Abstract
The aims of this clinical review are to: (i) highlight the importance of elevated baseline triglycerides (TG) in the setting of well-controlled low-density lipoprotein cholesterol (LDL-C) on statins as a major contributor to residual atherosclerotic cardiovascular disease (ASCVD) risk, particularly among patients with type 2 diabetes mellitus, metabolic syndrome, and obesity whose distinctive lipid phenotype cannot be optimally treated with LDL-C reduction therapy alone; (ii) describe the findings and clinical implications of the landmark REDUCE-IT trial in which ethyl eicosapentaenoic acid significantly improved ASCVD outcomes. While many genetic studies have shown that elevated TG are an independent causal factor for ASCVD, prior placebo-controlled trials using niacin, fibrates, omega-3 fatty acids, and dietary supplement fish oil preparations have failed to demonstrate significant CV event reduction when added to statin therapy. In contrast, the REDUCE-IT trial in 8179 participants showed convincingly that the administration of 4 g daily of icosapent ethyl (an ethyl ester of eicosapentaenoic acid) in patients at high risk for ASCVD with increased levels of baseline TG [median value, 2.44 mmol/L (216.0 mg/dL)] but well-controlled LDL-C [median value, 1.94 mmol/L (75.0 mg/dL)] reduced significantly incident events across both the trial primary endpoint and multiple prespecified secondary endpoints, including cardiovascular death, as well as both subsequent and total primary endpoint and key secondary endpoint events. Icosapent ethyl unequivocally contributed to ASCVD event reduction over and above statin therapy. The REDUCE-IT trial results should alter our approach to managing a growing population of hypertriglyceridaemic patients whose lipid phenotype requires more intensive treatment beyond LDL-C lowering alone.
Keywords: Hypertriglyceridaemia, Dyslipidaemia, Residual cardiovascular risk, Remnant lipoproteins, Icosapent ethyl, Omega-3 fatty acids
Introduction
For well over a decade, the pivotal role of high-intensity statins in reducing atherosclerotic cardiovascular disease (ASCVD) events has been firmly established in trials of both primary and secondary prevention. Yet, despite this incontrovertible scientific evidence of clinical event reduction, it has also been well-recognized that substantial residual risk persists despite significant reductions in low-density lipoprotein cholesterol (LDL-C) with high-intensity statins, with or without ezetimibe, as well as with the use of proprotein convertase subtilisin kexin type 91 inhibitors.1–5 In particular, residual ASCVD risk is especially noteworthy among high-risk subjects with type 2 diabetes mellitus (T2DM), metabolic syndrome, and obesity, where atherogenic dyslipidaemia is associated with not only elevated LDL-C and small dense LDL particles, but also with elevated levels of serum triglycerides (TG), low levels of high-density lipoprotein cholesterol (HDL-C), and increased remnant lipoproteins.1,3,6 It is also important to underscore that, despite intensive treatment with statins as well as newer and more powerful therapies directed at greater LDL-C reduction, the atherogenic dyslipidaemia induced by insulin resistance is not optimally treated in such high-risk patients in whom a high rate of clinical events occur despite intensive pharmacologic treatments directed at lowering LDL-C. This recognition has fuelled a decades-long search for additional lipid-modifying therapies that favourably modulate other lipids and lipoproteins that might, when added to statins, incrementally lower risk for ASCVD events.
Epidemiology and genetic basis for the association of hypertriglyceridaemia and incident atherosclerotic cardiovascular disease risk
Hypertriglyceridaemia is widely recognized as a highly prevalent metabolic disorder in adults.7 The prevalence of baseline levels of TG ≥1.69 mmol/L (≥150 mg/dL) in adults aged 20 years and older is approximately 25% based on estimates from the 1999–2014 US National Health and Nutrition Examination Survey (NHANES)8 and 36% based on an observational study of statin-treated patients ≥45 years of age in the UK.9 In a European cross-sectional cohort study of patients ≥ age 50 years, 21% had TG ≥2.30 mmol/L (204 mg/dL).10 In a more recent NHANES analysis spanning 2007–14, an estimated one-third of all patients on statin therapy have a serum TG ≥1.69 mmol/L in whom over 1 million ASCVD-related events are expected in the next decade.11 It is highly likely that the prevalence of elevated TG will continue to escalate in all regions of the world as the ‘triple-threat’ epidemics of T2DM, metabolic syndrome, and obesity continue to accelerate globally. While the observational epidemiology supporting the association between elevated baseline TG levels and low HDL-C levels with increased incident rates of cardiovascular events is strong and robust for both men and women,12 they do not provide unconfounded estimates of causality. In contrast, compelling scientific evidence from recent genetic studies using Mendelian randomization provides a causal role for hypertriglyceridaemia, rather than low baseline levels of HDL-C, in contributing directly to elevated ASCVD risk. In one such large multivariable Mendelian randomization study involving ∼20 000 myocardial infarction (MI) cases and 50 000 controls, for every 1 SD increase in baseline TG levels, there was a significant 54% increase in risk for coronary heart disease.13
