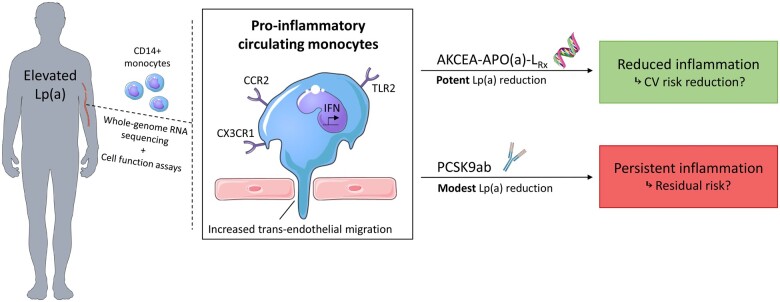
Abstract
Aims
Elevated lipoprotein(a) [Lp(a)] is strongly associated with an increased cardiovascular disease (CVD) risk. We previously reported that pro-inflammatory activation of circulating monocytes is a potential mechanism by which Lp(a) mediates CVD. Since potent Lp(a)-lowering therapies are emerging, it is of interest whether patients with elevated Lp(a) experience beneficial anti-inflammatory effects following large reductions in Lp(a).
Methods and results
Using transcriptome analysis, we show that circulating monocytes of healthy individuals with elevated Lp(a), as well as CVD patients with increased Lp(a) levels, both have a pro-inflammatory gene expression profile. The effect of Lp(a)-lowering on gene expression and function of monocytes was addressed in two local sub-studies, including 14 CVD patients with elevated Lp(a) who received apolipoprotein(a) [apo(a)] antisense (AKCEA-APO(a)-LRx) (NCT03070782), as well as 18 patients with elevated Lp(a) who received proprotein convertase subtilisin/kexin type 9 antibody (PCSK9ab) treatment (NCT02729025). AKCEA-APO(a)-LRx lowered Lp(a) by 47% and reduced the pro-inflammatory gene expression in monocytes of CVD patients with elevated Lp(a), which coincided with a functional reduction in transendothelial migration capacity of monocytes ex vivo (−17%, P < 0.001). In contrast, PCSK9ab treatment lowered Lp(a) by 16% and did not alter transcriptome nor functional properties of monocytes, despite an additional reduction of 65% in low-density lipoprotein cholesterol (LDL-C).
Conclusion
Potent Lp(a)-lowering following AKCEA-APO(a)-LRx, but not modest Lp(a)-lowering combined with LDL-C reduction following PCSK9ab treatment, reduced the pro-inflammatory state of circulating monocytes in patients with elevated Lp(a). These ex vivo data support a beneficial effect of large Lp(a) reductions in patients with elevated Lp(a).
Keywords: Lipoprotein(a), Apo(a)-antisense, PCSK9ab, Inflammation, Monocytes, Transcriptomics
See page 2272 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa420)
Introduction
Lipoprotein(a) [Lp(a)] is a lipoprotein consisting of a low-density lipoprotein (LDL)-like particle, covalently bound to apolipoprotein(a) [apo(a)] and is associated with cardiovascular disease (CVD) risk.1 Epidemiological and Mendelian randomization studies suggest an independent and causal relationship between elevated Lp(a) levels (above 50 mg/dL or ∼125 nmol/L) and CVD risk.2 , 3 Previously, we demonstrated that healthy subjects with elevated Lp(a) have an activated innate immune system, characterized by pro-inflammatory circulating monocytes. These findings coincided with an increased white blood cell influx in the arterial wall and a concomitant increase in arterial wall inflammation assessed by positron emission tomography/computed tomography (PET/CT).4 We identified oxidized phospholipids (OxPL), predominantly carried by Lp(a) in the plasma, as key intermediates in inducing the pro-inflammatory activation of monocytes.4 However, the mechanisms underlying monocyte activation in individuals with elevated Lp(a), as well as reversibility of this inflammatory state remains to be established.
Recently, we reported that a 14% reduction in Lp(a) following proprotein convertase subtilisin/kexin type 9 antibody (PCSK9ab) was not associated with a reduction of arterial wall inflammation on PET/CT in subjects with elevated Lp(a).5 The absence of an anti-inflammatory effect of modest Lp(a)-lowering concurs with negative results of modest Lp(a) reduction on CVD risk in both randomized clinical trials6 , 7 and Mendelian randomization studies.8 In the advent of antisense strategies targeting apo(a), with the potential to reduce Lp(a) by more than 90%,9 it provides an opportunity to test whether a greater absolute reduction in Lp(a) exerts a favourable biological effect in patients with elevated Lp(a). We hypothesize that potent Lp(a)-lowering conveys an anti-inflammatory effect as opposed to moderate Lp(a)-reducing strategies.
To test this hypothesis, we assessed the gene expression profile of circulating monocytes of healthy individuals and CVD patients, both with elevated Lp(a). Reversibility of an Lp(a) effect on monocyte activation was evaluated by comparing monocyte gene expression and function before and after treatment with either potent Lp(a)-lowering [antisense (AKCEA-APO(a)-LRx)] or moderate Lp(a)-lowering [PCSK9ab (evolocumab)] in two separate clinical intervention trials (NCT03070782 and NCT02729025, respectively).
Methods and materials
Study population and design
This monocentre study comprises four study groups in total: healthy individuals with normal Lp(a), healthy individuals with elevated Lp(a), and two groups of patients with elevated Lp(a) who participated in the AKCEA-APO(a)-LRx study (NCT03070782) and the ANITSCHKOW study (NCT02729025). An Lp(a) plasma level of ≥50 mg/dL or 125 nmol/L was defined as elevated. The healthy individuals with elevated Lp(a) were matched for age, sex, and body mass index (BMI) to healthy individuals with normal Lp(a). Inclusion and exclusion criteria and study design of the AKCEA-APO(a)-LRx and the ANITSCHKOW study have been published previously.5 , 10 In these intervention trials, blood sampling for the purpose of this monocyte study was performed at baseline and after 26 weeks of AKCEA-APO(a)-LRx or 16 weeks of PCSK9ab treatment, respectively. The protocol for the current sub-study was approved by the ethics committee of the Amsterdam UMC (location Academic Medical Center) and was conducted according to the principles of the Declaration of Helsinki. All participants provided written informed consent prior to enrolment.
