition of Gracilaria gracilis cultivated in Bizerte Lagoon and Bizerte Bay. Values are means ± SD of results obtained at different depth (from 0.25 m to 3.75 m); n = 45. Means in same row followed by same superscript letter are not significantly different according to the Duncn’s test (P > 0.05).
| BL | BB | |
|---|---|---|
| DGR(%) | 1.42a ± 0.65 | 1.19b ± 0.34 |
| Agar(%) | 19.19b ± 2.32 | 23.31a ± 2.64 |
| Sulfate(%) | 8.80a ± 0.86 | 6.00b ± 2.00 |
| 3,6-AG(%) | 23.30b ± 5.40 | 41.36a ± 3.68 |
| Proteins(%) | 20.74a ± 7.22 | 22.02a ± 6.34 |
| R-PE(mgg−1fw) | 0.33b ± 0.12 | 0.86a ± 0.31 |
BL: the Bizerte Lagoon; BB: the Bizerte Bay; DGR: Daily Growth Rate;3,6-AG(%):3,6-anhydrogalactose; R-PE: R-phycoerythrin; SD: Standard deviation.
The agar yields (Tables 3 and 4), which depend (p < 0.05) on the site of production, were 19.19 ± 2.32% and 23.31 ± 2.64% for the BL and the BB respectively. Inversely, the effect of the culture depth was not significant (p > 0,05) and homogeneous distribution in the whole studying water column was recorded. The 3,6-AG content and sulphate degree of agar varied significantly between the sites and the depths (p < 0.05). In the BB, the 3,6-AG content of agar was twice as high as that obtained in the BL (41.37 ± 3.68% vs 23.30 ± 5.40%) (Table 4). In addition, in the BL, the highest 3,6-AG content was recorded at 3.25 m, the lowest at 3.50 m and an irregular distribution between the other depths (Table 3). However, in the BB the highest amount of 3,6-AG (> 45%) was found near the water surface (<0.5 m), followed by a stationary phase between 0.5 m and 3 m; values varied between 40% and 45%. The lowest values (<40%) were recorded over 3 m (Table 3). The algae cultivated in the BL has the higher sulphate degree than those cultivated in the BB (6 ± 2.00% vs 8.80 ± 0.86%) (Table 3). Furthermore, in the BL the highest sulphate degree (10%) was attained over 3 m, and the lowest (7.50%) at 2.50 m. Nevertheless, in the BB, the lowest sulphate degree (3.40%) recorded at 3.25 m (Table 3).
The site and the depth does not affect the protein content of the alga (p > 0.05). Accordingly, the contents recorded were 22.02 ± 6.34% and 20.74 ± 7.22% for the BL and the BB respectively (Table 4). The R-PE contents in the BL (0.33 ± 0.12 mg.g−1 fwt) was lower (p < 0.05) than that obtained in the BB (0.86 ± 0.31 mg. g−1 fwt) and values were homogeneously distributed across the depths in both sites (Table 3).
Discussion
The physicochemical parametres of culture site
The lower Secchi depth (2 m) obtained in the BL is due to the turbidity. However, the muddy bottom and the wind increase the turbidity of the water. Likewise, the lagoon receives several urban and industrial discharges from the around cities, other than the sediments from the rivers24. Unlike the BL, Secchi depth obtained in the BB occurs in the water depths greater than 20 m as indicated by25. The salinity value in the BL is similar to that recorded by26,27. The water temperature values reported in our study were similar to that obtained by26–28, which were (15–23 °C), (15–25 °C) and (19–20 °C) respectively. In the BL, there is no vertical gradient of salinity or temperature as indicated for other lagoons29,30, probably due to the shallow depth. The salinity and temperature values recorded in the BB between March and May are consistent with those reported previously (19 °C and 37 psu)[84].
The nutrient concentrations in the BL are comparable to those of a Mediterranean Sea Lagoon, Lake Burullus in Egypt31. Accordingly, the authors reported nutrient concentrations in spring as follows: nitrate (7.41–28.90 µM), ammonium (7.34–34.30 µM) and phosphates (8.59–24.30 µM). In addition, a result of a physicochemical assessment of the Nador Lagoon water quality (Northeast Morocco), indicates a varied nitrate (1.08–29.28 µM) and phosphates (1–7 µM) concentrations32. Furthermore, a nitrate (0.35–52.4 µM) and phosphate (0.41–2.24 µM) concentrations were determined in Mar Chiquita, a coastal lagoon in Argentina (South Atlantic Ocean)33. According to34, there is no vertical stratification of different nutrients (ammonium, nitrate and orthophosphate) in the BL. The lagoon receives in winter and spring an important flow of nutrients (liquid and solid) from their watershed, containing a very high quantity of nitrate compared to the BB35. In conclusion, during the culture period, the BB has a higher transparency but a lower nitrogen (nitrate + ammonium) concentration compared to the BL.