Triglyceride levels in plasma may be influenced by multiple genes as well as environmental factors.14 Several genetic studies have likewise shown that genetically lower TG irrespective of the mechanism results in a lower risk of incident ASCVD events.14–17 In contrast, there was no association observed with genetically lower levels of HDL-C and incident ASCVD event rates.18 In another recent, very large Mendelian randomization study involving 654 783 participants, TG were shown to be an independent causal factor for ASCVD, with an effect that seemed to be modulated by ApoB100 levels.19 This latter finding likely reflects the fact that TG are carried in very low-density lipoproteins (VLDL) and VLDL remnants, such as small VLDL particles and intermediate-density lipoproteins, all of which contain ApoB100. Triglyceride-enriched lipoproteins correlate highly with increased risk for ASCVD events.20,21 In addition to their TG cargo, remnant lipoproteins may be proatherogenic because they also carry cholesterol and are proinflammatory.22,23 Multiple prior placebo-controlled trials in the statin era, which tested the co-administration of fibrates or niacin, have failed to demonstrate incremental ASCVD event reduction after ‘optimally controlled’ LDL-C levels have been achieved with statins.24 Notably, therapies such as fibrates (in particular gemfibrozil) and niacin also lower LDL-C and ApoB with benefits proportional to the reductions in ApoB.25
Current role of triglyceride-lowering drugs on cardiovascular outcomes beyond optimal low-density lipoprotein cholesterol reduction
In current practice, fibrates, niacin, and omega-3 fatty acids (OM3FA) are three major classes of lipid-altering agents available to treat elevated TG and/or low HDL-C with the related and important goal of reducing residual risk and incident ASCVD events. While data from the prior Veterans Affairs HDL Intervention Trial (VA-HIT) in the pre-statin era did show clinical event reduction among 2531 men pre-selected with low levels of baseline HDL-C treated with gemfibrozil, there was no clear association between TG-lowering and improved outcomes during a 5.1-year follow-up.26
More relevant to contemporary clinical practice in the statin era, there is little evidence that fibrates are associated with significant cardiovascular event reduction. In the Action to Control Cardiovascular Risk in Diabetes Lipid (ACCORD-LIPID) trial, the addition of fenofibrate to simvastatin 40 mg in the overall study population [median baseline TG = 1.82 mmol/L (162 mg/dL)] showed no significant benefit,24 though in a subgroup of 941 participants in the highest tertile of baseline TG [≥2.30 mmol/L (204 mg/dL)] and lowest tertile of HDL-C [≤0.88 mmol/L (≤34 mg/dL)], there was a 31% lower risk of major ASCVD events, which was of borderline statistical significance (P = 0.06). Similar findings were observed in a post hoc analysis of the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes (AIM-HIGH) Trial among 522 subjects (15%) who were in both the highest TG [>2.24 mmol/L (198 mg/dL)] and lowest HDL-C [<0.85 mmol/L (33 mg/dL)] tertiles, where extended-release niacin showed a trend toward benefit [hazard ratio (HR) = 0.74, P = 0.073].27 Clearly, additional outcomes studies in patients optimally treated with statins and pre-selected for hypertriglyceridaemia are needed. One such large randomized trial, Pemafibrate to Reduce Cardiovascular OutcoMes by Reducing Triglycerides IN patiENts with DiabeTes (PROMINENT), (NCT 03071692) is presently ongoing.
Omega-3 fatty acids and fish oil as therapeutic interventions
Omega-3 fatty acids are known to be efficacious in lowering TG levels by reducing VLDL production, stimulating lipoprotein lipase activity, and augmenting VLDL clearance.28 The OM3FA may also exert pleiotropic effects, such as attenuating atherogenesis and reducing plaque instability, which could likewise contribute to residual ASCVD risk reduction.29 The OM3FA are substrates for the formation of protectins and resolvins, molecules which promote the resolution of inflammation in a variety of disease states, including atherosclerosis.30 Convincing clinical outcomes data, however, have been lacking from prior randomized trials of various preparations, which mostly included combination preparations of ethyl eicosapentaenoic acid (EPA) plus docosahexaenoic acid (DHA), when added to a high-potency statin.31,32 The conflicting outcomes among studies that have used DHA may relate to a modest increase in LDL-C and which, in part, could potentially negate the overall clinical benefit of EPA, though this remains unproven. In contrast, no significant change in LDL-C has been consistently observed with EPA agents.31,32 Several studies, including primary and secondary prevention trials and meta-analyses of OM3FA, have so far yielded inconsistent results on ASCVD outcomes.33–37 One explanation for this apparent heterogeneity of treatment effect of OM3FA preparations, which applies also to the many dietary fish oil supplements that are in widespread use as dietary supplement, is that many OM3FA trials have used a low-dose that is likely insufficient to lower TG effectively, and hence clinical events.
