Abstract
Pericytes, the mural cells surrounding microcirculation, are gaining increasing attention for their roles in health and disease of the central nervous system (CNS). As an essential part of the neurovascular unit (NVU), pericytes are actively engaged in interactions with neighboring cells and work in synergy with them to maintain homeostasis of the CNS, such as maintaining the blood–brain barrier (BBB), regulating cerebral blood flow (CBF) and the glymphatic system as well as mediating immune responses. However, the dysfunction of pericytes may contribute to the progression of various pathologies. In this review, we discuss: (1) origin of pericytes and different pericyte markers; (2) interactions of pericytes with endothelial cells (ECs), astrocytes, microglia, oligodendrocytes, and neurons; (3) physiological roles of pericytes in the CNS; (4) effects of pericytes in different CNS diseases; (5) relationship of pericytes with extracellular vesicles (EVs) and microRNAs (miRs); (6) recent advances in pericytes studies and future perspective.
Keywords: Pericyte, extracellular vesicles, microRNA, central nervous system disease, glymphatic system
Introduction
Pericytes surround endothelial cells (ECs) of the microcirculation which includes pre-capillary arterioles, capillaries, and post-capillary venules.1 Here, we particularly focus on the pericytes of the capillaries within the central nervous system (CNS), where pericytes are sandwiched between ECs and astrocytes.2 Within the blood–brain barrier (BBB), pericytes and ECs are separated by the endothelial basement membrane (BM), and together are embedded within the vascular BM that is derived from astrocytes.3 Pericytes are most abundant in CNS where the EC to pericyte ratio can range from 1:1 to 3:1.4 Such high regional density and strategic position of pericytes with neighboring cells may indicate distinct roles for pericytes in CNS. However, the recognized role of pericytes is limited and has long been overshadowed by ECs, due to the paucity of means to identify and distinguish these cells.5 In recent years, advances in transgenic animal generation, in vivo microscopy techniques, and vascular single-cell transcriptomics have led to the identification of specific pericytes markers and visualization of pericytes in live animals.6,7 However, gaps in knowledge persist. Findings in pericyte expression profiles have expanded pericyte categorization, and yet the functions of each type of pericyte in health and disease are not fully determined.1,6 Also unclear, are pericyte contractile properties and their stem-cell potentials.8,9 Although both benefits and drawbacks of pericytes have been broadly explored, the therapeutic translation related to pericyte function remains. In this review, we summarize the current knowledge and recent findings in the area of pericyte origin, pericyte identification, the physiological effects of pericytes as well as their multi-faceted roles in CNS pathologies. We then review the interactions between pericytes and extracellular vesicles (EVs) and with microRNAs (miRs), which may provide potential therapeutic targets for the treatment of CNS diseases.10,11 Finally, we briefly discuss recent advances in pericyte studies and present future perspectives.
Pericyte origins
Pericytes vary in origin dependent on their anatomical location. Neural crest cells are the major source of pericytes in the forebrain.12,13 In the remaining brain, pericytes are largely derived from the mesenchymal stem cells (MSCs) of the mesoderm which also generates the pericyte counterparts in peripheral organs.14 Myeloid lineage cell is an alternative source of pericytes in the CNS,15 and appears more specific to the pericytes in ectodermal organs like brain and skin, since no striking pericyte deficiencies are found in the mutant heart and liver and there are no detectable pericyte changes in vascular smooth muscle cells (VSMCs) in myeloid-deficient transgenic mice.16
Pericyte identification
Both VSMCs and pericytes are the mural cells of the microcirculation.3 Protruding ovoid nuclei with thin extended processes is the widely accepted characteristic morphology of pericytes, and is distinct from the ring-shaped VSMCs with circumferential processes.1,3 This difference in morphology is very useful to distinguish pericytes from VSMCs within microcirculations, as many pericyte markers label both VSMCs and pericytes.6 However, a transitional cell appears to exist between VSMCs and pericytes at the arteriolar level, and both have protruding cell bodies and circumferential processes.1 Because the vessels they cover are arteriolar branches located between penetrating arterioles and capillaries, these transitional cells are referred to as “pre-capillary VSMCs.”8 Grant et al.1 recently redefined these hybrid cells as “ensheathing pericytes”. Therefore, how to identify and how to distinguish capillary pericytes from transitional cells are problematic. Here, we review pericyte markers and summarize these markers and related transgenic mouse lines in Table 1.
Table 1.
Pericyte identification.
| Classic pericyte markers | References | |
|---|---|---|
| PDGFR-β | (1) Surface protein that outlines the contour of the cell;(2) First existing pericyte marker during embryogenesis;(3) Labels relatively immature pericytes and VSMCs in CNS;(4) Dynamic expressions in brain injuries | Armulik et al.3Junget al.17Goritz et al.127Kyyriainen et al.18 |
| NG2 | (1) Integral membrane proteoglycan;(2) First appears as a pericyte marker at E11.5; Expression in OPCs starts from E16 ;(3) Stimulates proliferation and migration of pericytes. | Zhu et al.19Yotsumoto et al.112Jung et al.17 |
| α-SMA | (1) Microfilaments in VSMCs and a subset of pericytes;(2) Exists as a pericyte marker after birth;(3) Positive expression in pre-capillary mural cells. May negative in capillary pericytes.(4) In combination of morphology may help distinguish capillary pericytes from pre-capillary mural cells | Jung et al.17Grant et al.1Hartmann et al.21 |
| Desmin | (1) Intermediate microfilament;(2) As pericyte marker throughout embryogenesis;(3) Labels mature pericytes and VSMCs in CNS; canonical marker of muscle cells;(4) May also be expressed in activated astrocytes in brain injury; | Armulik et al.3Jung et al.17Jung et al.17Choi et al.26Kelly-Goss et al.25 |
| CD13 | (1) Aminopeptidase N;(2) Exists as a pericyte marker after birth at P6;(3) Does not label pericytes in lung. | Hartmann et al.21Jung et al.17Vanlandewijck et al.22 |
| RGS5 | Labels angiogenic capillary pericytes; | Mitchellet al.28 |
| Endosialin(CD248) receptor | Labels angiogenic capillary pericytes; | Simonavicius et al.35 |
| CD146 | Labels pericytes from E11, and may coordinate EC-pericyte interaction; | Chen et al.30 |
| New pericyte markers | ||
| VTN/Ifitm-1 | Labels capillary pericytes; | He et al.6 |
| NeuroTrace 500/525 | Absorbed by capillary pericytes ; | Damisah et al.27 and Grant et al.1 |
| Transgenicpericytesmice | ||
| Pdgfrbret/ret | PDGF-B-truncating mutation | Moura et al.151 |
| Pdgfrb-CreER mice | Tamoxifen-inducible Cre-recombinase under the control of the Pdgfrb promoter | |
| (1) Pdgfrb-P2A-CreERT2 | express Cre-ERT2 from the endogenous Pdgfrb promoter | Cuervo et al.152 |
| (2) Pdgfrb(BAC)-CreERT2 | Pdgfrb gene from BACf library | Eilken et al.36 |
| Pdgfrb-Cre mice + fluorescent protein reporter | Show the continuous contour of the mural cells | |
| (1) Pdgfrb-eGFP mice | Jung et al.17 and He et al.6 | |
| (2) Pdgfrb-tdTomato mice | Grant et al.1 | |
| (3) Pdgfrb-YFP mice | Berthiaume et al.82 | |
| Pdgfrb-Cre-ChR2 mice | Optogenetic stimulation cause capillary constriction | Hartmann et al.7 and Kisleret al.103 |
| NG2-CreER™ BAC transgenic mice | Eilken et al.36 | |
| NG2-CreER mice +fluorescent protein reporter | Display the structure of individual mural cells | Jung et al.17 and He et al.6 |
| (1) NG2-DsRed mice | ||
| (2) NG2-tdTomato | Grant et al.1 and Berthiaume et al.82 | |
| NG2-Cre-ChR2 mice | Optogenetic stimulation cause arteriole constriction | Hill et al.8 |
| Hey1-GFP (Tg(Hey1-EGFP)ID40Gsat) reporter mice | Label immature pericytes at angiogenic front | Eilken et al.36 |
| Tbx18 | Labels all PCs, regardless of subsets of pericytes; | Guimaraes-Camboa, et al.9 |
PDGFR-β: Platelet derived growth factor receptor beta; NG2: neural/glial antigen 2; RGS5: regulator of G-protein signaling 5; VTN/Ifitm-1:vitronectin/interferon-induced transmembrane protein 1; eGFP: estrogen-receptor; BAC: bacterial artificial chromosome; eGFP: enhanced green fluorescent protein; YFP: yellow fluorescent protein; ChR2: channelrhodopsin2.
Markers for both VSMCs and pericytes
Platelet-derived growth factor receptor beta
This is a surface protein, is the most widely used pericyte marker,3 and has been employed to identify pericytes during embryogenesis.17 Although PDGFR-β is a non-specific protein with expression in neuronal progenitor cells, glia, and fibroblast cells after injuries, the distinctive ovoid cell body and close proximity to microvessels permit specific identification of PDGFR-β+ pericytes.18
Neural/glial antigen 2
Neural/glial antigen 2 (NG2) is another commonly used pericyte marker,6 although it is also expressed in oligodendrocyte progenitor cells (OPCs) and astrocytes.19 However, during embryogenesis, NG2+ pericytes (E11.5) appear prior to OPC’s (E16), and its close proximity to endothelial cells (ECs) as well as the prominent ovoid cell body can easily distinguish it from OPCs.3,20
CD13, the aminopeptidase N
CD13, the aminopeptidase N is regarded as a more specific marker for mural cells compared with PDGFR-beta and NG2.21 However, CD13 cannot be employed to distinguish precapillary mural cells from capillary pericytes. In addition, CD13 cannot label pericytes of the lung.22
Desmin
Desmin is an intermediate filament of muscle cells.23 As pericytes have been visualized as smooth muscle cells at the capillary level, desmin can justifiably label pericytes in the BBB.3,17 However, in a perilesional zone, desmin-positive pericytes do not overlap with PDGFR-β+ pericytes and resemble GFAP+ astrocytes.24,25 Given the similarity in structure between the desmin and GFAP+ cells, the specificity of desmin is open to doubt in injured tissue regions.26 Whether desmin represents inflamed pericytes or activated glial cells needs further confirmation.
Alpha-smooth muscle actin
Alpha-smooth muscle actin (α-SMA) has long been used as marker for both VSMCs and pericytes and until recently it was reported that SMA is not suitable for the pericytes of terminal vessel branches. Pericytes that encompass the pre-capillary arterioles are defined as “ensheathing pericytes” and express SMA, while their counterparts that occupy the downstream branches-capillaries are SMA-negative.1 This finding may facilitate unveiling the distinct role of pericytes on different vessels.
