Abstract
Antipsychotic drugs target primarily dopaminergic system which makes catechol-O-methyltransferase (COMT) an interesting target in studies searching for treatment response predictors in schizophrenia. The study assessed the association of the COMT rs4680 and rs4818 polymorphisms with therapeutic response to olanzapine, risperidone, clozapine or other antipsychotic medication after 8 weeks of monotherapy in patients with schizophrenia. 521 Caucasian patients with schizophrenia received a monotherapy with olanzapine (10–20 mg/day; N = 190), risperidone (3–6 mg/day; N = 99), or clozapine (100–500 mg/day; N = 102). The fourth group (N = 130) consisted of patients receiving haloperidol (3–15 mg/day), fluphenazine (4–25 mg/day) or quetiapine (50–800 mg/day). Treatment response was defined as a 50% reduction from the baseline positive and negative syndrome scale (PANSS) total and subscale scores, but also as an observed percentage reduction from the initial PANSS0–6 total and subscale scores. Carriers of the COMT rs4680 A allele and carriers of the COMT rs4680–rs4818 C-A haplotype block had greater reduction in the PANSS total scores following olanzapine treatment, compared to carriers of the COMT rs4680 GG genotype and other COMT rs4680–rs4818 haplotypes. The COMT rs4680 A allele, and COMT rs4680–rs4818 C-A haplotype, were significantly associated with therapeutic response in patients treated with olanzapine, but not in patients treated with other antipsychotics.
Subject terms: Molecular biology, Neuroscience, Biomarkers, Diseases, Molecular medicine
Introduction
All antipsychotic drugs target primarily dopamine receptors1, and, therefore, the genes coding for components of dopaminergic system are the candidate genes that have been studied as valid predictors of treatment response to antipsychotics in schizophrenia. Catechol-O-methyltransferase (COMT), an enzyme responsible for methylation of catecholamines (dopamine, epinephrine, and norepinephrine), regulates dopamine degradation and therefore impacts prefrontal dopaminergic function. There are numerous polymorphisms of the COMT gene, but the most frequently studied is a functional polymorphism Val158Met (rs4680) that affects enzyme activity2–4, and rs4818 polymorphism that affects COMT expression5. The COMT Val158Met (rs4680) has been investigated extensively as a possible genetic marker of treatment response1, 4, treatment resistance7, 8, or symptomatic remission9 in schizophrenia, but its role is still not clear1, 10. COMT rs4680 polymorphism, a G/A substitution, results in the amino acid change from valine (Val) to methionine (Met) at codon 158 of membrane bound COMT (MB-COMT) and at codon 108 of soluble short form (S-COMT), and it leads to three or fourfold decrease in the enzymatic activity in the A (Met) carriers. Favorable response to antipsychotics was detected in patients, carriers of the COMT rs4680 AA (Met/Met) genotype in a meta-analysis1, whereas over-representation of the G (Val) allele was found in poor responders with schizophrenia11. On the other hand, several studies did not confirm the significant association between COMT rs4680 and treatment response to olanzapine or other typical and atypical antipsychotics12–16, or with remission in schizophrenia9. COMT rs4818 polymorphism, located in exon 4, is a synonymous polymorphism, consisting of a C/G substitution at codon 86 of the S-COMT and codon 136 of the MB-COMT, corresponding to a leucine residue. This polymorphism affects prefrontal dopamine function5. Carriers of the COMT rs4818 GG genotype have higher COMT activity than CC genotype carriers, and the presence of the G allele leads to reduced tonic prefrontal cortex dopamine signaling5. The COMT rs4680–rs4818 C-A haplotype has been associated with treatment response11, but also with treatment resistance7 in schizophrenia. COMT rs4818 polymorphism was also associated, in a haplotype analysis, with a treatment response to antidepressant medication in patients with depression17. Namely, regrading response to antidepressants, the COMT haplotype C-C-A (rs4633–rs4818–rs4680) was more frequent in the responders compared to non-responders17. These results suggest that, in addition to genotype- or allele-based approaches, haplotype-based association studies are also powerful tools in evaluating genetic underpinnings of treatment response in schizophrenia7, 11.
In our recent study, which used criteria for remission defined as a reduction to mild levels on the key 8 symptoms on the Positive and Negative Syndrome Scale (PANSS) (items P1, P2, P3, N1, N4, N6, G5, G9) for at least 6 months18, carriers of the COMT rs4680 GA genotype have shown a trend for achieving symptomatic remission9. Literature data reviewed in a meta-analysis suggest that carriers of one or two COMT rs4680 A alleles show better response than G allele carriers to treatment with atypical antipsychotics1. Haplotype data revealed that the presence of the C-A haplotype (rs4680–rs4818) is related to better response11. Therefore, in this longitudinal study, which evaluated treatment response, and not treatment resistance or remission, we expected that COMT rs4680 A allele, COMT rs4818 C allele, and C-A (rs4680–rs4818) haplotype, will be more frequently represented among patients with schizophrenia with a good response to treatment. There are contradictory and inconsistent data on the association between COMT genotype and haplotype variants and treatment response to different antipsychotics in schizophrenia, and criteria for the treatment response vary significantly19. Therefore, the aim of this study was to evaluate genotype- and haplotype-based association of the COMT rs4680 and rs4818 with the much better treatment response19 to monotherapy with olanzapine, risperidone, clozapine, or merged group treated with haloperidol, fluphenazine, or quetiapine, in ethnically homogeneous Caucasian subjects with chronic schizophrenia of both sexes.
Results
Significant differences (Kruskal–Wallis ANOVA followed by the Dunn post hoc test) were detected in age, PANSS total, positive, negative, and general psychopathology scores at baseline and after 8 weeks of treatment with specific antipsychotic between the group of patients treated with olanzapine, risperidone, clozapine or other antipsychotics (Table 1). Patients treated with olanzapine were significantly younger than the other patients (p = 0.005). Patients treated with olanzapine, risperidone or other antipsychotics had marginally to significantly lower (p = 0.014–0.001) total PANSS scores and PANSS positive and negative subscale scores at baseline and after 8 weeks of treatment (p = 0.006–0.001), compared to patients treated with clozapine (Table 1). At baseline, no significant differences (p = 0.045) were found in patients treated with clozapine, compared to patients treated with olanzapine, risperidone, and other antipsychotics, while after treatment, nominally higher (p = 0.013) PANSS general psychopathology scores were detected in patients receiving clozapine therapy (Table 1). There were no differences between the groups of patients treated with different antipsychotics in terms of disease duration and number of episodes (Table 1). However, patients treated with olanzapine were more frequently smokers (p < 0.001) then patients treated with other antipsychotics (Table 1).
Table 1.
Demographic and clinical data of 521 schizophrenic patients treated with olanzapine, risperidone, clozapine and other antipsychotics.
| Olanzapine n = 190 |
Risperidone n = 99 |
Clozapine n = 102 |
Other antipsychotics n = 130 |
Test statistics | ||
|---|---|---|---|---|---|---|
| Sex (male/female) | 144/46 (75.8/24.2) | 69/30 (69.7/30.3) | 71/31 (69.6/30.4) | 68/62 (52.3/47.7) | χ2 = 20.07 | p = 0.058 |
| Smoking (yes/no) | 132/58 (69.5/30.5) | 57/42 (57.6/42.4) | 59/43 (57.8/42.2) | 74/56 (56.9/43.1) | χ2 = 7.47 | p < 0.001 |
| Age (years) | 37.0 (19–71) | 40.0** (20–69) | 42.0** (19–71) | 41.5** (19–82) | H = 12.81 | p = 0.005 |
| Duration of disease (years) | 8 (1–31) | 10 (1–31) | 10 (4–25) | 7 (1–30) | H = 8.00 | p = 0.046 |
| Number of episodes | 4 (0–20) | 4 (1–20) | 5 (1–20) | 4 (0–31) | H = 5.76 | p = 0.124 |
| Total PANSS0–6 scores (week 0) | 85.5* (47–141) | 83.0*# (43–144) | 94.0# (47–141) | 80.5 (47–141) | H = 14.43 | p = 0.002 |
| Total PANSS0–6 scores (week 8) | 39.0* (15–93) | 36.0* (5–89) | 47.0 (15–93) | 32.0* (4–92) | H = 14.56 | p = 0.002 |
| PANSS0–6 positive scores (week 0) | 23.5# (9–38) | 22.0 (6–39) | 25.0 (9–38) | 21.0* (6–35) | H = 10.66 | p = 0.014 |
| PANSS0–6 positive scores (week 8) | 9.0* (0–25) | 8.0* (0–25) | 11.5 (0–25) | 6.5* (0–23) | H = 12.32 | p = 0.006 |
| PANSS0–6 negative scores (week 0) | 20.0* (11–36) | 20.0* (7–35) | 23.0 (11–36) | 18.5* (5–36) | H = 21.57 | p < 0.001 |
| PANSS0–6 negative scores (week 8) | 12.0*# (3–29) | 11.0* (2–28) | 14.0 (3–29) | 9.0* (1–29) | H = 17.86 | p < 0.001 |
| PANSS0–6 general psychopathology scores (week 0) | 41.0 (16–68) | 40.0 (17–73) | 44.0 (16–68) | 38.5* (15–72) | H = 8.05 | p = 0.045 |
| PANSS0–6 general psychopathology scores (week 8) | 18.0* (8–46) | 17.0* (2–44) | 21.0 (8–46) | 15.5* (0–48) | H = 10.85 | p = 0.013 |
Categorical data was analyzed with Chi-square test (df = 2) and shown as n (%). Numerical data was analyzed with Kruskal–Wallis ANOVA (df = 3) test and shown as median (range).