Growth
For G. gracilis growth, the DGR recorded in the BL and the BB are in the range of those recorded in outdoor culture, in Tunisia or in others regions, which generally varied between 1% and 4% day−1 8,9,36,37. In contrast, indoor culture the DGR attained 10% day−1 or higher38. The difference between the results could be due to the nitrogen concentration of the medium (nitrate + ammonium), which falls within the range of outdoor concentrations found in other studies. However, in the BL and the BB, the nitrogen concentration is too low (<50 µM) to sustain the high seaweed DGR required for biomass production as indoor culture (>1000 µM) due to the highly nitrophilic character of G. gracilis. Hence, the higher growth rate in the BL can be attributed to the nitrogen enrichment due to surface run off into the lagoon, which allows the alga to meet their nitrogen requirements compared to the BB. Moreover, the water transparency, act differently on G. gracilis growth39. Consequently, lower light and higher nitrogen in the BL enhance DGR in contrast to lower nitrogen and higher light in the BB, which reduce algae growth. Hence, the difference between the findings could be due to the significant effect of light and nitrogen interaction on G. gracilis growth as indicated by39. In addition, the lower DGR (<3%) obtained in the BL and the BB could be due to the temperature and salinity values, which were out of the optimum growth range. Wide temperature (0–35 °C) and salinity (10–40 psu) tolerance of Gracilaria spp. has been reported but the optimum growth has been recorded in a restricted ranges (20 °C–28 °C; 25 psu −30 psu)40–42. In contrast, the water temperature and salinity values in the BL and the BB, during the experimental period, are outside the optimum range of alga growth (<20 °C, >30 psu) or near to their lower growth limit. Finally, based on the DGR obtained, the plants reached a harvestable size after 90 days in the BL and 110 days in the BB, but it is only of 30 days in Chilika Lake in India43. The difference between results may be attributed to the physicochemical characteristics of the water in both sites.
Our data show a markedly difference in DGR of G. gracilis, across the depths in both sites. However, light intensity is a relevant factor, which affects algae growth. In the BL, the highest DGR values were obtained in the shallowest depth (<1 m) and the lowest in deeper one (>3 m). Our findings are consistent with those of44–47. Consequently, they indicate that the lower DGR of Gracilaria spp. obtained is explained by the reduced light intensity due to the high turbidity essentially in eutrophic lagoon. In contrast, the DGR of G. gracilis in the BB was not affected in a depth of 4 m, suggesting the availability of enough light quantities in the studying depths. According to48,49, Gracilaria can grow in the depths between 8 m and 10 m, but over 4 m their growth is largely affected; especially in a turbid environment that do not let light through.
In our study the PTL is low than that recorded in the same lagoon and culture period, using bottom planting methods50. The studies on G. gracilis farming carried out in the BL, by these methods, revealed that the PTL constituted a detrimental factor50. However, algae losses are related to difficulties in inserting tufts on ropes50, epiphytism51 and associated fauna, which contain zoological groups that use the genus Gracilaria for habitat and food52. In our study no epiphytes or epifauna associated to G. gracilis in both sites, which could explain the lower PTL. The problems of grazing and entanglement by other epiphytes were controlled upto higher level by the lantern net, which covered all sides of the thallus. The maximum loss was recorded at the shallow depths (<1.5 m) due to fragmentation of the thalli into small parts (<1 cm). However, this size facilitates their escape through the mesh of the net in addition to the decomposition of those that remain trapped. The Gracilaria culture like any other mariculture activity has an impact on the marine environment. According to53,54, there was a decrease in the growth rate of Zostera japonica but an increase in the abundance and diversity of invertebrates in the community under the cultivated ropes of Gracilaria spp. In the BL, there is no development of seaweeds over 5 m, which avoids any interaction between Gracilaria and other species. Lantern nets developed for our study have more advantages than the benthic or the suspended culture methods using thalli inserted on the ropes50. However, G. gracilis productivity is higher and the PTL is lower. This advantage is essentially linked to the attenuation of wave effects on G. gracilis thalli, which depend on the site and the depth. However, near the sea bottom, the wave velocity decrease (<0.2 m s−1), whereas near the surface increase (0.38 ms−1), which can explain the higher PTL recorded in this part of the water column. In addition, in our study an improvement in attachment to nets covering the hoops (0.5 m2) is a mechanism that enables alga to survive the wave action and currents occur in this zone, which result in higher biomass production. In the BL, the marine wave velocity is low (<0.2 m s−1), which could explain their little shearing actions on the thallus24,55. In conclusion, the lower PLT obtained, the better we need for G. gracilis culture success in these regions.
Based on DGR, after one month when we start from a stocking density of 7.5 kg m−2, we will reach a final density of 11.5 kg m-2, generating a multiplication factor of 1.5. However, this value is relatively higher than that obtained by the benthic culture system used in the BL50 but was similar to those obtained by the suspended culture system in the sea, for other Gracilaria species. Accordingly, the multiplication factor values obtained were 1.2 for Gracilaria caudata45; 2 for Gracilaria chilensis56; 2.03 for Gracilaria chilensis57; 2.16 for Gracilaria chilensis58 and 6.36 for Gracilaria sp.47.