Novel markers for capillary pericytes
Vitronectin and interferon-induced transmembrane protein 1
Vitronectin (VTN) and interferon-induced transmembrane protein 1(Ifitm-1) are two markers for capillary pericytes, which have been validated by single-cell RNA sequencing and fluorescent in situ hybridization.6 VTN, a glycoprotein, is expressed in capillary pericytes, and has reduced abundance in pericytes that surround the arterioles and veins. Ifitm-1 shows a weak but specific signal for capillary pericytes.6
NeuroTrace 500/525
NeuroTrace 500/525 is a Nissl dye, but does not label neurons in vivo. Surprisingly, it is exclusively absorbed by the capillary pericytes, as confirmed using both Pdgfrb-Cre:Tomato and NG2-Cre:Tomato transgenic mice.27 NeuroTrace 500/525 generates a robust signal overlapping with endogenous PDGFR-β and NG2-positive cells on the capillary level and is absent from immediate upstream arterioles. NeuroTrace 500/525 and α-SMA are mutually exclusive,1 which may provide a clear distribution of pericyte branches when using the two markers together.1
Pericyte markers with angiogenesis and BBB development
Regulator of G-protein signaling 5
Regulator of G-protein signaling 5 (RGS5) is particularly expressed in pericytes of angiogenic vessels. The RGS5 gene expression is more sensitive than morphological measurement. Its alteration is positively correlated with the number of activated pericytes and to a greater extent than that of NG2 and desmin during angiogenesis,28,29 indicating RGS5 is an effective tool for pericyte quantification during angiogenesis. Likewise, endosialin is also reported to be exclusively expressed by angiogenic pericytes. CD146 is a transmembrane immunoglobulin. During BBB development, CD146 is expressed in the ECs that compose nascent vessels without pericyte coverage. However, as pericytes are recruited to the vessel sprouts, CD146 expression is diminished in ECs and enhanced in the pericytes. From E11 onward, CD146 is predominately expressed by pericytes and not by ECs of mature vessels with stable pericyte coverage. This transition indicates a coordinating role of CD146 between ECs and pericytes during BBB formation.30
Pericyte effects in cell–cell interactions
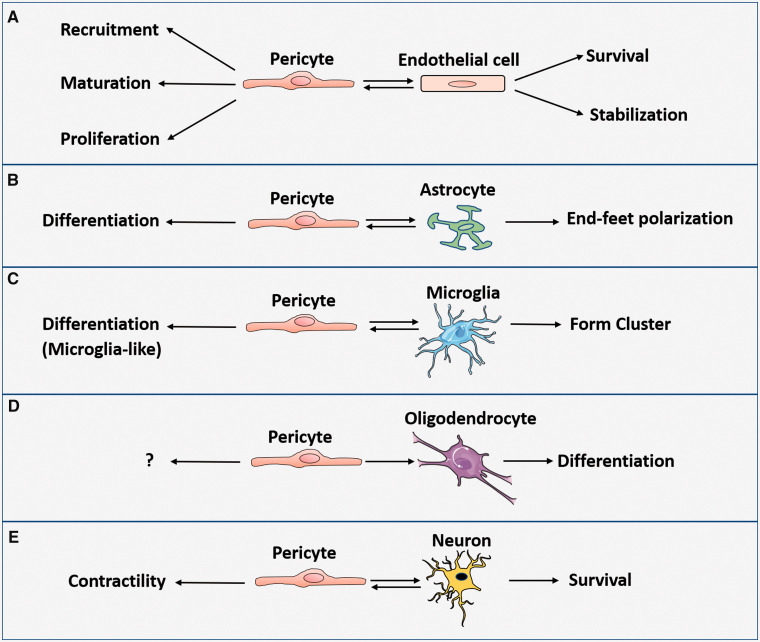
We review the interactions of pericytes with ECs, astrocytes, microglia, oligodendrocytes, and neurons (Figure 1).
Figure 1.
Pericyte effects in cell–cell interactions within NVU: (a) Pericyte promotes EC survival and stabilization, whereas EC enhances pericyte recruitment, promotes pericyte maturation and proliferation during embryogenesis and after birth. (b) Pericyte induces polarization of astrocytic end-feet. In turn, astrocyte help induce differentiation of pericyte. (c) Pericytes may differentiate into microglia. Pericyte and microglia can form clusters during injury. (d) Pericyte promotes OPC to differentiate into oligodendrocyte. (e) Pericyte secretes neuronal growth factor to enhance neuron survival, whereas neuron will send cues to control pericyte contractility.
Pericytes and EC interaction (Figure 1(a)
The interactions between pericytes and ECs are complex and have been widely studied.31 The platelet-derived growth factor (PDGF)-BB/PDGFR-β signaling pathway is vital for the survival, recruitment, and proliferation of pericytes during angiogenesis32 Angiopoitin1/tyrosine protein kinase receptor (Tie2) signaling pathway is required for vascular stabilization.33 These two pathways have been discussed extensively in previous reviews.32 Here we review four pathways as follows:
Vascular endothelial growth factor (VEGF)/VEGFR pathway
VEGF-VEGFR signaling pathways play an important role in angiogenesis where it promotes the survival and proliferation of ECs. The roles of pericytes in the responses of ECs to VEGF vary among different studies. A study of tumor angiogenesis shows that pericytes promote EC survival by inducing ECs to release autocrine VEGF which subsequently activates downstream anti-apoptotic factors to stabilize vessels.34 However, pericytes may also have destabilizing effects on vessel sprouting during both developmental and tumor angiogenesis.35 Endosialin that is exclusively expressed by the pericytes in the angiogenic vessels can decrease the phosphorylation of VEGF/VEGFR2 downstream pathways in ECs and lead to EC apoptosis. By doing so, pericytes promote selective vessel regression, a process of pruning undesired capillaries for an organized vascular remodeling.35 Pericytes are also required for the normal vessel branching during vessel sprouting. A study of retinal angiogenesis shows that deletion of pericyte-derived VEGFR-1 results in reduced vascular branch points and scattered vessel network, accompanied by an enhanced VEGF/VEGFR2 signaling pathway.36 Soluble VEGFR1 (sVEGFR1) released by the pericytes at the front of angiogenic vessels can bind the circulating VEGF to prevent from VEGF acting on VEGFR2, and thereby inhibit EC hyperplasia that thickens the sprouting without branching. VEGF may also negatively control pericytes via disrupting pericyte recruitment and maturation by forming a PDGFR-β and VEGFR-2 complex on pericytes.37 However, another study of cancer-related retinopathy shows that VEGF ablates the pericytes via VEGFR1 not VEGFR2, as blockade of VEGFR2 cannot restore pericytes.38 This discrepancy may be due to the different types of pericyte cell lines the studies tested. One used α-SMA-positive pericyte cell line,37 whereas the other one used NG2-positive retinal pericytes,38 which suggests that different subsets of pericytes may respond differently to VEGF.
Transforming growth factor beta (TGF-β)
TGF-β is normally latent in both ECs and pericytes, and mediates angiogenesis, inflammation, and fibrosis when activated.31 TGF-β regulates vessel stability by inducing pericyte differentiation.31 Deletion of TGF-β leads to remarkable reduction of mature pericyte coverage of vessels, which enhances vascular permeability and results in abnormal changes of retina that mimics those of diabetic retinopathy. Conversely, systemic deletion of platelet-derived growth factor receptor β (PDGFR-β) in pericytes leads to downregulation of TGF-β, which may form a vicious cycle.31 EC morphogenesis in developing brain partially relies on pericyte-expressed TGF-β type1 receptor kinase and tissue inhibitor of matrix metalloproteinase 3 (TIMP3).39 TGF-β/smad4 (Mothers against decapentaplegic homolog 4) pathway and Notch pathways in ECs cooperatively regulate the expression of N-cadherin, a key adhesion protein connecting ECs and pericytes.40
Sphingosine-1-phosphate (S1P) signaling pathway
S1P is a bioactive sphingolipid metabolite and mediates a myriad of physiological and pathological processes via binding to a family of five G protein-coupled receptors (S1PR1-S1PR5).41 Pericyte-derived S1P increases N-cadherin and VE-cadherin expression between pericytes and ECs and reduces Angpt-2 in ECs.31 A recent study shows that S1PR2 is upregulated in pericytes in experimental stroke.42 S1PR2 may reduce N-cadherin expression and promote pericyte migration through NF-κB p65 signaling, which increases BBB permeability and exacerbates neural injury.42
Ephrin–erythropoietin-producing hepatoma (Eph) pathway
EphrinB2 is vital for pericytes and ECs to assemble into a vascular structure.43 It regulates the internalization of PDGFR-β by pericytes.44 Mutant mice with no EphrinB2 expression in pericytes and VSMCs are prone to develop aneurysms,44,45 and suffer delayed neurovascular repair after stroke.46 By contrast, diabetes induces the expression of EphrinB2 in brain pericytes, which is concomitant with immature neovascularization characterized by large diameter and tortuous appearance.47
Pericyte and astrocyte interaction (Figure 1(b))
Pericytes regulate the polarization of astrocyte end-feet.2 Aquaporin 4 (AQP4), a principal water channel protein, is exclusively expressed by astrocytes, and mediates CNS water homeostasis, glymphatic system function, synaptic plasticity, and cognitive function.48,49 Aqp4 is strongly expressed on the side of astrocytes that connects with pericytes.50 Pericyte deficiency results in a redistribution of AQP4 on astrocyte end-feet where AQP4 moves away and is expressed on other side of astrocytes with no vessel contact.2,50 This redistribution may be related with α-syntrophin, the component of dystrophin complex that regulates AQP4 anchoring.51 Normally, α-syntrophin is expressed at increased density at astrocytic end-feet abutting pericytes.50 In pericyte-deficient mice, α-syntrophin is decreased with concomitantly reduced AQP4 expression in the perivascular site of the astrocyte end-foot.50,52 In addition, the laminin a2 chain (Lama2), an astrocyte-derived extracellular matrix (ECM) protein, is important for the regulation of cell growth, motility, and adhesion.53 Lama2 is greatly decreased in pericyte-deficient mice without affecting EC-derived basement membrane proteins.2 Conversely, astrocytic laminin may also regulate pericyte differentiation via the integrin α2 receptor.54
Pericyte and microglial interaction (Figure 1(c))
Not only do microglia acquire macrophage traits, a subset of pericytes share the same origin with macrophages.15 Observations in CNS diseases demonstrate the potential transitions between pericytes and microglia.55 The brain pericytes isolated from transgenic Alzheimer’s mice can spontaneously differentiate into a CD11b-positive microglial-like cell types.55 In both experimental seizures and human brain slice obtained from patients with seizure, pericytes appear with disorganized soma and ramifications and form a cluster with microglia around capillaries, yet without losing the markers NG2 and PDGFR-β.56 By contrast, in experimental stroke, angiogenic pericytes gradually lose their characteristic morphology as well as their featured markers, gaining the microglial phenotype (CD11b-positive) seven days post stroke.57 These transformed pericytes in stroke do not express CD68, a marker of perivascular macrophages, nor do they derive from the bone marrow, suggesting that the newly appeared microglia-like cells are local-derived.57 Notably, not all pericytes present with microglial appearance, and the microglia-like pericytes do not overlap with the fibroblast-like pericytes that also proliferate post stroke, suggesting the divergent fates of different subsets of pericytes.57 The microglial fate of pericytes may be attributed to the pericyte response to the compromised BBB and inflammation in AD, seizure, and stroke. These transformed pericytes may also acquire their own unique immune function, behaving differently from microglia.