In the large, open-label Japan EPA Lipid Intervention Study (JELIS) of over 18 000 subjects who received moderate-dose EPA (1.8 g daily) and low-dose statin therapy (pravastatin 10 mg or simvastatin 5 mg daily), there was a significant 19% reduction in incident ASCVD events (P = 0.011), albeit with a median baseline TG level of 1.72 mmol/L (153 mg/dL).37 While the trial met its primary composite endpoint, only hospitalization for unstable angina did so in a statistically significant manner. None of the reductions in other individual endpoints (non-fatal MI or stroke, revascularization, or CV mortality) were significant. However, in post hoc analyses, it was observed that among subjects with high baseline TG levels [≥1.69 mmol/L (150 mg/dL)] and low HDL-C levels [<1.03 mmol/L (40 mg/dL)] who exhibited the highest risk of coronary events, there was a nominally significant 53% reduction in events [HR 0.47, 95% confidence interval (CI) 0.23–0.98; P = 0.043].38 Of note, the benefits seen in JELIS were consistent in both the secondary and primary prevention cohorts enrolled. In addition, a small open-label study of 241 acute coronary syndrome patients who received moderate-dose EPA (1.8 g/day) plus pitavastatin 2 mg/day within 24 h of successful primary angioplasty showed up to a 58% reduction in cardiovascular events at 1 year, including cardiovascular mortality; however, both studies could only be considered, at best, hypothesis-generating.39
Now along comes REDUCE-IT and ethyl eicosapentaenoic acid in atherosclerotic cardiovascular disease event reduction: a new era in dyslipidaemia therapeutics
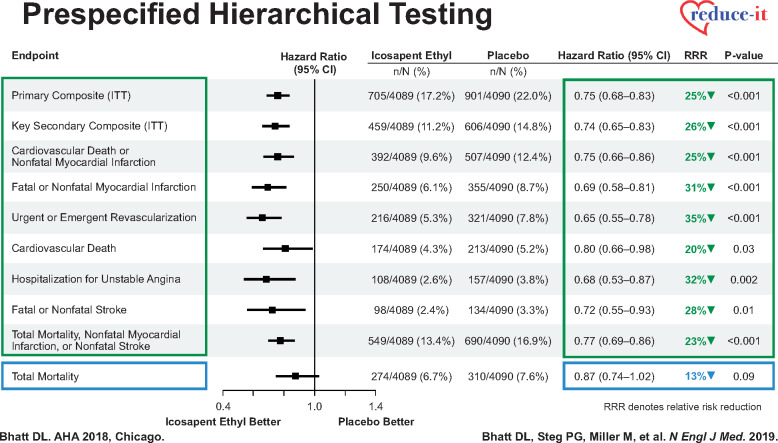
Against this backdrop of disappointing and inconclusive randomized secondary prevention trials directed at reducing ASCVD residual risk beyond LDL-C reduction and the inconsistent findings of prior studies of OM3FA, we now have the remarkable findings from the first, prospective, placebo-controlled, randomized trial (REDUCE-IT) of icosapent ethyl (an ethyl ester of eicosapentaenoic acid) showing a highly significant treatment effect on both the trial primary outcome measure and several prespecified secondary endpoints.40 Among the 8179 participants with high CV risk (of whom 71% had established cardiovascular disease, 29% comprised a primary prevention cohort, and 58% had T2DM), the baseline LDL-C levels were well-controlled with statins [median value, 1.94 mmol/L (75.0 mg/dL)], while TG levels were moderately elevated [median value, 2.44 mmol/L (216.0 mg/dL)].40 The comparative baseline characteristics between the icosapent ethyl and placebo groups are shown in Table 1. Several lipid and inflammatory biomarkers were reduced with icosapent ethyl, which are shown in Table 2. Compared with patients who took 4 g of icosapent ethyl per day (2 g twice daily with meals) vs. placebo, during a median follow-up of 4.9 years, the primary efficacy endpoint (a composite of cardiovascular death, non-fatal MI, non-fatal stroke, coronary revascularization, or hospitalization for unstable angina), in an intention-to-treat time to first event analysis, occurred in 22% of placebo-treated patients and in 17.2% of the icosapent ethyl-treated patients—corresponding to an absolute between-group difference of 4.8 percentage points in the rate of the endpoint (HR 0.75, 95% CI 0.68–0.83, with a statistically robust P = 1 × 10–8), and a number needed to treat of 21.40,41 The risk of the prespecified key secondary endpoint (a composite of cardiovascular death, non-fatal MI, or non-fatal stroke) was likewise significantly lower in the icosapent ethyl group (11.2%) vs. the placebo group (14.8%)—a 3.6% absolute between-group difference and a 26% lower endpoint risk, (HR 0.74, 95% CI 0.65–0.83; P < 0.001), respectively.40 The authors also performed detailed hierarchical statistical testing which showed concordant effects on all individual trial endpoints, except for all-cause mortality, which trended towards being lower (P = 0.09) (Figure 1).41 The prespecified subgroups described in the main publication showed consistent benefits, including by sex, age, region, baseline LDL-C, and baseline TG. Of note, even the subset of patients with baseline TG ≤ 150 mg/dL benefitted.40
Table 1.
| Icosapent ethyl (N = 4089) | Placebo (N = 4090) | |
|---|---|---|
| Age (years), median (Q1–Q3) | 64.0 (57.0–69.0) | 64.0 (57.0–69.0) |
| Female, n (%) | 1162 (28.4%) | 1195 (29.2%) |
| Non-White, n (%) | 398 (9.7%) | 401 (9.8%) |
| Westernized region, n (%) | 2906 (71.1%) | 2905 (71.0%) |
| CV risk category, n (%) | ||
| Secondary prevention cohort | 2892 (70.7%) | 2893 (70.7%) |
| Primary prevention cohort | 1197 (29.3%) | 1197 (29.3%) |
| Ezetimibe use, n (%) | 262 (6.4%) | 262 (6.4%) |
| Statin intensity, n (%) | ||
| Low | 254 (6.2%) | 267 (6.5%) |
| Moderate | 2533 (61.9%) | 2575 (63.0%) |
| High | 1290 (31.5%) | 1226 (30.0%) |
| Type 2 diabetes, n (%) | 2367 (57.9%) | 2363 (57.8%) |
| Triglycerides (mg/dL), median (Q1–Q3) | 216.5 (176.5–272.0) | 216.0 (175.5–274.0) |
| HDL-C (mg/dL), median (Q1–Q3) | 40.0 (34.5–46.0) | 40.0 (35.0–46.0) |
| LDL-C (mg/dL), median (Q1–Q3) | 74.0 (61.5–88.0) | 76.0 (63.0–89.0) |
| Triglycerides category | ||
| <150 mg/dL | 412 (10.1%) | 429 (10.5%) |
| 150 to <200 mg/dL | 1193 (29.2%) | 1191 (29.1%) |
| ≥200 mg/dL | 2481 (60.7%) | 2469 (60.4%) |
| Prior atherosclerotic coronary artery disease (%) | 58.40 | 58.50 |
| Prior atherosclerotic cerebrovascular disease (%) | 15.70 | 16.20 |
| Prior atherosclerotic peripheral artery disease (%) | 9.50 | 9.50 |
| Triglyceride category (by tertiles) | ||
| ≥81 to ≤190 mg/dL | Median 163 mg/dL | |
| >190 to ≤250 mg/dL | Median 217 mg/dL | |
| >250 to ≤1401 mg/dL | Median 304 mg/dL | |
Table 2.
