Abstract
Aging is accompanied by vascular and structural changes in the brain, which include decreased grey matter volume (GMV), cerebral blood flow (CBF), and cerebrovascular reactivity (CVR). Enhanced fitness in aging has been related to preservation of GMV and CBF, and in some cases CVR, although there are contradictory relationships reported between CVR and fitness. To gain a better understanding of the complex interplay between fitness and GMV, CBF and CVR, the present study assessed these factors concurrently. Data from 50 participants, aged 55 to 72, were used to derive GMV, CBF, CVR and VO2peak. Results revealed that lower CVR was associated with higher VO2peak throughout large areas of the cerebral cortex. Within these regions lower fitness was associated with higher CBF and a faster hemodynamic response to hypercapnia. Overall, our results indicate that the relationships between age, fitness, cerebral health and cerebral hemodynamics are complex, likely involving changes in chemosensitivity and autoregulation in addition to changes in arterial stiffness. Future studies should collect other physiological outcomes in parallel with quantitative imaging, such as measures of chemosensitivity and autoregulation, to further understand the intricate effects of fitness on the aging brain, and how this may bias quantitative measures of cerebral health.
Keywords: Aging, cerebrovascular reactivity, fitness, MRI, perfusion-weighted imaging
Introduction
Continuous and optimal blood flow is thought to be necessary for structural integrity and normal neuronal activity in the brain.1 During aging, the vascular system undergoes a cascade of events that negatively affect the integrity of the cerebrovascular system, leading to decreased perfusion. However, it may be possible to reduce these deficits, and in some instances, reverse them as a result of plasticity. Plasticity refers to the capacity of the brain to change its function, hemodynamics and microstructure in response to cognitive or physiological challenges.2,3 In aging, there is some indication that physical activity may be capable of inducing beneficial plastic changes.4,5 Notably, aerobic exercise has become a subject of particular interest for maintaining and even enhancing cognition and brain integrity.4,6,7 It is likely that these effects are mediated by changes in cerebrovascular health given the well-established positive influence of exercise on the cardiovascular system in aging.8,9 It has been demonstrated that more highly fit individuals have enhanced endothelial function10 and reduced arterial stiffness,9,11 both of which are impaired in aging.12–14 Of note, individuals who are more “highly fit” have higher cardiovascular fitness (VO2peak), which can be quantified in multiple ways. Specifically, VO2max is considered the gold standard measure.15 However, reaching a true VO2max is difficult to attain for many older adults, thus utilizing VO2peak is a more feasible option.16,17 These measurements will therefore be referred to as VO2peak for the remainder.
Given the positive relationship between the vascular system and exercise, there is an increasing body of work investigating the relationship amongst aging, VO2peak, cerebral structural integrity and hemodynamics. Magnetic resonance imaging (MRI) is the method of choice to study these relationships as it is a versatile technique which can be used to measure several of these parameters, including grey matter volume (GMV), cerebral blood flow (CBF) and cerebrovascular reactivity (CVR). In general, GMV and CBF are positively associated with VO2peak in cross-sectional18–20 and longitudinal studies.21–25 However, many of the existing studies showing this beneficial effect have used GMV as a marker of “structural integrity”. This is problematic because GMV has been shown to be mainly qualitative and physiologically non-specific,26,27 making a mechanistic interpretation of these effects difficult.
More physiologically specific approaches have involved looking at the relationship between CBF and VO2peak. In cross-sectional studies, it has been demonstrated that there is a positive relationship between VO2peak and CBF.20,28,29 This also seems partly supported by intervention studies. For instance, Chapman et al.21 found that individuals who completed a 12-week aerobic training program demonstrated significant increases in CBF compared to the passive control group. Yet, in a later study Chapman et al.30 found CBF to be unchanged after the same aerobic training program. While this could be due to an insufficient exercise dose, it is possible that CBF is not a sensitive enough marker of cerebrovascular health in isolation. This could be both due to its relatively limited signal-to-noise ratio31 and the fact that homeostasis seeks to maintain CBF to ensure adequate perfusion to maintain oxygen and glucose delivery.32 There are indications that while CBF does steadily decrease across the lifespan,33 more dynamic aspects of hemodynamics, such as CVR may change earlier than CBF in the course of aging.34–36
CVR is measured as the hemodynamic response (in terms of CBF or blood-oxygen-level dependent (BOLD) change for example) to a vasodilatory challenge, such as hypercapnia,37 breath-holds38 or acetazolamide.39 CVR is hypothesized to represent the health of the cerebral vasculature.40 If it is assumed that CO2-related local chemosensitivity is consistent across age and disease groups, it could be treated as a vascular vasodilatory capacity biomarker. Furthermore, if CVR is taken to be a marker of vascular health, it can be posited that those with higher VO2peak levels would have greater CVR, as their vascular system would be more compliant and therefore have an increased ability to respond to a vasodilatory stimulus. Consistent with this hypothesis, it has been demonstrated that CVR is decreased in aging,34,41 stroke42 and carotid artery stenosis.43 Yet, the literature has found conflicting results within healthy populations, where some have observed elevated CVR in relation to higher VO2peak levels,44–47 while others have found that lower CVR is related to increased VO2peak,28 and others have found no difference40–42 in aging. It is unclear, however, if this is due to differences in measurement method, spatial localization of the measurement or an interesting physiological interplay between multiple hemodynamic aspects of brain health.
In summary, there is an assortment of negative consequences that can occur due to an aging vascular system that causes deterioration of brain microstructure and hemodynamics. Importantly, there is evidence that exercise is capable of mitigating some of these adverse age-related complications. Yet, the fitness literature suggests that the effects of exercise on brain hemodynamics may be complex, so a more comprehensive imaging approach is necessary to understand the interplay between the effects of aging and VO2peakon cerebral hemodynamics. The present study explores the relationship between aging, VO2peak, cerebral hemodynamics and GMV using a cross-sectional dataset employing a comprehensive imaging approach in healthy younger and older adults of varying VO2peak.
Methods
Participants
A total of 28 young adults (seven females, mean age 24 ± 3 years) and 50 older adults (37 females, mean age 63 ± 5 years) participated in this study. Participants were recruited through participant databases at the Centre de recherche de l'Institut universitaire de gériatrie de Montréal and Laboratoire D'Etude de la Santé cognitive des Ainés.
Inclusion criteria were comprised of being between 18 and 40 years for young adults and 55 and 75 years for older adults; approval by a geriatrician to participate (older adults), non-smoker (for at least five years), no evidence of cognitive impairment as determined through cognitive tests conducted by a neuropsychologist, and ability to complete the peak oxygen uptake test (VO2peak) and MRI. Exclusion criteria included taking prescription medication that could be vasoactive (e.g. diuretics, calcium channel blockers, statins, thyroid replacement hormones, etc.), presence of cardiac disease, hypertensives, neurological or psychiatric illnesses, diabetes, asthma, thyroid disorders, or excessive drinking (more than two drinks per day). Finally, a neuropsychologist completed the Mini Mental Status Examination, a global cognitive screening tool for dementia; participants with scores less than 26 (out of 30) were excluded.48
All procedures were approved by Comité mixte d'éthique de la recherche du Regroupement Neuroimagerie/Québec and were conducted according to the Declaration of Helsinki. All participants provided written informed consent.