Agar yields and composition
Agar yields of G. gracilis obtained in the BL and the BB were comparable to those previously found for G. gracilis and others species, which varied from 20% to 30%59–61. In contrast, a lower agar yield of G. gracilis was obtained in a shallow part of the BL9. However, not all studies agree with this finding due to the difference between species and culture sites. The multiple environmental factors, such as nutrient status, light, salinity and water temperature, could affect agar yield2,39. The higher agar yields obtained in the BB, could be due to the high salinity level, light quantity and a lower nitrate concentrations than that recorded in the BL39. The culture depth of G.gracilis in the BL and the BB didn’t affect the agar yields in contrary to previous findings in the BL by9, which indicated a higher agar yields at a depth of 2 m compared to those obtained at 0.5 m. Unlike the previous work, this study was performed in the eastern part of the lagoon, characterized by a higher turbidity (Secchi depth <0.5 m). Consequently, the amount of light is insufficient (<70 µmol m2 s−1) to interact with other factors and allowing the alga to produce higher agar yields39,62. Accordingly, in the BL the lower light effect on G.gracilis at a depth over 2 m, which superior to a critical value indicated above, may be alleviated by the interaction of light and other factors. However, when agar yield was affected, interactions between abiotic factors (light, salinity and nitrogen) alleviate the negative impact and maintain the yield similar to that obtain at the shallow depths39. In contrast, light amount in the BB, which is independent of the depths, can explain the homogeneity of the agar yields along water column.
Agar composition (sulphate degree and 3,6-AG content) obtained in both sites (the BL and the BB) was consistent with those of63, which indicate that higher agar yields was accompanied by higher 3,6-AG content and lower sulphate degree. The ideal structure of agarocolloid is a non-substituted galactan backbone composed of repeating units of (1,3)-linked-D-galactose and (1,4)-linked 3,6-anhydro-α-L-galactose. However, native agarocolloids are generally a mixture of neutral, sulphated, methylated and pyruvated agarose, which influences their rheological properties. According to64,65, the gel properties (gel strength, gelling temperature and melting temperature), which are the most important criteria to evaluated agar, are highly dependent on the amount of sulphate groups as well as the 3,6-AG content. Accordingly, the higher the 3,6-AG content and the lower the sulphate degree, the better agar gel strength. However, the quality of agar from the BB (41.36 ± 3.68% of 3,6-AG and 6.00 ± 2.00% of sulphate) approach that of agarose (48% of 3,6-AG and 2% of sulphate). In contrast, the agar obtained in the BL is of lower quality due to the lower percentage of 3,6-AG and higher sulphate degree (23.30 ± 5.40% of 3,6-AG and 8.80 ± 0.86% of sulphate). Similar results were obtained in the BL9.
Proteins and R-PE contents
The protein contents of G. gracilis (>25%), are lower than that obtained by10,66 but are higher than those obtained by67,68, which indicates a value varying between 11% and 20%. The difference between results could be due to the environment factors and extraction methods. Hence, in both sites (the BL and the BB) ammonium is a limiting factor, which affects positively chlorophyll and proteins contents but negatively the carbohydrate69. The ammonium concentration in the lagoon and the bay does not meet the G. gracilis requirement to produce proteins contents higher than 20%. In addition, the higher proteins contents were obtained at a salinity lower than 30 psu. The similar proteins contents obtained in both sites despite the difference in nitrate concentration and light quantity, were due to the interaction between them39. In addition, the differences between the results could be attributed to the extraction methods. However, enzyme-assisted extraction of G. gracilis produces a high proteins contents compared to the native method13.
The R-PE is the most abundant phycoerythrins in Rhodophyta70, which does not exceed 10 mgg−1 in Gracilaria species71. However, their concentrations were inversely proportional to the growth rate and vary considerably with environmental factors; essentially light39,72. The lower pigments contents of G. gracilis in the BL compared to the BB, can be attributed to their higher growth. In addition, the difference between the results could be due to the amount of quantity of light available. However, the inverse relationship between R-PE contents and light intensity is well established72–74. Accordingly, a decrease in light intensity enhances the alga pigments accumulation75. Red algae grow under intense light, accumulate few phycobilisomes and lower phycobiliproteins content compared to those grow under a low light intensity76,77. However, the Secchi depth in the BB was higher than the depth of G.gracilis culture, which indicates that the amount of light is homogeneous. In contrast the homogeneity of R-PE in the BL despite the Secchi depth (<2.5 m) can be attributed to the effects of others factors. For this purpose, light effect on G.gracilis R-PE contents depend mainly on nitrogen concentration, salinity and their interaction39,78,79. Probably without the interaction mechanisms, there will be differences in the amount of R-PE between the depths.
