Abstract
The effect Helicobacter pylori (Hp) infection and small intestinal bacterial over growth (SIBO) in minimal hepatic encephalopathy (MHE) is not well understood. The aim of the study was to determine the effect of eradication of Hp infection and SIBO treatment on MHE in patients with cirrhosis. Patients with cirrhosis were enrolled and MHE was determined by psychometric tests and critical flicker frequency analysis. Hp infection and SIBO were assessed by urea breath and Hydrogen breath tests respectively in patients with cirrhosis and in healthy volunteers. Patients with Hp infection and SIBO were given appropriate treatment. At six weeks follow-up, presence of Hp infection, SIBO and MHE status was reassessed. Ninety patients with cirrhosis and equal number of healthy controls were included. 55 (61.1%) patients in the cirrhotic group were diagnosed to have underlying MHE. Among cirrhotic group, Hp infection was present in 28 with MHE (50.9%) vs. in 15 without MHE (42.8%) (p = 0.45). Similarly, SIBO was present in 17 (30.9%) vs. 11 (31.4%) (p = 0.95) in patients with and without MHE respectively. In comparison with healthy controls, patients with cirrhosis were more frequently harboring Hp and SIBO (47.7% vs. 17.7% (p < 0.001) and 31.1% vs. 4.4% (p < 0.001) respectively. On follow-up, all patients showed evidence of eradication of Hp and SIBO infection. Treatment of SIBO significantly improved the state of MHE in cirrhotics, however eradication of Hp infection did not improve MHE significantly. Additionally, patients with low Model for End-Stage Liver Disease (MELD) score and belonging to Child class B had significantly better improvement in MHE. A large number of patients with cirrhosis had either active Hp infection or SIBO with or without MHE, compared to healthy controls. Treatment of SIBO significantly improved MHE in patients with cirrhosis, whereas eradication of Hp did not affect the outcome of MHE in these patients.
Subject terms: Gastroenterology, Liver cirrhosis, Liver cirrhosis, Gastroenterology
Introduction
Hepatic encephalopathy (HE) is a frequent complication of chronic liver disease (CLD)1. HE can be precipitated by various factors like gastrointestinal bleeding, sepsis, azotemia, drugs (e.g. sedatives, diuretics), electrolyte imbalance and constipation. The most relevant substance considered in the pathogenesis of HE is ammonia, although the exact mechanisms of its neurotoxic effects are still under study2.
A substantial number of patients with advanced CLD have minimal hepatic encephalopathy (MHE), which is the earliest stage in the spectrum of HE. One study reported the occurrence of MHE to be as high as 50%in patients with CLD3. MHE, by definition, has no obvious clinical manifestations and is characterized by neurocognitive impairment in attention, vigilance and integrative function4. It develops in patients with significant liver function impairment or with porto-systemic shunting. MHE is associated not only with impaired daily functioning and quality of life, but is also considered as an occupational and public health hazard i.e. patient may be unfit to drive a car, operate a machinery, handle finances etc.5. However, current practice guidelines do not recommend treating MHE routinely6. MHE is not noticeable on clinical examination, but can be detected by various neuropsychological evaluations like Psychometric Hepatic Encephalopathy Score (PHES) and neurophysiological tests such as electro-encephalogram (EEG), spectral EEG, Critical Flicker Frequency (CFF), evoked potentials and computerized tests.
Various studies elucidating the relationship between active Helicobacter pylori (Hp) infection and hepatic encephalopathy have been published1,7. However, scanty data is available with regards to the same in MHE8.Work done by Schulz and his colleagues in a prospective clinical trial (though without healthy control group for comparison) failed to show an increased prevalence of Hp infection in patients presenting with MHE as opposed to those without MHE9. In addition to this, much interest has recently developed to define the role of small intestinal bacterial overgrowth (SIBO) among patients with MHE10,11. However, evidence of such a role in our patient population still seems to be lacking.