Biochemical measurements
Blood sampling was obtained in a fasting state (≥9 h). The lipid profiles of the healthy individuals were measured in the local clinical laboratory. Plasma total cholesterol, high-density lipoprotein cholesterol, triglycerides, and apolipoprotein B (apoB) were measured with commercially available enzymatic assays. Low-density lipoprotein cholesterol was calculated using the Friedewald formula. Lipoprotein(a) was measured by an isoform-independent immunoturbidometric assay (QUANTIA Lp(a) 7K00-01, Abbott, Wiesbaden, Germany). In the AKCEA-APO(a)-LRx study, lipid profiles were measured with commercially available kits at Medpace Reference Laboratories (Medpace Reference Laboratories, Leuven, Belgium), and Lp(a) levels were measured by an isoform-independent assay (Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, USA). In the ANITSCHKOW study, lipid profiles were assessed at the Medpace core lab (Medpace Reference Laboratories, Leuven, Belgium), and Lp(a) levels were measured using an isoform-independent immunoturbidometric assay (Polymedco, Cortlandt Manor, NY, USA).
Ex vivo monocyte experiments
All the laboratory experiments and statistical analyses regarding the monocyte characterization are available in detail in the Supplementary material online.
Statistical analyses
All data were analysed using GraphPad Prism 8 (La Jolla, CA, USA), SPSS version 25 (SPSS Inc., Chicago, IL, USA), and R version 3.5.3 (R Core Team, Vienna, Austria). Data are presented as mean ± standard deviation (SD) for normally distributed data, median [interquartile range (IQR)] for non-normally distributed data, or as a number (n) with percentage from total (%) for categorical variables. Since this study was not specifically designed to evaluate the effects of both AKCEA-APO(a)-LRx and PCSK9ab on biochemical measurements, P-values are not provided for the differences in lipid and inflammatory plasma markers but are stated as mean (SD), or median (IQR), absolute and/or percent change from baseline at Week 26 or 16, respectively.
Results
Clinical characteristics
Thirteen healthy individuals with normal Lp(a) [median Lp(a) 7 mg/dL (18 nmol/L)] and 12 age-, sex-, and BMI-matched healthy individuals with elevated Lp(a) [median Lp(a) 87 mg/dL (218 nmol/L)] were included (Table 1, Supplementary material online, Table S1). In the phase-2b AKCEA-APO(a)-LRx-trial, informed consent was obtained in 14 sequential patients who were randomized at the Amsterdam UMC site and were included in this site-specific sub-study (Table 1). According to study protocol, all patients had established CVD, in the vast majority based on coronary artery disease (86%). All patients received standard-of-care preventive therapy, including lipid-lowering therapy (86% statin therapy, 64% ezetimibe, and 14% PCSK9ab). Median Lp(a) at baseline was 82 mg/dL (205 nmol/L), and mean LDL-C 1.9 mmol/L. In the phase-3b ANITSCHKOW trial, informed consent was obtained in 18 patients who were randomized at the Amsterdam UMC site to evolocumab 420 mg once every 4 weeks (Q4W) and were included in this sub-study (Table 1). Four patients had established CVD based on coronary artery disease (three subjects) and stroke (one subject). Nine patients received lipid-lowering therapy in primary prevention setting, of which five patients had the diagnosis of familial hypercholesterolaemia. Seventy-two percent of the subjects used statins at baseline, and 22% ezetimibe. Median Lp(a) at baseline was 102 mg/dL (255 nmol/L), with a mean LDL-C of 3.3 mmol/L.
Table 1.
Baseline characteristics in healthy control and patient cohorts
| Healthy individuals normal Lp(a) (n = 13) | Healthy individuals elevated Lp(a) (n = 12) | AKCEA-APO(a)-LRx subjects elevated Lp(a) (n = 14) | PCSK9ab subjects elevated Lp(a) (n = 18) | |
|---|---|---|---|---|
| Age (years) | 44.4 (16.8) | 44.3 (13.1) | 53.0 (7.5) | 60.6 (7.4) |
| Sex male, n (%) | 6 (46) | 6 (50) | 12 (86) | 9 (50) |
| BMI (kg/m2) | 23.6 (2.8) | 25.4 (3.3) | 29.0 (5.4) | 25.8 (3.3) |
| Smoking active, n (%) | 0 (0) | 0 (0) | 1 (7) | 2 (11) |
| SBP (mmHg) | 122 (16) | 135 (15) | 132 (15) | 135 (16) |
| DBP (mmHg) | 79 (10) | 83 (7) | 82 (7) | 82 (9) |
| CVD, n (%) | 0 (0) | 0 (0) | 14 (100) | 4 (22) |
| Coronary artery disease | 0 (0) | 0 (0) | 12 (86) | 3 (17) |
| Stroke | 0 (0) | 0 (0) | 1 (7) | 1 (6) |
| Peripheral artery disease | 0 (0) | 0 (0) | 1 (7) | 0 (0) |
| Medication use, n (%) | 0 (0) | 0 (0) | 14 (100) | 14 (78) |
| Antihypertensives | 0 (0) | 0 (0) | 11 (79) | 7 (39) |
| Antidiabetics | 0 (0) | 0 (0) | 1 (7) | 0 (0) |
| Statins | 0 (0) | 0 (0) | 12 (86) | 13 (72) |
| Ezetimibe | 0 (0) | 0 (0) | 9 (64) | 4 (22) |
| PCSK9ab | 0 (0) | 0 (0) | 2 (14) | 0 (0) |
| Total cholesterol (mmol/L)a | 5.1 (0.9) | 5.5 (0.8) | 3.8 (0.7) | 5.4 (0.9) |
| LDL-cholesterol (mmol/L)a | 3.0 (0.8) | 3.4 (0.8) | 1.9 (0.6) | 3.3 (0.7) |
| HDL-cholesterol (mmol/L)a | 1.8 (0.4) | 1.6 (0.4) | 1.2 (0.3) | 1.4 (0.4) |
| Triglycerides (mmol/L)b | 0.8 (0.3) | 1.1 (0.5) | 1.3 (0.5) | 1.4 (0.3) |
| ApoB (g/L) | 0.9 (0.2) | 1.0 (0.2) | 0.8 (0.2) | 1.0 (0.1) |
| Lipoprotein(a) (mg/dL)c | 7 (3–17) | 87 (79–114) | 82 (62–121) | 102 (64–121) |
| Leucocytes (109/L) | 5.09 (1.41) | 5.55 (0.86) | 6.60 (2.19) | 5.66 (1.63) |
| Neutrophils (109/L) | 2.56 (1.25) | 3.00 (0.44) | 3.81 (1.44) | 3.38 (1.20) |
| Lymphocytes (109/L) | 1.92 (0.60) | 1.91 (0.48) | 1.96 (0.65) | 1.66 (0.39) |
| Monocytes (109/L) | 0.39 (0.13) | 0.43 (0.12) | 0.52 (0.187 | 0.40 (0.15) |
| hs-CRP (mg/L) | 0.5 (0.3–1.8) | 1.0 (0.5–1.3) | 0.5 (0.4–2.3) | 0.9 (0.5–1.3) |
Data are represented as mean (SD), median (interquartile range), or n (%).