Pericyte and oligodendrocyte interaction (Figure 1(d))
Oligodendrocytes derived from oligodendrocyte precursor cells (OPCs) myelinate the axon.58 Pericytes and OPCs are located in close proximity in the perivascular area of cerebral white matter, and are mutually promoted.59 Pericyte-derived Lama2 stimulates the differentiation of OPCs to oligodendrocytes during remyelination60 and also guides neuron stem cells (NSCs) to adopt an oligodendrocyte fate.61 All of these indicate that pericytes favor oligodendrocyte generation, which implies a role for pericytes in demyelination diseases.
Pericyte and neuron interaction (Figure 1(e))
Pericytes isolated from the cortex of adult mice can promote the differentiation of NSCs from the ventricular-subventricular zone (V-SVZ).62 Vitronectin (VTN) released by pericytes from the SVZ of adult mice can bind αvβ5 integrins of astrocytes, resulting in induction of astrocytic pro-neurogenic factors.63 Pericytes per se can also generate neurotrophic growth factors, i.e. pleiotrophin.64 When pericytes are challenged with diphtheria toxin in vivo, adult mice exhibit loss of pericytes as well as neuronal loss. This may due to the deprivation of pericyte-derived pleiotrophin, as re-administration of pleiotrophin in pericyte-ablated mice rescues the neuronal loss, and pleiotrophin is mostly expressed in CD13-positive pericytes within NVU.64 In addition, rapid loss of pericytes also leads to acute brain circulatory collapse including BBB breakdown, vasogenic edema, and poor blood flow, with all of these events contributing to neuronal loss.64 Therefore, pericytes not only promote neurogenesis after birth, but also support mature neuronal survival, suggesting a potential role of pericytes in neurodegenerative diseases.
Physiological roles of pericytes in the CNS
Pericytes in relationship to the BBB (Figure 2(a) and (b))
Figure 2.
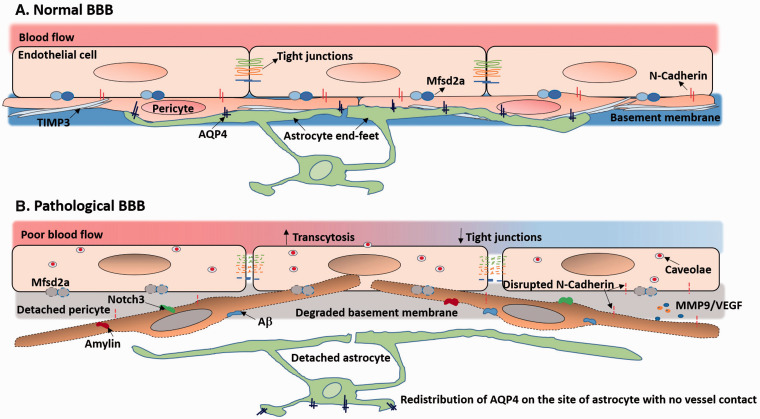
Role of pericytes in BBB function under normal and pathophysiological conditions: (a) Normally, pericyte coverage enables low transcytosis of ECs and fosters the expression of tight junction proteins and adhesion molecules between pericytes and ECs. Pericytes induce the polarization of astrocytic end-feet that AQP4 anchors at the perivascular site of astrocyte end-feet. Also, pericytes secrete ECM molecules such as TIMP3 to maintain the basement membrane. (b) In diseases, degenerated and migrated pericytes lead to high transcytosis of ECs due to loss of Mfasd2a and disrupted tight junctions. Aβ, amylin, and Notch3 deposition can lead to pericyte dysfunction and loss. Dysfunctional pericytes release MMP9 and VEGF and disrupt N-cadherin to further destabilize the BBB. The basement membrane is degraded as well.
ECs, pericytes, and astrocyte end-feet form a sandwich-like structure that provides the basic form of BBB.3 During rodent embryogenesis, genesis of the cortical barrier (E13.5–E15.5) requires pericytes and astrocytes to cover the immature vessels where pericyte recruitment occurs prior to astrocyte generation.65 Genetic deletion of pericytes in mouse embryos results in defective BBB development and postnatal BBB leakiness, the extent of which is proportional to the degree of pericyte deficiency and aging.66 Pericytes maintain BBB integrity through several approaches. We will review these approaches as follows:
Pericytes preserve the tight junctions of ECs
Pericytes are necessary for functional tight junctions.2 Pericyte loss leads to aberrant morphology of tight junctions that exhibit random alignment with convoluted appearance.2 Pericyte detachment from vessels progressively decreases tight junction expression with aging.66 N-cadherin is an integral component of heterotypic adhesion between pericytes and ECs.67 Silencing N-cadherin in pericytes results in barrier defects, whereas overexpression of N-cadherin promotes barrier function.67
Pericytes regulate transcytosis in ECs
Vesicular trafficking, known as transcytosis, of brain ECs is much lower than that of ECs in other organs,68 a phenomenon maintained by pericytes.65 Pericyte-loss-related BBB leakiness does not result mainly from disruption of tight junction but from high transcytosis of ECs.69,70 Suppression of transcytosis requires the major facilitator super family domain containing 2a (Mfsd2a) that is expressed by ECs and in proportion to pericyte coverage.68–70 However, the ECs of blood–retina barrier of day1 postnatal mice (P1) still exhibit bulk transcytosis and high permeability, though they are covered by pericytes with functional tight junctions.70 This inconsistency may due to the differentiation stages of pericytes which may be more crucial to transcytosis than the physical presence or the density of cells. The cytokines from mature pericytes may impact barrier integrity more than those from immature pericytes. Another interesting indication is that as transcytosis is the vesicular transport of macromolecules,71 it may also serve as a means for molecules to shuttle between the peripheral blood and the CNS, and potentially be a target for drug delivery to the CNS.
Pericytes and regulation of cerebral blood flow (CBF) (Figure 3(a))
Figure 3.
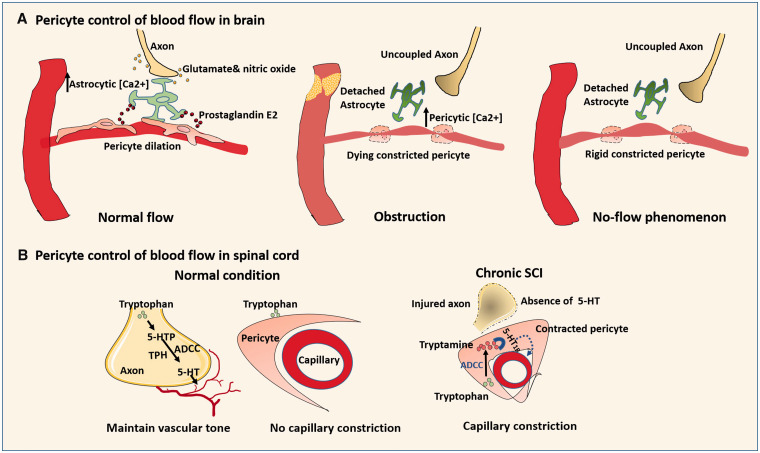
Role of pericytes in blood flow control under normal and pathophysiological conditions: (a) Normally, neurons may first send signals (glutamate and nitric oxide) to astrocytes to increase astrocytic [Ca2+], triggering the release of prostaglandin E2 to relax pericytes. When obstruction occurs in pre-capillary arterioles, the low ATP increases the intercellular [Ca2+] of distant capillary pericytes, resulting in irreversible pericyte constriction even after restoration of blood flow. (b) In normal spinal cord, the vessel constrictor 5-HT is released by axons via enzymes TPH and ADCC to maintain the basal vascular tone. Since TPH and ADCC are minimally expressed by capillary cells, there is no capillary constriction with tryptophan treatment. In chronic SCI, the compromised axon loss the ability to generate 5-HT. Blood flow is controlled by pericytes where ADCC is highly expressed. Within the pericyte, tryptamine converted from tryptophan by ADCC activates 5-HT1B that causes pericyte to contract and capillary constriction.
Since VSMCs control the blood flow within large vessels,72 it is likely that pericytes also control the blood flow within the microcirculation, as pericytes are regarded as microvascular VSMCs.8,73–80 However, the role of pericytes in the regulation of CBF has been debated among different studies. Pericyte contraction with segmental narrowing of capillaries results from prolonged impairment of the microcirculation in experimental ischemic stroke.74 During hypoxia, pericytes dilate capillaries, an event that precedes arteriolar dilation and accounts for more than 80% of increased blood flow.73 Hypoxia increases astrocytic [Ca2+], triggering the release of prostaglandin E2 that works on pericytes to evoke capillary dilation.81 Hartmann et al.7 showed that selective optogenetic stimulation of individual pericytes generates a ∼20% reduction in capillary diameter as well as red blood cell velocity. Other studies further find that the microvascular constriction can spread from the proximal constricted pericytes to the distant non constricted pericytes. Selective removal of one pericyte can trigger the extension of adjacent pericyte processes that facilitates regaining the control of blood flow.1,80,82 When obstruction occurs in pre-capillary arterioles, the ensuing low ATP will increase the intracellular [Ca2+] of distant capillary pericytes, resulting in irreversible pericyte constriction even after blood reperfusion.74 The rigidly constricted pericytes will further lead to neurovascular uncoupling, which thereby exacerbates neural injury.74 In contrast, some studies found that: (1) capillary pericytes exhibit contractile property, and cannot mediate functional hyperemia8; (2) The changes of capillary diameter are independent of pericyte coverage.83 (3) Capillary pericytes do not contain SMA, and have no detectable contractility in response to optogenetic stimulation.8 It is the arteriolar VSMCs that control the regional blood flow, not the downstream pericytes.76 One possible explanation for these divergent findings is the different stimulation approaches. First, the chemical stimulating agents vary in kind and dose among studies. Pericytes may be able to constrict capillaries as long as capillaries are subjected to more potent and/or higher dose of chemical stimuli. Second, different transgenic mouse lines were used in these noted studies. The membrane-bound reporter protein in different transgenic mice may exhibit different results. For example, the fragmented fluorescent protein expression of α-SMA in transgenic mice after tamoxifen injection may contribute to the poor detection of α-SMA.84,8 Additionally, transgenic mouse line carries different reporter genes that may also influence the results. For example, in NG2-Cre ChR2 mice, pericytes do not display contractile property for CBF regulation, whereas in Pdgfrb-Cre ChR2 mice, pericytes contract under two-photon stimulation, shrink capillary lumen, and block red blood cell flow, which indicate differential roles of different pericyte subtypes in regulating cerebral blood flow.1 Next, the way that a chemical stimulant acts on regional capillary is quite different from optogenetic stimulation. Chemical stimulants influence the entire local region, whereas optogenetic stimulation directly targets the pericytes. Optogenetic stimulation has the advantage of avoiding the influence of other neighboring cells on CBF change.85 However, this highly selective stimulation may not fully represent the natural occurrence of CBF change, as the CBF change is only part of neurovascular coupling and pericyte activity can also be affected by other CNS cells.81 Therefore, additional investigations are warranted before concluding a definitive role for pericytes in the regulation of CBF.