H. pylori are non-invasive, gram-negative bacteria recognized as a pathogen of upper gastrointestinal diseases, such as acute and chronic gastritis, gastro-duodenal ulcers and mucosa-associated lymphoid tissue (MALT) lymphoma. Additionally, H. pylori has also been characterized as group 1 (definitive) carcinogen by the International Agency for Research on Cancer (IARC) because of its association with gastric adenocarcinoma12. Studies on animal models have shown a significant increase in the liver fibrotic score and aminotransferase activity (and hence promote the development of CLD) in a group inoculated with Hp and CCl4 (carbon tetrachloride) compared with a CCl4treated group13. Moreover, by producing large amounts of highly active enzyme urease, Hp can convert urea to ammonia. This phenomenon results in alteration of neurotransmission, and hence affects consciousness and behavior, which has been implicated in causation of HE in patients with liver cirrhosis1.
SIBO is described as a proliferation of the bacterial population in the small intestine, particularly distal gut. CLD is associated with reduced gut motility and decreased gastric acid secretion in the stomach, resulting in gastric enteropathy. Both these factors can predispose to development of SIBO14. In addition, SIBO may facilitate translocation of bacteria or bacterial components (antigens) across the intestinal barrier, with harmful consequences to health, one of which could be HE15.
The aim of our study was to determine whether treatment of active Hp infection and SIBO play a role in improving MHE in cirrhotic patients.
Methods
Study design
This was a prospective cohort study.
Setting
The study was conducted in the outpatient department of the Aga Khan University Hospital, Karachi, Pakistan.
Sample size
It is estimated that about 55% of patients with cirrhosis would have MHE based on a positive result for both Psychometric Hepatic Encephalopathy Scoring (PHES) and Critical Flicker Frequency (CFF) analysis16. Assuming the CFF to have a sensitivity of 87% and specificity of 82% for MHE, and a confidence interval of 5%, the estimated sample size for this study was calculated to be 16417. To achieve the study objectives, the sample size was inflated by 10% to 180.
Sampling technique
Non-probability convenient sampling was used to identify the subjects.
Patients selection
Inclusion criteria
Patients with liver cirrhosis (irrespective of cause), 18 years and above, and without prior history of overt hepatic encephalopathy were included in the study.
Exclusion criteria
Patients currently receiving Hp eradication therapy, those on antibiotics for spontaneous bacterial peritonitis (SBP) prophylaxis or any other infection within last 4 weeks, or those who were on sedatives, rifaximin and/or lactulose (within 1 week) were excluded. Patients who had overt encephalopathy, severe cardiac, pulmonary, renal or cerebral disease, as well as those who had a history of recent upper gastrointestinal (GI) bleed (in last 6 weeks) were excluded from the study.
Ethical clearance
Ethical approval was obtained from the Ethical Review Committee (ERC) of the Aga Khan University, Karachi, Pakistan (ERC Reference #: 2873-Med-ERC-13). Written informed consent was obtained from all participants. Patients’ identification remained anonymous throughout the study. Patients were informed that the data will be used for research purpose and publication without revealing individual identification and information. Study was performed in accordance with the principles of good clinical practice from the Declaration of Helsinki.
Data collection procedure
All patients already diagnosed to have cirrhosis based on clinical signs and symptoms, laboratory parameters and imaging studies, meeting the inclusion criteria and agreeing to participate, were enrolled after written informed consent. The informed consent was translated in Urdu (native) language as well. Child Turcotte Pugh (CTP) and Model of End-stage Liver Disease (MELD) scores of individual patients were calculated in order to determine disease severity. These patients then underwent psychometric hepatic encephalopathy scoring (PHES) and critical flicker frequency (CFF) analysis to detect MHE. For the study purpose, a patient was diagnosed as having MHE if he tested positive for both CFF analysis and PHES testing. CFF analysis was incorporated as the examination is reliable, simple, easy to apply and can be performed without difficulty by patients with low educational background, such as patients from a country with relatively low levels of literacy.