ApoB, apolipoprotein B; BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); PCSK9ab, proprotein convertase subtilisin/kexin type 9 antibody; SBP, systolic blood pressure.
To convert to mg/dL, multiply by 38.7.
To convert to mg/dL, multiply by 88.6.
To convert to nmol/L, multiply by 2.5.
Effect of AKCEA-APO(a)-LRx and PCSK9ab treatment on lipid levels and inflammatory plasma markers
The 14 CVD patients participating in the AKCEA-APO(a)-LRx trial were randomized to one of the five dose regimens with the investigational product AKCEA-APO(a)-LRx [three subjects (21%) in 20 mg/Q4W group, two subjects (14%) in 40 mg/Q4W group, two subjects (14%) in 20 mg/Q2W group, four subjects (29%) in 60 mg/Q4W group, and three subjects (21%) in 20 mg/QW group]. Compared to baseline, a pooled mean absolute reduction of 51 (53) mg/dL [or 128 (132) nmol/L] and a pooled mean percent reduction of 47 (18) % was achieved after a treatment duration of 26 weeks (Table 2). Minute reductions in apoB and LDL-C were seen [mean percent change of −6.7% (14.4), and −7.1% (20.4), respectively] after AKCEA-APO(a)-LRx treatment, as well as small absolute changes in C-reactive protein (CRP) [−0.1 (−0.2 to 0.5) mg/L] and monocyte count [−0.02 (0.07) *109/L] (Table 2). These results were generally comparable with the main phase-2b trial.10
Table 2.
Effect of AKCEA-APO(a)-LRx and PCSK9ab treatment on lipid levels and inflammatory plasma markers
| AKCEA- APO(a)-LRx | PCSK9ab | |
|---|---|---|
| Lipoprotein(a) | ||
| Mean absolute change Lp(a) (mg/dL), (SD)a | −50.6 (52.6) | −18.9 (19.5) |
| Mean percent change Lp(a) (%), (SD) | −46.6 (18.3) | −16.1 (18.7) |
| Median post-treatment Lp(a) (mg/dL), (IQR)a | 35.0 (26.1–84.6) | 83.3 (59.1–105.4) |
| Change in other lipid levelsb | ||
| Total cholesterol | −2.2 (13.4) | −40.5 (8.9) |
| LDL-cholesterol | −7.1 (20.4) | −64.5 (14.4) |
| HDL-cholesterol | 8.8 (13.8) | 11.2 (10.6) |
| Triglycerides | −9.3 (−14.4) | −29.7 (14.1) |
| ApoB | −6.7 (14.4) | −53.1 (9.8) |
| Change in inflammatory markersc | ||
| hs-CRP (mg/L) | −0.1 (−0.2 to 0.5) | −0.2 (−1.3 to 0.5) |
| Leucocytes (*109/L) | −0.19 (1.35) | −0.17 (1.05) |
| Monocytes (*109/L) | −0.02 (0.07) | −0.00 (0.15) |
ApoB, apolipoprotein B; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); PCSK9ab, proprotein convertase subtilisin/kexin type 9 antibody.
To convert to nmol/L, multiply by 2.5.
Change in other lipid levels is defined as mean percent change (SD) from baseline.
Change in inflammatory markers is defined as median (IQR) or mean absolute change (SD) from baseline for non-normally and normally distributed data, respectively.
The 18 subjects participating in the ANITSCHKOW trial received monthly subcutaneous injections of evolocumab 420 mg, reaching a mean absolute reduction in Lp(a) of 19 (20) mg/dL [or 48 (49) nmol/L] and a mean percent reduction of 16 (19) % (Table 2) after 16 weeks of treatment. Low-density lipoprotein cholesterol was reduced by 65 (14) %. Almost no effect on both CRP and monocyte count was seen [−0.2 (−1.3 to 0.5) mg/L, and −0.00 (0.15) *109/L, respectively].