Pericytes and the glymphatic system
The glymphatic system is a pathway within the CNS that removes brain waste and neurotoxins in cerebrospinal fluid (CSF) and extracellular interstitial fluid.86 In the glymphatic system, CSF enters the paravascular spaces around penetrating vessels, travels along the downstream arteries, reaches the basal lamina surrounding capillaries, continues along the paravenous routes, and finds its drainage outlet via cervical lymphatics.48 Along this brain-throughout journey, AQP4 on end-feet is important for the mediation of CSF clearance.48 As noted above, pericytes regulate AQP4 distribution of astrocytes end-feet.50 Advancing age is not only related to the loss of astrocytic AQP4 polarization, but also of pericytes.48,66 Therefore, it is highly likely that pericyte loss undermines glymphatic function by reducing AQP4 polarization, thereby decreasing the clearance of toxic wastes such as amyloid beta (Aβ). Pericyte loss also alters abluminal structure along the capillary bed, which may interfere with the glymphatic clearance system.3 In this regard, preventing pericyte loss may be beneficial for renormalizing glymphatic system and waste clearance.
Pericytes in CNS immune responses
Inflammation responses (Figure 4(a))
Figure 4.
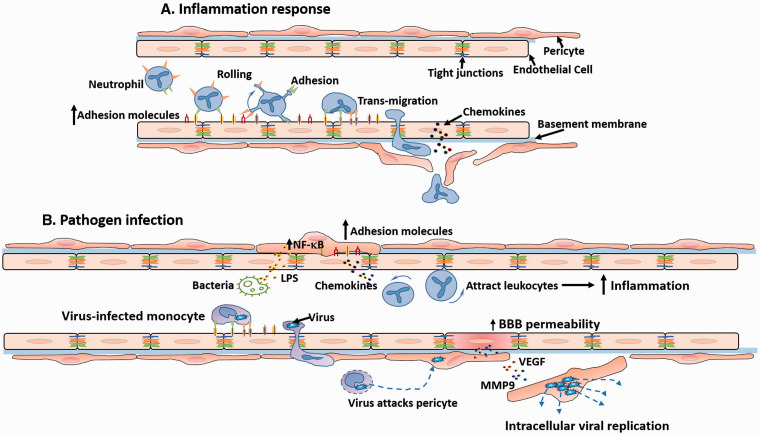
Role of pericytes in inflammatory response and pathogen infection: (a) During inflammation, pericytes sense the inflammatory cue and enhance the expression of adhesion molecules to guide and facilitate the activation of leukocytes. Then, pericytes promote an efficient trafficking of leukocytes to inflammatory foci. (b) Pericytes are permissive for virus invasion and replication, and promote virus-infected monocyte trafficking through vessels. The virus released from the monocytes may directly attack pericytes and replicate within them. The infected pericytes increase secretion of MMP9 and VEGF to increase BBB permeability. In bacterial infection, the LPS released by bacteria increase the NF-κB and thereby induce pericyte expression of adhesion molecules and chemokines that further enhance leukocyte migration.
The close proximity of pericytes to microvessels fosters a role of pericytes in immune responses.87 During inflammation, NG2+ pericytes sense the inflammatory cue early on.87 Stimulated by proinflammatory factors, like TNF-α, pericytes enhance expression of surface adhesion molecules to gain more interplay with leukocytes, which facilitate activation of leukocytes. Then, pericytes promote an efficient trafficking of leukocytes to inflammatory foci.87 Hence, pericytes develop a niche-like environment to guarantee a fast and precise inflammatory reaction, which also provides an alternative target for anti-inflammatory therapies, in addition to leukocyte-endothelium reactions.
Pathogen infections (Figure 4(b))
Pericytes are susceptible to certain pathogens (bacteria and viruses) and are more permissive for virus invasion and replication than other CNS cells.88,89 Pericytes express the receptors of human immunodeficiency virus (HIV) and enhance transcytosis of HIV into cells.90 HIV-derived proteins can promote pericyte migration,88 which may be the reason for reduced brain pericyte coverage found in both experimental HIV infection and HIV-infected patients.88 Likewise, pericytes can be the reservoir for cytomegalovirus replication, further escalating inflammation.91 Escherichia coli-attacked pericytes promote the bacterial invasion of ECs.89 The toxins produced after infection also severely affect pericytes. Lipopolysaccharide (LPS) produced by most gram-negative bacteria affects the luminal side of ECs,71 which then activates the NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) signaling pathway in pericytes, provoking the cells to release a myriad of proinflammatory cytokines.92 Instead of being a defender, the infected pericytes are fragile and may actually contribute to disease progression, though pericytes may express anti-inflammatory cytokines,93 and even transform into macrophages during infections or other pathologies.55 Yet the exact role of these transformed pericytes is still unknown.
Role of pericytes in CNS diseases
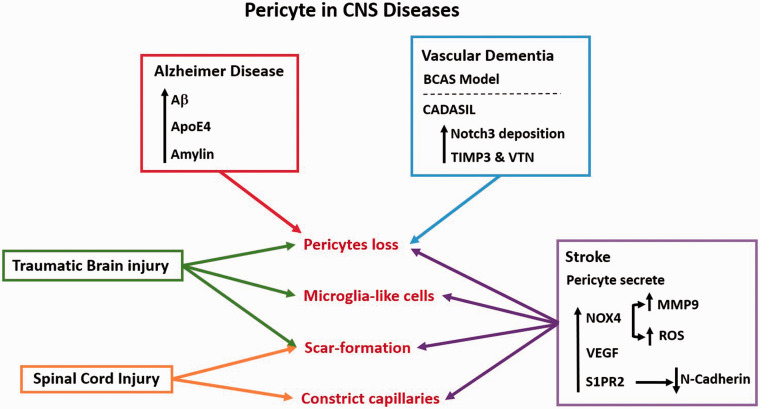
Normal pericytes are critical for maintaining CNS homeostasis, and dysfunction of pericytes may lead to pathologies (Figure 5). Here, we review the effects of pericytes in selected CNS diseases, as follows.
Figure 5.
The role of pericytes in different CNS diseases: There are many factors that lead to pericyte loss. Included among the causes of pericyte loss in Alzheimer disease are genetic ApoE4 mutation and Aβ deposition and amylin aggregation within pericytes. Pericytes are decreased in experimental chronic vascular dementia induced by BCAS. In genetic vascular dementia and CADASIL, mutant Notch3 deposition leads to pericyte dysfunction and loss. In traumatic brain injury, pericytes appear to initially decline. A subset of pericytes may transform into microglia-like cells, while another subset of pericyte act as activated astrocytes to form scar. In spinal cord injury, pericytes contract and limit the blood flow below the spinal lesion, and a subset of pericytes forms scar around the lesion. Stroke causes pericyte dysfunction and loss, and dysfunctional pericytes in stroke produce NOX4 which induces oxidative stress, and release MMP9 and VEGF which further destabilizes the BBB. Elevated levels of S1PR2 within pericytes lead to the loss of N-cadherin. After stroke, pericytes may also constrict capillaries and transform into microglia-like cells and scar-forming cells.
Pericytes in ischemic stroke
Pericytes play multifaceted roles in ischemic stroke. Pericytes may exert unfavorable effects post stroke depending on timing and their location. Pericytes react rapidly to experimental ischemic stroke. In response to oxidative-nitrative stress, pericytes firmly constrict capillaries within 1 h after ischemia, causing a long-lasting no-flow phenomenon even after reperfusion.73,74 This reaction suggests a distinct role of pericytes in pathological blood flow control, contradicting the expected mechanism that vessels tend to dilate to allow more oxygen delivery to the organ in response to hypoxia.94 If so, timely rescue the pericytes may prevent this untoward blood redistribution after ischemic stroke, an area of research that warrants further exploration.
Pericytes also aggravate BBB disruption after stroke. Pericyte soma cover only 7% of cortical capillary network, yet occupy 80% of the area where BBB leakage occur post-ischemia.73 As early as 30 min after experimental ischemic stroke, vascular leakage is preferentially located at pericyte soma and develops at a faster rate compared with non-pericyte areas. This is accompanied by increased activity of matrix metallopeptidase 9 (MMP9) around pericyte soma.95 Twelve hours post ischemic stroke, the S1P receptor (S1PR)2 of pericytes is significantly upregulated, which disrupts N-cadherin and induces pericyte detachment, leading to severe BBB leakage that reaches a zenith at 24 h post stroke.42,96 Between day 1 and day 7 post stroke, Nox4 (reduced nicotinamide adenine dinucleotide phosphate oxidase 4), a source of ROS, is upregulated in pericytes and drives BBB opening via activating NFκB and MMP9.97 However, mice with systemic knockout of pericytes suffer more severe brain edema and neurological deficits compared with control mice in focal ischemic stroke.98 The interactions of PDGF/PDGFR-β and TGF-β signaling pathways may generate mature pericytes that work in the late phase of angiogenesis to stabilize nascent vessels in this scenario.98 Additionally, a mouse study of ischemic stroke shows that long-term local administration of low-dose VEGF to brain before stroke increases pericyte coverage and thereby benefits neurological outcome after stroke.99 This suggest that as long as the dose of VEGF kept under a threshold, a VEGF prophylactic supplement may contribute to healthy robust vasculature, which primes brain to better weather impending injuries. Intriguingly, the pericytes isolated form C57BL6/j mouse brain express neither VEGFR1 nor VEGFR2.99 Other signaling pathways beyond VEGF/VEGFRs may exist and be involved in the interactions between pericytes and VEGF. VEGF may potentiate Angpt-Tie signaling or enhance S1P-mediated eNOS activation, both of which cause vasorelaxation.100 Therefore, further investigations are warranted on the interaction of pericytes with multifunctional factors, like VEGF, after stroke.
In addition, pericytes may transform into multi-potential cells post stroke.57,101 Pericytes can be transformed into stromal cells that proliferate in the ischemic core and have a clear demarcation with glial scar.102 Pericytes may also transform into microglia post stroke and the transformed cells do not overlap with the pericyte forming the scar (for more detailed discussion please see the Pericytes and Microglia interaction section). This suggests the divergent fates of different subsets of pericytes under various conditions, and thus individual consideration of these subtypes is necessary when therapeutically manipulating the pericytes.