MRI acquisition
All acquisitions were completed on a Siemens TIM Trio 3T MRI system (Siemens Medical Solutions, Erlangen, Germany). A 32-channel head coil was used for all brain acquisitions. An anatomical 1 mm3 MPRAGE acquisition (TR/TE/flip angle = 300 ms/3 ms/90°, 256 × 240 matrix) was acquired for registration and GMV estimation. A fluid attenuation inversion recovery (FLAIR) acquisition with parameters: TR/TE/flip angle 9000 ms/107 ms/120° with echo train length of 15, an inversion time of 2500 ms, 512 × 512 matrix for an in-plane resolution of 0.43 × 0.43 mm and 25 slices of 4.8 mm was used to estimate the presence and severity of white-matter lesions in older adults. A pseudo-continuous arterial spin labeling (pCASL) acquisition,49 providing simultaneous BOLD contrast using dual-echo readouts (TR/TE1/TE2/flip angle = 2000 ms/10 ms/30 ms/90°, 4 × 4×7 mm voxels, 64 × 64 matrix and 11 slices, post-label delay = 900 ms, tag duration = 1.5 s, and a 100 mm gap) was acquired during a hypercapnia challenge.
Aortic exam
As described in Gauthier et al.,50 during the MRI session a thoracic aortic exam was also acquired using simultaneous brachial pressure recording (Model 53,000, Welch Allyn, Skaneateles Falls, NY USA) using a 24-element spine matrix coil. Black blood turbo spin echo sagittal oblique images were acquired to visualize aortic arch (TR/TE/flip angle: 700 ms/6.5 ms/180°, 1.4 × 1.4 mm in-plane resolution, 2 slices at 7.0 mm). A perpendicular plane to the ascending and descending aorta was defined from these images. A cine phase-contrast velocity encoded series was collected (TR/TE/flip angle: 28.6 ms/1.99 ms/30°, 1.5 × 1.5 mm × 5.5 mm) during 60 cardiac cycles in three segments, with velocity encoding of 180 cm/s. A series of cine FLASH images were also acquired in the same plane (TR/TE/flip angle: 59 ms, 3.44 ms, 15°; 1.2 ×1.2 mm × 6 mm) over 60 cardiac cycles in eight segments.
Hypercapnia
The hypercapnic manipulation used here has been described previously.34,50 Briefly, it was completed with a computer-controlled gas system with a consecutive gas delivery circuit (Respiract™ GEN3, Thornhill Research Inc., Toronto Canada).51 End tidal O2 was targeted to be 100 mmHg throughout the manipulation, while CO2 had a target of 45 mmHg during the hypercapnia blocks and 40 mmHg during normocapnia. More specifically, two hypercapnia blocks, of 2 min each in duration, were completed after, and followed by 2 min blocks of breathing room air. Participants breathed through a soft plastic mask (Tegaderm 3M Healthcare, St. Paul MN) that was secured on their face with adhesive tape to ensure that no leaks were present. Participants completed the breathing manipulation once prior to being in the scanner (to ensure comfort and tolerance to procedure), and once during the MRI session.
VO2peak
Participants completed a maximal oxygen consumption test (VO2peak) to approximate their VO2peak, where a greater amount of oxygen consumed indicates enhanced VO2peak.52 The test was completed on a stationary cycle ergometer and was monitored throughout by an electrocardiogram under medical supervision to ensure participant safety. Initial workload was set based on the body weight of the individual (1 watt (W)/kg) and then increased incrementally by 15 W every minute until voluntary exhaustion. Oxygen uptake was determined using an automated system that averaged in 30-s increments (Moxus, AEI Technologies, Naperville, IL). The highest oxygen uptake over a 30-s period during the test was considered as the VO2peak (ml/kg/min).
Data analysis
GMV
T1-weighted MPRAGE images were preprocessed using SPM's Computational Anatomy Toolbox (CAT)1253–55 to calculate voxel-based morphometry (VBM) after data were segmented into grey matter, white matter and cerebrospinal fluid (CSF). VBM calculates the difference between the volume estimated for tissue from an individual compared to the expected volume of tissue from a template. This provided a statistical map for each voxel type which is then classified into the structural category with the highest probability, allowing for analysis between participants.
The registration matrix was calculated as part of the VBM pipeline and was then applied to the GMV, CBF and CVR maps (described below) to bring them from native to MNI space. Individual BOLD-CVR, resting CBF and CBF-CVR were produced for each participant. Co-registration of these maps from native to native T1 space was performed using a non-linear rigid registration with ANTS56 with a b-spline interpolation. CAT1253–55 was used to register from T1 to standard space using a uniform non-linear registration with 12 degrees of freedom and smoothing of the data employed a Gaussian filter of 8 mm. An average grey matter mask from each age group was also created to restrict voxel-wise analyses to the grey matter.
CVR analysis
Preprocessing of the BOLD-CVR has been described previously and was performed using Neurolens2 (www.neurolens.org).34 All raw images were preprocessed with motion correction52 and spatial smoothing with a 6 mm Gaussian kernel. The BOLD signal was extracted from the second echo series with a linear surround addition.57–59 The BOLD fractional change during hypercapnia was obtained by fitting a general linear model to the BOLD signal and dividing the estimated effect size by the estimated constant term. Glover's60 parameters for a single-gamma hemodynamic response function were used when fitting the linear models, which also included linear, quadratic and third-order polynomials representing baseline signal and drifts. The BOLD percent change obtained was then divided by the average end-tidal CO2 change during the hypercapnia manipulation to yield BOLD-CVR. CBF-CVR was calculated in the same way as BOLD-CVR, but the CBF signal was isolated from the first series of echoes using linear surround subtraction.57
Resting CBF analysis
Resting CBF was quantified using the first echo of the whole pCASL data time series, using the first 2 min of the time series, before the beginning of the first hypercapnia block. The average of the control images was used for each participant with modeling of the T1-recovery to obtain the fully recovered magnetization (M0) using AFNI, FSL and in-house scripts. CSF masks were created for each older adult participant to use as CSF M0 in the CBF quantification. To do this, 10 voxels were chosen in the same axial slice for all older participants where the lateral ventricles were clearly located, except for four participants where a more superior, or inferior slice was required to clearly identify the ventricles from the pCASL scans. All individual masks were visually inspected to ensure the ROIs were located in the ventricles. For the younger adults, one participant was chosen at random and the same method was used to identify 10 voxels. A single CSF mask was used for all younger participants as this mask was confirmed to be located in the ventricles in all participants upon visual inspection. However, this was not possible with the older adults due to varying anatomical structures. FSL's BASIL61 toolkit was then utilized to quantify CBF, with the following standard parameters: labelling: cASL/pcASL; bolus duration: constant (1.5 s), post label delay: 0.9 s; calibration image: average of the control images; reference tissue type: CSF; mask: CSF mask for each participant; CSF T1: 4.3 s; TE: 10 ms; T2 : 750 ms; blood T2: 150 ms; arterial transit time: 1.3 s, T1: 1.3 s , T1 blood: 1.65 s, inversion efficiency: 0.85.
Vascular lesion quantification
The volume of white matter hyperintensities (WMHs) in the brain was estimated in a semi-automatic way as described in Gauthier et al.50 Briefly, a single trained rater, who was blinded to clinical information, visually identified WMH on the FLAIR images, which were then delineated using Jim image analysis package, version 6.0 (Xinapse Systems Ltd, Northants, UK).
Pulse wave velocity data
The aortic data were analyzed using the ARTFUN software,62 where pulse wave velocity (PWV) was computed between the ascending and descending aorta using the cine phase contrast images for blood velocity and the cine images for aorta delineation. PWV was calculated as described in Gauthier et al.50 These data were included as a covariate, so that any relationships that might be present between VO2peak and the hemodynamic brain outcomes were not due to differences in arterial stiffness in large arteries among the older adults but rather to brain-specific properties.