Aquaculture and biotechnology relevance of results
We stated that the lagoon was characterized by a higher nitrogen concentration and a lower amount of light. In contrast the opposite was happened in the bay. The red alga G. gracilis grow well under higher nitrogen concentrations and light quantity, as in many coasts of Asian countries. Accordingly, the DGR will be limited in our conditions compared to those in India coasts43. However, in those countries, with the same period of culture, we obtain two production cycles (between 30 and 45 days each one) and only one in Tunisia (between 90 and 110 days). In addition, in the Asian regions there are two growth periods of this alga; it is feasible to obtain four production cycles per year43. Contrarily, in Tunisia and the Mediterranean region in general, there is only one period of growth (spring) and only one development cycle per year4,8,50. Accordingly, what we presented as a result allows us to prepare a pilot scale up, which is necessary before starting Gracilaria aquaculture in the Mediterranean region to boost their productivity by suspended methods. The pilot scale up can spend two years (one cycle/year) and a socioeconomic study can be started. If we consider this to be our ultimate goal, the results obtained in this study allowed us to gather maximum information’s on the subject and facilitates the starting alga culture in an industrial way in the near future.
In the world, the market price of Gracilaria is related to the agar yields and quality. The Gracilaria gracilis, is used in several industrials sectors mainly food due to their agar yields. As the agar yields of alga harvested from the BB was higher with better quality, compared to the BL, the total economic value might remain the same. If the physicochemical parameters of the BL and the BB generate a low DGR, which limit the alga quantities, we propose to improve the total economic value of the biomass by the extraction of others interested molecules such as proteins and R-PE. In our study protein content can be compared to other high-protein foods such as soya (30%), beef (25%) or salmon (20%)80. In addition, a valuable phytochemical may be co-extracted with proteins such us polyphenols, pigments and enzymes having higher additional values, which may be of interest13,14,81. A sequential extraction of agar and other compounds was developed with fresh G. verrucosa14, but it is possible to use dried algae instead of fresh. Increasing interest is being given in the last decades to Gracilaria drying. Consequently, a drying technologies were developed allowing the recovery of proteins, R-PE and agar in sufficient quantity82–84.
Tunisian aquaculture sector have highly improved during the last two decades; indeed, the national production passed from 2000 tons in 2000 to 20 000 tons in 2017 and the number of aquaculture societies was ten times higher85. The marine fish farming production represents 90% of the total production. However, we have interested to minimize negative environmental impacts of this activity. The integrated aquaculture systems (IMTA) based on integrated culturing of finfish, seaweed and mussels, have to contribute to the sustainability of aquacultures, but their development requires further research to optimize the technique, which depends on the selected seaweed species and the system of fish farming. In IMTA, the red algae Gracilaria utilize photosynthesis to convert inorganic nutrients into organic molecules and reduce the negative environmental impact of aquaculture activity. Consequently, based on the results of growth and biochemical composition obtained in the BB, Gracilaria gracilis could be used in IMTA systems due to the homogenous growth and chemical composition obtained. Furthermore, it can absorb a higher quantity of nitrogen, under changing abiotic factors, which can be produced by the system39.
Conclusions
Our results highlight that G. gracilis, cultivated in the BL and the BB using suspended method, possess active growth and interesting concentrations of chemical components (agar, proteins, and R-PE). However, we obtain a better productivity, a higher tufts recovery and larger exploited surface than the culture on the substrate. Furthermore, no direct interaction between the alga and the flora. The light was the key factor; the water depth acts differently. However, in the BL the DGR was affected over the depth of 1.5 m but in the BB is not affected up to 4 m. The choice of the depth and site culture may depend on the growth and the final use of biomass (agar, proteins and R-PE). With the higher agar yields in the BL and the BB, suspended culture of G. gracilis in the BB is much more attractive due to the higher protein content, R-PE amount and the better agar quality.
Supplementary information
Acknowledgements
This study was funded by Ministry of Higher Education and Scientific Research, Tunisia. Special thanks to Superior Institute of Fishing and Aquaculture of Bizerte responsible, for sharing facilities and logistic support. The authors would like to thank the head of B3Aqua laboratory, INSTM – Salammbo, for providing the reagent. We are grateful to Hammami wahiba for preparing all algae culture activities.
Author contributions
F. Mensi wrote the main manuscript text. F. Mensi, S. Nasraoui and S. Bouguerra were responsible for the experimental design. F. Mensi, S. Nasraoui, S. Bouguerra and M. Chalghaf were responsible for the field experimentation. S. Nasraoui, S. Bouguerra and A. Ben Ghedifa carried out the biochemical analyses. All authors reviewed the manuscript.
Data availability
The datasets generated during the current study are available on request to the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-66003-y.
References
- 1.F.A.O. The state of world fisheries and aquaculture 2018 - Meeting the sustainable development goals (Rome, 2018).