Effects of icosapent ethyl vs. placebo on various biomarkers in REDUCE-IT40
| Biomarker a | Icosapent ethyl (N = 4089) | Placebo (N = 4090) | Median between-group difference at Year 1 |
||||
|---|---|---|---|---|---|---|---|
| Median |
Median |
||||||
| Baseline | Year 1 | Baseline | Year 1 | Absolute change from baseline | % change from baseline | % change | |
| P-value | |||||||
| Triglycerides (mg/dL) | 216.5 | 175.0 | 216.0 | 221.0 | −44.5 | −19.7 | <0.001 |
| Non-HDL-C (mg/dL) | 118.0 | 113.0 | 118.5 | 130.0 | −15.5 | −13.1 | <0.001 |
| LDL-C (mg/dL) | 74.0 | 77.0 | 76.0 | 84.0 | −5.0 | −6.6 | <0.001 |
| HDL-C (mg/dL) | 40.0 | 39.0 | 40.0 | 42.0 | −2.5 | −6.3 | <0.001 |
| ApoB (mg/dL) | 82.0 | 80.0 | 83.0 | 89.0 | −8.0 | −9.7 | <0.001 |
| hsCRP (mg/L) | 2.2 | 1.8 | 2.1 | 2.8 | −0.9 | −39.9 | <0.001 |
| Log hsCRP (mg/L) | 0.8 | 0.6 | 0.8 | 1.0 | −0.4 | −22.5 | <0.001 |
| EPA (µg/mL) | 26.1 | 144.0 | 26.1 | 23.3 | +114.9 | +385.8 | <0.001 |
ApoB and hsCRP were measured at Year 2.
Figure 1.
Prespecified hierarchical testing of a variety of individual and composite ischaemic endpoints, showing significant reductions in endpoints such as cardiovascular death (with permission from Bhatt et al.40).
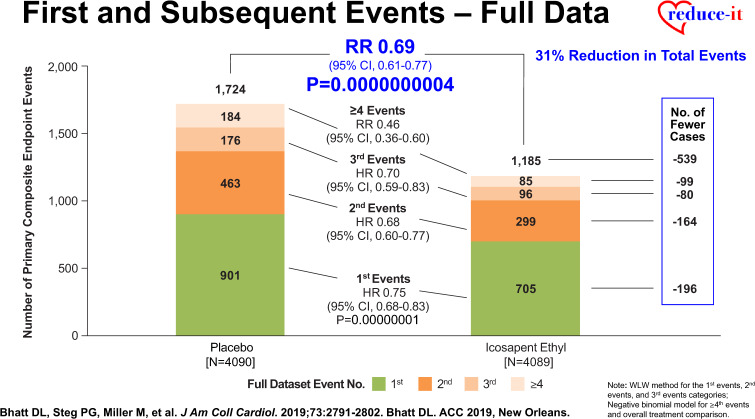
Effects of icosapent ethyl on subsequent and total atherosclerotic cardiovascular disease events
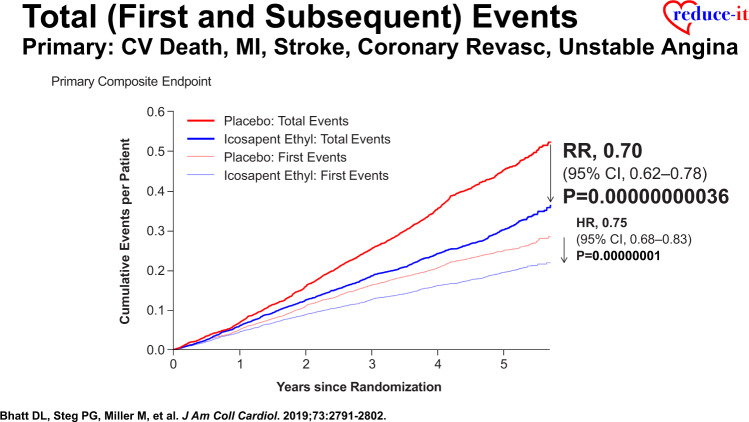
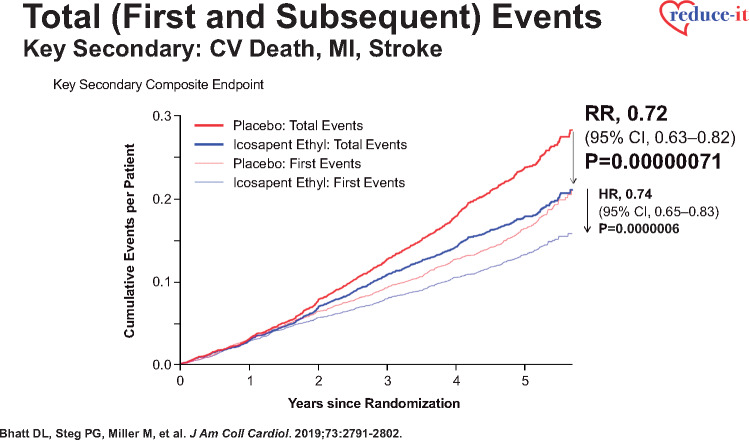
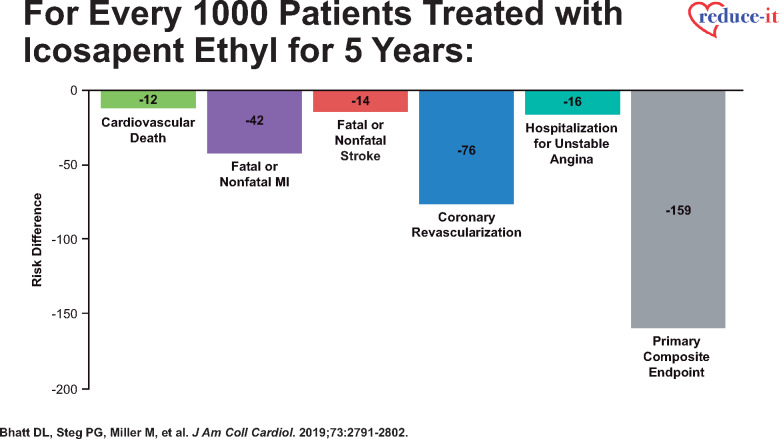
More recent analyses from the REDUCE-IT Trial demonstrate that there were 1606 (55.2%) first primary endpoint events as well as 1303 (44.8%) subsequent primary endpoint events, which included 762 second events and 541 third or more events.43 Importantly, as compared with placebo, icosapent ethyl decreased total primary endpoint events by 30% from 89 to 61 per 1000 patient-years (RR 0.70, 95% CI 0.62–0.78; P < 0.0001) (Figure 2), while total key secondary endpoint events were significantly reduced by 28% from 44 to 32 events per 1000 patient-years (RR 0.72, 95% CI 0.63–0.82; P < 0.0001) (Figure 3).43 The reason why such reductions in not only the time to first event but also in subsequent and total events is so important clinically is that such patients have significantly increased risk for both recurrent and fatal events (Figure 4).43 For this reason, a more comprehensive assessment of all aggregate CV events provides a more accurate and robust quantification of the total atherosclerotic event burden that such patients face over the time course of their disease process that may span many years’ (Figure 5).43 Thus, a therapeutic intervention like icosapent ethyl has the capacity to favourably alter the trajectory and occurrence rates of ASCVD in subjects with high residual risk (Take home figure).40,41,44,45 Furthermore, the results of REDUCE-IT appear to be quite generalizable to routine clinical practice.46 Outcomes were shown to be independent of both baseline serum TG and LDL-C levels.41