n number of subjects, PANSS positive and negative syndrome scale.
*p < 0.05 vs. clozapine; #p < 0.05 vs. other antipsychotics; **p < 0.05 vs. olanzapine (Dunn’s test).
The frequency of responders and non-responders did not differ in patients treated with olanzapine, risperidone, clozapine or other antipsychotics, when evaluated according to the 50% reduction in the PANSS total (χ2 = 7.200; df = 3; p = 0.066), positive (χ2 = 2.121 df = 3; p = 0.548) and general psychopathology (χ2 = 4.931; df = 3; p = 0.177) scores. However, a significant difference in the distribution of responders and non-responders was found when comparing all four groups of patients evaluated according to the reduction in the PANSS negative scores (χ2 = 29.95; df = 3; p < 0.001).
The frequency of the COMT rs4680 (χ2 = 0.046; df = 1; p = 0.829) or the COMT rs4818 (χ2 = 0.523; df = 1; p = 0.469) genotypes did not deviate from HWE in patients with schizophrenia. The distribution of the COMT rs4680 AA, AG and GG genotypes (χ2 = 1.654; df = 2; p = 0.437), or the COMT rs4818 CC, CG and GG genotypes (χ2 = 0.076; df = 2; p = 0.963) did not differ significantly between male and female patients. However, we evaluated the possible association of treatment response with COMT rs4680 and COMT rs4818 genotypes or haplotypes, separately in male and female patients, and we have observed no significant associations (Supplementary Tables S1–12).
Therefore, in the further analyses, patients with schizophrenia were not subdivided according to the gender. At baseline, there were no significant differences in the frequency of the COMT rs4680 (χ2 = 1.432; df = 2; p = 0.964) or COMT rs4818 (χ2 = 5.548; df = 2; P = 0.476) genotypes between patients treated with olanzapine, risperidone, clozapine or other antipsychotics.
Table 2 demonstrates the PANSS-derived response rates in steps of 25% in patients treated for 8 weeks with adequate monotherapy19. The frequency of response rates differed nominally between the groups treated with different antipsychotic (p = 0.035). In all treatment groups, the highest treatment response, in 43.1–51.1% of patients, was detected in the 50–74% symptom reduction category. These results confirmed that our primary cut-off, defined a priori, as a 50% reduction in the baseline PANSS scores19, was a good choice to detect clinically meaningful improvement.
Table 2.
PANSS-derived response rates in patients with schizophrenia after 8 weeks of treatment with olanzapine, risperidone, clozapine or other antipsychotics.
| Antipsychotic | Total n | PANSS-reduction | χ2 test | |||
|---|---|---|---|---|---|---|
| < 25% | 25–49% | 50–74% | 75–100% | |||
| Olanzapine | 190 | 18 (9.5) | 58 (30.5) | 96 (50.5) | 18 (9.5) | χ2 = 17.98; df = 9; p = 0.035 |
| Risperidone | 99 | 11 (11.1) | 27 (27.3) | 49 (49.5) | 12 (12.1) | |
| Clozapine | 102 | 7 (6.9) | 47 (46.1) | 44 (43.1) | 4 (3.9) | |
| Other antipsychotics | 130 | 17 (13.1) | 31 (23.8) | 67 (51.5) | 15 (11.5) | |
Frequencies (%) are shown in parenthesis.
n number of subjects, PANSS positive and negative syndrome scale.
After 8 weeks of antipsychotic treatment, when patients were subdivided according to the treatment response and according to the COMT rs4680 and rs4818 genotypes, no significant differences were found in the distribution of the COMT rs4680 (Table 3) or COMT rs4818 (Table 4) genotypes in responders and non-responders treated with olanzapine, risperidone, clozapine or other antipsychotics.
Table 3.
The COMT rs4680 genotype count and frequencies in schizophrenia patients treated with olanzapine, risperidone, clozapine or other antipsychotics, subdivided into responders (R) and non-responders (NR) according to the 50% reduction in the baseline PANSS0–6 total and subscale scores.
| COMT rs4680 | Olanzapine n = 190 |
Risperidone n = 99 |
Clozapine n = 102 |
Other antipsychotics n = 130 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | AA | AG | GG | AA | AG | GG | AA | AG | GG | ||
| Total PANSS0–6 score reduction at week 8 | NR | 17 (22.4) | 35 (46.1) | 24 (31.6) | 13 (34.2) | 15 (39.5) | 10 (26.3) | 13 (24.1) | 26 (48.1) | 15 (27.8) | 13 (27.1) | 21 (43.8) | 14 (29.2) |
| R | 29 (25.4) | 64 (56.1) | 21 (18.4) | 13 (21.3) | 31 (50.8) | 17 (27.9) | 13 (27.1) | 22 (45.8) | 13 (27.5) | 17 (20.7) | 44 (53.7) | 21 (25.6) | |
| χ2 = 4.40; p = 0.111 | χ2 = 2.15; p = 0.341 | χ2 = 0.12; p = 0.940 | χ2 = 1.27; p = 0.753 | ||||||||||
| PANSS0–6 positive scores reduction at week 8 | NR | 12 (23.1) | 22 (42.3) | 18 (34.6) | 9 (32.1) | 12 (42.9) | 7 (25.0) | 8 (22.2) | 16 (44.4) | 12 (33.3) | 10 (25.6) | 17 (43.6) | 12 (30.8) |
| R | 34 (34.6) | 77 (55.8) | 27 (19.6) | 17 (23.9) | 34 (47.9) | 20 (28.2) | 18 (27.3) | 32 (48.5) | 16 (24.2) | 20 (22.0) | 48 (52.7) | 23 (25.3) | |
| χ2 = 4.97; p = 0.083 | χ2 = 0.70; p = 0.706 | χ2 = 1.02; p = 0.602 | χ2 = 0.92; p = 0.630 | ||||||||||
| PANSS0–6 negative scores reduction at week 8 | NR | 31 (25.2) | 57 (46.3) | 35 (28.5) | 17 (30.9) | 23 (41.8) | 15 (27.3) | 22 (25.3) | 41 (47.1) | 24 (27.6) | 16 (23.2) | 33 (47.8) | 20 (29.0) |
| R | 15 (22.4) | 42 (62.7) | 10 (14.9) | 9 (20.5) | 23 (52.3) | 12 (27.3) | 4 (26.7) | 7 (46.7) | 4 (26.7) | 14 (23.0) | 32 (52.5) | 15 (24.6) | |
| χ2 = 5.72; p = 0.057 | χ2 = 1.59; p = 0.451 | χ2 = 0.01; p = 0.993 | χ2 = 0.37; p = 0.830 | ||||||||||
| PANSS0–6 general psychopathology scores reduction at week 8 | NR | 22 (26.5) | 36 (43.4) | 25 (30.1) | 13 (31.0) | 17 (40.5) | 12 (28.6) | 11 (19.3) | 29 (50.9) | 17 (29.8) | 17 (28.8) | 25 (42.4) | 17 (28.8) |
| R | 24 (22.4) | 63 (58.9) | 20 (18.7) | 13 (22.8) | 29 (50.9) | 15 (26.3) | 15 (33.3) | 19 (42.2) | 11 (24.4) | 13 (18.3) | 40 (56.3) | 18 (25.4) | |
| χ2 = 5.06; p = 0.080 | χ2 = 1.22; p = 0.544 | χ2 = 2.61; p = 0.271 | χ2 = 2.94; p = 0.230 | ||||||||||
Frequencies (%) are shown in parenthesis.
n number of subjects, NR non-responders, PANSS positive and negative syndrome scale, R responders.