Voxel-wise analyses
Using FSL's toolbox Randomise, 63permutation-based threshold-free cluster enhancement (TFCE),64 using 10,000 permutations, was employed to test for spatial relationships between VO2peak and structural or hemodynamic outcomes. These analyses were restricted to GM using a group mask of all GM voxels present in all participants (i.e. the intersection of all participants GM segmentation mask in MNI space). A separate group GM mask was created for younger and older adults. Voxel-wise general linear models were used to identify the relationship within GM between: (i) VO2peak and GMV; (ii) VO2peak and BOLD-CVR; (iii) VO2peak and resting CBF; and (iv) VO2peak and CBF-CVR data for young and older adults. Age and sex were included for both young and older adults as covariates. For the older adults, we also included sex-specific estimated absolute multivariate risk scoring with the Framingham cardiovascular risk factor, as proposed by Ralph et al.,65 to estimate general cardiovascular risk and future cardiovascular risk as a confound. Volume of WMH and PWV was also used as potential confounds in the older adults to remove the potential effects of existing WM lesions and central arterial stiffness.
Regions of interest
Voxels that exhibited a significant relationship between BOLD-CVR and VO2peak were extracted and binarized to be used as regions of interest (ROI) for further analysis. Specifically, this ROI was then used to further investigate if VO2peak and GMV, resting CBF, or CBF-CVR were related to each other in these regions, in an attempt to disentangle the physiological relationship amongst these factors in aging and VO2peak. This ROI was then multiplied by each individual's VBM map to create individual ROI masks. The values from each participant for this individual ROI were then extracted for all maps using weighted average with FSLmeants to correct for possible GM atrophy.
Finally, a finite impulse response (FIR) analysis was completed as reported in Gauthier et al.34 to estimate the temporal course of the BOLD response to hypercapnia in order to identify whether dynamic aspects of the response could be linked to VO2peak. Briefly, the average time course for BOLD during hypercapnia was determined, where the temporal response was measured starting 15 s prior to and after the end of each hypercapnic block. The beginning of the upward phase of the response, as well as the plateau were identified manually and the linear fit for the values between these two points (slope of the upward response) was identified using a linear regression in the SPSS 20.0 software (IBM, Armonk, New York, USA) for each participant. The BOLD time course was averaged within the BOLD-CVR VO2peak ROI for both young and older adults, and these values were then extracted. With the exclusive intent of facilitating visualization, the older adult group was then rank ordered according to their VO2peak. Once rank ordered, they were subdivided into five bins based on VO2peak to further visualize the relationship between response shape and VO2peak.
Statistical analysis
Statistical analysis of the behavioural data was completed using SPSS to identify if relationships were present between VO2peak and demographic data with correlational analyses. A partial correlation was used to identify if there were relationships between VO2peak and values extracted from the significant CVR-BOLD VO2peak ROI, while accounting for the covariates described above (e.g. age, sex, PWV, Framingham Risk Factors, white matter hyperintensity volume). As the white matter hyperintensity volumes were found to be non-normal, it was necessary to log transform this data to allow for parametric statistical analyses with the data. All other data were found to be normally distributed. Finally, a partial correlation analysis was also utilized to investigate potential relationships between VO2peak and the slope of the BOLD upward response where age was included as a covariate. Statistical significance was set to p ≤ 0.05 for all outcomes and Tukey's post hoc analysis was used where applicable.
Results
Younger versus older adults
A total of 50 older adults and 26 young adults participated in this study, and demographics for both are listed in Table 1.
Table 1.
Participant demographics separated by age group.
| Demographic | Young adults (n = 26) | Older adults (n = 50) |
|---|---|---|
| Sex (M/F) | 19/7* | 17/33* |
| Age (years) | 23.7 (2.9)* | 63.4 (4.9)* |
| Education (years) | 16.7 (1.4) | 16.4 (3.6) |
| VO2peak (ml/kg/min) | 42.7 (7.6)* | 29.1 (7.0)* |
| MMSE (out of 30) | – | 28.8 (0.9) |
| Framingham risk factor score | – | 8.8 (2.6) |
| Log WMH volume | – | 0.367 (0.162) |
| Grey matter volume (mm3) | 0.551 (0.038)* | 0.466 (0.034)* |
| BOLD-CVR (%change/mmHg CO2) | 0.261 (0.094)* | 0.176 (0.041)* |
| Resting CBF (ml/100 g/min) | 48.6 (10.7)* | 42.4 (9.9)* |
| CBF-CVR (ml/100 g/min/mmHg CO2) | 5.13 (1.22) | 4.63 (2.35) |
Note: Independent samples t-test was used to identify differences between young and older adults.
Statistically different p < 0.05; all values reported are mean (±SD).
VO2peak, brain structure and hemodynamics in GM
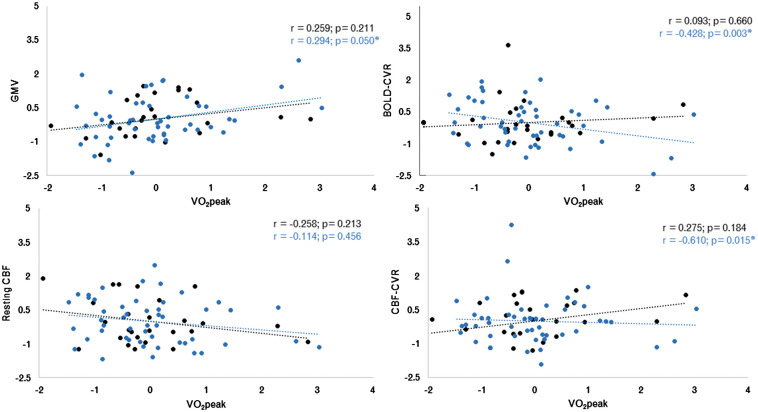
The mean values over all GM for hemodynamic parameters in each participant versus VO2peak for both young and older adults are shown in Figure 1. It was found that younger adults had a significantly higher GMV (p = 4.43 × 10−15), BOLD-CVR (p = 1.4 × 10−4) and resting CBF (p = 0.015) in whole GM than older adults. There were no differences between age groups for CBF-CVR in whole GM (p = 0.315) (Table 1).
Figure 1.
Results from voxel-wise analysis for relationships between structural and hemodynamic outcomes with VO2peak z-scores (ml/kg/min) for young adults (black dots) and older adults (blue dots). (a) grey matter volume z-score (mm3); (b) BOLD-CVR (%BOLD/mmHg CO2) z-score; (c) resting CBF (ml/100g/min) z-score and; (d): CVR CBF (ml/100g/min/mmHg CO2) z-score. The regression line for each group is plotted in their corresponding colours. *Indicates significant correlation (p ≤ 0.05).
Young adults
Correlations found no significant relationships between VO2peak and the mean extracted values for GMV, BOLD-CVR, resting CBF or CBF-CVR in GM for young adults (p > 0.05).
Older adults
A partial correlation in older adults including all covariates (e.g. age, sex, Framingham Risk Factor score, WMH and PWV) revealed a significant relationship between VO2peak and: (i) all GMV (r = 0.294; p = 0.05); (ii) BOLD-CVR in GM (r = −0.428; p = 0.003); and (iii) CBF-CVR in GM (r = −0.361; p = 0.015). No significant correlation was found between VO2peak and resting CBF in all GM (p > 0.05).