Figure 2.
Reduction in cardiovascular death, myocardial infarction, stroke, coronary revascularization, and hospitalization for unstable angina in time to first event and total event analyses in REDUCE-IT (with permission from Bhatt et al.43).
Figure 3.
Reduction in cardiovascular death, myocardial infarction, and stroke in time to first event and total event analyses in REDUCE-IT (with permission from Bhatt et al.43).
Figure 4.
Reduction in first and subsequent ischaemic events in REDUCE-IT (with permission from Bhatt et al.43).
Figure 5.
Number of ischaemic events prevented by icosapent ethyl for every 1000 patients treated for 5 years in REDUCE-IT (with permission from Bhatt et al.43).
Take home figure.
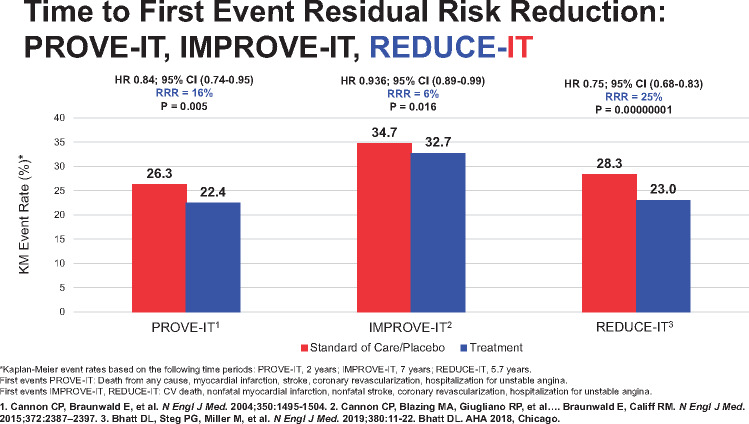
Landmark trials in cardiovascular risk reduction and the associated degrees of residual risk reduction.40,41,44,45
Potential limitations and uncertainties
There has been some criticism of the REDUCE-IT trial that warrants clarification. Some have highlighted that the purported CV benefits of icosapent ethyl were out of proportion to what would have been predicted based on changes in on-treatment TG levels alone.47 Viewed purely from the perspective that the entirety of icosapent ethyl benefit was TG-mediated, the observed median placebo-corrected reductions in both TG [0.50 mmol/L (44.5 mg/dL)] and non-HDL-C [0.40 mmol/L (15.5 mg/dL)] from baseline to 1 year with this pure EPA intervention would have been expected to reduce incident ASCVD events by only ∼6–8%, which is considerably less than the 25% observed reduction in the primary composite outcome or the 30% reduction in total events. Some have also argued that the mineral oil-containing placebo may have raised on-treatment LDL-C levels and increased both atherogenic lipoproteins and C-reactive protein which, in turn, potentially magnified the observed treatment difference between the icosapent ethyl and placebo groups.47 However, even if true, the 5 mg/dL difference in LDL-C in the placebo arm relative to the EPA arm would likely explain at most 3% of the relative risk reduction, which still leaves unexplained a large observed 20–25% risk reduction with EPA. Of note, the authors are presently unaware of any data proving that mineral oil in the minute quantities used in this trial (∼2 mL twice daily) would cause cardiovascular harm.