Table 4.
The COMT rs4818 genotype count and frequencies in schizophrenia patients treated with olanzapine, risperidone, clozapine or other antipsychotics, subdivided into responders (R) and non-responders (NR) according to the 50% reduction in PANSS0–6 total and subscale scores.
| COMT rs4818 | Olanzapine n = 190 |
Risperidone n = 99 |
Clozapine n = 102 |
Other antipsychotics n = 130 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | GC | GG | CC | GC | GG | CC | GC | GG | CC | GC | GG | ||
| Total PANSS0–6 scores reduction at week 8 | NR | 29 (38.2) | 29 (38.2) | 18 (23.7) | 18 (47.4) | 14 (36.8) | 6 (15.8) | 16 (29.6) | 29 (53.7) | 9 (16.7) | 22 (45.8) | 17 (35.4) | 9 (18.8) |
| R | 36 (31.6) | 62 (54.4) | 16 (14.0) | 22 (36.1) | 30 (49.2) | 9 (14.8) | 15 (31.3) | 25 (52.1) | 8 (16.7) | 34 (43.1) | 36 (40.8) | 12 (14.6) | |
| χ 2 = 5.46; p = 0.065 | χ2 = 1.56; p = 0.459 | χ2 = 0.04; p = 0.983 | χ2 = 0.97; p = 0.611 | ||||||||||
| PANSS0–6 positive scores reduction at week 8 | NR | 19 (36.5) | 21 (40.4) | 12 (23.1) | 12 (42.9) | 11 (39.3) | 5 (17.9) | 8 (22.2) | 20 (55.6) | 8 (22.2) | 18 (37.5) | 16 | 5 (62.5) |
| R | 46 (33.3) | 70 (50.7) | 22 (15.9) | 28 (39.4) | 33 (46.5) | 10 (14.1) | 23 (34.8) | 34 (51.5) | 9 (13.6) | 38 (41.8) | 37 (40.7) | 16 (17.6) | |
| χ2 = 2.03; p = 0.362 | χ2 = 0.48; p = 0.786 | χ2 = 2.32; p = 0.313 | χ2 = 0.51; p = 0.776 | ||||||||||
| PANSS0–6 negative scores reduction at week 8 | NR | 43 (35.0) | 54 (43.9) | 26 (21.1) | 24 (43.6) | 23 (41.8) | 8 (14.5) | 27 (31.0) | 46 (52.9) | 14 (16.1) | 33 (47.8) | 26 (37.7) | 10 (14.5) |
| R | 22 (32.8) | 37 (55.2) | 8 (11.9) | 16 (36.4) | 21 (47.7) | 7 (15.9) | 4 (26.7) | 8 (53.3) | 3 (20.0) | 23 (37.7) | 27 (44.3) | 11 (18.0) | |
| χ2 = 3.27; p = 0.195 | χ2 = 0.54; p = 0.763 | χ2 = 0.20; p = 0.906 | χ2 = 1.37; p = 0.505 | ||||||||||
| PANSS0–6 general psychopathology scores reduction at week 8 | NR | 32 (38.6) | 32 (38.6) | 19 (22.9) | 19 (45.2) | 15 (35.7) | 8 (19.0) | 13 (22.8) | 34 (59.6) | 10 (17.5) | 27 (45.8) | 21 (35.6) | 11 (18.6) |
| R | 33 (30.8) | 59 (55.1) | 15 (14.0) | 21 (36.8) | 29 (50.9) | 7 (12.3) | 18 (40.0) | 20 (44.4) | 7 (15.6) | 29 (40.8) | 32 (45.1) | 10 (14.1) | |
| χ2 = 5.55; p = 0.062 | χ2 = 2.40; p = 0.301 | χ2 = 3.60; p = 0.165 | χ2 = 1.31; p = 0.521 | ||||||||||
Frequencies (%) are shown in parenthesis.
n number of subjects, NR non-responders, PANSS positive and negative syndrome scale, R responders.
To further evaluate this negative finding, we calculated the percentage of the reduction from the initial PANSS0–6 total and subscale scores after 8 weeks of treatment with olanzapine, risperidone, clozapine or other antipsychotics in schizophrenic patients subdivided according to the COMT rs4680 (Table 5) and COMT rs4818 (Table 6) genotypes. Nominally significant differences were detected between olanzapine-treated patients carrying COMT rs4680 AA, GA and GG genotypes in the total PANSS0–6 scores (p = 0.019; Kruskal Wallis ANOVA and Dunn’s test), and in the PANSS0–6 positive subscale scores (p = 0.027). However, this significance was lost due to the Bonferroni correction. Namely, carriers of the COMT rs4680 GA genotype had more pronounced reduction in the total PANSS0–6 scores compared to GG carriers (Table 5). To further evaluate this result, we additionally subdivided responders and non-responders into COMT rs4680 A carriers (the combined group of AA and AG genotype carriers) and GG homozygous genotype carriers. Mann–Whitney test revealed a significant difference in the PANSS0–6 total scores (U = 2,375.5; p < 0.006) and a trend of significance in PANSS0–6 positive scores (U = 2,394.5; p = 0.007), but no significant differences in the PANSS0–6 general psychopathology (U = 2,666.0; p = 0.043) or PANSS0–6 negative (U = 2,611.0; p = 0.064) scores between COMT rs4680 A carriers vs. GG carriers treated with olanzapine. Collectively, COMT rs4680 A allele carriers displayed larger percentage of the reduction in all PANSS0–6 scores when compared to GG carriers. Other PANSS0–6 scores did not differ significantly between schizophrenic patients, carriers of the COMT rs4680 genotypes treated with risperidone, clozapine or other antipsychotics (Table 5). Carriers of the COMT rs4818 genotypes had similar PANSS0–6 scores in all treatment’s groups (Table 6).
Table 5.
Percentage of reduction from the initial PANSS0–6 total and subscale scores after 8 weeks of treatment with olanzapine, risperidone, clozapine or other antipsychotics in schizophrenia patients subdivided according to the COMT rs4680 genotypes.
| Olanzapine n = 190 |
Risperidone n = 99 |
Clozapine n = 102 |
Other antipsychotics n = 130 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | IR | Median | Range | IR | Median | Range | IR | Median | Range | IR | |
| Total PANSS0–6 scores reduction after 8 weeks of treatment | ||||||||||||
| COMT rs4680 | ||||||||||||
| AA | 56.0 | 83 | 33 | 52.4 | 83 | 32 | 50.1 | 55 | 23 | 54.3 | 83 | 40 |
| AG | 58.2 | 81 | 24 | 58.0 | 70 | 28 | 49.2 | 66 | 22 | 60.0 | 84 | 28 |
| GG | 49.5 | 71 | 23 | 63.2 | 76 | 37 | 45.5 | 53 | 27 | 54.2 | 79 | 33 |
| Kruskal–Wallis ANOVA | H = 7.93; p = 0.019 | H = 0.37; p = 0.832 | H = 0.04; p = 0.981 | H = 1.37; p = 0.505 | ||||||||
| PANSS0–6 positive symptom scores reduction after 8 weeks of treatment | ||||||||||||
| COMT rs4680 | ||||||||||||
| AA | 64.5 | 81 | 34 | 62.2 | 82 | 30 | 52.9 | 75 | 21 | 67.6 | 88 | 47 |
| AG | 63.6 | 79 | 31 | 61.4 | 86 | 31 | 57.7 | 89 | 27 | 64.3 | 83 | 30 |
| GG | 52.9 | 84 | 26 | 68.8 | 84 | 27 | 53.3 | 74 | 28 | 60.9 | 90 | 40 |
| Kruskal–Wallis ANOVA | H = 7.26; p = 0.027 | H = 0.18; p = 0.912 | H = 0.33; p = 0.850 | H = 0.54; p = 0.763 | ||||||||
| PANSS0–6 negative symptom scores reduction after 8 weeks of treatment | ||||||||||||
| COMT rs4680 | ||||||||||||
| AA | 37.7 | 80 | 33 | 37.2 | 89 | 47 | 28.9 | 73 | 13 | 37.9 | 88 | 55 |
| AG | 43.5 | 86 | 37 | 48.1 | 189 | 41 | 28.9 | 67 | 23 | 47.6 | 86 | 55 |
| GG | 36.4 | 96 | 29 | 44.0 | 92 | 42 | 28.2 | 72 | 22 | 44.4 | 80 | 47 |
| Kruskal–Wallis ANOVA | H = 3.88; p = 0.144 | H = 1.85; p = 0.398 | H = 0.15; p = 0.930 | H = 0.12; p = 0.944 | ||||||||
| PANSS0–6 general psychopathology scores reduction after 8 weeks of treatment | ||||||||||||
| COMT rs4680 | ||||||||||||
| AA | 51.2 | 95 | 34 | 48.4 | 86 | 26 | 51.9 | 58 | 24 | 44.4 | 83 | 30 |
| AG | 55.9 | 93 | 25 | 56.8 | 86 | 30 | 45.4 | 74 | 23 | 56.3 | 87 | 28 |
| GG | 48.3 | 75 | 17 | 53.6 | 78 | 39 | 43.9 | 46 | 25 | 50.0 | 106 | 34 |
| Kruskal–Wallis ANOVA | H = 5.218; p = 0.074 | H = 0.22; p = 0.897 | H = 1.60; p = 0.449 | H = 3.61; p = 0.164 | ||||||||
Values are given as median, range and interquartile range (IR).