Inflammatory gene expression profile in monocytes of healthy individuals with elevated lipoprotein(a)
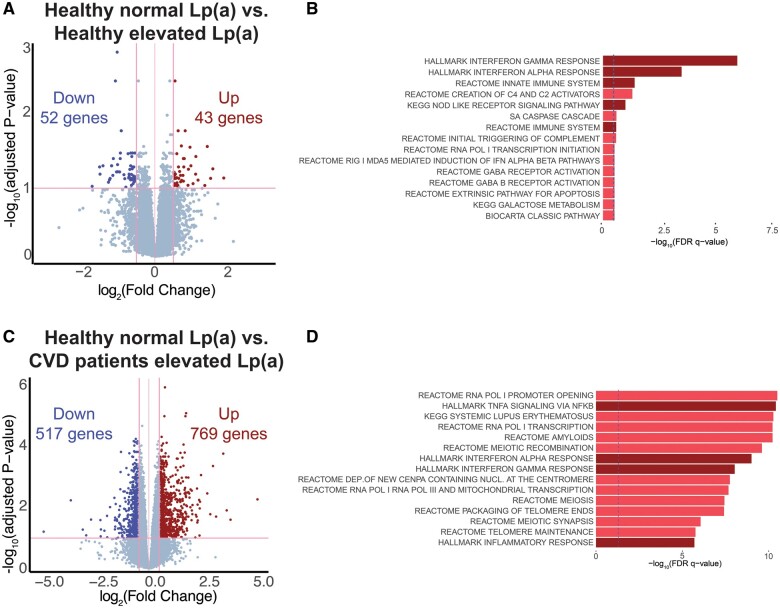
To get more insight into the molecular pathways underlying monocyte activation in subjects with elevated Lp(a), we first compared the gene expression profile of circulating monocytes from healthy individuals with normal Lp(a) and healthy individuals with elevated Lp(a). Transcriptome analysis revealed 95 significantly differentially expressed genes (DEGs), of which 43 genes were up-regulated and 52 genes were down-regulated in the healthy individuals with elevated Lp(a) (Figure 1A). Canonical pathway and Hallmark (CP&H) pathway enrichment analysis showed a significant increase in several pathways related to the innate immune response (Figure 1B). The interferon alpha (IFNα) and interferon gamma (IFNγ) pathways were the most pronounced amongst the significantly up-regulated pathways.
Figure 1.
Inflammatory gene expression in circulating monocytes of healthy individuals with elevated Lp(a) and cardiovascular disease patients with elevated Lp(a). (A) Volcano plot showing the difference in gene expression between healthy individuals with normal Lp(a) vs. healthy individuals with elevated Lp(a). (B) Canonical and Hallmark pathway analysis of top 1000 up-regulated genes. Dark red bars indicate inflammatory pathways. Blue dotted line at P = 0.05. (C) Volcano plot showing the difference in gene expression between healthy individuals with normal Lp(a) vs. cardiovascular disease patients with elevated Lp(a). (D) Canonical and Hallmark pathway analysis of top 1000 up-regulated genes. Dark red bars indicate inflammatory pathways. Blue dotted line at P = 0.05.
Monocytes of cardiovascular disease patients with elevated lipoprotein(a) show a robust pro-inflammatory transcriptome profile
Next, we compared the gene expression profile of monocytes of CVD patients with elevated Lp(a) and healthy individuals with normal Lp(a). Whereas healthy individuals with elevated Lp(a) show a modest number of DEG (95), CVD patients with Lp(a) elevation display a larger number of DEG (1286), of which 769 genes are significantly up-regulated (Figure 1C). CP&H pathway enrichment analysis revealed a pronounced up-regulation of multiple immune response-related pathways including the TNFA signalling pathway and, similar to the transcriptome profile of healthy individuals with Lp(a) elevation, IFNα, and IFNγ response pathways (Figure 1D). Collectively, this data implies that elevated Lp(a) contributes to a pro-inflammatory gene expression signature in monocytes of both healthy individuals and CVD patients.
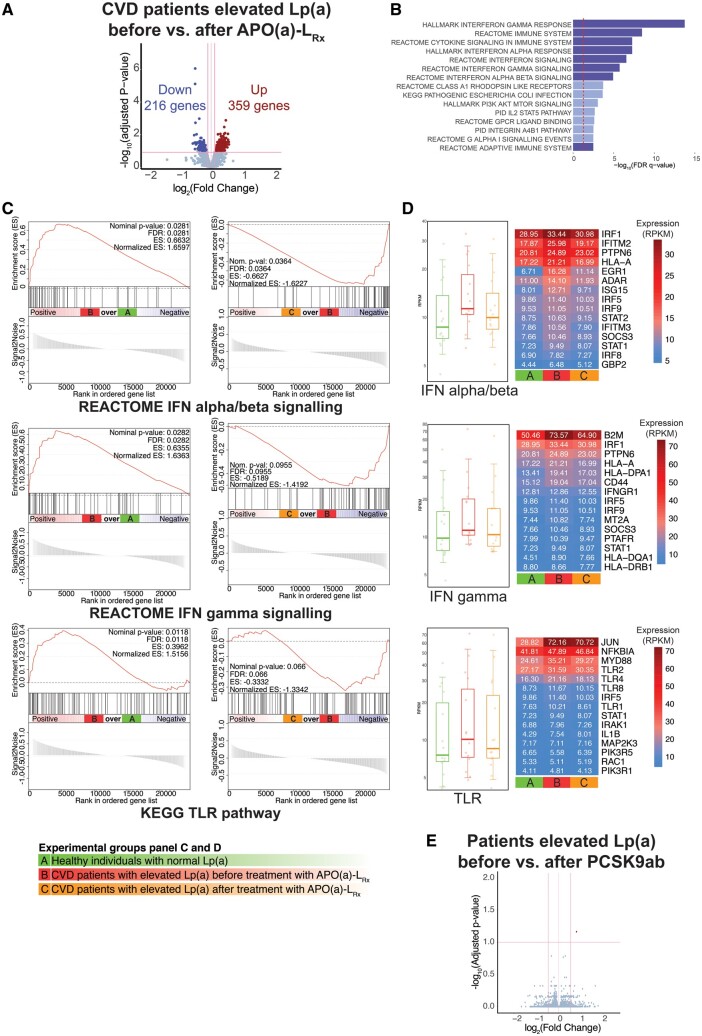
Potent Lp(a)-lowering by AKCEA-APO(a)-LRx leads to down-regulation of inflammatory gene expression in monocytes of cardiovascular disease patients with elevated lipoprotein(a)
Subsequently, we investigated the effect of potent Lp(a)-lowering by AKCEA-APO(a)-LRx on the gene expression profile in monocytes of CVD patients with elevated Lp(a) in a paired fashion. Compared to baseline, transcriptome analysis showed 575 significantly DEG, of which 359 genes were up-regulated, and 216 genes were down-regulated following AKCEA-APO(a)-LRx treatment (Figure 2A). CP&H pathway enrichment analysis demonstrated a distinct reduction of multiple pathways regulating the immune response in monocytes, including IFNα, IFNγ, and Toll-like receptor (TLR) pathways (Figure 2B). In line with these findings, gene set enrichment analysis showed significant enrichment of genes involved in IFNα/β [normalized enrichment scores (NES) = 1.7, false discovery rate (FDR) 0.03], IFNγ (NES = 1.6, FDR = 0.03), and the TLR signalling pathway (NES = 1.5, FDR = 0.01) in CVD patients with elevated Lp(a) compared to healthy individuals with normal Lp(a) (Figure 2C), which were reduced after AKCEA-APO(a)-LRx treatment (IFNα/β: NES = −1.6, FDR = 0.04; IFNγ: NES = −1.4, FDR = 