VO2peak, structure and hemodynamics
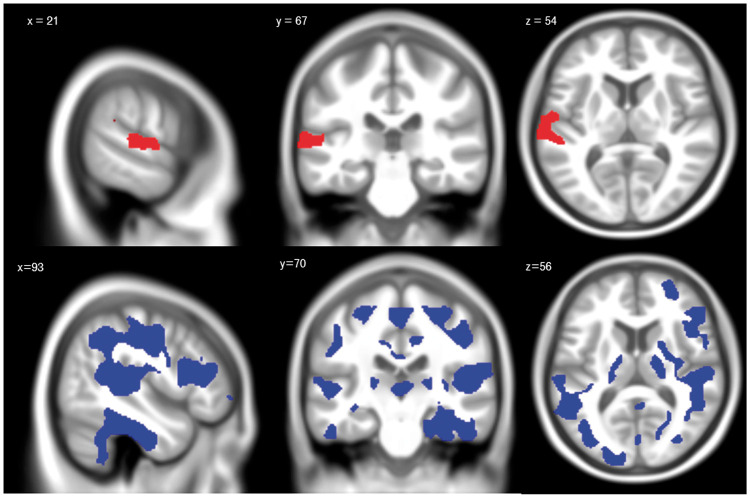
Voxel-wise analyses within the younger adults did not reveal any significant relationships between VO2peak and GMV or between VO2peak and the hemodynamic outcomes (p > 0.05) within GM. In older adults, voxel-wise analyses within GM revealed a significant positive relationship between GMV and VO2peak (r = 0.320; p = 0.025) and a significant negative association between BOLD-CVR and VO2peak (r = −0.392; p = 0.005). The positive relationship between GMV and VO2peak was present within the superior temporal gyrus (see Figure 2(a)). The negative association between BOLD-CVR and VO2peak was found in large portions of temporal, parietal cortices and smaller amounts of the frontal lobes (see Figure 2(b)). No relationship was found between VO2peak and resting CBF or CBF-CVR (p > 0.05).
Figure 2.
Voxel-wise analyses results in older adults. Significant regions identified with the voxel-wise analysis between VO2peak z-score and GMV z-score (a). This figure shows areas of the brain where there is a positive association between VO2peak z-score and GMV z-score (red), indicating that in these areas, those with higher fitness have significantly higher GMV compared to those with lower fitness (p < 0.05). Significant regions identified with the voxel-wise analysis between VO2peak z-score and BOLD-CVR z-score. Areas of the brain where there is a negative association between VO2peak and BOLD-CVR (blue), indicating that in these areas, those with higher fitness have significantly reduced BOLD-CVR compared to those with lower fitness (p < 0.05).
Region of interest analysis
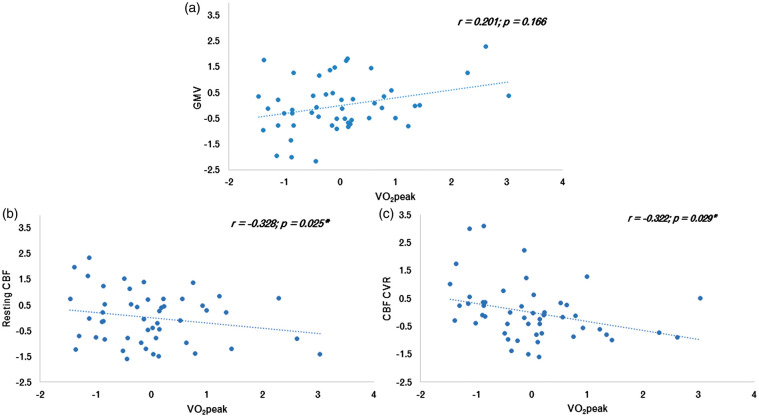
To understand whether the relationship between VO2peak and other structural or hemodynamic parameters could help to explain the negative association between VO2peak and BOLD-CVR, other parameters were averaged in the areas significantly negatively related between BOLD-CVR and VO2peak. Within these ROI, a significant negative relationship was identified between VO2peak and resting CBF (r = −0.328, p = 0.025), and VO2peak and CBF-CVR (r = −0.322, p = 0.029). The relationships between all structural or hemodynamics outcomes within these ROI with VO2peak are shown in Figure 3.
Figure 3.
Association between fitness, structure and hemodynamics in the BOLD-CVR vs VO2peak ROI. Relationships from the CVR VO2peak z-score ROI in; (a): GMV z-score; (b): Resting CBF z-score; and (c): CBF-CVR z-score. Graphs demonstrate the relationship between each of these parameters and fitness in older adults. *Represents significant correlation (p < 0.05).
FIR
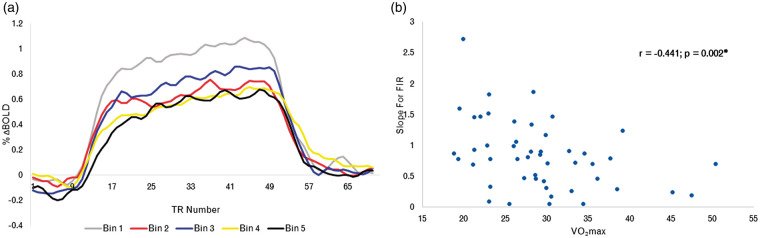
Finally, to identify potential relationships between VO2peak and BOLD response dynamics, a FIR analysis was run within the ROI derived from the voxel-wise analysis of CVR and VO2peak. For visualization purposes, the BOLD time course to hypercapnia in these areas for older adults consisted of rank ordering based on VO2peak, then creating five different bins according to their rank order (see Figure 4(a)). The partial correlation analysis revealed that there was a significant negative correlation between VO2peak and the slope of the linear regression (r = −0.441; p = 0.002). This relationship is shown in Figure 4(b).
Figure 4.
Time course of the BOLD response to hypercapnia. (a) Time course showing the percent BOLD response to hypercapnia in BOLD-CVR VO2peak ROI. Where the fitness level for older adults was binned into five categories; Bin 1 representing the lowest VO2peak bin, and increasing until Bin 5, which includes the data from those with the highest binned VO2peak in this sample. (b) Linear regression of the relationship between the slope of the upward portion of the response and fitness in older adults.
Discussion
This study investigated the relationship between VO2peak, GMV and brain hemodynamics in a population of healthy older adults. This group showed the expected pattern of reduced GMV, BOLD-CVR and resting CBF as compared to healthy younger adults. Voxel-wise analyses over all GM demonstrated a significant positive relationship between VO2peak and GMV and a somewhat surprising significant inverse relationship between BOLD-CVR and VO2peak in older adults in a number of GM regions throughout the cortex. A more in-depth review of hemodynamics within these regions demonstrated that the relationship between VO2 and other hemodynamic parameters also exhibited this inverse relationship. Specifically, there was no relationship between VO2peak and GMV, but a significant negative relationship between VO2peak and resting CBF, and between VO2peak and CBF-CVR. To determine whether these relationships between VO2peak and BOLD-CVR were confined to response amplitude or if it was also present in response dynamics, we performed an analysis of the time course of the BOLD response to hypercapnia. This analysis revealed a slower ramp-up towards a plateau in those with higher VO2peak, regardless of age as demonstrated in Figure 4(b). Together, these results indicate that the relationship between VO2peak and hemodynamics in aging is more complex than previously thought and that BOLD-CVR in particular may be biased by physiological mechanisms affected by exercise.