- 2.Lee WK, et al. Factors affecting yield and gelling properties of agar. J. Appl. Phycol. 2017;29:1527–1540. [Google Scholar]
- 3.Sfriso A, Maistro S, Andreoli C, Moro I. First record of Gracilaria vermiculophylla (gracilariales, rhodophyta) in the Po delta lagoons, Mediterranean sea (italy) J. Phycol. 2010;46:1024–1027. [Google Scholar]
- 4.Ksouri J, Ben Said R. Potentialités en macroalgues: cartographie et biomasse de l’agarophyte Gracilaria dans le lac de Bizerte. Bull. Inst. Natn. Scien. Tech. Mer de Salammbô. 1998;25:17–34. [Google Scholar]
- 5.Menéndez M, Comin FA. Spring and summer proliferation of floating macroalgae in a Mediterranean coastal lagoon (Tancada Lagoon, Ebro Delta, NE Spain) Estuar. Coast. Mar. Sci. 2000;51:215–226. [Google Scholar]
- 6.Sfriso A, Wolf MA, Maistro S, Sciuto K, Moro I. Spreading and autoecology of the invasive species Gracilaria vermiculophylla (Gracilariales, Rhodophyta) in the lagoons of the north-western Adriatic Sea (Mediterranean Sea, Italy) Estuar. Coast. Shelf Sci. 2012;114:192–198. [Google Scholar]
- 7.Khaled A, Hessein A, Abdel-Halim AM, Morsy FM. Distribution of heavy metals in seaweeds collected along Marsa-Matrouh beaches, Egyptian Mediterranean Sea. Egypt. J. Aquat. Res. 2014;40:363–371. [Google Scholar]
- 8.Mensi F, Ksouri J, Hammami W, Romdhane MS. État des connaissances et perspectives de recherches sur la culture de Gracilariales (Gracilaria et Gracilariopsis):application a la lagune de Bizerte. Bull. Inst. Natn. Scien. Tech. Mer de Salammbô. 2014;41:101–119. [Google Scholar]
- 9.Ben Said R, et al. Effects of depth and initial fragment weights of Gracilaria gracilis on the growth, agar yield, quality, and biochemical composition. J. Appl. Phycol. 2018;30:2499–2512. [Google Scholar]
- 10.Francavilla M, Franchi M, Monteleone M, Caroppo C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs. 2013;11:3754–76. doi: 10.3390/md11103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francavilla M, Manara P, Kamaterou P, Monteleone M, Zabaniotou A. Cascade approach of red macroalgae Gracilaria gracilis sustainable valorization by extraction of phycobiliproteins and pyrolysis of residue. Bioresour. Technol. 2015;184:305–313. doi: 10.1016/j.biortech.2014.10.147. [DOI] [PubMed] [Google Scholar]
- 12.Van Hal JW, Huijgen W, López-Contreras A. Opportunities and challenges for seaweed in the biobased economy. Trends Biotechnol. 2014;32:231–233. doi: 10.1016/j.tibtech.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Mensi F, Ksouri J, Seale E, Romdhane MS, Fleurence J. A statistical approach for optimization of R-phycoerythrin extraction from the red algae Gracilaria verrucosa by enzymatic hydrolysis using central composite design and desirability function. J. Appl. Phycol. 2012;24:915–926. [Google Scholar]
- 14.Mensi F. Agar yield from R-phycoerythrin extraction by-product of the red alga Gracilaria verrucosa. J. Appl. Phycol. 2019;31:741–751. [Google Scholar]
- 15.Dawes CJ, Lluisma AD, Trono CG. Clonal propagation of Eucheuma denticulatum and Kappaphycus alvarezii for Philippine seaweed farms. Hydrobiologia. 1993;260/261:379–383. [Google Scholar]
- 16.Solorzano L. Determination of ammonia in natural waters by the phenolhypochloride method. Limnol. Oceanogr. 1969;14:799–801. [Google Scholar]
- 17.Murphy J, Riley JP. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962;27:21–36. [Google Scholar]
- 18.Preisendorfer RW. Secchi disk science: visual optics of natural waters. Limnol. Oceanogr. 1986;31:909–926. [Google Scholar]
- 19.Fonseca, M. S. Physical Measurements in Seagrass Research Methods (ed Phillips, R. C. and McRoy, C. P.). UNESCO, (Paris 1990).
- 20.Lloyd AG, Dodgson KS, Price RG, Rose FAI. Infrared studies on sulphate esters. I. Polysaccharide sulphates. Biochim. Biophys. Acta. 1961;46:108–115. doi: 10.1016/0006-3002(61)90652-7. [DOI] [PubMed] [Google Scholar]
- 21.Yaphe W, Arsenault GP. Improved resocinol reagent for the the determination of fructose,and of 3,6-anhydrogalactose in polysaccharides. Anal. Biochem. 1965;13:143–148. [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 23.Statoft. Statistica Electronic Manual. Version 5.1. Statsoft Inc., Tulsa, USA (1995).