Pericytes in Alzheimer’s disease
Pericyte loss is involved in pathologies of AD (Figure 5)
Alzheimer’s disease (AD) is a common cause of cognitive decline and its progression is influenced by the neurovascular milieu.103,104 Pericyte deficiency can cause neurovascular uncoupling,103 and impairs the blood–axon barrier of white matter, exposing the brain tissues to the blood-derived toxins.103 Large amounts of pericyte loss are found in brain tissue of both animal models of AD and AD patients,105,106 and implantation of pericytes in brain can attenuate AD pathology.107
Factors causing pericyte loss in AD
Aβ
Aβ exclusively constricts brain capillaries at pericyte sites with no changes in diameter of arterioles and venules in both AD patient brain tissue and a mouse model of AD.108 In AD mice, the higher load of Aβ deposition, the more rapid capillary constriction.108 Aβ deposition may induce NOX4-derived ROS which then triggers potent vessel constrictor endothelin-1(ET) to evoke pericyte constriction.108Pericyte injury is positively correlated with the ratio of Aβ1-42/Aβ1-40, two most abundant forms of Aβ.109,110 Oligomeric Aβ1-42 drives pericyte detachment by activating MMP9 to shed NG2 on pericytes, whereas the fibril-enriched preparations of Aβ1-42 reduce the decline of NG2.111 The shedding of NG2 loosens the connection between pericytes and ECs, resulting in vascular leakiness. Additionally, since pericytes regulate AQP4 distribution on astrocyte end-feet that is necessary for glymphatic clearance of Aβ,2,48,112 we may speculate that impairment of glymphatic clearance of Aβ via pericyte loss is another contributor to Aβ deposition in AD.
Apolipoprotein E
Isoforms of Apolipoprotein E (ApoE) determine the genetic risk of late-onset AD.113 Pericyte degeneration is more severe in AD ApoE4 carriers than in AD ApoE3 carriers. The proinflammatory lipoprotein receptor-related protein-1 (LRP1)-cyclophilin A(CypA)-MMP9 pathway in pericytes is also upregulated in AD ApoE4 carriers, which causes severe BBB damage.105
Amylin
Amylin is an amyloid polypeptide hormone derived from pancreatic β-cells.114 Amylin-inclusions is found in AD patients and non-dementia patients, and deposits in brain microvascular pericytes.115 The amylin afflicted pericytes exhibit blebbed or fragmented nuclei.115 The toxic oligomeric amylin enhances caspase3/7 activity, decreases NG2 expression, and impairs autophagy.115 The aggregated amylin acts faster and stronger than oligomeric Aβ1-42.115 In addition, amylin accumulates in pancreas and brain hippocampus in patients with type 2 diabetes mellitus (DM).116 Cytoplasmic tau and Aβ protein deposits are found in both pancreas and brain in the same subjects. Amylin may serve as a potential link between the two diseases,116 and pericytes may be the target of amylin in both these diseases, and important in the interaction between DM and AD. Another study found that plasma amylin level is reduced in AD and in patients with mild cognitive deficits compared with non-cognitively impaired patients.117 Administration of amylin analog in AD mice decreases oxidative stress and inflammation in hippocampus and improves neurological outcome.117 It is not clear whether there exists a causal relation between the decreased plasma level of amylin and the deposition of amylin in pericytes of the brain. Further studies are therefore warranted.
Pericytes in vascular dementia
Vascular dementia (VD) is another common cause of dementia that is closely related with aging.118 VD is a broad umbrella term referring to various subtypes of cognitive deficits due to vascular dysfunctions, such as microinfarcts, microbleeds, and hypoperfusion.119 Pericyte dysfunction may render capillaries prone to ischemia and hemorrhage that contribute to cognitive decline.
Pericytes in chronic cerebral hypoperfusion-induced dementia
In the animal model of chronic cerebral hypoperfusion secondary to bilateral carotid artery stenosis (BCAS), the expression of pericytes reaches a nadir in the corpus collosum on the third day post-surgery, and is accompanied by increased BBB permeability (Figure 5).120 This occurs prior to any evident white matter damage, which is consistent with other studies demonstrating that pericyte degeneration may lead to white matter dysfunction and be a cause of the cognitive impairment.121 In addition, the poor brain circulation induced by BCAS may lead to pericyte contraction as seen in ischemic stroke, which will further compromise the blood flow supply to brain.74,103 Also, the pericyte loss may interfere with the glymphatic system and thereby exacerbate neuronal toxicity.122 Another rodent model of vascular dementia generated from administration of cholesterol crystals into the external carotid artery to form multiple microinfarcts (MMI), animals exhibit reduced cognitive function and impaired glymphatic system,123 which may also be related to pericytes.
Pericytes in genetic vascular dementia-CADASIL
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL is the most common cause of inherited monogenic stroke with cognitive impairment, characterized by the mutations of Notch3 receptor with unpaired cysteines in the epidermal growth factor-repeats.124 Normally, Notch3 is required for pericyte development and pericyte proliferation. In CADASIL model mice, aggregated Notch3 deposits on pericytes results in age-dependent pericyte loss and associated BBB dysfunction.124 Although Notch3 is expressed in both VSMCs and pericytes, no detectable changes are found in VSMCs, highlighting the imperative role of pericytes in the progression of CADASIL.124 The aberrant extracellular domain of Notch3 receptor promotes the formation of Notch3/TIMP3 complex and Notch3/VTN complex, thereby impairing the homeostasis of ECM, a possible cause of vessel fibrosis.125 Pericytes are an important source of both TIMP3 and VTN.6,126 Dysfunctional pericytes in small-vessel diseases, like CADASIL, may cause vascular function by secreting excessive ECM, along with BBB hyperpermeability and circulatory collapse.
Pericytes in spinal cord injury
Pericytes regulate blood flow in SCI (Figure 3(b))
Pericytes impair blood flow and motor function in chronic SCI.79 Normally, the vessel tone of spinal cord is maintained by brainstem neuron-derived monoamine serotonin (5-HT) and noradrenaline (NA) that are converted by enzymes, like tryptophan hydroxylase (TPH) and aromatic l-amino acid decarboxylase (AADC). In chronic SCI, this neuronal regulation is compromised, and pericytes take control of vessel tone and blood flow. Without 5-HT and NA, pericytes reside caudal to spinal cord transection could automatically activate 5-HT1B receptor by expressing ADCC which then constricts pericytes and curbs capillary blood flow.79 This phenomenon may also explain the prolonged pericyte constriction in ischemic stroke after reperfusion.73 The stressed pericytes may generate autocrine “clenching substances,” like ADCC, to shift the blood from the dying vessels to the healthier ones. Yet these possible mechanisms may be organ-dependent and differ between chronic ischemia and acute no/low-flow.
Pericyte-derived scar in SCI
Pericyte is another important source for scar tissues in experimental SCI, along with astrocytes.127 From day 3 after SCI, pericytes-derived cells start to proliferate in the lesion center, which reach a peak on day 14 and then decline to a stable level for up to seven months.127 These pericyte progeny lose their CD13 identity at day 9 and afterwards gradually present with PDGFR-β and fibroblast marker, fibronectin. Instead of tightly covering nascent vessels like pericytes, the pericyte-derived cells detach from blood vessels, migrate through ECM and fill up the lesioned cavity with a clear demarcation with astrogliosis that surrounds the lesioned cavity. The scar densely populated by pericytes progeny may also hinder the outgrowing axons penetrating into the lesion in a murine model of SCI.128 After moderate reduction of pericytes prior to SCI, the mice exhibit high axon regeneration and improved sensorimotor function.128 This is accompanied by the suppression of astrogliosis and inflammation. However, severe reduction of pericytes in mice results in undesired inflammatory reactions, indicating the importance of an appropriate number of pericytes for the recovery of SCI.128 The proliferation of pericytes may be induced by S1P/SIPR3 interaction, inhibition of which may significantly reduce glial scar formation and promote locomotor recovery.129
Pericytes in traumatic brain injury
Pericytes react rapidly during the acute phase of TBI.130 One hour after TBI, a subset of pericytes start migrating away from blood vessels in the perilesional zone, whereas the remaining immobile ones undergo degeneration (Figure 5).130 The degenerated pericytes display enlarged endoplasmic reticulum and swollen mitochondria as well as increased transcytosis. Some exhibit hypertrophic or necrotic changes and a tendency to transform into phagocytes.131 Pericytes decrease in perilesional zone 6 to 12 h after TBI and then re-increase from day 3 to reach a peak level on day 5.132 The repopulated pericytes surround the lesion with a clear demarcation with gliosis.132 Another study observed elevated pericyte death 24 h after TBI, a phenomenon which becomes more pronounced on day 3.133 Administration of carbon monoxide (CO) after TBI significantly improves pericyte survival and promotes the neurogenesis, which may due to the ability of CO to strengthen the cross-talk between pericytes and NSCs as observed in in vitro.133 In addition, after TBI, the ApoE4 mice exhibit a delay of pericyte increase compared with wild type and ApoE3 mice, which is accompanied by a postponed spontaneous recovery of BBB integrity.134,135
Pericytes and miRs
miRs are a family of small non-coding RNAs containing 20–30 nucleotides, and they post-transcriptionally regulate a wide spectrum of genes that are involved in cell fate determination, organ development, and disease progression, etc.136 Hypoxia significantly increases miR-345-5p and miR − 336, but decreases miR − 140, miR − 222, miR − 24, and miR − 145 expression in cultured CNS pericytes.137 MiR-345-5p is associated with anti-apoptosis and cell proliferation, whereas miR-140 inhibits growth and migration of cells, the change of which underlies changes in proliferation and migration of pericytes under hypoxia.138,139 MiR-24, a pro-apoptotic factor, is decreased in pericytes in response to hypoxia, which is consistent with upregulation of anti-apoptotic miR-345-5p.137 MiR-145 is exclusively expressed in the pericytes of brain and kidney.137,140 It may regulate the fate and plasticity of pericytes, and thus may be alternative targets for pathologies with over-proliferating pericytes that are seen in the scar formation in brain injury and renal fibrosis. Hypoxia induces miR-126 in pericytes which may be the compensatory mechanism for pericytes to maintain vessel integrity in response to low oxygen delivery.137 MiR-126 deficiency contributes to cardiac defects after stroke compared with stroke control mice, suggesting the potential role of miR-126 in brain–heart interactions.141 As pericytes reside in both brain and heart, the abnormality of miR-126 may participate pericyte-related pathologies in both brain and heart.