n number of subjects, PANSS positive and negative syndrome scale.
Table 6.
Percentage reduction from the initial PANSS0–6 total and subscale scores after 8 weeks of treatment with olanzapine, risperidone, clozapine or other antipsychotics in schizophrenic patients subdivided according to the COMT rs4818 genotypes.
| Olanzapine n = 190 |
Risperidone n = 99 |
Clozapine n = 102 |
Other antipsychotics n = 130 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | IR | Median | Range | IR | Median | Range | IR | Median | Range | IR | |
| Total PANSS0–6 scores reduction after 8 weeks of treatment | ||||||||||||
| COMT genotype | ||||||||||||
| CC | 53.9 | 83 | 32 | 56.5 | 79 | 32 | 49.5 | 59 | 27 | 55.5 | 84 | 32 |
| CG | 59.0 | 81 | 23 | 58.1 | 72 | 22 | 48.3 | 61 | 23 | 58.4 | 78 | 30 |
| GG | 49.5 | 60 | 16 | 62.3 | 76 | 47 | 45–1 | 53 | 26 | 63.9 | 71 | 37 |
| Kruskal–Wallis ANOVA | H = 5.32; p = 0.070 | H = 0.07; p = 0.965 | H = 0.01; p = 0.994 | H = 1.94; p = 0.378 | ||||||||
| PANSS0–6 positive symptom scores reduction after 8 weeks of treatment | ||||||||||||
| COMT genotype | ||||||||||||
| CC | 62.1 | 82 | 33 | 61.9 | 84 | 30 | 52.2 | 75 | 25 | 64.6 | 90 | 35 |
| CG | 66.7 | 88 | 32 | 65.4 | 81 | 25 | 59.7 | 79 | 28 | 63.0 | 86 | 33 |
| GG | 53.4 | 79 | 25 | 68.8 | 84 | 40 | 51.6 | 74 | 27 | 77.8 | 85 | 41 |
| Kruskal–Wallis ANOVA | H = 4.44; p = 0.108 | H = 0.03; p = 0.984 | H = 1.27; p = 0.531 | H = 2.25; p = 0.325 | ||||||||
| PANSS0–6 negative symptom scores reduction after 8 weeks of treatment | ||||||||||||
| COMT genotype | ||||||||||||
| CC | 38.9 | 86 | 34 | 39.4 | 89 | 43 | 28.6 | 73 | 12 | 42.1 | 88 | 49 |
| CG | 42.3 | 104 | 37 | 45.5 | 189 | 49 | 28.6 | 67 | 24 | 50.0 | 85 | 47 |
| GG | 36.1 | 79 | 28 | 47.1 | 92 | 48 | 34.8 | 72 | 24 | 50.0 | 72 | 52 |
| Kruskal–Wallis ANOVA | H = 1.93; p = 0.381 | H = 0.62; p = 0.734 | H = 1.56; p = 0.460 | H = 1.37; p = 0.504 | ||||||||
| PANSS0–6 general psychopathology scores reduction after 8 weeks of treatment | ||||||||||||
| COMT genotype | ||||||||||||
| CC | 51.2 | 91 | 30 | 51.0 | 84 | 29 | 52.3 | 68 | 23 | 50.0 | 83 | 31 |
| CG | 55.9 | 93 | 26 | 57.7 | 86 | 26 | 43.7 | 66 | 25 | 53.7 | 87 | 28 |
| GG | 48.7 | 70 | 11 | 48.7 | 78 | 46 | 44.7 | 46 | 25 | 47.6 | 106 | 41 |
| Kruskal–Wallis ANOVA | H = 4.53; p = 0.104 | H = 0.09; p = 0.957 | H = 3.20; p = 0.202 | H = 2.08; p = 0.354 | ||||||||
Values are given as median, range and interquartile range (IR)n number of subjects, PANSS positive and negative syndrome scale.
Haplotype analysis
LD plot for two analyzed COMT SNPs was determined with Haploview software v. 4.2 and shown in Fig. 1.
Figure 1.
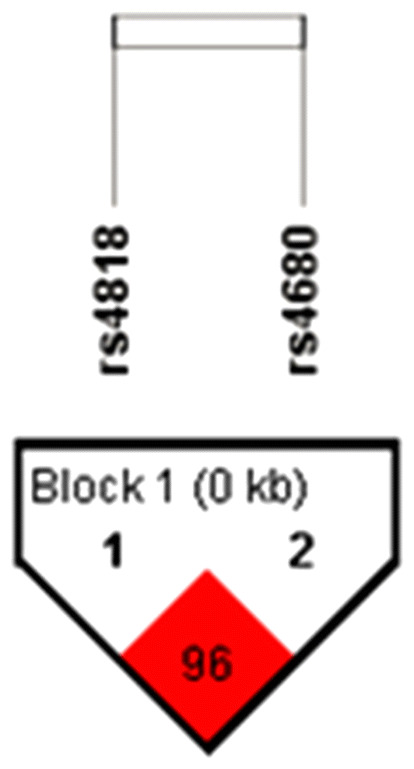
Linkage disequilibrium (LD) plot for two COMT SNPs in schizophrenia patients. Pairwise LD value (D’ × 100) is calculated using 4-gamete rule and represented in red square. Revealed D’ value indicates a strong link between rs4818 and rs4680 loci.
Since COMT rs4818 and rs4680 loci were highly linked (D′ = 0.96; LOD > 2), the frequencies of four possible haplotypes were calculated (Fig. 1). Frequencies of various COMT (rs4818–rs4680) haplotypes in patients with schizophrenia, estimated with Haploview v 4.2., were C-A (0.488); G-G (0.369); C-G (0.124) and G-A (0.008, less than 1%). Rare haplotype (< 1%) was excluded from the analysis.
To further evaluate this significant association between the COMT rs4680 A allele and better response to olanzapine treatment, we compared the frequency of the most common COMT C-A haplotype with other haplotype carriers (Table 7), subdivided into responders and non-responders. Nominally significant differences were found in the frequency of the COMT C-A haplotype carriers and other haplotype carriers between responders and non-responders to olanzapine (Table 7), defined according to the reduction in the PANSS0–6 total scores (p = 0.036), PANSS0–6 positive subscale scores (p = 0.021), and PANSS0–6 negative subscale scores (p = 0.050). These findings did not remain significant after correction for multiple testing. In the case of the PANSS0–6 general psychopathology scores, there was no difference (p = 0.064) in the frequency of COMT C-A haplotype carriers vs. other haplotype carriers in patients who responded well to the therapy with olanzapine and non-responders. Similar distribution of the COMT rs4680–rs4818 haplotypes was found in responders and non-responders to risperidone, clozapine and other antipsychotic medication (Table 7).