0.1; TLR: NES = −1.3, FDR = 0.07) (Figure 2C). Several genes of the Interferon Regulatory Factor (IRF) family, including IRF1, IFITM2, and GBP2 are amongst the top 15 most significantly up-regulated genes in the IFN α/β and γ signalling pathways in CVD patients, which were down-regulated after AKCEA-APO(a)-LRx treatment (Figure 2D, Supplementary material online, Figure S1A). JUN, MYD88, TLR2, TLR4, and TLR8 were amongst the top 15 most up-regulated genes in the TLR pathway in CVD patients before AKCEA-APO(a)-LRx and were down-regulated after AKCEA-APO(a)-LRx treatment (Figure 2D, Supplementary material online, Figure S1B). In contrast, modest Lp(a)-lowering following PCSK9ab did not alter gene expression in monocytes of subjects with elevated Lp(a), despite a concomitant robust LDL-C reduction (Figure 2E).
Figure 2.
Potent, but not modest, Lp(a)-lowering reduces inflammatory gene expression in circulating monocytes. (A) Volcano plot showing the difference in gene expression between cardiovascular disease patients before AKCEA-APO(a)-LRx treatment vs. cardiovascular disease patients after AKCEA-APO(a)-LRx treatment. (B) Canonical and Hallmark pathway analysis of top 1000 down-regulated genes. Dark blue bars indicate inflammatory pathways. Red dotted line at P = 0.05. (C) Gene set enrichment analysis enrichment plots of interferon alpha/beta (up), interferon gamma (middle), and TLR pathway (bottom). Left: cardiovascular disease patients before AKCEA-APO(a)-LRx (red) vs. healthy individuals with normal Lp(a) (green). Right: cardiovascular disease patients before AKCEA-APO(a)-LRx (red) vs. after AKCEA-APO(a)-LRx (orange). (D) Boxplot and heatmap showing average expression of the top 15 most expressed genes in cardiovascular disease patients before AKCEA-APO(a)-LRx vs. healthy individuals with normal Lp(a) for the interferon alpha/beta signalling, gamma signalling, and TLR pathways. Column colours: green, healthy individuals with normal Lp(a), red, cardiovascular disease patients before AKCEA-APO(a)-LRx treatment, orange: cardiovascular disease patients after AKCEA-APO(a)-LRx treatment. (E) Volcano plot showing the difference in gene expression between patients before PCSK9ab treatment vs. patients after PCSK9ab treatment.
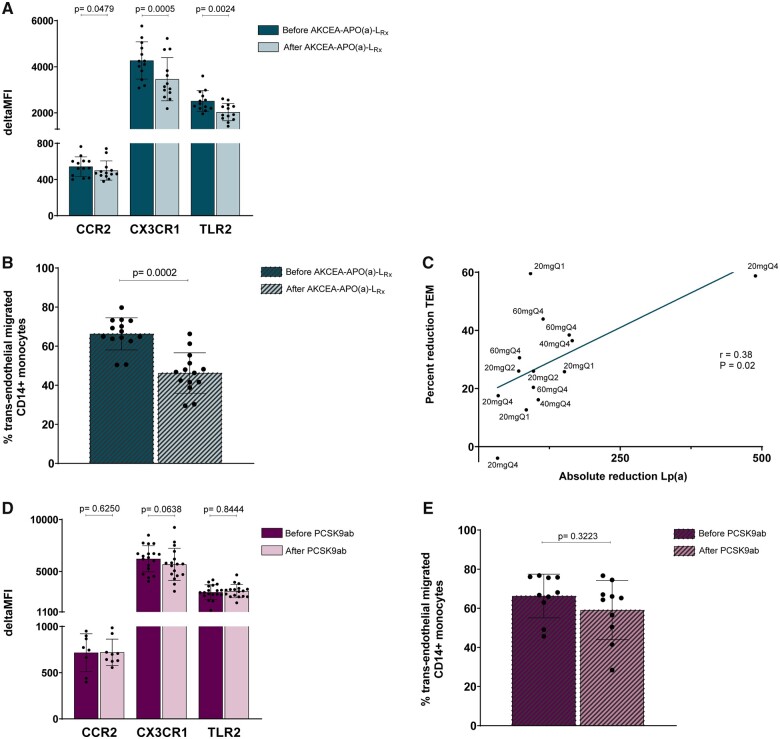
Down-regulation of inflammatory gene expression following potent lipoprotein(a)-lowering coincides with reduced inflammatory monocyte function
To examine whether the relatively modest effect sizes in pro-inflammatory gene expression after potent Lp(a)-lowering resulted in dampening of pro-inflammatory monocyte function, we performed flow cytometry experiments to functionally assess the expression of chemokine and TLRs on the monocyte cell surface. In line with the down-regulation of their respective genes, the expression of C-C chemokine receptor type 2 (CCR2), CX3C chemokine receptor 1 (CX3CR1), and TLR2 was significantly reduced after Lp(a)-lowering (Figure 3A, Supplementary material online, Figures S1C and S2). Furthermore, we performed a standardized transendothelial migration (TEM) assay to assess the migratory capacity of monocytes through a layer of arterial endothelial cells ex vivo. In accordance with the reduction in inflammatory signature in the transcriptome and flow cytometry data, the trans-endothelial migration activity of monocytes was significantly reduced by 22% (P < 0.0001) after potent Lp(a)-lowering (Figure 3B). The percent reduction in TEM was positively correlated with the absolute reduction in Lp(a) (r = 0.38, P = 0.02) (Figure 3C). A trend towards a correlation was observed between percent reduction in TEM and absolute reduction in OxPL-apoB (r = 0.26, P = 0.07) (Supplementary material online, Figure S3). We performed the same flow cytometry and TEM experiments in the ANITSCHKOW subjects, and both experiments did not show significant differences before vs. after PCSK9ab treatment (Figure 3D and E).