Age-group comparisons
The impact of healthy aging on brain structure and hemodynamics is an active field of research and the age group comparisons performed as part of this study are consistent with these existing results. In comparison to young adults, older adults were found to have lower: GMV,45,66,67 CVR,34,35,68–70 and resting CBF.34,35
Regional relationships between VO2peak, structure and hemodynamics
The main result from this study is the finding that BOLD-CVR in GM is negatively correlated with VO2peak in older adults. Voxel-wise analysis revealed large sections of GM including temporal, parietal and frontal regions were responsible for this negative relationship. Given that higher CVR is typically interpreted as being related to better cerebral health,40 this reverse relationship was counter-intuitive. Interestingly, to date, only one other published study (in addition to our own work50) has also demonstrated a negative relationship between VO2peak and BOLD-CVR in older adults.28,50 Specifically, Thomas et al.28 found that Master athletes had significantly lower BOLD-CVR compared to their sedentary counterparts over most of cerebral cortex, including the parietal and temporal cortices. Notably, most studies investigating VO2peak and hemodynamics have used transcranial Doppler (TCD) to identify a positive relationship between CVR and VO2peak.44–47 To the best of our knowledge, MRI studies have only identified a negative relationship, perhaps reflecting the different vascular compartments and properties imaged with both techniques. TCD images flow velocity in major arteries, while the BOLD signal reflects a mixture of blood flow, blood volume and oxidative metabolism arising from the parenchyma and veins. Therefore, it is possible that changes in the venous system, such as venous collagenosis, or related to the parenchymal vasculature lead to the BOLD-CVR results measured using MRI.
The voxel-wise analysis also revealed a positive relationship between VO2peak and GMV within the superior temporal gyrus. This is consistent with other studies pointing to a general association between VO2peak and GMV,6 and specifically within the superior temporal gyrus.71 However, as mentioned previously, GMV should be interpreted with caution as it is qualitative, not physiologically specific, and may be biased by differences in blood volume.26,27
Notably, the lack of relationship with other hemodynamic parameters could be attributable to the fact that the present study involved a very healthy group of older adults. Exclusion criteria were numerous, including but not limited to, taking most medication regularly, suffering from chronic diseases, or cardiovascular risk factors. Moreover, the Framingham scores for this group is low, with the average (8.8) just below the score expected solely due to the average age of the group (9), indicating overall absence of cardiovascular risk factors within the group. Furthermore, participants in this study had VO2peak values that were greater than the 50th percentile for their age and sex, thus demonstrating higher than average VO2peak levels.72 Therefore, it is possible that the relationship between VO2peak and these other hemodynamic parameters is below the detection limit in this healthy group of older adults, especially in the context of the limited SNR provided by ASL and the stringent thresholding required by the numerous multiple comparisons performed in voxel-wise analyses. Our findings suggest that CVR may be one of the first hemodynamic properties to decline in aging and indicate that it may be more sensitive to aging-related changes in the cerebral vasculature, than CBF and GMV, in line with previous published work.34
Physiological underpinnings of BOLD-CVR and fitness association
To better understand the physiological underpinnings of this negative relationship between VO2peak and BOLD-CVR, a more in-depth investigation of the relationship between VO2peak and other hemodynamic parameters and GMV within these regions was performed. No relationship between GMV and VO2peak was revealed; however, resting CBF and CBF-CVR had significantly negative associations with VO2peak, indicating that lower fit individuals had higher CBF and CBF-CVR. These findings are in opposition to those reported in the extant literature by Tarumi et al.,29 where endurance-trained older adults showed a higher CBF in the occipitoparietal area as compared to their sedentary counterparts. These results are difficult to compare to those of the present study. Firstly, because the coordinates for the areas used in Tarumi et al. are not available, so that any putative overlap between region and ROI used in the present study is impossible to determine. Secondly, the endurance trained group had considerably higher VO2peak than this cohort. Zimmerman et al.20 also found higher global, frontal and parietal CBF in those with greater VO2peak levels. However, 39% of their participants were taking blood pressure medication, thus it could be that medication impacted these results given the vasoactive nature of these molecules. Future studies, with both larger VO2peak and cardiovascular health ranges, are therefore necessary to determine whether non-linear effects in the link between CBF and VO2peak can account for these contradictions in the literature.
While hyperperfusion is not typically associated with aging, one area of research that has identified a pattern of hyperperfusion in similar areas is the APoE4 literature.73–75 Scarmeas et al.73 found that both young and older individuals with the APoE4 gene demonstrated hyperperfused areas of the brain compared to non-carriers.74 Furthermore, a longitudinal study in older adults found that areas that were hyperperfused at baseline in carriers as compared to non-carriers, had significantly lower CBF at the eight-year follow-up.75 Given the similarity in the hyperperfusion pattern, it is possible that part of our results could be explained by putative over-representation of APoE4 carriers in the low VO2peak participants. However, as we did not measure APoE expression in our participants, we cannot assess whether this is the case. In general, however, one could speculate that these patterns of hyperperfusion in APoE4 carriers and in the less fit individuals included in this study could indicate that there exists a set of physiological compensatory mechanisms which initially seem like preserved hemodynamics, but that are in fact associated with poorer health or greater damage over time.
Chemosensitivity and autoregulation
In addition to the relationship between BOLD-CVR and VO2peak already discussed (Figure 4(a)), we also identified a relationship between the slope of the upswing of the response to hypercapnia and VO2peak. The slower BOLD response to hypercapnia in more highly fit older adults could be indicative of a local desensitization to CO2, or to pre-dilation76; however, the latter is unlikely here as lower resting CBF was found to be linked to higher VO2peak within these same regions. Further studies including additional measurement of autoregulation and the respiratory response to exercise, for example, could help untangle the physiological underpinning of these response dynamics.
Overall, there are a few rationales that could explain our results of low BOLD-CVR in higher fit individuals. For example, the first, hypothesized by Thomas et al.,28 is that perhaps higher fit individuals have decreased local sensitivity to CO2, likely from a lifetime of increased exposure because of increased aerobic activity. This idea is consistent with studies showing that endurance training reduces the ventilatory response at a given workload, indicating a decrease in local chemosensitivity.77,78 Nitric oxide is the primary mechanism to respond to changing pH levels from CO2 in an attempt to maintain homeostasis in the brain.79,80 It is thus possible that higher fit individuals could have lower levels of nitric oxide in response to hypercapnia than lower fit individuals, which in turn would explain the reduced blood flow response to hypercapnia. Moreover, it has also been proposed that cerebral inflammation could lead to increased nitric oxide signaling,79 thus lower fit individuals could have increased nitric oxide due to the presence of inflammation. This would also be consistent with the higher resting CBF observed in lower fit individuals. The presence of inflammation is, however, unlikely to be the main explanation for our results, given the overall health of this cohort. Lastly, nitric oxide and CO2 have an effect on cerebral autoregulation80; when present, it increases the ability of the cerebral blood vessels to dilate or constrict in response to a sudden change in blood pressure, allowing for sufficient blood to flow. For example, it has been found that those with arterial hypertension, have greater central chemosensitivity than those without.77,81 Moreover, during exhaustive exercise, cerebral autoregulation was decreased compared to rest,82 and was observed to be reduced in young master athletes compared to sedentary counterparts.83 It is therefore possible, given the interplay between CO2 sensitivity (local vs. central), nitric oxide presence and cerebral autoregulation, that in combination this may account for the reduced BOLD-CVR in higher fit individuals in aging.