Table 7.
Haplotype frequencies of COMT rs4680 and rs4818 polymorphisms in schizophrenia patients treated with olanzapine, risperidone, clozapine or other antipsychotics, subdivided into responders (R) and non-responders (NR) according to the 50% reduction in PANSS0–6 total and subscale scores.
| COMT rs4680–rs4818 | Olanzapine n = 190 |
Risperidone n = 99 |
Clozapine n = 102 |
Other antipsychotics n = 130 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| C-A haplotype carriers | C-A carriers | Non-carriers | C-A carriers | Non-carriers | C-A carriers | Non-carriers | C-A carriers | Non-carriers | |
| Total PANSS0–6 scores reduction at week 8 | NR | 51 (61.7) | 25 (32.9) | 28 (73.7) | 10 (26.3) | 39 (72.2) | 15 (27.8) | 32 (66.7) | 16 (33.3) |
| R | 92 (80.7) | 22 (19.3) | 44 (72.1) | 17 (27.9) | 35 (72.9) | 13 (27.1) | 60 (73.2) | 22 (26.8) | |
| χ2 = 4.53; p = 0.033 | χ2 = 0.03; p = 0.866 | χ2 = 0.01; p = 0.937 | χ2 = 0.62; p = 0.431 | ||||||
| PANSS0–6 positive scores reduction at week 8 | NR | 33 (63.5) | 19 (36.5) | 21 (75.0) | 7 (25.0) | 24 (66.7) | 12 (33.3) | 26 (66.7) | 13 (33.3) |
| R | 110 (79.7) | 28 (20.3) | 51 (71.8) | 20 (28.2) | 50 (75.8) | 16 (24.2) | 66 (72.5) | 25 (27.5) | |
| χ2 = 5.36; p = 0.021 | χ2 = 0.11; p = 0.750 | χ2 = 0.97; p = 0.326 | χ2 = 0.45; p = 0.501 | ||||||
| PANSS0–6 negative scores reduction at week 8 | NR | 87 (70.7) | 36 (29.3) | 40 (72.7) | 15 (27.3) | 63 (72.4) | 24 (27.6) | 47 (68.1) | 22 (31.9) |
| R | 56 (83.6) | 11 (16.4) | 32 (72.7) | 12 (27.3) | 11 (73.3) | 4 (26.7) | 45 (73.8) | 16 (26.2) | |
| χ2 = 3.85; p = 0.050 | χ2 = 0.00; p = 1.000 | χ2 = 0.01; p = 0.941 | χ2 = 0.50; p = 0.479 | ||||||
| PANSS0–6 general psychopathology scores reduction at week 8 | NR | 57 (68.7) | 26 (31.3) | 30 (71.4) | 12 (28.6) | 40 (70.2) | 17 (29.8) | 40 (67.8) | 19 (32.2) |
| R | 86 (80.4) | 21 (19.6) | 42 (73.7) | 15 (26.3) | 34 (75.6) | 11 (24.4) | 52 (73.2) | 19 (26.8) | |
| χ2 = 3.44; p = 0.064 | χ2 = 0.06; p = 0.803 | χ2 = 0.37; p = 0.545 | χ2 = 0.46; p = 0.497 | ||||||
Frequencies (%) are shown in parenthesis.
n number of subjects, NR non-responders, PANSS positive and negative syndrome scale, R responders.
Results presented in Table 8 revealed significantly greater reduction of total PANSS0–6 scores (Mann Whitney test; p < 0.006) and a trend towards larger reduction of PANSS0–6 positive subscale scores (p = 0.007) in COMT C-A haplotype carriers in olanzapine-treated patients. Slight reduction was detected in the PANSS0–6 general psychopathology scores (p = 0.037), when comparing COMT C-A haplotype carriers to the carriers of other haplotypes in patients treated with olanzapine. Other results were not significant, showing that reduction in the PANSS0–6 total and subscale scores did not differ between the carriers of the COMT C-A haplotype compared to other haplotype carriers in patients treated with risperidone, clozapine or other antipsychotics (Table 8).
Table 8.
Percentage reduction from the initial PANSS0–6 total and subscale scores after 8 weeks of treatment with olanzapine, risperidone, clozapine or other antipsychotics in schizophrenic patients subdivided according to the COMT rs4680–rs4818 haplotypes into C-A haplotype carriers and carriers of the other haplotypes (non-carriers).
| Olanzapine n = 190 |
Risperidone n = 99 |
Clozapine n = 102 |
Other antipsychotics n = 130 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | IR | Median | Range | IR | Median | Range | IR | Median | Range | IR | |
| Total PANSS0–6 scores reduction after 8 weeks of treatment | ||||||||||||
| COMT rs4680–rs4818 | ||||||||||||
| C-A carriers | 57.7 | 86 | 26 | 57.3 | 83 | 30 | 49.5 | 66 | 23 | 57.9 | 85 | 32 |
| Non-carriers | 49.5 | 71 | 23 | 63.2 | 76 | 37 | 45.5 | 53 | 27 | 53.6 | 79 | 34 |
| Mann–Whitney U test | U = 2,463.5; p = 0.006 | U = 927.5; p = 0.727 | U = 1,016.5; p = 0.884 | U = 1701.0; p = 0.810 | ||||||||
| PANSS0–6 positive symptom scores reduction after 8 weeks of treatment | ||||||||||||
| COMT rs4680–rs4818 | ||||||||||||
| C-A carriers | 64.0 | 81 | 31 | 61.4 | 86 | 30 | 54.6 | 90 | 27 | 65.9 | 86 | 33 |
| Non-carriers | 52.9 | 84 | 26 | 68.8 | 84 | 27 | 53.3 | 74 | 28 | 58.3 | 90 | 40 |
| Mann–Whitney U test | U = 2,474.0; p = 0.007 | U = 943.0; p = 0.820 | U = 984.5; p = 0.699 | U = 1736.5. p = 0.953 | ||||||||
| PANSS0–6 negative symptom scores reduction after 8 weeks of treatment | ||||||||||||
| COMT rs4680–rs4818 | ||||||||||||
| C-A carriers | 42.9 | 86 | 37 | 43.35 | 198 | 48 | 28.9 | 73 | 19 | 47.4 | 88 | 56 |
| Non-carriers | 36.4 | 96 | 31 | 44.0 | 92 | 42 | 28.2 | 72 | 22 | 44.2 | 80 | 46 |
| Mann–Whitney U test | U = 2,747.0; p = 0.061 | U = 900.0; p = 0.571 | U = 988.0; p = 0.719 | U = 1714.0; p = 0.862 | ||||||||
| PANSS0–6 general psychopathology scores reduction after 8 weeks of treatment | ||||||||||||
| COMT rs4680–rs4818 | ||||||||||||
| C-A carriers | 55.0 | 95 | 25 | 54.2 | 95 | 28 | 47.3 | 74 | 21 | 53.8 | 92 | 27 |
| Non-carriers | 48.3 | 75 | 19 | 53.6 | 78 | 39 | 43.9 | 46 | 25 | 49.5 | 106 | 35 |
| Mann–Whitney U test | U = 2,679.0; p = 0.037 | U = 950.0; p = 0.863 | U = 1,014.5; p = 0.872 | U = 1621.0; p = 0.516 | ||||||||
Values are given as median, range and interquartile range (IR).
n number of subjects, PANSS positive and negative syndrome scale.
Discussion
This longitudinal study detected a significant association of COMT rs4680 genotype and COMT rs4680–rs4818 haplotype with the much-improved therapeutic response to 8-weeks monotherapy with olanzapine in Caucasian patients with schizophrenia. Our results revealed that olanzapine treated patients, carriers of the COMT rs4680 A allele, or carries of the COMT rs4680–rs4818 C-A haplotype, had greater reduction in the PANSS0–6 total scores. Similar, but statistically non-significant trend, was observed in the COMT rs4680 GA homozygous genotype carriers. A trend towards more significant reduction in the PANSS0–6 positive subscale scores was also detected for the COMT rs4680 A allele carriers and the COMT rs4680–rs4818 C-A haplotype carriers, when compared to the carriers of the COMT rs4680 GG genotype or the carriers of other COMT rs4680–rs4818 haplotypes. Treatment response was determined using a priori cut-off, based on criteria suggested by Leucht et al.19 and based on our previous studies and clinical experience. However, treatment response was also determined by the observed percentage reduction from the initial PANSS0–6 total and subscale scores. This was done in order to avoid potential error by determining the treatment response only by a specific cut-off. From our results it is visible that both approaches yielded similar results and trends. This was observed in the case of COMT rs4680 and treatment response to olanzapine (Tables 3,5), but also in the case of haplotype analysis (Tables 7,8). However, most of these nominally significant differences were lost after correcting for multiple testing, but the detected trend in a treatment response was the same when we used either a priori cut-off or the percentage reduction from the initial PANSS0–6 total and subscale scores.