It is also possible that there may be other important non-lipid mechanisms at play that explain the strikingly positive and consistent endpoint findings observed in REDUCE-IT, which may include an anti-inflammatory effect of icosapent ethyl (as depicted by the reduction in C-reactive protein levels), potential stabilization or regression of coronary plaques, improvement in endothelial dysfunction, or a membrane-stabilizing effect, any of which might potentially explain the lower rates of cardiac arrest and sudden cardiac death observed with icosapent ethyl vs. the placebo group.40,47 Moreover, the significant reductions in diverse clinical endpoints such as stroke and elective revascularization do suggest that multiple therapeutic mechanisms may be at play. While the precise mechanism(s) by which icosapent ethyl provided endpoint reductions in the REDUCE-IT trial may not be fully understood, this does not diminish the importance of the study findings. It is also worth emphasizing that statins are believed to exert pleiotropic effects, and it is by no means implausible that icosapent ethyl has similar, multifaceted mechanisms that explain its clinical effectiveness beyond TG reduction alone. Finally, regarding safety and adverse events noted in REDUCE-IT, a larger percentage of patients in the icosapent ethyl group than in the placebo group were hospitalized for atrial fibrillation or flutter (3.1% vs. 2.1%, P = 0.004), while serious bleeding events occurred in 2.7% of the patients in the icosapent ethyl group and in 2.1% in the placebo group (P = 0.06).40 The significance of these observations is uncertain, and further analyses are ongoing. However, it is important to point out that the atrial fibrillation result does not necessarily imply that icosapent ethyl is arrhythmogenic, since sudden death was significantly reduced by 52%.40
Are the REDUCE-IT trial findings unique, or is this likely a class effect?
In their editorial to the REDUCE-IT trial, ‘FISHing for the Miracle of Eicosapentaenoic Acid,’ Kastelein and Stroes stated that they ‘welcome these results with surprise, speculation, and hope’.47 What remains unsettled until the completion of the ongoing Statin Residual Risk Reduction With EpaNova in High Cardiovascular Risk Patients with Hypertriglyceridaemia (STRENGTH; NCT02104817) trial is whether a different branded formulation of EPA and DHA (Epanova®), employed in a placebo-controlled, randomized outcomes trial in over 13 000 subjects with a design like REDUCE-IT, will have the same or different findings.48 Because there are fundamental differences between EPA and DHA in the duration of antioxidant activity as well as effects on membrane lipid structure and function within peripheral cells, it cannot be assumed that the same clinical findings observed in REDUCE-IT will be found in other trials using different formulations containing DHA and EPA.49 Moreover, two large meta-analyses of OM3FA therapy,36,50 as well as the recent Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus (ASCEND) trial testing 1-g capsules of fish oil51 and the Vitamin D and Omega-3 Trial (VITAL), failed to show any CV risk reduction with combination DHA/EPA preparations compared to placebo.52 Thus, it would be premature to suggest that the findings of REDUCE-IT will be necessarily replicated in trials of other OM3FA formulations, in much the same way that it is increasingly clear that low-dose dietary fish oil supplement products have been shown to be ineffective in reducing incident ASCVD events.
Conclusion
REDUCE-IT convincingly demonstrated that the administration of high-dose prescription icosapent ethyl 4 g daily (2 g twice daily with meals) in patients at high risk for ASCVD who have increased baseline TG but well-controlled LDL-C levels have a highly significant clinical benefit and reduced incident events across both the trial primary endpoint and multiple prespecified secondary endpoints, including cardiovascular death. In addition, more recent REDUCE-IT trial data confirm a highly significant treatment effect of icosapent ethyl on reducing both subsequent and total primary endpoint and key secondary endpoint events which, in the aggregate, likely reflect a favourable effect of prescription EPA on ameliorating total atherosclerotic event burden. The therapeutic implications of the REDUCE-IT trial findings should substantially alter our approach to managing a growing population of hypertriglyceridaemic patients with T2DM and metabolic syndrome whose lipid phenotype requires treatment beyond LDL-C lowering alone. Finally, both the updated 2019 Lipid Guidelines of the European Society of Cardiology Congress and the U.S. National Lipid Association have explicitly referenced and endorsed icosapent ethyl as a Class IIa and Class IB dyslipidaemic therapy, respectively, for patients with hypertriglyceridaemia.53,54 We believe these recommendations underscore that icosapent ethyl and the profoundly positive findings of REDUCE-IT herald a new era in dyslipidaemia therapeutics, and one in which the previously unfulfilled promise of residual cardiovascular risk reduction in these high-risk patients can now be achieved.