Pericytes and EVs
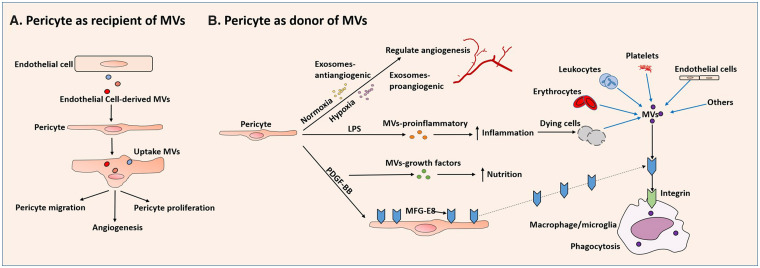
EVs are a wide spectrum of nanoparticles that include exosomes and microvesicles (MVs), both of which are able to mediate cell–cell communications.142 Exosomes are nanosized vesicles (∼30–100 nm in diameter) that intracellularly package biomolecules from the early endosome and transfer nucleic acids, proteins and lipids.142 MVs, by contrast, have a wider range of size (100–1000 nm in diameter) and directly bud from the plasma membrane. MVs also carry and deliver nucleic acid and proteins to cells.142
Pericytes can be donors of EVs or recipients. As a donor, pericytes secrete vesicles with different contents according to stimuli (Figure 6(b)). Stimulated by PDGF-BB, pericyte-derived MVs carry growth factors, whereas inflamed by LPS, pericyte-derived MVs predominantly contain proinflammatory cytokines.143 In the hypoxic milieu, pericytes can release exosomes that encompass proangiogenic factors to enhance vessel density.11 In normoxic condition, pericytes may generate exosomes that incorporate anti-angiogenic factors to maintain vascular stability.31
Figure 6.
The relation between pericytes and EVs: Pericytes can be donors or recipients of EVs. (a) As a recipient, pericytes may receive EVs from multiple cell sources. Pericytes can engulf various ECs-derived EVs that contain miRs, and then migrate, proliferate, and promote angiogenesis. (b) As a donor, pericytes secrete vesicles context-dependently. Under normoxic conditions, pericytes may secrete exosomes that contain antiangiogenic factors (Exosomes-antiangiogenic) to maintain vascular stability. In hypoxia, pericytes secrete exosomes with proangiogenic factors (exosomes-proangiogenic). Stimulated by PDGF-BB, pericyte-derived MVs carry growth factors (MVs-growth factors), whereas inflamed by LPS, pericyte-derived MVs contain proinflammatory cytokines (MVs-proinflammatory). Conversely, pericytes may negatively regulate the transportation of EVs. Stimulated by PDGF-BB, pericytes secrete scavenger MGF-E8 that promotes phagocytes engulfment of MVs produced by cells that are coated with phosphatidylserines.
As a recipient, pericytes may receive EVs from multiple cell sources. In diabetic wound tissue, pericytes decrease the expression of ephrin-B2 and VEGF after uptake of EC-derived MVs enriched with miR-503 (Figure 6(a)). This reduces pericyte migration and proliferation.144 By incorporation of EC-derived MVs loaded with miR-328-3p and let-7d-3p, pericytes enhance their expression of VEGF-B associated with neuronal degeneration.10,145 In addition to ECs, the origin of EVs delivered to pericytes are from a variety of cells. Therefore, additional investigations are required to investigate the dynamic interactions between pericytes and other cell-derived EVs.
Pericytes may negatively regulate the transportation of EVs (Figure 6(b)). First, pericyte coverage guarantees a low rate of transcytosis of ECs.65 This may block the exosomal and MV shuttle of RNAs, proteins, and cytokines. Second, pericytes per se can secrete proteins to scavenge MVs.146 Milk fat globule-epidermal growth factor 8 protein (MGF-E8, also known as lactadherin) is found to be produced by phagocytes and can assist the clearance of MVs coated with phosphatidylserines.146 In brain injury, brain-derived MVs exacerbate BBB leakage and trigger systemic coagulopathy.146 Exogenous administration of MGF-E8 significantly preserves BBB integrity via facilitating phagocytosis of the MVs.146 Pericytes are a source of MGF-E8 under PDGF-BB stimulation.147 A subset of pericytes may function as phagocytes in brain injury as noted above.15 Therefore, it is highly likely that beneficial effects of MGF-E8 are partly attributed to pericytes. It appears paradoxical that pericytes can generate both MVs and MV-scavenger proteins. Temporal and spatial sequences may be present context-dependently between the two processes. Pericyte-derived MVs and MGF-E8 may work synergistically to guarantee a balance between producing particles and cleaning debris. Pathologies that reinforce one side may tilt the scale, and disrupt CNS homeostasis. Although the interactions between the MVs and pericytes presently are enigmatic, they may bear enormous therapeutic potential for certain CNS pathologies.
Approaches to studying the pericytes
Progress in experimental tools allows more extensive and thorough aspects of pericytes to be unveiled. Here, we briefly review these approaches that have been used in the recent studies of pericytes.
Genetic engineering technique
With the development of Cre-recombination techniques, pericyte-deficient transgenic mice permit tracking pericyte development from embryonic to aging periods.65,66 Identification of the target genes specific to the pericytes at retinal angiogenic microvessels may offer a clearer picture of pericyte activities in angiogenesis with less noise from the angiogenic-quiescent pericytes.35,36 The offspring of the transgenic mice designed to mimic CNS diseases and pericyte-deficient transgenic mice may help investigate the roles of pericytes in CNS pathological scenarios.106 In combination with two-photon microscopy and 3D techniques, transgenic mice carrying fluorescent reporter markers are available to visualize the pericyte behavior in live animals.17 Additionally, optogenetic techniques by using light and genetically encoded light-sensitive proteins in novel transgenic mouse lines may permit highly selective activation of pericytes that can be employed to distinguishe the role of pericytes in CBF regulation from other cells in NVU.
Single-cell RNA sequence
The RNA sequence of individual cells isolated from the brain of transgenic reporter mice provide genome-wide quantitative transcriptomes, and thus facilitate insight into functional differences between individual cells.148 The transcriptomes of pericytes, fibroblast-like cells and ECs can identify their respective phenotypic evolution along the arteriovenous axis (database of the website http://betsholtzlab.org/VascularSingleCells/database.html),22 and identify novel capillary pericyte markers as well as the subcellular location of pericyte-enriched genes and related genomic network.6 The single-cell RNA sequence can also be used to compare the genes of pericytes among organs, which may help evaluate pericytes from a systemic view.149
Future perspective and conclusion
There are a number of important aspects of pericytes that warrant further investigation: (1) Stem cell potential of pericytes. In vitro and in vivo study shows that pericytes have stem-cell like potential and express markers for mesenchymal stem cells (MSC).150 However, this view has been challenged by a recent in vivo loss-of-function study. Guimaraes-Camboa et al.9 found that pericytes do not behave as MSCs or as tissue-resident progenitors in multiple organs, suggesting the pericyte plasticity observed in previous studies may result from the manipulation of cells ex vivo.9 (2) The role of pericytes on post-capillary venules remains enigmatic. Investigations on venular pericytes in identification, physiology, pathology are warranted. (3) The role of pericytes in peripheral organs. In addition to brain, pericytes are also necessary for heart, gut, and kidney function.14 Systemic study of pericytes may help clarify the relationships of inflammatory response, blood flow regulation, and coagulopathy between brain and peripheral organs. (4) Therapies that maximize the benefits and minimize the toxicity of pericytes may provide therapeutic opportunities not only for CNS diseases but also for peripheral comorbidities.
To summarize, pericytes are indispensable for the normal development and the maturation of the CNS. Dysfunction of pericytes greatly aggravates CNS pathologies. With further advances in bio-techniques, additional functions of pericytes as well as their untoward effects can be investigated.
Acknowledgements
We are thankful to Dr. Jianning Zhang for many thoughtful discussions of pericytes in brain injuries.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Heart, Lung, and Blood Institute R01HL143432 (JC).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
ZZ: manuscript writing; MC and JC: manuscript writing, final approval of manuscript, financial support.