The association between COMT rs4680 A allele and better response to treatment with olanzapine (greater reduction in the PANSS0–6 total and a similar trend in PANSS0–6 positive subscale scores), detected in our study, agrees with findings showing that homozygous COMT rs4680 AA genotype carriers with schizophrenia had faster therapeutic response to olanzapine20, 21. Two studies with small sample sizes, evaluating olanzapine monotherapy, reported that the COMT rs4680 AA carriers more effectively reduced PANSS symptoms compared to G carriers22. Patients with schizophrenia, carriers of the COMT rs4680 AA genotype had faster and better response to atypical antipsychotics than G allele carriers11, 22–27. Similar results to ours were reported for the Japanese patients with schizophrenia, carriers of the COMT rs4680 AA genotype, treated with aripiprazole for 6 weeks, who showed greater improvement in the PANSS total and general psychopathology scores28. In line with our data, the recent-meta analysis demonstrated that in patients treated with atypical antipsychotics, the COMT rs4680 AA carriers were significantly more likely to respond well to therapy compared to G allele carriers1. Although there are differences in the treatment response definition between our (50% reduction) and the cited study (30% reduction), the results are similar.
On the other hand, in 107 Italian patients with schizophrenia, treated with clozapine, carriers of the COMT rs4680 GG genotype showed a greater improvement in the PANSS negative subscale score (but not in other PANSS subscales scores), compared to both GA or AA genotype carriers29. In our study, including 102 patients treated with clozapine, no significant association was detected between the COMT rs4680 genotype and treatment response or improvement in negative symptoms after clozapine monotherapy. These differences might be explained by the use of different criteria and different design, since we subdivided all our patients according to the treatment response into responders and non-responders, and into carriers of the COMT rs4680 genotypes, while the other study evaluated treatment response in resistant patients with the regression analysis, using COMT and HTR1A genotypes as predictors29. An additive effect of COMT and HTR1A genotypes on the improvement in the PANSS negative symptoms subscale score was suggested, and a better reduction in negative symptoms, after clozapine treatment, was found in patients who were carriers of both COMT rs4680 GG and HTR1A GG genotypes29.
The type of antipsychotic medication might be a possible predictor of the treatment response1. On the other hand, our results, showing a trend towards beneficial therapeutic effect of olanzapine in carriers of the COMT rs4680 GA genotype, are in line with the results of the previous study that revealed a trend, a 3 times greater prevalence of the heterozygous COMT rs4680 GA genotype, compared to AA or GG genotypes, in patients who achieved symptomatic remission7. Our results do not agree with the lack of association between COMT rs4680 and clinical response to antipsychotics, including olanzapine15, 16. Opposed to our data, higher frequency of the COMT rs4680 G allele was found in the responders compared to ultra-resistant patients of the Mexican origin30. The discrepancies might be due to the ethnic origin of the patients, as well as to duration and definition of the response, remission and ultra-resistance.
In a haplotype analysis we have detected a significant association of the COMT C-A haplotype (rs4818–rs4680) and treatment response to olanzapine, but not to risperidone, clozapine or other antipsychotics. This COMT haplotype (C-A) was reported to be significantly related to a good response to risperidone11. In contrast to these results, in our study, including 99 patients treated with risperidone, the COMT C-A haplotype was not significantly associated with treatment response. The differences might be due to different ethnicity and treatment response definition, since we included Caucasian patients, with treatment response as 50% reduction of the PANSS baseline scores, while the other study included subjects of South Indian origin and defined treatment response as the reduction to the scores of two or less on the CGI Global Improvement scale11. In our previous study on the treatment resistant schizophrenia7, the G alleles of the COMT rs4680 and rs4818, as well as the high activity COMT G-G/G-G haplotype, had lower risk to become treatment resistant only in female but not in male patients with schizophrenia. In contrast to these data7, in the present study the genotype distribution for the COMT rs4680 and rs4818 did not differ according to gender. However, when male and female subjects were evaluated separately, no significant association was detected between the COMT rs4680 and rs4818 genotypes and haplotypes and treatment response to olanzapine, risperidone, clozapine or other antipsychotics (Supplementary Tables S1–S12). In addition, in contrast to our previous study7, in the present study we evaluated treatment response and not treatment resistance. The differences between these studies are in the design, since present study was longitudinal while our previous study was cross-sectional, and, unlike the current investigation, previous study did not exclude patients who received ECT, as well as different antipsychotic combinations7. Large heterogeneity across studies, adherence to treatment, population stratification6, as well as influence of other functional variants in the COMT gene7, 31, 32, interactions with other gene polymorphisms, such as DRD4 (120–bp duplication)33, might explain some of the inconsistent findings. In addition, recent studies34, 35 discussed the disadvantages of the candidate gene association studies compared to genome-wide association studies (GWAS), pointing to the problems of high false discovery rate, low replication rates and insufficient knowledge to correctly identify possible candidate genes. However, in the case of COMT polymorphisms, meta-analyses demonstrated the consistent effects on the treatment outcome in schizophrenia1, 6, but with small effect sizes and limited predictive power. Due to these inconsistent data on the association between COMT variants and treatment response to different antipsychotics in schizophrenia, we evaluated genotype- and haplotype-based association of the COMT rs4680 and rs4818 polymorphisms with the much better treatment response in schizophrenia.
The limitations of the study should be acknowledged. Although the sample size was respectable (N = 521), when stratified according to the individual antipsychotic medication, the study included 190 olanzapine-, 99 risperidone-, 102 clozapine-treated patients and 130 patients treated with other antipsychotics, which is lower than the calculated required sample size for each medication group. Moreover, the association between treatment response and only two COMT polymorphisms (rs4680 and 4818) was analyzed, while not taking into account that other COMT polymorphisms or polymorphisms of other dopaminergic genes might affect treatment response in a polygenic multi-factorial disorder, such as schizophrenia. On the other hand, COMT rs4680 was recently3 confirmed to be a functional polymorphism, since it significantly affects abundance, stability, and activity of the COMT enzyme36. There is also evidence of a large inter-individual variation in the pharmacokinetics of olanzapine, leading to multiple differences in drug exposure between subjects at a given dose, which might explain the concentration-dependent therapeutic failures between studies. However, in our study we were unable to determine plasma concentration of olanzapine in patients with schizophrenia. Non-replication of the pharmacogenetic data is common, due to different study designs, small sample sizes, lack of statistical power, ethnic and racial differences, small effects of the most individual genes, variety of environmental and clinical confounders, differences in definition of response, remission or resistance, and lack of evaluation of the possible effects of other gene polymorphisms4, 6, 36.
Strengths of the present study are in both genotype and haplotype analyses, olanzapine, risperidone, and clozapine monotherapy, inclusion of ethnically homogenous Caucasian patients with schizophrenia, usage of a priori cut-off point of 50% reduction from the baseline PANSS total and subscale scores19 and the percentage reduction from the initial PANSS0–6 scores for the treatment response, corrected p-value, evaluation of the possible sex differences, and the longitudinal study design (including 8 weeks follow up). Unlike previous studies, which investigated treatment response to different antipsychotics, the present investigation focused on the substantial response to olanzapine, risperidone or clozapine monotherapy. Therefore, our results confirmed a significant association of the COMT rs4680 A allele, and the COMT rs4680–rs4818 C-A haplotype, with a good therapeutic response to olanzapine. These data offer pharmacogenetic information for clinicians, with a predictive value to choose responsive patients for the treatment with olanzapine, in a quest to find reliable genetic markers of treatment outcome in schizophrenia.