Figure 3.
Potent, but not modest, Lp(a)-lowering reduces inflammatory receptor expression, with a concomitant functional improvement. (A) Flow cytometry results of inflammatory markers on circulating monocytes before and after AKCEA-APO(a)-LRx treatment, expressed as delta Median Fluorescence Intensity (MFI). Data are represented as mean ± standard deviation. (B) Percentage trans-endothelial migrated CD14+ monocytes before and after AKCEA-APO(a)-LRx treatment. Data are represented as mean ± standard deviation. (C) Correlation between absolute reduction in Lp(a) and percent reduction in trans-endothelial migration. (D) Flow cytometry results of inflammatory markers on circulating monocytes before and after PCSK9ab treatment, expressed as delta MFI. Data are represented as mean ± standard deviation. (E) Percentage trans-endothelial migrated CD14+ monocytes before and after PCSK9ab treatment. Data are represented as mean ± standard deviation and were analysed by Wilcoxon signed rank test (P-values < 0.05 were considered statistically significant).
Discussion
Using transcriptome analysis, we show that elevated Lp(a) promotes a distinct pro-inflammatory gene expression profile in circulating monocytes, which is more pronounced in patients with established CVD compared to healthy individuals. We also show that potent Lp(a)-lowering by the apo(a) antisense AKCEA-APO(a)-LRx markedly attenuates the inflammatory changes on transcriptional as well as functional level in monocytes of CVD patients with elevated Lp(a). In contrast, modest Lp(a)-lowering combined with robust LDL-C lowering following PCSK9ab had no effect on the gene expression profile nor on function of monocytes. Collectively, this data lends further support to Lp(a)-mediated pro-inflammatory effects on the innate immune system, which is reversible only following large reductions in Lp(a) (Take home figure).
Take home figure.
Unbiased whole-genome RNA sequencing and functional analyses of circulating monocytes of individuals with elevated Lp(a) show a strong pro-inflammatory and pro-migratory profile. Only potent Lp(a) reduction demonstrated a profound reduction in the reported pro-inflammatory and pro-migratory profile, as opposed to modest Lp(a) reduction, indicating the promising potential of potent Lp(a)-lowering strategies in reducing cardiovascular risk.
As pointed out, we observed a marked, reversible pro-inflammatory Lp(a) signature in circulating monocytes, but we made several other key observations as well. First, we found a more pronounced inflammatory effect in CVD patients, which likely pertains to the fact that CVD patients are generally hallmarked by multiple cardiovascular (CV) risk factors, potentiating the pro-inflammatory gene expression signature.11 Equally important is the observation that these CVD patients with elevated Lp(a) are still characterized by an inflammatory profile of monocytes, despite the use of medication comprising statins and platelet inhibition, both with documented anti-inflammatory effects.12 , 13 Yet, potent and highly selective Lp(a)-lowering by AKCEA-APO(a)-LRx reduced the augmented inflammatory gene expression in circulating monocytes of CVD patients with elevated Lp(a). This underscores a strong contributory inflammatory effect mediated by Lp(a), that seems to be independent from other risk factors.14 Although the effect size on inflammatory gene expression was modest after Lp(a) lowering, it was associated with a marked reduction in TEM, a functional property of circulating monocytes regulated by inflammatory stimuli. Hence, prolonged and more potent absolute reductions of Lp(a) using optimally dosed apo(a)-antisense is expected to be associated with a more robust anti-inflammatory effect. A potent effect of Lp(a) on monocyte activation is substantiated by the absence of any change in monocyte gene expression or monocyte migratory capacity following PCSK9ab in patients with Lp(a) elevation. The latter concurs with our previous observation that PCSK9ab did not attenuate arterial wall inflammation in subjects with elevated Lp(a), in whom post-treatment Lp(a) levels were still elevated.5 Taken together, these results support a causal role of Lp(a) in circulating monocyte activation in vivo.