The results of this and other studies have shown that quantitative techniques such as MRI measurements of CVR and CBF may be biased by health components not typically taken into account in MRI studies (e.g. local or central chemosensitivity and cerebral autoregulation). This is problematic as it may lead to bias in group comparisons or longitudinal studies. Though we were not able to test these additional parameters in the present study, it highlights the need for comprehensive studies that seek to measure all the components of the complex relationship between cerebral hemodynamics and VO2peak. These studies are necessary to make these techniques truly quantitative and reveal the physiological changes that occur in aging and disease.
Limitations
Although we found that higher VO2peak is related to decreased BOLD-CVR in aging, it is difficult to interpret our results in comparison to other studies due to the high level of variability of BOLD-CVR in the aging literature. For example, some report 0.19% BOLD/mmHg in line with our results,84 yet another study has reported higher levels at 0.28%85 and lower levels at 0.13%.86 This indicates that there is physiological variability within individuals and between studies, and potentially technical variability (i.e. type of scanner, delivery of CO2, amount of CO2 inhaled). Moreover, given that our CO2 challenge was 5 mmHg, it could be that our data suffer from worse SNR than what would be expected with a greater amount of CO2 delivered, such as 10 mmHg. Therefore, more work is necessary to further comprehend inter-individual variability, and to implement more robust study designs with a greater breadth of outcome measures and to implement progressive hypercapnia in addition to block designs.
A limitation of the use of ASL for measuring CBF is that extensive coverage of the entire brain is typically not possible without advanced parallel imaging techniques. Given that the original aim of this study at the time of data collection was more focused on executive functions and the frontal lobes, it was not possible to capture structural and hemodynamics of the hippocampus. Therefore, while the hippocampus is a structure associated with VO2peak-related changes, we are unable to test for associations between the hippocampus and VO2peak here. Furthermore, the post-label delay chosen here is suboptimal for older adults, so that lower perfusion measured could be the result of slower transit time, rather than lower perfusion. Moreover, given that blood flow velocity is likely increased during hypercapnia, and it is known that labeling efficiency decreases as blood velocity increases,87 there is a potential for our CBF data during hypercapnia to be underestimated as we assumed a consistent labeling efficiency which is likely not the case. Thus, future work aiming to disentangle the relationships among aging, cognition and VO2peak should optimize the acquisition, using a multi-band acquisition approach for example, to capture both the entire cerebral cortex and the hippocampus, and multiple post-label delays to better capture perfusion across age and VO2peak.
Another limitation to this study is its cross-sectional design, which makes it difficult to draw clear conclusions about the relationships between VO2peak, aging and brain health. While large longitudinal cohorts exist, none have so far also included measurement of CVR and VO2peak, likely because these techniques are challenging to implement. On the other hand, ASL acquisitions are becoming more common and future studies could attempt to use cohorts of older adults for studying the relationship between physical activity, CBF and other measures of vascular health. Dedicated longitudinal studies over several years including VO2peak, CBF and CVR would however be necessary to truly understand these relationships. Although VO2max is considered the gold standard of cardiovascular fitness, there are some indications from the literature that a true VO2max may not be practically attainable in older adults.17 Thus, we used VO2peak here. It is also noteworthy that there are inherent limitations to using either as an outcome, as they can be influenced by genetics, pulmonary function, skeletal muscle limitations, cardiac output, to name a few (see Bassett and Howley88 for in-depth review), which were not measured in this study.
Conclusions
Overall, this paper identified a negative relationship between BOLD-CVR and VO2peak in a very healthy older adult sample. Within the ROI's that demonstrated a significant relationship, other hemodynamic outcomes also showed negative relationships with VO2peak. These negative relationships could be the result of changes in CO2 sensitivity, or autoregulation. In addition, our findings suggest that quantitative measures of CVR and CBF could be biased by unknown physiological changes in these autoregulatory and chemosensitivity properties, and that studies using these markers in aging and disease may underestimate their effects on cerebral hemodynamics. Thus, to further understand and attempt to disentangle the modulatory effect that VO2peak has on hemodynamics in aging, more comprehensive studies of physiological outcomes are necessary.
Acknowledgements
The authors thank Carollyn Hurst and André Cyr for their help with data acquisition, Élie Mousseaux, Alban Redheuil, Muriel Lefort, Frédérique Frouin, and Alain Herment for their help with the aortic protocol and analysis, Cécile Madjar, Mélanie Renaud and Élodie Boudes for their help with logistics, Fatemeh Razavipoor and Julia Huck for helpful discussions, Saïd Mekary for his help with VO2peak testing, Ellen Garde, Arnold Skimminge and Pernille Iversen for their help with vascular lesion segmentation, and Céline Denicourt for performing the blood draws. They thank Jiongjiong Wang of the Department of Neurology at UCLA who provided the pCASL sequence.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Canadian Institutes of Health Research (MOP 84378, Banting and Best Scholarship held by C.J. Gauthier), the Canada Foundation for Innovation (Leaders Opportunity Fund 17380), the Ministère du développement économique, de l'innovation et de l'exportation (PSR-SIIRI-239), the Canadian National Sciences and Engineering Research Council (R0018142, RGPIN 2015-04665), the Heart and Stroke Foundation of Canada (New Investigator Award held by C.J. Gauthier) and the Michal and Renata Hornstein Chair in Cardiovascular Imaging (held by C.J. Gauthier).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
Brittany Intzandt provided substantial contribution to this project through data analysis and interpretation; original drafting of manuscript and revised it for essential intellectual content and approved the version to be published. Dalia Sabra provided substantial contribution to this project through data analysis and interpretation; revised it critically for essential intellectual content and approved the version to be published. Catherine Foster provided substantial contribution to this project through data analysis and interpretation; revised it critically for essential intellectual content and approved the version to be published. Laurence Desjardins-Crépeau made a substantial contribution to this project through her acquisition of the data, intellectual feedback and approved the final version to be published. Richard D Hoge had a substantial contribution to the concept and design of this study; provided feedback on interpretation and approved the final version to be published. Christopher J Steele had a substantial contribution to the data analysis and interpretation of data; provided critical intellectual feedback and approved the final version to be published. Louis Bherer made a substantial contribution to the concept and design; interpretation of data; was essential to revisions for intellectual content and approved the version to be published. Claudine J Gauthier made a substantial contribution to all components of this manuscript including: concept and design; acquisition, analysis and interpretation of data; essential to the drafting and intellectual content; and approved the version to be published.