Methods
Participants
The clinical characteristics of the study sample were described in detail in our previous study37. Diagnosis of schizophrenia was conducted using a structured clinical interview38 based on the DSM-IV criteria. The present study included 521 patients (67.6% males) who were 40.3 ± 12.0 years old (range 19–82), and part of them (N = 87) were included in our previous longitudinal 6 months study evaluating remission and not therapeutic response9. Before the study, patients with schizophrenia were treated with different antipsychotics: olanzapine (5–20 mg/day), clozapine (100–800 mg/day), risperidone (2–6 mg/day), fluphenazine (5–15 mg/day), haloperidol (4–15 mg/day), promazine (50–300 mg/day), quetiapine (300–800 mg/day), ziprasidone (80–160 mg/day), amisulpride (200–600 mg/day), sulpiride (200–800 mg/day), sertindole (12–16 mg/day), zuclopenthixol (20–40 mg/day), alone or in combination with benzodiazepines, i.e. diazepam (5–30 mg/day). At some point, some patients were also previously treated with long-acting antipsychotics (LAI): olanzapine 210–405 mg monthly, risperidone LAI 25–50 mg monthly, fluphenazine LAI 25–50 mg monthly, haloperidol LAI 50–100 mg monthly, zuclopenthixol depot (150–300 mg monthly). All depot preparations were discontinued at least a month prior to inclusion in the study, whilst majority of such patients stopped receiving LAIs several months before entering this trial. After inclusion in the study, patients were subdivided, under the discretion of their psychiatrist, into groups treated with olanzapine (10–20 mg/day); N = 190 (36.5%), risperidone (3–6 mg/day); N = 99 (19.0%), or clozapine (100–500 mg/day); N = 102 (19.6%) monotherapy for 8 weeks. The fourth group, due to the small sample sizes was merged into one group designated as “other antipsychotics” (N = 130; 25%), consisted of patients receiving monotherapy with haloperidol (3–15 mg/day) or fluphenazine (4–25 mg/day) or quetiapine (50–800 mg/day). All patients received monotherapy for 8 weeks. During the study no concomitant medication was allowed except benzodiazepines when needed. Patients were excluded from the study in the case of exacerbation of the illness, or the need to add additional antipsychotic or switching to another antipsychotic medication. Patients were evaluated with structured interview for the Positive and Negative Syndrome Scale (PANSS) including the PANSS positive, PANSS negative and PANSS general psychopathology subscales39. Patients were included if they were in- and out-patients diagnosed with schizophrenia for at least 5 years; treated with monotherapy with the above listed antipsychotic drugs, with added benzodiazepines when needed; patients who finished 8 weeks of treatment; who were ≥ 18 years old and who signed informed consent. Exclusion criteria were the use of antidepressants and polytherapy with antipsychotics, intellectual disabilities, patients with first-episode psychosis and patients with mild symptoms at baseline (baseline PANSS1–7 score ≤ 58), substance abuse and dependence in the previous 3 months, patients with any comorbid severe somatic or neurological disorder and patients who had no available detailed medical records with complete psychiatric medication history. After inclusion, all patients underwent complete diagnostic evaluation. Evaluation of the treatment response was conducted using the PANSS total and subscale scores at baseline (during the first few days of admission) and after 8 weeks of treatment. All raters were blind to genotyping data. Interrater reliability was 97%. Patients were sampled from the two centers (University Hospital Center Zagreb and Clinics for Psychiatry Vrapce, Zagreb) and were all ethnically homogenous unrelated Caucasians of European ancestry (of Croatian origin). Before the study, treatment response was defined as ≥ 50% reduction from the baseline PANSS total and subscale scores19. The scoring system of the PANSS was corrected from values of 1–7 to values of 0–6 as suggested19. At baseline, median corrected PANSS0–6 total score was 86.0 (range 43–144), while after 8 weeks of treatment, it was 39.0 (range 4–116).
The local Ethics committees from University Hospital Center Zagreb and Clinics for Psychiatry Vrapce, Zagreb, approved the study. After explaining the aims and procedures of the study, all participants signed the informed consent. The study did not include the patients that needed legally authorized representative for signing the informed consent. All human studies were performed with the full cooperation and understanding of the participants. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Blood collection and genotyping
Blood samples were collected during routine laboratory visits. Genomic DNA was extracted from peripheral blood using a salting out method40. The genotyping of the COMT rs4680 (assay ID: C_25746809_50) and COMT rs4818 (assay ID: C_2538750_10) was performed according to the procedures described by Applied Biosystems. Researchers were blind to clinical data. We used the primers and probes from the TaqMan R Drug Metabolism Genotyping Assays (Applied Biosystems, Foster City, CA, United States), and detection was performed on ABI Prism 7300 Real time PCR System apparatus (Applied Biosystems, Foster City, CA, United States). The reaction volume of 10 mL contained 30–100 ng of DNA. As a quality control, we randomly selected up to 10% of samples and genotyped them again.
Statistical evaluation
Data were analyzed using Sigma Stat 3.5 (Jandel Scientific Corp. San Rafael, CA, USA) and Microsoft Excel. Data distribution normality was determined with the Kolmogorov–Smirnov normality test. Due to the lack of a normal distribution, Kruskal–Wallis analysis of variance (ANOVA) and Dunn post hoc were used to assess differences in age, PANSS total, positive, negative, and general psychopathology scores between different groups of patients. The Hardy–Weinberg equilibrium (HWE), as well as genotype and haplotype distributions, were determined using χ2-test41. Haploview software v. 4.242 was used to determine LD pairwise values for COMT rs4818 and rs4680 polymorphisms. Loci were considered to be in linkage disequilibrium if D’ coefficient was > 0.80. Haplotype was estimated for every patient by PLINK v. 1.07 software using the expectation–maximization algorithm43.
For individual SNP analysis the p-value (0.05/8 = 0.00625) was corrected because two SNPs were analyzed and treatment response was tested in 4 medication groups. The results were considered significant if p < 0.00625.
G ∗ Power 3 Software44 was used to conduct the power analysis. For a χ2-test [with α = 0.006; with expected medium effect size = 0.3; power (1 − β) = 0.800] the required sample size was N = 188 for df = 3; N = 169 for df = 2; or N = 144 for df = 1. For ANOVA [with α = 0.006; with expected medium effect size = 0.25; and power (1 − β) = 0.800] the required sample size was N = 276 for 4 compared groups and N = 249 for 3 compared group. For t-test [with α = 0.006; with expected medium effect size = 0.5; power (1 − β) = 0.800] the required sample size was N = 184.
Supplementary information
Acknowledgements
This work has been supported by the project sponsored by the University of Zagreb, Croatia “Predictors of treatment response in schizophrenia”, BM1.45, PI: Marina Sagud; and by the project sponsored by Ministry of Sciences and Education “Molecular basis and treatment of psychiatric and stress related disorders” No. 098-0982522-2455, PI: Nela Pivac.
Author contributions
All authors contributed to the study. N.P. and M.S. contributed to study design and conceptualization. M.S., M.Z., S.U., O.K., N.M. and A.M.P. were responsible for patient enrollment and evaluation. MNP designed the experiments and performed the experimental procedures. N.P., M.S. and M.N.P. analyzed and interpreted the results. N.P., M.S., M.N.P., G.N.E. and D.S.S. wrote the initial draft of the manuscript. All authors participated in revising and editing of the manuscript and they have all read and approved the final manuscript.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-67351-5.