The mechanisms by which Lp(a) elicits inflammation have remained incompletely understood. The discordance of a distinct anti-inflammatory effect of AKCEA-APO(a)-LRx, and the lack of this effect following PCSK9ab treatment, suggests that Lp(a) and LDL-C mediate monocyte activation via independent pathways. We previously reported that intracellular accumulation of LDL-C in circulating monocytes of patients with severe hypercholesterolaemia is associated with monocyte activation.15 Accordingly, LDL-C lowering following PCSK9ab reversed the observed pro-inflammatory activation of circulating monocytes in these patients, which coincided with less intracellular lipid accumulation. Lipoprotein(a), on the other hand, is on an equimolar basis more atherogenic than LDL-C, which is attributed to its additional apo(a)-tail and OxPL content.16 Lipoprotein(a)-carried OxPL, has been identified as a critical signalling source for pro-inflammatory monocyte activation in subjects with elevated Lp(a).4 OxPL are a signalling source for danger-associated molecular pattern-mediated monocyte activation via TLR recognition,17 , 18 which is in line with our observation that genes involved in the TLR pathway are up-regulated at baseline in CVD patients with elevated Lp(a) and are down-regulated after Lp(a) lowering by AKCEA-APO(a)-LRx. Also, the observed trend in a positive correlation between reduction in TEM and reduction in OxPL-apoB levels suggests a role of OxPL in monocyte activation. Another intriguing finding in this study is the distinct up-regulation of the IFNα/β and IFNγ pathways in both healthy subjects with elevated Lp(a) as well as CVD patients with elevated Lp(a). Since IFNγ in vivo is predominantly produced by natural killer cells and T-lymphocytes rather than monocytes themselves,19 this finding could imply that other immune cells such as lymphocytes may act as intermediates in Lp(a)-induced monocyte activation in humans.
Clinical implications
The advent of potent Lp(a)-lowering strategies has ignited the debate whether Lp(a)-lowering strategies are capable of lowering CVD risk. In contrast to the linear relationship between LDL-C reduction and CV benefit, the mandatory Lp(a) changes potentially mediating CVD risk reduction remain to be established. Mendelian randomization studies8 suggest that absolute reductions as high as 100 mg/dL may be required in order to achieve clinically relevant CV risk reductions. In support, randomized controlled trials with moderate Lp(a)-lowering compounds (percent reduction ranging from 20% to 25% following nicotinic acid derivates and cholesteryl ester transfer protein (CETP)-inhibitors, respectively6 , 7 , 20) failed to convey CV benefit that can be attributed to Lp(a) reduction. In this ex vivo study, we showed a marked anti-inflammatory effect on circulating monocytes only after potent Lp(a)-lowering strategies in patients, in absence of any change in inflammatory profile following moderate Lp(a)-lowering. In accordance with the cumulating data supporting a strong and independent effect of inflammatory activation on CVD risk,21 , 22 our data support the benefit of potent Lp(a)-lowering in CVD prevention strategies. Moreover, the absence of an anti-inflammatory effect following potent LDL-C lowering implies that solely targeting other CV risk factors than Lp(a) is unlikely to fully attenuate the increased CVD risk in patients with Lp(a) elevation. These findings require validation in the planned CV outcomes study for AKCEA-APO(a)-LRx (NCT04023552).
Study limitations
Several limitations merit closer attention. First, this study includes the results of two separate intervention studies, and therefore direct comparison of the transcriptomic data between all groups was not feasible. However, since the included studies were intervention trials, patients served as their own control for the transcriptome analysis. Secondly, we only focused on the inflammatory changes in transcriptome profile and monocyte phenotype and function. However, multiple pathways have been suggested to contribute to Lp(a)’s atherogenicity, also comprising pro-coagulant and pro-thrombotic effects, which we did not address in the current study. Thirdly, in the AKCEA-APO(a)-LRx trial patients were treated for 26 weeks, whereas in the ANITSCHKOW trial subjects were treated for 16 weeks. This may have contributed to an underestimation of the effect of PCSK9ab treatment. However, no trend towards reduction of the inflammatory activity of circulating monocytes following PCSK9ab treatment was observed, making this an unlikely confounder.
Conclusion
This study supports the hypothesis that Lp(a) contributes to the pro-inflammatory activation of circulating monocytes in both healthy individuals and CVD patients, which is reversible only by large absolute reductions in Lp(a). Collectively, these ex vivo findings provide indirect translational evidence that treatment with AKCEA-APO(a)-LRx could lead to CV benefit in CVD patients with elevated Lp(a).
Supplementary Material
Acknowledgements
The authors thank M. Versloot for her assistance in the lab experiments, and Servier Medical Art for using their image bank to create the Take home figure.
Funding
This investigator-initiated project has received support from the European Union’s Horizon 2020 research and innovation program under grant agreement No 667837 (REPROGRAM). The ANITSCHKOW trial (NCT02729025) was funded by Amgen Inc. The AKCEA-APO(a)-LRx trial (NCT03070782) was funded by Ionis Pharmaceuticals Inc.
Conflict of interest: L.C.A.S., K.H.M.P., R.M.H., S.L.V., J.G.S., K.E.D., A.J.C., and M.B. have nothing to disclose. J.K. received a postdoctoral grant from Amsterdam Cardiovascular Sciences and a VENI grant from ZonMW (91619098). S.T. is a co-inventor and receives royalties from patents owned by UCSD on oxidation-specific antibodies and of biomarkers related to oxidized lipoproteins, has a dual appointment at UCSD and Ionis Pharmaceuticals, is a co-founder of Oxitope, Inc. and Kleanthi LLC and is a consultant to Boston Heart Diagnostics. E.S.G.S. reports that his institution has received lecturing fees and advisory board fees from Amgen Inc., Regeneron, Sanofi, Akcea, Mylan and Esperion, and a grant from Athera. M.P.J.W. is supported by the Netherlands Heart Foundation (CVON 2011/B019, CVON 2017-20); Spark-Holding BV (2015B002); the European Union (ITN-grant EPIMAC), and Foundation Leducq (LEAN-Transatlantic Network Grant).