- 24.Harzallah A. Transport de polluants dans la lagune de Bizerte simule par un modèle de circulation de l’eau. Bull. Inst. Natn. Scien. Tech. Mer de Salammbô. 2003;30:121–133. [Google Scholar]
- 25.Nishijima W, Umehara A, Sekito S, Okuda T, Nakai S. Spatial and temporal distributions of Secchi depths and chlorophyll a concentrations in the Suo Nada of the Seto Inland Sea, Japan, exposed to anthropogenic nutrient loading. Sci. Total Environ. 2016;571:543–550. doi: 10.1016/j.scitotenv.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Béjoui B, Harzallah A, Moussa M, Chapelle A, Solidoro C. Analysis of hydrobiological pattern in the Bizerte lagoon (Tunisia) Estuar. Coast. Shelf. Sci. 2008;80:21–129. [Google Scholar]
- 27.Ben Said R, Ouini H, Akrout F. Gracilaria bursa-pastoris of Bizerte lagoon (North of Tunisia): a spatio-temporal study of some hydro-biological parameters, agar yield and quality. Bull. Inst. Natn. Scien. Tech. Mer de Salammbô. 2017;44:165–175. [Google Scholar]
- 28.Mensi F, Ksouri J, Hammami W, Romdhane MS. L’algue rouge Gracilaria verrucosa (hudson) papenfuss de la lagune de Bizerte (tunisie septentrionale):essai de culture en mode suspendu et composition biochimique. Bull. Inst. Natn. Scien. Tech. Mer de Salammbô. 2010;36:125–137. [Google Scholar]
- 29.Gikas GD, Yiannakopoulou T, Tsihrintzis VA. Water quality trends in a coastal lagoon impacted by non-point source pollution after implementation of protective measures. Hydrobiologia. 2006;563:385–406. [Google Scholar]
- 30.Mc Carthy MJ, et al. Nitrogen dynamics and microbial food web structure during a summer cyanobacterial bloom in a subtropical, shallow, well-mixed, eutrophic lake (Lake Taihu, China) Hydrobiologia. 2006;581:195–207. [Google Scholar]
- 31.Okbah M, Hussein N. Impact of environmental conditions on the phytoplankton structure in Mediterranean Sea Lagoon, Lake Burullus, Egypt. Water Air Soil. Pollut. 2006;172:129–150. [Google Scholar]
- 32.Mostar MMM, et al. Physico-chemical assessment of the Nador lagoon’s water quality – North of the Eastern Morocco after the opening of the new inlet Mater. J. Mater. Environ. Sci. 2016;7:4795–5809. [Google Scholar]
- 33.Marcovecchio J, et al. Seasonality of hydrographic variables in a coastal lagoon: Mar Chiquita, Argentina. Aquatic Conserv: Mar. Freshw. Ecosyst. 2006;16:335–347. [Google Scholar]
- 34.Sakka AH, et al. The planktonic food web of the Bizerte lagoon south-western Mediterranean during summer: I. Spatial distribution under different anthropogenic pressures. Estuar. Coast Shelf Sci. 2008;78:61–77. [Google Scholar]
- 35.Ben Garali A, Ouakad M, Gueddari M. Bilans hydrologiques de la lagune de Bizerte Nord-Est de la Tunisie. Rev. Sci. Eau. 2009;22:525–534. [Google Scholar]
- 36.Rejeki S, Ariyati RW, Widowati LL, Bosma RH. The effect of three cultivation methods and two seedling types on growth, agar content and gel strength of Gracilaria verrucosa. Egypt. J. Aquat. Res. 2018;44:65–70. [Google Scholar]
- 37.Kassila J, et al. Opportunities for the development of seaweed farming as a supplementary income for small-scale fishermen in Nador lagoon: experimental cultivations of Gracilaria gracilis (Stackhouse) Med. Far. 2019;2:12–26. [Google Scholar]
- 38.Araño KG, Trono GC, Montaño NE, Hurtado AQ, Villanueva RD. Growth, agar yield and quality of selected agarophyte species from the Philippines. Bot. Mar. 2000;43:517–524. [Google Scholar]
- 39.Mensi F, Ben Ghedifa A. Optimum ranges of combined abiotic factor for Gracilaria gracilis aquaculture. J. Appl. Phycol. 2019;31:3025–3040. [Google Scholar]
- 40.Daughtery BK, Bird KT. Salinity and temperature effects on agar production from Graccilaria Verrucosa strain G-16. Aquaculture. 1988;1-2:105–116. [Google Scholar]
- 41.Kumar M, Kumari P, Reddy CR, Jha B. Salinity and desiccation induced oxidative stress acclimation in seaweeds. Adv. Bot. Res. 2014;71:91–123. [Google Scholar]
- 42.Choi HG, et al. Effects of temperature and salinity on the growth of Gracilaria verrucosa and G. chorda, with the potential for mariculture in Korea. J. Appl. Phycol. 2006;18:269–277. [Google Scholar]
- 43.Padhi S, et al. Cultivation of Gracilaria verrucosa (Huds) papenfuss in Chilka Lake for livelihood generation in coastal areas of Orissa state. J. Appl. Phycol. 2011;23:151–155. [Google Scholar]