References
- 1.Huang, E. et al. Catechol-O-methyltransferase Val158Met polymorphism and clinical response to antipsychotic treatment in schizophrenia and schizo-affective disorder patients: a meta-analysis. Int. J. Neuropsychopharmacol.19, pyv132 (2016). [DOI] [PMC free article] [PubMed]
- 2.Lotta T, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 3.Scanlon PD, Raymond FA, Weinshilboum RM. Catechol-O-methyltransferase: thermolabile enzyme in erythrocytes of subjects homozygous for allele for low activity. Science. 1979;203:63–65. doi: 10.1126/science.758679. [DOI] [PubMed] [Google Scholar]
- 4.Tunbridge EM, et al. Which dopamine polymorphisms are functional? Systematic review and meta-analysis of COMT, DAT, DBH, DDC, DRD1-5, MAOA, MAOB, TH, VMAT1, and VMAT2. Biol. Psychiatry. 2019;86:608–620. doi: 10.1016/j.biopsych.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Roussos P, Giakoumaki SG, Pavlakis S, Bitsios P. Planning, decision-making and the COMT rs4818 polymorphism in healthy males. Neuropsychologia. 2008;46:757–763. doi: 10.1016/j.neuropsychologia.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida, K. & Müller, D. J. Pharmacogenetics of antipsychotic drug treatment: update and clinical implications. Mol. Neuropsychiatry 1–26, doi: 10.1159/000492332 (2018). [DOI] [PMC free article] [PubMed]
- 7.Sagud M, et al. Haplotypic and Genotypic Association of Catechol-O-Methyltransferase rs4680 and rs4818 Polymorphisms and Treatment Resistance in Schizophrenia. Front. Pharmacol. 2018;9:705. doi: 10.3389/fphar.2018.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terzic T, Kastelic M, Dolžan V, Plesničar BK. Genetic polymorphisms in dopaminergic system and treatment-resistant schizophrenia. Psychiatr. Danub. 2016;28:127–131. [PubMed] [Google Scholar]
- 9.Zivkovic M, et al. The lack of association between COMT rs4680 polymorphism and symptomatic remission to olanzapine monotherapy in male schizophrenic patients: a longitudinal study. Psychiatry Res. 2019;279:389–390. doi: 10.1016/j.psychres.2019.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Sagud M, et al. Catechol-O-methyl transferase and schizophrenia. Psychiatr. Danub. 2010;22:270–274. [PubMed] [Google Scholar]
- 11.Gupta M, et al. Association studies of catechol-O-methyltransferase (COMT) gene with schizophrenia and response to antipsychotic treatment. Pharmacogenomics. 2009;10:385–397. doi: 10.2217/14622416.10.3.385. [DOI] [PubMed] [Google Scholar]
- 12.Illi A, et al. Interaction between angiotensin-converting enzyme and catechol-O-methyltranferase genotypes in schizophrenics with poor response to conventional neuroleptics. Eur. Neuropsychopharmacol. 2003;13:147–151. doi: 10.1016/S0924-977X(02)00176-1. [DOI] [PubMed] [Google Scholar]
- 13.Illi A, et al. Catechol-O-methyltransferase val108/158met genotype and response to antipsychotic medication in schizophrenia. Hum. Psychopharmacol. 2007;22:211–215. doi: 10.1002/hup.841. [DOI] [PubMed] [Google Scholar]
- 14.Inada, T., Nakamura, A. & Iijima, Y. Relationship between catechol-O-methyltransferase polymorphism and treatment-resistant schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet.120B, 35–39 (2003). [DOI] [PubMed]
- 15.Tybura P, et al. Pharmacogenetics of adverse events in schizophrenia treatment: Comparison study of ziprasidone, olanzapine and perazine. Psychiatry Res. 2014;219:261–267. doi: 10.1016/j.psychres.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 16.Vehof J, et al. Clinical response to antipsychotic drug treatment: association study of polymorphisms in six candidate genes. Eur. Neuropsychopharmacol. 2012;22:625–631. doi: 10.1016/j.euroneuro.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Kocabas NA, et al. The impact of catechol-O-methyltransferase SNPs and haplotypes on treatment response phenotypes in major depressive disorder: a case–control association study. Int. Clin. Psychopharmacol. 2010;25:218–227. doi: 10.1097/YIC.0b013e328338b884. [DOI] [PubMed] [Google Scholar]
- 18.Andreasen NC, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am. J. Psychiat. 2005;162:441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- 19.Leucht S, Davis JM, Engel RR, Kissling W, Kane JM. Definitions of response and remission in schizophrenia: recommendations for their use and their presentation. Acta Psychiatr. Scand. 2009;119:7–14. doi: 10.1111/j.1600-0447.2008.01308.x. [DOI] [PubMed] [Google Scholar]
- 20.Molero P, Ortuño F, Zalacain M, Patiño-García A. Clinical involvement of catechol-O-methyltransferase polymorphisms in schizophrenia spectrum disorders: influence on the severity of psychotic symptoms and on the response to neuroleptic treatment. Pharmacogenomics J. 2007;7:418–426. doi: 10.1038/sj.tpj.6500441. [DOI] [PubMed] [Google Scholar]
- 21.Stefanis NC, et al. Variation in catechol-o-methyltransferase val158 met genotype associated with schizotypy but not cognition: a population study in 543 young men. Biol. Psychiatry. 2004;56:510–515. doi: 10.1016/j.biopsych.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Bertolino A, et al. COMT Val158Met polymorphism predicts negative symptoms response to treatment with olanzapine in schizophrenia. Schizophr. Res. 2007;95:253–255. doi: 10.1016/j.schres.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Anttila S, et al. Interaction between NOTCH4 and catechol-O-methyltransferase genotypes in schizophrenia patients with poor response to typical neuroleptics. Pharmacogenetics. 2004;14:303–307. doi: 10.1097/00008571-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Fijal BA, et al. Candidate-gene association analysis of response to risperidone in African American and white patients with schizophrenia. Pharmacogenomics J. 2009;9:311–318. doi: 10.1038/tpj.2009.24. [DOI] [PubMed] [Google Scholar]
- 25.Weickert TW, et al. Catechol-O-methyltransferase val108/158met genotype predicts working memory response to antipsychotic medications. Biol. Psychiatry. 2004;56:677–682. doi: 10.1016/j.biopsych.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Woodward ND, Jayathilake K, Meltzer HY. COMT val108/158met genotype, cognitive function, and cognitive improvement with clozapine in schizophrenia. Schizophr. Res. 2007;90:86–96. doi: 10.1016/j.schres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Zhao QZ, et al. Association between a COMT polymorphism and clinical response to risperidone treatment: a pharmacogenetic study. Psychiatr. Genet. 2012;22:298–299. doi: 10.1097/YPG.0b013e328358629a. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko H, et al. COMT Val 108/158 Met polymorphism and treatment response to aripiprazole in patients with acute schizophrenia. Neuropsychiatr. Dis. Treat. 2018;14:1657–1663. doi: 10.2147/NDT.S164647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosia M, et al. COMT Val158Met and 5-HT1A-R –1019 C/G polymorphisms: effects on the negative symptom response to clozapine. Pharmacogenomics. 2015;16:35–44. doi: 10.2217/pgs.14.150. [DOI] [PubMed] [Google Scholar]
- 30.Escamilla R, et al. Association study between COMT, DRD2, and DRD3 gene variants and antipsychotic treatment response in Mexican patients with schizophrenia. Neuropsychiatr. Dis. Treat. 2018;14:2981–2987. doi: 10.2147/NDT.S176455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong Y, et al. Polymorphisms in microRNA target sites influence susceptibility to schizophrenia by altering the binding of miRNAs to their targets. Eur. Neuropsychopharmacol. 2013;23:1182–1189. doi: 10.1016/j.euroneuro.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Nackley AG, et al. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- 33.Rajagopal VM, Rajkumar AP, Jacob KS, Jacob M. Gene-gene interaction between DRD4 and COMT modulates clinical response to clozapine in treatment-resistant schizophrenia. Pharmacogenet. Genomics. 2018;28:31–35. doi: 10.1097/FPC.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 34.Johnson EC, Border R, Melroy-Greif WE, de Leeuw C, Ehringer MA, Keller MC. No evidence that schizophrenia candidate genes are more associated with schizophrenia than non-candidate genes. Biol Psychiatry. 2017;82:702–708. doi: 10.1016/j.biopsych.2017.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherva R, Farrer LA. Power and pitfalls of the genome-wide association study approach to identify genes for Alzheimer's disease. Curr. Psychiatry. Rep. 2011;13:138–146. doi: 10.1007/s11920-011-0184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandl EJ, Kennedy JL, Müller DJ. Pharmacogenetics of antipsychotics. Can. J. Psychiatry. 2014;59:76–88. doi: 10.1177/070674371405900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikolac Perkovic, M. et al. Association between the brain-derived neurotrophic factor Val66Met polymorphism and therapeutic response to olanzapine in schizophrenia patients. Psychopharmacology231, 3757–3764 (2014). [DOI] [PubMed]
- 38.First MB, Spitzer RL, Williams JBW, Gibbons M. Structured clinical interview for DSM-IV-patient edition (SCID-P) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 39.Kay SR, Fisbein A, Opler LA. The Positive and negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 40.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez S, Gaunt TR, Day IN. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am. J. Epidemiol. 2009;169:505–514. doi: 10.1093/aje/kwn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 43.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;8:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