Conflict of interest: Dr W.E.B. discloses the following relationships: Executive steering committee member of the TRAVERSE Trial, funded by Abbvie, Inc.; .Research grant support: Clinical Trials Network, Massachusetts Veterans Epidemiology, Research, and Information Center, VA New England Healthcare System; National Heart, Lung, and Blood Institute as National co–principal investigator for the ISCHEMIA trial; Axio Research, Inc, Seattle, WA; AbbVie; Amarin Pharmaceuticals, Inc; Amgen; Astra-Zeneca; and Sanofi Aventis. Board of Directors: Boston VA Research Institute, Inc.; and CardioDx, Mountain View, CA. Data monitoring committee: VA Cooperative Studies Program; National Coordinator, STRENGTH trial, with honoraria from the Cleveland Clinic Clinical Coordinating Center. Journal of the American College of Cardiology (Associate Editor, Clinical Cardiology); Speaking honoraria: Amgen; AstraZeneca; Janssen Pharmaceuticals; and Regeneron. Dr D.L.B. serves as Chair and International Principal Investigator of REDUCE-IT and received research funding from the trial sponsor, Amarin Pharma, Inc. Dr D.L.B. discloses the following relationships—Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, PLx Pharma Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. Dr K.K.R. reports personal fees from Abbvie, grants and personal fees from Amgen, personal fees from Astra Zeneca, grants and personal fees from Sanofi, grants and personal fees from Regeneron, grants and personal fees from MSD, grants and personal fees from Pfizer, personal fees from Resverlogix, personal fees from Akcea, personal fees from Boehringer Ingelheim, personal fees from Novo Nordisk, personal fees from Takeda, personal fees from Kowa, personal fees from Algorithm, personal fees from Cipla, personal fees from Cerenis, personal fees from Dr Reddys, personal fees from Lilly, personal fees from Zuellig Pharma, personal fees from Bayer, personal fees from Daiichi Sankyo, personal fees from The Medicines Company; personal fees from Esperion outside the submitted work. Dr P.P.T. is a consultant to Amarin, Amgen, AstraZeneca, Kowa, Regeneron, and Theravance; he is a member of the speakers’ bureau for Amarin, Amgen, Kowa, Novo-Nordisk, Regeneron, and Sanofi; he is a member of the steering committee for the BETonMACE trial sponsored by Resverlogix. Dr M.J.C. has received research grants from Merck, Kowa, Pfizer, and Randox and honoraria for Advisory Boards, consultancy, or speaker activities from Alexion, Amarin, Amgen, Daiichi-Sankyo, Kowa, Merck, Pfizer, Regeneron, Sanofi, Servier, and Unilever. Dr T.F.L. has nothing to disclose. Amarin paid for the figure permissions for this article and for open access.
References
- 1. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 2. Hong KN, Fuster V, Rosenson RS, Rosendorff C, Bhatt DL.. How low to go with glucose, cholesterol, and blood pressure in primary prevention of CVD. J Am Coll Cardiol 2017;70:2171–2185. [DOI] [PubMed] [Google Scholar]
- 3. Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, LaRosa JC, Waters DD, Demicco DA, Simes RJ, Keech AC, Colquhoun D, Hitman GA, Betteridge DJ, Clearfield MB, Downs JR, Colhoun HM, Gotto AM Jr, Ridker PM, Grundy SM, Kastelein JJ.. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol 2014;64:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R.. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–2561. [DOI] [PubMed] [Google Scholar]
- 5. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 6. Xiao C, Dash S, Morgantini C, Hegele RA, Lewis GF.. Pharmacological targeting of the atherogenic dyslipidemia complex: the next frontier in CVD prevention beyond lowering LDL cholesterol. Diabetes 2016;65:1767–1778. [DOI] [PubMed] [Google Scholar]
- 7. Ganda OP, Bhatt DL, Mason RP, Miller M, Boden WE.. Unmet need for adjunctive dyslipidemia therapy in hypertriglyderidemia management. J Am Coll Cardiol 2018;72:330–343. [DOI] [PubMed] [Google Scholar]
- 8. Rosinger A, Carroll MD, Lacher D, Ogden C.. Trends in total cholesterol, triglycerides, and low-density lipoprotein in US adults, 1999-2014. JAMA Cardiol 2017;2:339–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amber V, Kotseva K, Turner EL, Jennings C, Atrey A, Jones J, Connolly S, Bowker TJ, Wood DA.. Dyslipidaemia and atherosclerotic vascular disease: DYSIS results in the UK. Br J Cardiol 2013;20(suppl 3): S9–S10. [Google Scholar]
- 10. Halcox JP, Banegas JR, Roy C, Dallongeville J, De Backer G, Guallar E, Perk J, Hajage D, Henriksson KM, Borghi C.. Prevalence and treatment of atherogenic dyslipidemia in the primary prevention of cardiovascular disease in Europe: EURIKA, a cross-sectional observational study. BMC Cardiovasc Disord 2017;17:160.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan W, Philip S, Granowitz C, Toth PP, Wong ND.. Hypertriglyceridemia in statin-treated US adults: the National Health and Nutrition Examination Survey. J Clin Lipidol 2019;13:100–108. [DOI] [PubMed] [Google Scholar]