- 44.Yoshimura CY, Cunha SR, Oliveira EC. Testing open-water cultivation techniques for Gracilaria domingensis (Rhodophyta, Gracilariales) in Santa Catarina, Brazil. J. Coast. Res. 2006;39:1290–1293. [Google Scholar]
- 45.Xu J, Gao K. Growth, pigments, UV-absorbing compounds and agar yield of the economic red seaweed Gracilaria lemaneiformis (Rhodophyta) grown at different depths in the coastal waters of the South China Sea. J. Appl. Phycol. 2007;20:681–686. [Google Scholar]
- 46.Marinho-Soriano E, Panucci RA, Carneiro MAA, Pereira DC. Evaluation of Gracilaria caudata J. Agardh for bioremediation of nutrients from shrimp farming wastewater. Bioresour. Technol. 2009;100:6192–6198. doi: 10.1016/j.biortech.2009.06.102. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Chai Z, Wang Q, Chen W, He Z, Jiang S. Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res. 2015;9:236–244. [Google Scholar]
- 48.Buschmann AH, Westermeier R, Retamales CA. Cultivation of Gracilaria on the sea-bottom in southern Chile: a rerview. J. Appl. Phycol. 1995;7:291–301. [Google Scholar]
- 49.Middelboe AL, Markager S. Depth limits and minimum light requirements of freshwater macrophytes. Freshw. Biol. 1997;37:553–568. [Google Scholar]
- 50.Ksouri J, Ben Said R, Pellegrini M. Résultats des cultures experimentales de la rhodophycée Gracilaria verrucosa papenfuss dans le lac Bizerte, Tunisie septentrionale. Bull. Inst. Natn. Scien. Tech. Mer de Salammbô. 1999;26:13–127. [Google Scholar]
- 51.Fletcher RL. Epiphytism and fouling in Gracilaria cultivation: an overview. J. Appl. Phycol. 1995;7:325–333. [Google Scholar]
- 52.Cruz-Rivera E, Friedlander M. Feeding preferences of mesograzers on aquacultured Gracilaria and sympatric algae. Aquaculture. 2011;322:218–222. doi: 10.1016/j.aquaculture.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skriptsova AV. The influence of rope cultivation of Graciliaria on a wild Zostera japonica bed in the lagoons of Southern Primorye, Sea of Japan. Russ. J. Mar. Biol. 2000;26:37–41. [Google Scholar]
- 54.Thomsen MS, Stæhr PA, Nejrup L, David R. Effects of the invasive macroalgae Gracilaria vermiculophylla on two co-occurring foundation species and associated invertebrate. Aquat. Invasions. 2013;8:133–145. [Google Scholar]
- 55.Béjaoui B, et al. 3D modeling of phytoplankton seasonal variation and nutrient budget in a southern Mediterranean Lagoon. Mar. Pollut. Bull. 2016;114:962–976. doi: 10.1016/j.marpolbul.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Westermeier R, Iván Gómez W, Rivera P. Suspended farming of Gracilaria chilensis (Rhodophyta, Gigartinales) at Cariquilda River, Maullín, Chile. Aquaculture. 1993;113:215–229. [Google Scholar]
- 57.Troell M, et al. Integrated marine cultivation of Gracilaria chilensis (Rhodophyta) and salamon cages for reduced environnemental impact and increased economic outpout. Aquaculture. 1997;156:45–61. [Google Scholar]
- 58.Halling C, Aroca G, Cifuentes M, Buschmann AH, Troell M. Comparison of spore inoculated and vegetative propagated cultivation methods of Gracilaria chilensis in an integrated seaweed and fish cage culture. Aquac. Int. 2005;13:409–422. [Google Scholar]
- 59.Pondevida HB, Hurtado-Ponce AQ. Assessment of some agarophytes from the coastal areas of Iloilo, Philippines. II. Seasonal variations in the agar quality of Gracilaria changii, Gracilaria manilaensis and Gracilariopsis bailinae (Gracilariales, Rhodophyta) Bot. Mar. 1996;39:123–127. [Google Scholar]
- 60.Marinho-Soriano E. Agar polysaccharides from Gracilaria species (Rhodophyta, Gracilariaceae) J. Biotechnol. 2001;89:81–84. doi: 10.1016/s0168-1656(01)00255-3. [DOI] [PubMed] [Google Scholar]
- 61.Marinho-Soriano E, Bourret E. Effects of season on the yield and quality of agar from Gracilaria species (Gracilariaceae, Rhodophyta) Bioresour. Technol. 2003;90:329–333. doi: 10.1016/s0960-8524(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 62.Mensi F, Ksouri J, Hammami W, Romdhane MS. Choix du site de culture de l’algue rouge Gracilaria verrucosa (hudson) papenfuss dans la lagune de bizerte: Caracteristiques physico-chimiques de l’eau. Bull. Inst. Natn. Scien. Tech. Mer de Salammbô. 2011;37:133–145. [Google Scholar]
- 63.Mollet J-C, Rahaoui A, Lemoine Y. Yield, chemical composition and gel strengh of agarocolloids of Gracilaria gracilis, Gracilariopsis longissima and the newly reported Gracilaria cf. vermiculophylla from Roscoff (Brittany, France) J. Appl. Phycol. 1998;10:59–66. [Google Scholar]