References
- 1.Grant RI, Hartmann DA, Underly RG, et al. Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J Cereb Blood Flow Metab 2017; 39: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–561. [DOI] [PubMed] [Google Scholar]
- 3.Armulik A, Genove G, Betsholtz C.Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011; 21: 193–215. [DOI] [PubMed] [Google Scholar]
- 4.Sims DE.The pericyte – a review. Tissue Cell 1986; 18: 153–174. [DOI] [PubMed] [Google Scholar]
- 5.Ferland-McCollough D, Slater S, Richard J, et al. Pericytes, an overlooked player in vascular pathobiology. Pharmacol Ther 2017; 171: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He L, Vanlandewijck M, Raschperger E, et al. Analysis of the brain mural cell transcriptome. Sci Rep 2016; 6: 35108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmann D, Grant RI, Harrill SA, et al. Optogenetic stimulation of pericytes lacking alpha smooth muscle actin produces a decrease in capillary blood flow in the living mouse brain. FASEB J 2018; 32. [Google Scholar]
- 8.Hill RA, Tong L, Yuan P, et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 2015; 87: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guimaraes-Camboa N, Cattaneo P, Sun Y, et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 2017; 20: 345–359.e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto S, Niida S, Azuma E, et al. Inflammation-induced endothelial cell-derived extracellular vesicles modulate the cellular status of pericytes. Sci Rep 2015; 5: 8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayo JN, Bearden SE.Driving the hypoxia-inducible pathway in human pericytes promotes vascular density in an exosome-dependent manner. Microcirculation 2015; 22: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korn J, Christ B, Kurz H.Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J Comp Neurol 2002; 442: 78–88. 2002/01/05. [DOI] [PubMed] [Google Scholar]
- 13.Etchevers HC, Vincent C, Le Douarin NM, et al. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 2001; 128: 1059–1068. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki T, Mukouyama YS.Tissue specific origin, development, and pathological perspectives of pericytes. Front Cardiovasc Med 2018; 5: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto S, Muramatsu M, Azuma E, et al. A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci Rep 2017; 7: 3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki T, Nalbandian A, Uchida Y, et al. Tissue myeloid progenitors differentiate into pericytes through TGF-beta signaling in developing skin vasculature. Cell Rep 2017; 18: 2991–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung B, Arnold TD, Raschperger E, et al. Visualization of vascular mural cells in developing brain using genetically labeled transgenic reporter mice. J Cereb Blood Flow Metab 2017; 38: 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyyriainen J, Ekolle Ndode-Ekane X, Pitkanen A.Dynamics of PDGFRbeta expression in different cell types after brain injury. Glia 2017; 65: 322–341. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, Bergles DE, Nishiyama A.NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 2007; 135: 145–157. [DOI] [PubMed] [Google Scholar]
- 20.Yamanishi E, Takahashi M, Saga Y, et al. Penetration and differentiation of cephalic neural crest-derived cells in the developing mouse telencephalon. Dev Growth Differ 2012; 54: 785–800. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann DA, Underly RG, Grant RI, et al. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2015; 2: 041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanlandewijck M, He L, Mae MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018; 554: 475–480. [DOI] [PubMed] [Google Scholar]
- 23.Munoz-Marmol AM, Strasser G, Isamat M, et al. A dysfunctional desmin mutation in a patient with severe generalized myopathy. Proc Natl Acad Sci U S A 1998; 95: 11312–11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi X.Cochlear pericyte responses to acoustic trauma and the involvement of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. Am J Pathol 2009; 174: 1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly-Goss MR, Sweat RS, Stapor PC, et al. Targeting pericytes for angiogenic therapies. Microcirculation 2014; 21: 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi JH, Riew TR, Kim HL, et al. Desmin expression profile in reactive astrocytes in the 3-nitropropionic acid-lesioned striatum of rat: characterization and comparison with glial fibrillary acidic protein and nestin. Acta Histochem 2017; 119: 795–803. [DOI] [PubMed] [Google Scholar]
- 27.Damisah EC, Hill RA, Tong L, et al. A fluoro-Nissl dye identifies pericytes as distinct vascular mural cells during in vivo brain imaging. Nat Neurosci 2017; 20: 1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell TS, Bradley J, Robinson GS, et al. RGS5 expression is a quantitative measure of pericyte coverage of blood vessels. Angiogenesis 2008; 11: 141–151. [DOI] [PubMed] [Google Scholar]
- 29.Bondjers C, Kalén M, Hellström M, et al. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol 2003; 162: 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Luo Y, Hui H, et al. CD146 coordinates brain endothelial cell-pericyte communication for blood-brain barrier development. Proc Natl Acad Sci U S A 2017; 114: E7622–E7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armulik A, Abramsson A, Betsholtz C.Endothelial/pericyte interactions. Circ Res 2005; 97: 512–523. [DOI] [PubMed] [Google Scholar]
- 32.Sweeney MD, Ayyadurai S, Zlokovic BV.Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 2016; 19: 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hori S, Ohtsuki S, Hosoya K, et al. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem 2004; 89: 503–513. [DOI] [PubMed] [Google Scholar]
- 34.Franco M, Roswall P, Cortez E, et al. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood 2011; 118: 2906–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonavicius N, Ashenden M, van Weverwijk A, et al. Pericytes promote selective vessel regression to regulate vascular patterning. Blood 2012; 120: 1516–1527. [DOI] [PubMed] [Google Scholar]
- 36.Eilken HM, Dieguez-Hurtado R, Schmidt I, et al. Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat Commun 2017; 8: 1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenberg JI, Shields DJ, Barillas SG, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 2008; 456: 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao R, Xue Y, Hedlund EM, et al. VEGFR1-mediated pericyte ablation links VEGF and PlGF to cancer-associated retinopathy. Proc Natl Acad Sci U S A 2010; 107: 856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dave JM, Mirabella T, Weatherbee SD, et al. Pericyte ALK5/TIMP3 axis contributes to endothelial morphogenesis in the developing brain. Dev Cell 2018; 44: 665–678.e666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li F, Lan Y, Wang Y, et al. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell 2011; 20: 291–302. [DOI] [PubMed] [Google Scholar]
- 41.Rosen H, Stevens RC, Hanson M, et al. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu Rev Biochem 2013; 82: 637–662. [DOI] [PubMed] [Google Scholar]
- 42.Wan Y, Jin HJ, Zhu YY, et al. MicroRNA-149-5p regulates blood-brain barrier permeability after transient middle cerebral artery occlusion in rats by targeting S1PR2 of pericytes. FASEB J 2018; 32: 3133–3148. [DOI] [PubMed] [Google Scholar]
- 43.Foo SS, Turner CJ, Adams S, et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 2006; 124: 161–173. [DOI] [PubMed] [Google Scholar]
- 44.Nakayama A, Nakayama M, Turner CJ, et al. Ephrin-B2 controls PDGFRbeta internalization and signaling. Genes Dev 2013; 27: 2576–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stratman AN, Schwindt AE, Malotte KM, et al. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood 2010; 116: 4720–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghori A, Freimann FB, Nieminen-Kelha M, et al. EphrinB2 activation enhances vascular repair mechanisms and reduces brain swelling after mild cerebral ischemia. Arterioscler Thromb Vasc Biol 2017; 37: 867–878. [DOI] [PubMed] [Google Scholar]
- 47.Coucha M, Barrett AC, Elgebaly M, et al. Inhibition of Ephrin-B2 in brain pericytes decreases cerebral pathological neovascularization in diabetic rats. PLoS One 2019; 14: e0210523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin M, Pu T, Wang L, et al. Astroglial water channel aquaporin 4-mediated glymphatic clearance function: a determined factor for time-sensitive treatment of aerobic exercise in patients with Alzheimer’s disease. Med Hypotheses 2018; 119: 18–21. [DOI] [PubMed] [Google Scholar]
- 49.Hubbard JA, Szu JI, Binder DK.The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res Bull 2018; 136: 118–129. [DOI] [PubMed] [Google Scholar]
- 50.Gundersen GA, Vindedal GF, Skare O, et al. Evidence that pericytes regulate aquaporin-4 polarization in mouse cortical astrocytes. Brain Struct Funct 2014; 219: 2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puwarawuttipanit W, Bragg AD, Frydenlund DS, et al. Differential effect of alpha-syntrophin knockout on aquaporin-4 and Kir4.1 expression in retinal macroglial cells in mice. Neuroscience 2006; 137: 165–175. [DOI] [PubMed] [Google Scholar]
- 52.Anderova M, Benesova J, Mikesova M, et al. Altered astrocytic swelling in the cortex of alpha-syntrophin-negative GFAP/EGFP mice. PLoS One 2014; 9: e113444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menezes MJ, McClenahan FK, Leiton CV, et al. The extracellular matrix protein laminin alpha2 regulates the maturation and function of the blood-brain barrier. J Neurosci 2014; 34: 15260–15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao Y, Chen ZL, Norris EH, et al. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun 2014; 5: 3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hutter-Schmid B, Humpel C.Primary mouse brain pericytes isolated from transgenic Alzheimer mice spontaneously differentiate into a CD11b(+) microglial-like cell type in vitro. Exp Gerontol 2018; 112: 30–37. [DOI] [PubMed] [Google Scholar]
- 56.Klement W, Garbelli R, Zub E, et al. Seizure progression and inflammatory mediators promote pericytosis and pericyte-microglia clustering at the cerebrovasculature. Neurobiol Dis 2018; 113: 70–81. [DOI] [PubMed] [Google Scholar]
- 57.Ozen I, Deierborg T, Miharada K, et al. Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol 2014; 128: 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franklin RJM, Ffrench-Constant C.Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci 2008; 9: 839–855. [DOI] [PubMed] [Google Scholar]
- 59.Maki T, Maeda M, Uemura M, et al. Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci Lett 2015; 597: 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De La Fuente AG, Lange S, Silva ME, et al. Pericytes stimulate oligodendrocyte progenitor cell differentiation during CNS remyelination. Cell Rep 2017; 20: 1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silva ME, Lange S, Hinrichsen B, et al. Pericytes favor oligodendrocyte fate choice in adult neural stem cells. Front Cell Neurosci 2019; 13: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crouch EE, Liu C, Silva-Vargas V, et al. Regional and stage-specific effects of prospectively purified vascular cells on the adult V-SVZ neural stem cell lineage. J Neurosci 2015; 35: 4528–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jia C, Keasey MP, Malone HM, et al. Vitronectin from brain pericytes promotes adult forebrain neurogenesis by stimulating CNTF. Exp Neurol 2019; 312: 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nikolakopoulou AM, Montagne A, Kisler K, et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci 2019; 22: 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468: 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gautam J, Cao Y, Yao Y.Pericytic laminin maintains blood-brain barrier integrity in an age-dependent manner. Transl Stroke Res 2019; 11: 228–242 [DOI] [PubMed] [Google Scholar]
- 68.Ben-Zvi A, Lacoste B, Kur E, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014; 509: 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Villasenor R, Kuennecke B, Ozmen L, et al. Region-specific permeability of the blood-brain barrier upon pericyte loss. J Cereb Blood Flow Metab 2017; 37: 3683–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chow BW, Gu C.Gradual suppression of transcytosis governs functional blood-retinal barrier formation. Neuron 2017; 93: 1325–1333.e1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dohgu S, Banks WA.Brain pericytes increase the lipopolysaccharide-enhanced transcytosis of HIV-1 free virus across the in vitro blood-brain barrier: evidence for cytokine-mediated pericyte-endothelial cell crosstalk. Fluids Barriers CNS 2013; 10: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Z, Chen Y, Zhang T, et al. Role of myoendothelial gap junctions in the regulation of human coronary artery smooth muscle cell differentiation by laminar shear stress. Cell Physiol Biochem 2016; 39: 423–437. [DOI] [PubMed] [Google Scholar]
- 73.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yemisci M, Gursoy-Ozdemir Y, Vural A, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009; 15: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 75.Vates GE, Takano T, Zlokovic B, et al. Pericyte constriction after stroke: the jury is still out. Nat Med 2010; 16: 959; author reply 960. [DOI] [PubMed] [Google Scholar]