Diagnosis of MHE
Psychometric hepatic encephalopathy scoring (PHES)
PHES is a battery of neuropsychological tests which has long been regarded as a ‘gold standard’ for the assessment of MHE18,19. Patients were classified as having MHE when the PHES score was less than −4 points. PHES comprises of five components:4
Number connection test A
The patient was instructed to join numbered circles in order on a piece of paper. The time required to complete the task was scored.
Number connection test B
The patient was instructed to join numbered circles and alphabets e.g. 1, A, 2, B and so on.
Line tracing (trail drawing test)
The patient was asked to trace a path, 5 mm wide, as fast as possible without touching the borders.
Serial dotting test
The patient was asked to dot the center of circles on a piece of paper.
Digit symbol test
The patient was asked to learn a code in which a digit is represented by a symbol. He/she then had to reproduce the symbol corresponding to the digit.
For calculation of PHES, individuals are supposed to complete all five components.
Critical flicker frequency (CFF) testing
The neurophysiologic CFF analysis is a tool that measures the ability of the central nervous system to detect flickering light, which is directly influenced by cortical activity5,20,21. In this method, an intermittent light stimulus appears as a flicker which is dependent on the frequency of light pulse presentation. The rate at which flicker just disappears is termed the critical flicker frequency (CFF). Patients were provided a CFF analyzer (a pair of spectacles shielding against outside light), and they were asked to concentrate on a red light, which was initially flickering. The frequency of the light was then gradually decreased by the operator until the patients perceived it as flickering, and they had to press a handgrip button when this happened. A lower level of 38 Hz was used as a cut-off for impaired CFF (and hence positive MHE).
Diagnosis of active H. pylori infection and SIBO
Diagnosis of active H. pylori infection was made by urea breath test (UBT) using Carbon 1322. Patients were asked to swallow 50 mg capsule of 13 C-urea. Breath samples were collected by exhaling into a 200 ml gas storage bag to be analyzed by an infrared spectrometer, and this was performed before and 15 minutes after the consumption of capsule. For the diagnosis of SIBO, hydrogen breath test (HBT) was utilized, in which 50 gram of lactulose was given to the subject, and an increase of ≥20 parts per million (p.p.m) in hydrogen production at 90 min from base-line was taken as a positive diagnosis for SIBO.
Treatment of active H. pylori infection and SIBO
In our study, all patients were subjected to UBT and HBT. Patients who were tested positive were given appropriate therapy. Triple medicine regimen which includes clarithromycin, amoxicillin (for 10 days) and a proton pump inhibitor (for another 4 weeks) was given for the eradication of H. pylori infection23. We chose the triple regime as it is easier to administer and because low recurrence rates of H. pylori infection (despite considerable resistance rates of approximately 36%) have been reported from our part of the world24.
With regards to treatment of patients with SIBO, rifaximin 1200 mg/day for 1 week, which has shown good efficacy for SIBO in various studies, was administered25,26. This dosage was selected as there had been substantial variation in the dose and duration of rifaximin at the time this study was conducted27. UBT and HBT were repeated after 6 weeks of completion of therapy28. CFF analysis and PHES were also repeated at the same time (i.e. after 6 weeks of completion of triple therapy and SIBO treatment) to detect an improvement in MHE. Rifaxamin was given to patients who came out as SIBO positive. Of note, none of the patients were given rifaximin as prophylaxis for hepatic encephalopathy during the follow-up period.