- 64.Duckworth M, Hong KC, Yaphe W. The agar polysaccharides of Gracilaria species. Carbohydr. Res. 1971;18:1–9. [Google Scholar]
- 65.Friedlander M, Zelikovitch N. Growth rates, phycocolloid yield and quality of the red seaweeds, Gracilaria sp., Pterocladia capillacea, Hypnea musciformis and Hypnea cornuta, in field studies in Israel. Aquaculture. 1984;40:57–66. [Google Scholar]
- 66.Sfriso A, Marcomini A, Pavoni B. Gracilaria distribution, production and composition in the lagoon of Venice. Bioresour. Technol. 1994;50:165–173. [Google Scholar]
- 67.Marinho-Soriano E, Fonseca PC, Carneiro MA, Moreira WS. Seasonal variation in the chemical composition of two seaweeds. Bioresour. Technol. 2006;97:2402–2406. doi: 10.1016/j.biortech.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 68.Ortiz J, et al. Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur. J. Lipid Sci. Technol. 2009;111:320–327. [Google Scholar]
- 69.Boulus A, Spaneir E, Friedlander M. Effect of outdoor conditions on growth rate and chemical composition of Gelidium crinale in culture. J. Appl. Phycol. 2007;19:471–478. [Google Scholar]
- 70.Glazer AN. Phycobilisome a macromolecular complex optimized for light energy transfer. Biochim. Biophys. Acta BBA – Rev. Bioenerg. 1984;768:29–51. [Google Scholar]
- 71.Dummy, J. & Morançais, M. Proteins and Pigments in Seaweed in Health and Disease Prevention (ed Fleurence, J. & Levine, I.) 275–318 (Amsterdam, 2016).
- 72.Lapointe BE. The effects of light and nitrogen on growth, pigment content, and biochemical composition of Gracilaria foliieera v. angustissima (gigartinales, rhodophyta) J. Phycol. 1981;17:90–95. [Google Scholar]
- 73.Chopin T, Gallant T, Davison I. Phosphorus and nitrogen nutrition in Chondrus crispus (Rhodophyta): effects on total phosphorus and nitrogen content, carrageenan production, and photosynthetic pigments and metabolism. J. Phycol. 1995;31:283–293. [Google Scholar]
- 74.Gomez I, Figueroa FL, Huovinen P, Ulloaa N, Morales V. Photosynthesis of the red alga Gracilaria chilensis under natural solar radiation in an estuary in southern Chile. Aquaculture. 2005;244:369–382. [Google Scholar]
- 75.Gomez I, et al. Patterns of photosynthesis in 18 species of intertidal macroalgae from southern Chile. Mar. Ecol. Prog. Ser. 2004;270:103–116. [Google Scholar]
- 76.Wehmeyer, W. Phycobilisomes: structure and function in Experimental Phycology. Cell Walls and Surfaces, Reproduction, Photosynthesis (ed Wiesser, W.D.G. & Robinson, R.C.S.), Springer, (Heidelberg, 1990).
- 77.Grossman AR, Schaffer MR, Chiang GG, Collier JL. The phycobilisome, a light harvestin complex response to environ mental conditions. Microbiol. Rev. 1993;57:725–49. doi: 10.1128/mr.57.3.725-749.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lobban, C. S., Harisson, P. J., Duncan, M. J. The physilogical ecology of seaweeds. Cambridge University Press, (Landon, 1985).
- 79.Kumar M, Kumari P, Gupta V, Reddy CRK, Jha B. Biochemical responses of red alga Gracilaria corticata (Gracilariales, Rhodophyta) to salinity induced oxidative stress. J. Exp. Mar. Biol. Ecol. 2010;391:27–34. [Google Scholar]
- 80.Fleurence J. The enzymatic degradation of algal cell walls: a useful approach for improving protein accessibility? J. Appl. Phycol. 1999;11:313–314. [Google Scholar]
- 81.Sanz-Pintos N, et al. Macromolecular Antioxidants and Dietary Fiber in Edible Seaweeds. J. Food Sci. 2017;82:289–295. doi: 10.1111/1750-3841.13592. [DOI] [PubMed] [Google Scholar]
- 82.Freile-Pelegrín Y. Does storage time influence yield and agar properties in the tropical agarophyte Gracilaria cornea? J. Appl. Phycol. 2000;12:153–158. [Google Scholar]
- 83.Tello-Ireland C, Lemus-Mondaca R, Vega-Gálvez A, López J, Di Scala K. Influence of hot-air temperature on drying kinetics, functional properties, colour, phycobiliproteins, antioxidant capacity, texture and agar yield of alga Gracilaria chilensis. LWT-Food Sci. Technol. 2011;44:2112–2118. [Google Scholar]
- 84.Bennamoun L, Afzal MT, Léonard A. Drying of alga as a source of bioenergy feedstock and food supplement-a review. Renew. Sust. Energ. Rev. 2015;50:1203–1212. [Google Scholar]
- 85.F.A.O. Département des pêches et de l’aquaculture, www.fao.org/fishery/facp/TUN/fr (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available on request to the corresponding author.