- 76.Fernandez-Klett F, Offenhauser N, Dirnagl U, et al. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A 2010; 107: 22290–22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Farrell FM, Mastitskaya S, Hammond-Haley M, et al. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. Elife 2017; 6: e29280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Almaca J, Weitz J, Rodriguez-Diaz R, et al. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab 2018; 27: 630–644.e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y, Lucas-Osma AM, Black S, et al. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat Med 2017; 23: 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peppiatt CM, Howarth C, Mobbs P, et al. Bidirectional control of CNS capillary diameter by pericytes. Nature 2006; 443: 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mishra A, Reynolds JP, Chen Y, et al. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci 2016; 19: 1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berthiaume AA, Grant RI, McDowell KP, et al. Dynamic remodeling of pericytes in vivo maintains capillary coverage in the adult mouse brain. Cell Rep 2018; 22: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei HS, Kang H, Rasheed ID, et al. Erythrocytes are oxygen-sensing regulators of the cerebral microcirculation. Neuron 2016; 91: 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alarcon-Martinez L, Yilmaz-Ozcan S, Yemisci M, et al. Capillary pericytes express alpha-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. Elife 2018; 7: e34861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferenczi EA, Tan X, Huang CL.Principles of optogenetic methods and their application to cardiac experimental systems. Front Physiol 2019; 10: 1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bacyinski A, Xu M, Wang W, et al. The paravascular pathway for brain waste clearance: current understanding, significance and controversy. Front Neuroanat 2017; 11: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stark K, Eckart A, Haidari S, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol 2013; 14: 41–51. [DOI] [PubMed] [Google Scholar]
- 88.Niu F, Yao H, Zhang W, et al. Tat 101-mediated enhancement of brain pericyte migration involves platelet-derived growth factor subunit B homodimer: implications for human immunodeficiency virus-associated neurocognitive disorders. J Neurosci 2014; 34: 11812–11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salmeri M, Motta C, Anfuso CD, et al. VEGF receptor-1 involvement in pericyte loss induced by Escherichia coli in an in vitro model of blood brain barrier. Cell Microbiol 2013; 15: 1367–1384. [DOI] [PubMed] [Google Scholar]
- 90.Nakagawa S, Castro V, Toborek M.Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J Cell Mol Med 2012; 16: 2950–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilkerson I, Laban J, Mitchell JM, et al. Retinal pericytes and cytomegalovirus infectivity: implications for HCMV-induced retinopathy and congenital ocular disease. J Neuroinflammation 2015; 12: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guijarro-Munoz I, Compte M, Alvarez-Cienfuegos A, et al. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-kappaB signaling pathway and proinflammatory response in human pericytes. J Biol Chem 2014; 289: 2457–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rustenhoven J, Scotter EL, Jansson D, et al. An anti-inflammatory role for C/EBPdelta in human brain pericytes. Sci Rep 2015; 5: 12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cohen PJ, Alexander SC, Smith TC, et al. Effects of hypoxia and normocarbia on cerebral blood flow and metabolism in conscious man. J Appl Physiol 1967; 23: 183–189. [DOI] [PubMed] [Google Scholar]
- 95.Underly RG, Levy M, Hartmann DA, et al. Pericytes as inducers of rapid, matrix metalloproteinase-9-dependent capillary damage during ischemia. J Neurosci 2017; 37: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim GS, Yang L, Zhang G, et al. Critical role of sphingosine-1-phosphate receptor-2 in the disruption of cerebrovascular integrity in experimental stroke. Nat Commun 2015; 6: 7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nishimura A, Ago T, Kuroda J, et al. Detrimental role of pericyte Nox4 in the acute phase of brain ischemia. J Cereb Blood Flow Metab 2016; 36: 1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen J, Xu G, Zhu R, et al. PDGFR-beta restores blood-brain barrier functions in a mouse model of focal cerebral ischemia. J Cereb Blood Flow Metab 2018; 39: 1501–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zechariah A, ElAli A, Doeppner TR, et al. Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke 2013; 44: 1690–1697. [DOI] [PubMed] [Google Scholar]
- 100.Igarashi J, Erwin PA, Dantas APV, et al. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci U S A 2003; 100: 10664–10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sakuma R, Kawahara M, Nakano-Doi A, et al. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J Neuroinflamm 2016; 13: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fernandez-Klett F, Potas JR, Hilpert D, et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J Cereb Blood Flow Metab 2013; 33: 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kisler K, Nelson AR, Rege SV, et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci 2017; 20: 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zlokovic BV.Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 2011; 12: 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Halliday MR, Rege SV, Ma Q, et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab 2016; 36: 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sagare AP, Bell RD, Zhao Z, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 2013; 4: 2932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Tachibana M, Yamazaki Y, Liu CC, et al. Pericyte implantation in the brain enhances cerebral blood flow and reduces amyloid-beta pathology in amyloid model mice. Exp Neurol 2018; 300: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nortley R, Korte N, Izquierdo P, et al. Amyloid beta oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 2019; 365: eaav9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dahlgren KN, Manelli AM, Stine WB, Jr., et al. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem 2002; 277: 32046–32053. [DOI] [PubMed] [Google Scholar]
- 110.Verbeek MM, de Waal RM, Schipper JJ, et al. Rapid degeneration of cultured human brain pericytes by amyloid beta protein. J Neurochem 1997; 68: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 111.Schultz N, Nielsen HM, Minthon L, et al. Involvement of matrix metalloproteinase-9 in amyloid-beta 1-42-induced shedding of the pericyte proteoglycan NG2. J Neuropathol Exp Neurol 2014; 73: 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yotsumoto F, You WK, Cejudo-Martin P, et al. NG2 proteoglycan-dependent recruitment of tumor macrophages promotes pericyte-endothelial cell interactions required for brain tumor vascularization. Oncoimmunology 2015; 4: e1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Verghese PB, Castellano JM, Holtzman DM.Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol 2011; 10: 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoppener JW, Oosterwijk C, Visser-Vernooy HJ, et al. Characterization of the human islet amyloid polypeptide/amylin gene transcripts: identification of a new polyadenylation site. Biochem Biophys Res Commun 1992; 189: 1569–1577. [DOI] [PubMed] [Google Scholar]
- 115.Schultz N, Byman E, Fex M, et al. Amylin alters human brain pericyte viability and NG2 expression. J Cereb Blood Flow Metab 2017; 37: 1470–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martinez-Valbuena I, Valenti-Azcarate R, Amat-Villegas I, et al. Amylin as a potential link between type 2 diabetes and Alzheimer’s disease. Ann Neurol 2019; 86: 539–551. [DOI] [PubMed] [Google Scholar]
- 117.Adler BL, Yarchoan M, Hwang HM, et al. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer’s disease pathogenesis and cognition. Neurobiol Aging 2014; 35: 793–801. [DOI] [PubMed] [Google Scholar]
- 118.O’Brien JT, Thomas A.Vascular dementia. Lancet 2015; 386: 1698–1706. [DOI] [PubMed] [Google Scholar]
- 119.Iadecola C.The pathobiology of vascular dementia. Neuron 2013; 80: 844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu Q, Radwanski R, Babadjouni R, et al. Experimental chronic cerebral hypoperfusion results in decreased pericyte coverage and increased blood-brain barrier permeability in the corpus callosum. J Cereb Blood Flow Metab 2017; 39: 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Montagne A, Nikolakopoulou AM, Zhao Z, et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med 2018; 24: 326–337. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012; 4: 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu P, Venkat P, Chopp M, et al. Role of microRNA-126 in vascular cognitive impairment in mice. J Cereb Blood Flow Metab 2018; 39: 2497–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ghosh M, Balbi M, Hellal F, et al. Pericytes are involved in the pathogenesis of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Ann Neurol 2015; 78: 887–900. [DOI] [PubMed] [Google Scholar]
- 125.Monet-Lepretre M, Haddad I, Baron-Menguy C, et al. Abnormal recruitment of extracellular matrix proteins by excess Notch3 ECD: a new pathomechanism in CADASIL. Brain 2013; 136: 1830–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saunders WB, Bohnsack BL, Faske JB, et al. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol 2006; 175: 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goritz C, Dias DO, Tomilin N, et al. A pericyte origin of spinal cord scar tissue. Science 2011; 333: 238–242. [DOI] [PubMed] [Google Scholar]
- 128.Dias DO, Kim H, Holl D, et al. Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell 2018; 173: 153–165.e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tang HB, Jiang XJ, Wang C, et al. S1P/S1PR3 signaling mediated proliferation of pericytes via Ras/pERK pathway and CAY10444 had beneficial effects on spinal cord injury. Biochem Biophys Res Commun 2018; 498: 830–836. [DOI] [PubMed] [Google Scholar]
- 130.Dore-Duffy P, Owen C, Balabanov R, et al. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res 2000; 60: 55–69. [DOI] [PubMed] [Google Scholar]
- 131.Castejon OJ.Ultrastructural pathology of cortical capillary pericytes in human traumatic brain oedema. Folia Neuropathol 2011; 49: 162–173. [PubMed] [Google Scholar]
- 132.Zehendner CM, Sebastiani A, Hugonnet A, et al. Traumatic brain injury results in rapid pericyte loss followed by reactive pericytosis in the cerebral cortex. Sci Rep 2015; 5: 13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Choi YK, Maki T, Mandeville ET, et al. Dual effects of carbon monoxide on pericytes and neurogenesis in traumatic brain injury. Nat Med 2016; 22: 1335–1341. [DOI] [PubMed] [Google Scholar]
- 134.Casey CS, Atagi Y, Yamazaki Y, et al. Apolipoprotein E inhibits cerebrovascular pericyte mobility through a RhoA protein-mediated pathway. J Biol Chem 2015; 290: 14208–14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Main BS, Villapol S, Sloley SS, et al. Apolipoprotein E4 impairs spontaneous blood brain barrier repair following traumatic brain injury. Mol Neurodegener 2018; 13: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ivey KN, Srivastava D.MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell 2010; 7: 36–41. [DOI] [PubMed] [Google Scholar]
- 137.Truettner JS, Katyshev V, Esen-Bilgin N, et al. Hypoxia alters MicroRNA expression in rat cortical pericytes. MicroRNA 2013; 2: 32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tang JT, Wang JL, Du W, et al. MicroRNA 345, a methylation-sensitive microRNA is involved in cell proliferation and invasion in human colorectal cancer. Carcinogenesis 2011; 32: 1207–1215. [DOI] [PubMed] [Google Scholar]
- 139.Gu R, Sun YF, Wu MF, et al. Biological roles of microRNA-140 in tumor growth, migration, and metastasis of osteosarcoma in vivo and in vitro. Tumour Biol 2016; 37: 353–360. [DOI] [PubMed] [Google Scholar]
- 140.Larsson E, Fredlund Fuchs P, Heldin J, et al. Discovery of microvascular miRNAs using public gene expression data: miR-145 is expressed in pericytes and is a regulator of Fli1. Genome Med 2009; 1: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen J, Cui C, Yang X, et al. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Transl Stroke Res 2017; 8: 374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Raposo G, Stoorvogel W.Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013; 200: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gaceb A, Ozen I, Padel T, et al. Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-BB. J Cereb Blood Flow Metab 2018; 38: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Caporali A, Meloni M, Nailor A, et al. p75(NTR)-dependent activation of NF-kappaB regulates microRNA-503 transcription and pericyte-endothelial crosstalk in diabetes after limb ischaemia. Nat Commun 2015; 6: 8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Poesen K, Lambrechts D, Van Damme P, et al. Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its ligand VEGF-B in motor neuron degeneration. J Neurosci 2008; 28: 10451–10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhou Y, Cai W, Zhao Z, et al. Lactadherin promotes microvesicle clearance to prevent coagulopathy and improves survival of severe TBI mice. Blood 2018; 131: 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Motegi S, Leitner WW, Lu M, et al. Pericyte-derived MFG-E8 regulates pathologic angiogenesis. Arterioscler Thromb Vasc Biol 2011; 31: 2024–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saliba AE, Westermann AJ, Gorski SA, et al. Single-cell RNA-seq: advances and future challenges. Nucl Acids Res 2014; 42: 8845–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.He L, Vanlandewijck M, Mae MA, et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci Data 2018; 5: 180160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008; 3: 301–313. [DOI] [PubMed] [Google Scholar]
- 151.Moura DAP, Lemos RR, Oliveira JRM. New Data from Pdfgb (ret/ret) Mutant Mice Might Lead to a Paradoxical Association Between Brain Calcification, Pericytes Recruitment and BBB Integrity. J Mol Neurosci 2017; 63: 419–421. [DOI] [PubMed] [Google Scholar]
- 152.Cuervo H, Pereira B, Nadeem T, et al. PDGFRbeta-P2A-CreER(T2) mice: a genetic tool to target pericytes in angiogenesis. Angiogenesis 2017; 20: 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]