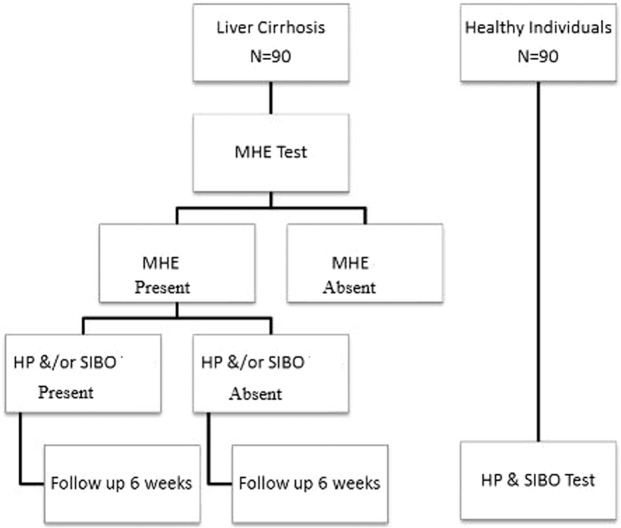
For comparison with healthy subjects, 90 age and gender-matched controls (e.g. patients’ relatives, healthy hospital employees, etc.) were also enrolled in the study after written informed consent and underwent UBT and HBT. Those who were found positive for Hp and SIBO were treated accordingly (Fig. 1).
Figure 1.
Study Methodology- Flowchart. HP: Helicobacter pylori. SIBO: Small Intestinal Bacterial Overgrowth. MHE: Minimal Hepatic Encephalopathy.
Statistical analysis
Data analysis was done using IBM SPSS Statistics for Windows, version 19.0 (Armonk, NY: IBM Corp. IBM Corp. Released 2011). Initially, the frequency was generated for all the variables. Continuous variables were presented as mean ± SD and categorical variables were presented as a percentage. Continuous variables between groups were compared by using unpaired t test or Mann Whitney test where appropriate. Categorical variables were compared by using the chi-square test or Fisher exact test. A p-value <0.05 was considered significant.
Results
A total of 90 patients were included in the study. Mean age was 44.9 ± 11.6 years, and the majority were males (53.3%). The clinical and laboratory parameters of cirrhotic patients with and without MHE are shown in Table 1.
Table 1.
Characteristics of patients with or without MHEΩ.
| With MHEn = 55 (%) | Without MHEn = 35 (%) | |
|---|---|---|
| Age (years) | 44.6 ± 11.9 | 45.5 ± 11.8 |
| Gender | ||
| Male | 29(52.7) | 19(54.3) |
| Female | 26(47.3) | 16(45.7) |
| Ascites | ||
| None | 35(63.6) | 26(74.3) |
| Mild to Moderate | 19(34.5) | 9(25.7) |
| Severe | 1(1.8) | 0 |
| Concomitant HCCµ | 4(7.3) | 0 |
| Hemoglobin (g/dl) | 10.3 ± 1.6 | 10.1 ± 1.7 |
| Platelets (x10E9/L) | 92.6 ± 34 | 103.7 ± 38.1 |
| Total Bilirubin (mg/dl) | 2.6 ± 0.66 | 2.5 ± 0.78 |
| Direct Bilirubin (mg/dl) | 1.5 ± 0.53 | 1.4 ± 0.61 |
| Indirect Bilirubin (mg/dl) | 1.1 ± 0.44 | 1.04 ± 0.46 |
| Albumin (g/dl) | 2.7 ± 0.32 | 2.8 ± 0.39 |
| Prothrombin time (sec) | 15.4 ± 3.0 | 15.4 ± 2.5 |
| INR∞ | 1.4 ± 0.28 | 1.3 ± 0.25 |
| Creatinine (mg/dl) | 1.05 ± 0.22 | 1.02 ± 0.22 |
| CTPα score | 8.5 ± 0.99 | 8.05 ± 0.83* |
| MELDβ score | 14.4 ± 3.06 | 13.8 ± 3.1 |
*p < 0.05.
ΩMHE: Minimal Hepatic Encephalopathy.
µHCC: hepatocellular carcinoma,
∞INR: International normalization ratio.
αCTP: Child-Turcotte-Pugh,
βMELD: Model for End-Stage Liver Disease.