References
- 1.Erecińska M, Silver I. ATP and brain function. J Cereb Blood Flow Metab 1989; 9: 2–19. [DOI] [PubMed] [Google Scholar]
- 2.Knaepen K, Goekint M, Heyman EM, et al. Neuroplasticity – exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med 2010; 40: 765–801. [DOI] [PubMed] [Google Scholar]
- 3.Kraft E. Cognitive function, physical activity, and aging: possible biological links and implications for multimodal interventions. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2012; 19: 248–263. [DOI] [PubMed] [Google Scholar]
- 4.Erickson K, Kramer A. Aerobic exercise effects on cognitive and neural plasticity in older adults. Brit J Sport Med 2009; 43: 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sexton C, Betts J, Demnitz N, et al. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage 2016; 131: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol Aging 2014; 35: S20–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes-Osman J, Cabral DF, Morris TP, et al. Exercise for cognitive brain health in aging: a systematic review for an evaluation of dose. Neurol Clin Pract 2018; 8: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heckman GA, McKelvie RS. Cardiovascular aging and exercise in healthy older adults. Clin J Sport Med 2008; 18: 479–485. [DOI] [PubMed] [Google Scholar]
- 9.Seals DR, Walker AE, Pierce GL, et al. Habitual exercise and vascular ageing. J Physiology 2009; 587: 5541–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashor A, Lara J, Siervo M, et al. Exercise modalities and endothelial function: a systematic review and dose–response meta-analysis of randomized controlled trials. Sports Med 2015; 45: 279–296. [DOI] [PubMed] [Google Scholar]
- 11.Monahan K, Tanaka H, Dinenno F, et al. Central arterial compliance is associated with age- and habitual exercise-related differences in cardiovagal baroreflex sensitivity. Circulation 2001; 104: 1627–1632. [DOI] [PubMed] [Google Scholar]
- 12.Meyer M, Tanaka H, Palta P, et al. Correlates of segmental pulse wave velocity in older adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertension 2015, pp. 29: 114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pugh K, Wei J. Clinical implications of physiological changes in the aging heart. Drugs Aging 2001; 18: 263–276. [DOI] [PubMed] [Google Scholar]
- 14.Zieman S, Melenovsky V, Kass D. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005; 25: 932–943. [DOI] [PubMed] [Google Scholar]
- 15.Saltin B, Strange S. Maximal oxygen uptake: ‘old’ and ‘new’ arguments for a cardiovascular limitation. Med Sci Sports Exerc 1992; 24: 30–37. [PubMed] [Google Scholar]
- 16.Hollenberg M, Ngo LH, Turner D, et al. Treadmill exercise testing in an epidemiologic study of elderly subjects. J Gerontol A Biol Sci Med Sci 1998; 53: B259–267. [DOI] [PubMed] [Google Scholar]
- 17.Huggett DL, Connelly DM, Overend TJ. Maximal aerobic capacity testing of older adults: a critical review. J Gerontol A Biol Sci Med Sci 2005; 60: 57–66. [DOI] [PubMed] [Google Scholar]
- 18.Thomas AG, Dennis A, Bandettini PA, et al. The effects of aerobic activity on brain structure. Front Psychol 2012; 3: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng BY, Uh J, Rossetti HC, et al. Masters athletes exhibit larger regional brain volume and better cognitive performance than sedentary older adults. J Magn Reson Imaging 2013; 38: 1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmerman B, Sutton BP, Low KA, et al. Cardiorespiratory fitness mediates the effects of aging on cerebral blood flow. Front Aging Neurosci 2014; 6: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman SB, Aslan S, Spence JS, et al. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci 2013; 5: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colcombe S, Erickson K, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci 2003; 58: 176–180. [DOI] [PubMed] [Google Scholar]
- 23.Colcombe S, Erickson K, Scalf P, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 2006; 61: 1166–1170. [DOI] [PubMed] [Google Scholar]
- 24.Erickson K, Raji C, Lopez O, et al. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology 2010; 75: 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maass A, Düzel S, Goerke M, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry 2015; 20: 585–593. [DOI] [PubMed] [Google Scholar]
- 26.Tardif C, Steele C, Lampe L, et al. Investigation of the confounding effects of vasculature and metabolism on computational anatomy studies. Neuroimage 2017; 149: 233–243. [DOI] [PubMed] [Google Scholar]
- 27.Tardif C, Gauthier C, Steele C, et al. Advanced MRI techniques to improve our understanding of experience-induced neuroplasticity. Neuroimage 2016; 131: 55–72. [DOI] [PubMed] [Google Scholar]
- 28.Thomas B, Yezhuvath U, Tseng B, et al. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging 2013; 38: 1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarumi T, Gonzales MM, Fallow B, et al. Central artery stiffness, neuropsychological function, and cerebral perfusion in sedentary and endurance-trained middle-aged adults. J Hypertens 2013; 31: 2400–2409. [DOI] [PubMed] [Google Scholar]
- 30.Chapman SB, Aslan S, Spence JS, et al. Distinct brain and behavioral benefits from cognitive vs. physical training: a randomized trial in aging adults. Front Hum Neurosci 2016; 10: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petcharunpaisan S, Ramalho J, Castillo M. Arterial spin labeling in neuroimaging. World J Radiol 2010; 2: 384–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willie C, Tzeng Y, Fisher J, et al. Integrative regulation of human brain blood flow. J Physiol 2014; 592: 841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JJ, Pike BG. MRI measurement of the BOLD-specific flow–volume relationship during hypercapnia and hypocapnia in humans. Neuroimage 2010; 53: 383–391. [DOI] [PubMed] [Google Scholar]
- 34.Gauthier CJ, Madjar C, Desjardins-Crépeau L, et al. Age dependence of hemodynamic response characteristics in human functional magnetic resonance imaging. Neurobiol Aging 2013; 34: 1469–1485. [DOI] [PubMed] [Google Scholar]
- 35.de Vis J, Hendrikse J, Bhogal A, et al. Age-related changes in brain hemodynamics: a calibrated MRI study. Hum Brain Mapp 2015; 36: 3973–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jefferson A, Cambronero F, Liu D, et al. Higher aortic stiffness is related to lower cerebral blood flow and preserved cerebrovascular reactivity in older adults. Circulation 2018; 138: 1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu P, De Vis JB, Lu H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: a technical review. Neuroimage 2019; 187: 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bright MG, Murphy K. Reliable quantification of BOLD fMRI cerebrovascular reactivity despite poor breath-hold performance. Neuroimage 2013; 83: 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue Y, Tanaka Y, Hata H, et al. Arterial spin-labeling evaluation of cerebrovascular reactivity to acetazolamide in healthy subjects. Am J Neuroradiol 2014; 35: 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandell D, Han J, Poublanc J, et al. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in patients with arterial steno-occlusive disease: comparison with arterial spin labeling. Stroke 2008; 39: 2021–2028. [DOI] [PubMed] [Google Scholar]
- 41.Lu H, Xu F, Rodrigue KM, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex 2011; 21: 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krainik A, Hund-Georgiadis M, Zysset S, et al. Regional impairment of cerebrovascular reactivity and BOLD signal in adults after stroke. Stroke 2005; 36: 1146–1152. [DOI] [PubMed] [Google Scholar]
- 43.Hartkamp NS, Hendrikse J, van der Worp HB, et al. Time course of vascular reactivity using repeated phase-contrast MR angiography in patients with carotid artery stenosis. Stroke 2012; 43: 553–556. [DOI] [PubMed] [Google Scholar]
- 44.Bailey DM, Marley CJ, Brugniaux JV, et al. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 2013; 44: 3235–3238. [DOI] [PubMed] [Google Scholar]
- 45.Braz I, Flück D, Lip G, et al. Impact of aerobic fitness on cerebral blood flow and cerebral vascular responsiveness to CO2 in young and older men. Am J Physiol Heart Circ Physiol 2017; 27: 634–642. [DOI] [PubMed] [Google Scholar]