Contributor Information
Lotte C A Stiekema, Department of Vascular Medicine, Amsterdam University Medical Centers, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Koen H M Prange, Department of Medical Biochemistry, Amsterdam University Medical Centers, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Renate M Hoogeveen, Department of Vascular Medicine, Amsterdam University Medical Centers, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Simone L Verweij, Department of Vascular Medicine, Amsterdam University Medical Centers, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Jeffrey Kroon, Department of Vascular Medicine, Amsterdam University Medical Centers, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Johan G Schnitzler, Department of Vascular Medicine, Amsterdam University Medical Centers, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Kim E Dzobo, Department of Vascular Medicine, Amsterdam University Medical Centers, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Arjen J Cupido, Department of Vascular Medicine, Amsterdam University Medical Centers, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Sotirios Tsimikas, Ionis Pharmaceuticals, 2855 Gazelle Ct, Carlsbad, CA 92008, USA; Sulpizio Cardiovascular Center, Vascular Medicine Program, University of California San Diego, 9434 Medical Center Dr, La Jolla, CA 92037, USA.
Erik S G Stroes, Department of Vascular Medicine, Amsterdam University Medical Centers, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Menno P J de Winther, Department of Medical Biochemistry, Amsterdam University Medical Centers, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Institute for Cardiovascular Prevention, IPEK, LMU, Pettenkoferstrasse 9, Munich 80336, Germany.
Mahnoush Bahjat, Department of Vascular Medicine, Amsterdam University Medical Centers, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
References
- 1. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol 2017;69:692–711. [DOI] [PubMed] [Google Scholar]
- 2. Kamstrup PR, Tybjærg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 2009;301:2331–2339. [DOI] [PubMed] [Google Scholar]
- 3. Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med 2009;361:2518–2528. [DOI] [PubMed] [Google Scholar]
- 4. van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, Ravandi A, Nederveen AJ, Verberne HJ, Scipione C, Nieuwdorp M, Joosten LAB, Netea MG, Koschinsky ML, Witztum JL, Tsimikas S, Riksen NP, Stroes ES. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation 2016;134:611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stiekema LCA, Stroes ESG, Verweij SL, Kassahun H, Chen L, Wasserman SM, Sabatine MS, Mani V, Fayad ZA. Persistent arterial wall inflammation in patients with elevated lipoprotein(a) despite strong low-density lipoprotein cholesterol reduction by proprotein convertase subtilisin/kexin type 9 antibody treatment. Eur Heart J 2019;40:2775–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.HPS2-THRIVE Collaborative GroupLandray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–212. [DOI] [PubMed] [Google Scholar]
- 7.HPS3/TIMI55–REVEAL Collaborative GroupBowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 2017;377:1217–1227. [DOI] [PubMed] [Google Scholar]
- 8. Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, Willeit P, Young R, Surendran P, Karthikeyan S, Bolton TR, Peters JE, Kamstrup PR, Tybjærg-Hansen A, Benn M, Langsted A, Schnohr P, Vedel-Krogh S, Kobylecki CJ, Ford I, Packard C, Trompet S, Jukema JW, Sattar N, Di Angelantonio E, Saleheen D, Howson JMM, Nordestgaard BG, Butterworth AS, Danesh J; European Prospective Investigation Into Cancer and Nutrition–Cardiovascular Disease (EPIC-CVD) Consortium. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a Mendelian randomization analysis.. JAMA Cardiol 2018;3:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, Marcovina SM, Hughes SG, Graham MJ, Crooke RM, Crooke ST, Witztum JL, Stroes ES, Tsimikas S. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016;388:2239–2253. [DOI] [PubMed] [Google Scholar]
- 10. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif J-C, Baum SJ, Steinhagen-Thiessen E, Shapiro MD, Stroes ES, Moriarty PM, Nordestgaard BG, Xia S, Guerriero J, Viney NJ, O’Dea L, Witztum JL. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med 2020;382:244–255. [DOI] [PubMed] [Google Scholar]
- 11. Bekkering S, van den Munckhof I, Nielen T, Lamfers E, Dinarello C, Rutten J, de Graaf J, Joosten LA, Netea MG, Gomes ME, Riksen NP. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis 2016;254:228–236. [DOI] [PubMed] [Google Scholar]
- 12. Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JH, Farkouh ME, Nunes IO, Beals CR, Shankar SS. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol 2013;62:909–917. [DOI] [PubMed] [Google Scholar]
- 13. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973–979. [DOI] [PubMed] [Google Scholar]
- 14. Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, Schwartz GG, Olsson AG, Colhoun HM, Kronenberg F, Drechsler C, Wanner C, Mora S, Lesogor A, Tsimikas S. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet 2018;392:1311–1320. [DOI] [PubMed] [Google Scholar]
- 15. Bernelot Moens SJ, Neele AE, Kroon J, van der Valk FM, Van den Bossche J, Hoeksema MA, Hoogeveen RM, Schnitzler JG, Baccara-Dinet MT, Manvelian G, de Winther MPJ, Stroes ESG. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J 2017;38:1584–1593. [DOI] [PubMed] [Google Scholar]
- 16. Que X, Hung M-Y, Yeang C, Gonen A, Prohaska TA, Sun X, Diehl C, Määttä A, Gaddis DE, Bowden K, Pattison J, MacDonald JG, Ylä-Herttuala S, Mellon PL, Hedrick CC, Ley K, Miller YI, Glass CK, Peterson KL, Binder CJ, Tsimikas S, Witztum JL. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018;558:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm 2010;2010:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YHC, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JSM, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008;133:235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schoenborn JR, Wilson CB. Regulation of interferon‐γ during innate and adaptive immune responses. Adv Immunology 2007;96:41–101. [DOI] [PubMed] [Google Scholar]
- 20. Cenarro A, Puzo J, Ferrando J, Mateo-Gallego R, Bea AM, Calmarza P, Jarauta E, Civeira F. Effect of Nicotinic acid/Laropiprant in the lipoprotein(a) concentration with regard to baseline lipoprotein(a) concentration and LPA genotype. Metabolism 2014;63:365–371. [DOI] [PubMed] [Google Scholar]
- 21. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002;347:1557–1565. [DOI] [PubMed] [Google Scholar]
- 22. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.