Out of 90 cirrhotic patients, 55 (61.1%) were diagnosed to have underlying MHE based on a positive CFF analysis and PHES. Patients with MHE had a significantly higher CTP score than those without MHE (p = 0.02). Among patients who had MHE, 28 (50.9%) were positive for UBT (representing active Hp infection) as opposed to 15 (42.8%) in the non-MHE group (p = 0.45). Similarly, in the MHE group, 17 (30.9%) patients were diagnosed to have SIBO (based on a positive HBT), while 11 (31.4%) were SIBO positive in the non-MHE group (p = 0.95).
In comparison with healthy controls, overall, 43/90 (47.7%) patients with cirrhosis were positive on UBT as compared to 16/90 (17.7%) in age and gender-matched controls (p < 0.001). Likewise, there were 28/90 (31.1%) patients with cirrhosis who were positive on HBT, as opposed to only 4/90 (4.4%) positive among controls (p < 0.001) (Table 2).
Table 2.
Frequency of active Hp* infection and SIBOΩ in patients and controls.
| Healthy Controlsn = 90 | Patients with liver cirrhosis | |||
|---|---|---|---|---|
| MHE∞ present Patientsn = 55 | MHE absent Patientsn = 35 | p value | ||
| SIBO present | 4 (4.4) | 17 (30.9) | 11 (31.4) | <0.001 |
| SIBO absent | 86 (95.6) | 38 (69.1) | 24 (68.6) | |
| Hp infection present | 16 (17.7) | 28 (50.9) | 15 (42.9) | <0.001 |
| Hp infection absent | 74 (93.3) | 27 (49.1) | 20 (57.1) | |
*Hp: Helicobacter pylori
ΩSIBO: Small Intestinal Bacterial Overgrowth
∞MHE: Minimal Hepatic Encephalopathy.
All subjects received appropriate therapy for active Hp and SIBO (as mentioned earlier). Six weeks after therapy, UBT and HBT were repeated in all patients irrespective of their baseline status, and interestingly those individuals who initially tested positive on either UBT and/ or HBT were now found to be negative on repeat testing, indicating complete eradication of both the conditions. Seven cirrhotic patients who had underlying MHE remained positive for it even after treatment with anti-Hp therapy and/ or rifaximin. Among such patients, 6 had active Hp infection, whereas 1 patient had SIBO. Six of the 12 individual patients with MHE, who did not have either active Hp infection or SIBO, were still found to have MHE at 6 weeks follow-up testing.
The overall improvement in MHE among patients who initially had SIBO versus those who did not have SIBO was found to be statistically significant. However, MHE among patients who had Hp infection did not show any significant improvement in their MHE. Furthermore, the improvement in the level of MHE was more prominent in patients with relatively less advanced liver disease i.e. those with lower MELD score and belonging to Child-Turcotte-Pugh (CTP) class B (Table 3).
Table 3.
Outcome of MHE∞ after six weeks of treatment of active Hp* infection and SIBOΩ..
| Cirrhotic patients with MHE n = 55 | MHE improved | MHE not improved | p value |
|---|---|---|---|
| SIBO positive | 16 (38.1) | 1 (7.7) | 0.03 |
| SIBO negative | 26 (61.9) | 12 (92.3) | |
| Hp positive | 22 (52.4) | 6 (46.2) | 0.69 |
| Hp negative | 20 (47.6) | 7 (53.8) | |
| Meld score | 14.02 ± 2.80 | 16.0 ± 3.46 | 0.04 |
| CTP classBC | 40 (95.2) 2(4.8) | 8 (61.5) 5 (38.5) | 0.001 |
∞MHE: Minimal Hepatic Encephalopathy
*Hp: Helicobacter pylori
πSIBO: Small Intestine Bacterial Overgrowth.
Discussion
It is a well-known fact that SIBO is common in patients with liver cirrhosis29, which most likely occurs as a result of delayed small bowel transit in such individuals. Our study elucidated a significant improvement in the state of MHE in cirrhotic patients after treatment with SIBO therapy compared to those who had MHE without SIBO. Similar observations were also noted in a previously published study in which 26 out of 60 patients with cirrhosis who had MHE were treated by rifixamin for one week and found a significant improvement in MHE after treatment10.