- 46.Barnes JN, Taylor JL, Kluck BN, et al. Cerebrovascular reactivity is associated with maximal aerobic capacity in healthy older adults. J Appl Physiol 2013; 114: 1383–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarumi T, Gonzales M, Fallow B, et al. Cerebral/peripheral vascular reactivity and neurocognition in middle-age athletes. Med Sci Sports Exerc 2015; 47: 2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurlowicz L, Wallace M. The mini-mental state examination (MMSE). J Gerontol Nurs 1999; 25: 8–9. [DOI] [PubMed] [Google Scholar]
- 49.Wu W, Fernández-Seara M, Detre JA, et al. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med 2007; 58: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 50.Gauthier C, Lefort M, Mekary S, et al. Hearts and minds: linking vascular rigidity and aerobic fitness with cognitive aging. Neurobiol Aging 2015; 36: 304–314. [DOI] [PubMed] [Google Scholar]
- 51.Slessarev M, Han J, Mardimae A, et al. Prospective targeting and control of end-tidal CO2 and O2 concentrations. J Physiol 2007; 15: 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Storer T, Davis J, Caiozzo V. Accurate prediction of VO2max in cycle ergometry. Med Sci Sports Exerc 1990; 22: 704–712. [DOI] [PubMed] [Google Scholar]
- 53.Gaser C and Robert D. CAT a computational anatomy toolbox for the analysis of structural MRI data. HBM2016. http://www.neuro.uni-jena.de/hbm2016/GaserHBM2016.pdf. [DOI] [PMC free article] [PubMed]
- 54.Penny W, Friston K, Ashburner J, et al. Statistical parametric mapping: The analysis of functional brain images 2011; 92–100. [Google Scholar]
- 55.Ashburner J, Friston KJ. Voxel-based morphometry – the methods. Neuroimage 2000; 11: 805–821. [DOI] [PubMed] [Google Scholar]
- 56.Avants B, Epstein C, Grossman M, et al. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Analy 2008; 12: 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu T, Wong E. A signal processing model for arterial spin labeling functional MRI. Neuroimage 2005; 24: 207–215. [DOI] [PubMed] [Google Scholar]
- 58.Gauthier CJ, Hoge RD. Magnetic resonance imaging of resting OEF and CMRO2 using a generalized calibration model for hypercapnia and hyperoxia. Neuroimage 2012; 60: 1212–1225. [DOI] [PubMed] [Google Scholar]
- 59.Gauthier CJ, Desjardins-Crépeau L, Madjar C, et al. Absolute quantification of resting oxygen metabolism and metabolic reactivity during functional activation using QUO2 MRI. Neuroimage 2012; 63: 1353–1363. [DOI] [PubMed] [Google Scholar]
- 60.Glover GH. Deconvolution of impulse response in event-related BOLD fMRI1. Neuroimage 1999; 9: 416–429. [DOI] [PubMed] [Google Scholar]
- 61.Chappell M, Groves A, Whitcher B, et al. Variational Bayesian inference for a nonlinear forward model. IEEE Transac Signal Process 2009; 57: 223–236. [Google Scholar]
- 62.Herment A, Kachenoura N, Lefort M, et al. Automated segmentation of the aorta from phase contrast MR images: validation against expert tracing in healthy volunteers and in patients with a dilated aorta. J Magn Reson Imaging 2010; 31: 881–888. [DOI] [PubMed] [Google Scholar]
- 63.Winkler A, Ridgway G, Webster M, et al. Permutation inference for the general linear model. Neuroimage 2014; 92: 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009; 44: 83–98. [DOI] [PubMed] [Google Scholar]
- 65.Ralph BD, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117: 743–753. [DOI] [PubMed] [Google Scholar]
- 66.Farokhian F, Yang C, Beheshti I, Matsuda H, et al. Age-related gray and white matter changes in normal adult brains. Aging Dis 2017; 8: 899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maillet D, Rajah NM. Association between prefrontal activity and volume change in prefrontal and medial temporal lobes in aging and dementia: a review. Ageing Res Rev 2013; 12: 479–489. [DOI] [PubMed] [Google Scholar]
- 68.Flück D, Braz I, Keiser S, et al. Age, aerobic fitness, and cerebral perfusion during exercise: role of carbon dioxide. Am J Physiol Heart Circ Physiol 2014; 307: H515–H523. [DOI] [PubMed] [Google Scholar]
- 69.Catchlove SJ, Parrish TB, Chen Y, et al. Regional cerebrovascular reactivity and cognitive performance in healthy aging. J Exp Neurosci 2018; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng S-L, Chen X, Li Y, et al. Age-related changes in cerebrovascular reactivity and their relationship to cognition: a four-year longitudinal study. Neuroimage 2018; 174: 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng G, Ye B, Zheng Y, et al. The effects of exercise on the structure of cognitive related brain regions: a meta-analysis of functional neuroimaging data. Int J Neurosci 2019; 129: 406–415. [DOI] [PubMed] [Google Scholar]
- 72.Kaminsky LA, Imboden MT, Arena R, et al. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing using cycle ergometry: data from the fitness registry and the importance of exercise national database (FRIEND) registry. Mayo Clin Proc 2017; 92: 228–233. [DOI] [PubMed] [Google Scholar]
- 73.Thambisetty M, Lori B-H, An Y, et al. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol 2010; 67: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scarmeas N, Habeck CG, Stern Y, et al. APOE genotype and cerebral blood flow in healthy young individuals. JAMA 2003; 290: 1581–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wierenga CE, Clark LR, Dev SI, et al. Interaction of age and APOE genotype on cerebral blood flow at rest. J Alzheimers Dis 2013; 34: 921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Halani S, Kwinta JB, Golestani AM, et al. Comparing cerebrovascular reactivity measured using BOLD and cerebral blood flow MRI: the effect of basal vascular tension on vasodilatory and vasoconstrictive reactivity. Neuroimage 2015; 110: 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Katayama K, Sato Y, Morotome Y, et al. Ventilatory chemosensitive adaptations to intermittent hypoxic exposure with endurance training and detraining. J Appl Physiol 1999; 86: 1805–1811. [DOI] [PubMed] [Google Scholar]
- 78.McConnell A, Semple E. Ventilatory sensitivity to carbon dioxide: the influence of exercise and athleticism. Med Sci Sports Exerc 1996; 28: 685–691. [DOI] [PubMed] [Google Scholar]
- 79.Haas J, Brigitte S-H, Biessmann A, et al. Inducible nitric oxide synthase and argininosuccinate synthetase: co-induction in brain tissue of patients with Alzheimer's dementia and following stimulation with beta-amyloid 1-42 in vitro. Neurosci Lett 2002; 322: 121–125. [DOI] [PubMed] [Google Scholar]
- 80.White R, Vallance P, Markus H. Effect of inhibition of nitric oxide synthase on dynamic cerebral autoregulation in humans. Clin Sci 2000; 99: 555–560. [PubMed] [Google Scholar]
- 81.Malenfant S, Brassard P, Paquette M, et al. Compromised cerebrovascular regulation and cerebral oxygenation in pulmonary arterial hypertension. J Am Heart Assoc 2017; 6: e006126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ogoh S, Dalsgaard MK, Yoshiga CC, et al. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Heart Circ Physiol 2005; 288: H1461–H1467. [DOI] [PubMed] [Google Scholar]
- 83.Mikkel L-H, Cotter JD, Helge JWW, et al. Cerebral autoregulation dynamics in endurance-trained individuals. J Appl Physiol 2011; 110: 1327–1333. [DOI] [PubMed] [Google Scholar]
- 84.Bhogal AA, Vis JB, Siero J, et al. The BOLD cerebrovascular reactivity response to progressive hypercapnia in young and elderly. Neuroimage 2016; 139: 94–102. [DOI] [PubMed] [Google Scholar]
- 85.Mcketton L, Sobczyk O, Duffin J, et al. The aging brain and cerebrovascular reactivity. Neuroimage 2018; 181: 132–141. [DOI] [PubMed] [Google Scholar]
- 86.Leoni RF, Oliveira IAF, Pontes-Neto O, et al. Cerebral blood flow and vasoreactivity in aging: an arterial spin labeling study. Braz J Med Biol Res 2017; 50: e5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aslan S, Xu F, Wang PL, Uh J, et al. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn Reson Med 2010; 63: 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bassett DR, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 2000; 32: 70–84. [DOI] [PubMed] [Google Scholar]