On the other hand, we could not extrapolate the same results of improvement in MHE with regards to those patients with MHE who had Hp infection compared to those who have no Hp infection. Effect of Hp infection eradication over MHE in patients with cirrhosis remained debatable and earlier studies have also demonstrated controversial results; one such study has shown that anti-Hp therapy results in reducing ammonia levels in blood and hence improvement in MHE8. On the contrary, another study showed that Hp eradication does not induce any improvement in the psychometric and/or electrophysiological tests, which are used to diagnose MHE30. One plausible explanation for the above phenomenon is the fact that PPI use (given as part of Hp eradication regimen) has itself been linked to development of MHE, by inducing changes in the gut flora and subsequently leading to increased ammonia production31. We also use PPI in our study for an extended period of 4 week after triple therapy. Therefore beneficial effect of eradication of Hp infection over MHE in present study is not evident hence we do not suggest treatment and eradication of Hp solely for improvement in MHE.
Our study showed that the frequency of Hp and SIBO was considerably higher in cirrhotic patients as compared to the healthy controls. This observation has been elucidated by previous studies as well32,33. An Italian study34 demonstrated Hp antibodies to be positive in 89% of cirrhotic patients as compared to 59% controls (p < 0.0001). Later, in another study it was discovered that Hp infection along with an elevated transforming growth factor- β1 (TGF-β1) may accelerate hepatic fibrosis through increased TGF-β1-induced pro-inflammatory signaling pathways in hepatic stellate cells35. A recent study also found Hp infection to be more frequent among patients with cirrhosis secondary to chronic viral hepatitis, which happens to be the most prevalent cause of cirrhosis in our part of the world36. However, the cause and effect relationship between Hp infection and cirrhosis is still a matter of dispute.
Our study had a few limitations. Firstly, we did not measure serum ammonia levels of the subjects, as was performed in earlier similarly conducted studies on MHE16. However, as previous work has shown that arterial ammonia does not seem to play a major role in the diagnosis of MHE, we think that performing this test will not contribute substantial information37. The diagnosis of SIBO in our patients was not based on jejunal aspiration and culture, which is considered the gold standard. This method is invasive, as it requires small bowel intubation and laboratory skills in isolating anaerobes. Therefore, the inexpensive and non-invasive HBT has been utilized as has been done previously38 as a sole diagnostic tool for SIBO.
Conclusion
Compared to healthy controls a significantly large number of cirrhotic patients had either active Hp infection or SIBO. Treatment of SIBO improves MHE in patients with cirrhosis, while eradication of Hp infection was not associated with improvement in MHE. Moreover, significant improvement in MHE was evident in patients with low MELD scores and belonging to CTP class B as compared to those with higher MELD scores and CTP class C respectively.
We suggest to treat SIBO in cirrhotic patients with MHE, however Hp eradication is not beneficial in such patients.
Acknowledgements
Aga Khan University. URC #:70292. We thanked the Department of Medicine- Aga Khan University, Karachi for their support thoughout the duration of the study.
Author contributions
Shahab Abid: Conception of idea, study design, acquiring funding, supervising data collection and overall study and reviewed draft manuscripts. Muhammad Kamran: Conducted the study and writing first draft. Adeel Abid:Participated in conducting study, writing and editing the draft manuscripts. Nazish Butt:Contributed by providing patients for the study and reviewing manuscripts. Safia Awan: Performed statistical analysis. Zaigham Abbas: Contributed by providing patients for the study and reviewing manuscripts.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abdel-Hady H, et al. Helicobacter pylori infection in hepatic encephalopathy: Relationship to plasma endotoxins and blood ammonia. Hepatol Res. 2007;37:1026–33. doi: 10.1111/j.1872-034X.2007.00146.x. [DOI] [PubMed] [Google Scholar]
- 2.Gorg B, et al. Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology. 2010;52:256–265. doi: 10.1002/hep.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauridsen MM, Jepsen P, Vilstrup H. Critical flicker frequency and continuous reaction times for the diagnosis of minimal hepatic encephalopathy: a comparative study of 154 patients with liver disease. Metab. Brain Dis. 2011;26:135–139. doi: 10.1007/s11011-011-9242-1. [DOI] [PubMed] [Google Scholar]
- 4.Stinton LM, Jayakumar S. Minimal hepatic encephalopathy. Can J Gastroenterol. 2013;27:572–574. doi: 10.1155/2013/547670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez MR, et al. Value of the Critical Flicker Frequency in Patients with Minimal Hepatic Encephalopathy. Hepatology. 2007;45:879–885. doi: 10.1002/hep.21586. [DOI] [PubMed] [Google Scholar]
- 6.Vilstrup H, et al. Hepatic Encephalopathy in Chronic Liver Disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–35. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 7.Dasani BM, Sigal SH, Lieber CS. Analysis of risk factors for chronic hepatic encephalopathy: the role of Helicobacter pylori infection. Am. J. Gastroenterol. 1998;93:726–31. doi: 10.1111/j.1572-0241.1998.214_a.x. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal A, et al. Role of Helicobacter pylori infection in the pathogenesis of minimal hepatic encephalopathy and effect of its eradication. Indian J. Gastroenterol. 2011;30:29–32. doi: 10.1007/s12664-011-0087-7. [DOI] [PubMed] [Google Scholar]
- 9.Schulz C, et al. Prevalence of Helicobacter pylori infection in patients with minimal hepatic encephalopathy. J. Gastrointestin Liver Dis. 2016;25:191–195. doi: 10.15403/jgld.2014.1121.252.hpy. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. Effects of SIBO and rifaximin therapy on MHE caused by hepatic cirrhosis. Int. J. Clin. Exp. Med. 2015;8:2954–2957. [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A, et al. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J. Hepatol. 2010;53:849–55. doi: 10.1016/j.jhep.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Schistosomes, Liver Flukes and Helicobacter Pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC. Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 13.Goo MJ, et al. Helicobacter pylori promotes hepatic fibrosis in the animal model. Lab Invest. 2009;89:1291–1303. doi: 10.1038/labinvest.2009.90. [DOI] [PubMed] [Google Scholar]
- 14.Bellot P, Francés R, Such J. Pathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implications. Liver Int. 2013;33:31–39. doi: 10.1111/liv.12021. [DOI] [PubMed] [Google Scholar]
- 15.Othman M, Aguerob R, Lin HC. Alterations in intestinal microbial flora and human disease. Curr Opin Gastroenterol. 2008;24:11–16. doi: 10.1097/MOG.0b013e3282f2b0d7. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado-Garza HJ, et al. Prevalence of minimal hepatic encephalopathy in cirrhotic patients. Ann Hepatol. 2011;10:S40–4. doi: 10.1016/S1665-2681(19)31605-9. [DOI] [PubMed] [Google Scholar]
- 17.Montoliu C, et al. IL-6 and IL-18 in Blood May Discriminate Cirrhotic Patients with and without Minimal Hepatic Encephalopathy. J. Clin. Gastroenterol. 2009;43:272–279. doi: 10.1097/MCG.0b013e31815e7f58. [DOI] [PubMed] [Google Scholar]
- 18.Ferenci P, et al. Hepatic encephalopahy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congress of Gastroenterology, Vienna, 1998. J. Hepatol. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz M, Jacas C, Cordoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J. Hepatol. 2005;42:S45–S53. doi: 10.1016/j.jhep.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Romero GM. Critical flicker frequency: It is time to break down barriers surrounding minimal hepatic encephalopathy. J. Hepatol. 2007;47:10–1. doi: 10.1016/j.jhep.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Torlot FJ, McPhail MJ, Taylor-Robinson SD. Meta-analysis: The diagnostic accuracy of critical flicker frequency in minimal hepatic encephalopathy. Aliment Pharmacol Ther. 2013;37:527–36. doi: 10.1111/apt.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logan RPH. R.P.H. et al. Simplified single sample 13Carbon urea breath test for Helicobacter Pylori: comparison with histology, culture and ELISA serology. Gut. 1991;32:1461–1464. doi: 10.1136/gut.32.12.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malfertheiner P, et al. Management of Helicobacter pylori infection – the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 24.Yakoob J, et al. Low rate of recurrence of Helicobacter Pylori infection in spite of high clarithromycin resistance in Pakistan. BMC Gastroenterol. 2013;13:33. doi: 10.1186/1471-230X-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauritano EC, et al. Rifaximin dose-finding study for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2005;22:31–35. doi: 10.1111/j.1365-2036.2005.02516.x. [DOI] [PubMed] [Google Scholar]
- 26.Lauritano EC, et al. Antibiotic therapy in small intestinal bacterial overgrowth: rifaximin versus metronidazole. Euro. Rev. Med. Pharmacol Sci. 2009;13:111–6. [PubMed] [Google Scholar]
- 27.Gatta L, Scarpignato C. Systematic review with meta-analysis: Rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment. Pharmacol. Ther. 2017;45:604–16. doi: 10.1111/apt.13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attumi TA, Graham DY. Follow-up testing after treatment of helicobacter pylori infections in those who were initially tested positive, to document eradication of the infection: Cautions, caveats and recommendations. Clin. Gastroenterol. Hepatol. 2011;9:373–5. doi: 10.1016/j.cgh.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 29.Morencos FC, et al. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig. Dis. Sci. 1996;41:552–556. doi: 10.1007/BF02282340. [DOI] [PubMed] [Google Scholar]
- 30.Miquel J, et al. Role of helicobacter pylori infection and its eradication in patients with subclinical hepatic encephalopathy. Eur. J. Gastroenterol. Hepatol. 2001;13:1067–72. doi: 10.1097/00042737-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Nardelli S, et al. Proton pump inhibitors are associated with minimal and overt hepatic encephalopathy and increased mortality in patients with cirrhosis. Hepatol. 2019;70:640–649. doi: 10.1002/hep.30304. [DOI] [PubMed] [Google Scholar]
- 32.Huang J, Cui J. Evaluation of helicobacter pylori infection in patients with chronic hepatic disease. Chin. Med. J. (Engl). 2017;130:149–154. doi: 10.4103/0366-6999.197980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao J, et al. Nutrition status and small intestinal bacterial overgrowth in patients with viral-related cirrhosis. Asia Pac. J. Clin. Nutr. 2016;25:283–291. doi: 10.6133/apjcn.2016.25.2.06. [DOI] [PubMed] [Google Scholar]
- 34.Pellicano R, et al. Helicobacter pylori seroprevalence in hepatitis C virus positive patients with cirrhosis. J. Hepatol. 2000;33:648–650. doi: 10.1016/S0168-8278(00)80018-5. [DOI] [PubMed] [Google Scholar]
- 35.Ki MR, et al. Helicobacter pylori accelerates hepatic fibrosis by sensitizing transforming growth factor-β1-induced inflammatory signaling. Lab Invest. 2010;90:1507–16. doi: 10.1038/labinvest.2010.109. [DOI] [PubMed] [Google Scholar]
- 36.Pogorzelska J, et al. Helicobacter pylori infection among patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2017;29:1161–1165. doi: 10.1097/MEG.0000000000000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bragagnolo MA, Jr., et al. Minimal hepatic encephalopathy detection by neuropsychological and neurophysiological methods and the role of ammonia for its diagnosis. Arq. Gastroenterol. 2009;46:43–9. doi: 10.1590/S0004-28032009000100013. [DOI] [PubMed] [Google Scholar]
- 38.Gupta A, et al. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J. Hepatology. 2010;53:849–53. doi: 10.1016/j.jhep.2010.05.017. [DOI] [PubMed] [Google Scholar]