- 12. Libby P. Triglycerides on the rise: should we swap seats on the seesaw? Eur Heart J 2015;36:774–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Musunuru K, Kathiresan S.. Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circ Res 2016;118:579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hegele RA, Ginsberg HN, Chapman MJ, Nordestgaard BG, Kuivenhoven JA, Averna M, Borén J, Bruckert E, Catapano AL, Descamps OS, Hovingh GK, Humphries SE, Kovanen PT, Masana L, Pajukanta P, Parhofer KG, Raal FJ, Ray KK, Santos RD, Stalenhoef AFH, Stroes E, Taskinen M-R, Tybjærg-Hansen A, Watts GF, Wiklund O; European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014;2:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, McCarthy S, Van Hout CV, Bruse S, Dansky HM, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Habegger L, Lopez A, Penn J, Zhao A, Shao W, Stahl N, Murphy AJ, Hamon S, Bouzelmat A, Zhang R, Shumel B, Pordy R, Gipe D, Herman GA, Sheu W, Lee I-T, Liang K-W, Guo X, Rotter JI, Chen Y-D, Kraus WE, Shah SH, Damrauer S, Small A, Rader DJ, Wulff AB, Nordestgaard BG, Tybjærg-Hansen A, van den Hoek AM, Princen HMG, Ledbetter DH, Carey DJ, Overton JD, Reid JG, Sasiela WJ, Banerjee P, Shuldiner AR, Borecki IB, Teslovich TM, Yancopoulos GD, Mellis SJ, Gromada J, Baras A.. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med 2017;377:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 2014;371:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Nordestgaard BG, Ray KK, Reiner Z, Taskinen M-R, Tokgözoglu L, Tybjærg-Hansen A, Watts GF; European Atherosclerosis Society Consensus Panel. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 2011;32:1345–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khera AV, Kathiresan S.. Genetics of coronary artery disease: discovery, biology and clinical translation. Nat Rev Genet 2017;18:331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ference BA, Kastelein JJP, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, Laufs U, Oliver-Williams C, Wood AM, Butterworth AS, Di Angelantonio E, Danesh J, Nicholls SJ, Bhatt DL, Sabatine MS, Catapano AL.. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA 2019;321:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joshi PH, Khokhar AA, Massaro JM, Lirette ST, Griswold ME, Martin SS, Blaha MJ, Kulkarni KR, Correa A, D'Agostino RB Sr, Jones SR, Toth PP; Lipoprotein Investigators Collaborative (LIC) Study Group. Remnant lipoprotein cholesterol and incident coronary heart disease: the Jackson Heart and Framingham Offspring Cohort Studies. J Am Heart Assoc 2016;5.[CVOCROSSCVO] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Varbo A, Benn M, Nordestgaard BG.. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol Ther 2014;141:358–367. [DOI] [PubMed] [Google Scholar]
- 22. Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG.. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol 2013;61:427–436. [DOI] [PubMed] [Google Scholar]
- 23. Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG.. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013;128:1298–1309. [DOI] [PubMed] [Google Scholar]
- 24. Ginsberg HN, Elam MB, Lovato LC, Crouse JR III, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC Jr, Cushman WC, Simons-Morton DG, Byington RP.. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robinson JG, Wang S, Jacobson TA.. Meta-analysis of comparison of effectiveness of lowering apolipoprotein B versus low-density lipoprotein cholesterol and nonhigh-density lipoprotein cholesterol for cardiovascular risk reduction in randomized trials. Am J Cardiol 2012;110:1468–1476. [DOI] [PubMed] [Google Scholar]
- 26. Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J.. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol: Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999;341:410–418. [DOI] [PubMed] [Google Scholar]
- 27. Guyton JR, Slee AE, Anderson T, Fleg JL, Goldberg RB, Kashyap ML, Marcovina SM, Nash SD, O'Brien KD, Weintraub WS, Xu P, Zhao XQ, Boden WE.. Relationship of lipoproteins to cardiovascular events: the AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes). J Am Coll Cardiol 2013;62:1580–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shearer GC, Savinova OV, Harris WS.. Fish oil—how does it reduce plasma triglycerides? Biochim Biophys Acta 2012;1821:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacobson TA, Glickstein SB, Rowe JD, Soni PN.. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: a review. J Clin Lipidol 2012;6:5–18. [DOI] [PubMed] [Google Scholar]
- 30. Kohli P, Levy BD.. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol 2009;158:960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhatt DL, Steg PG, Brinton EA, Jacobson TA, Miller M, Tardif J-C, Ketchum SB, Doyle RT, Murphy SA, Soni PN, Braeckman RA, Juliano RA, Ballantyne CM.. Rationale and design of REDUCE-IT: reduction of cardiovascular events with icosapent ethyl-intervention trial. Clin Cardiol 2017;40:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alexander DD, Miller PE, Van Elswyk ME, Kuratko CN, Bylsma LC.. A meta-analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long-chain omega-3 fatty acids and coronary heart disease risk. Mayo Clin Proc 2017;92:15–29. [DOI] [PubMed] [Google Scholar]
- 33. Siscovick DS, Barringer TA, Fretts AM, Wu JHY, Lichtenstein AH, Costello RB, Kris-Etherton PM, Jacobson TA, Engler MB, Alger HM, Appel LJ, Mozaffarian D.. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation 2017;135:e867–e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS.. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 2012;308:1024–1033. [DOI] [PubMed] [Google Scholar]
- 35. Bucher HC, Hengstler P, Schindler C, Meier G.. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med 2002;112:298–304. [DOI] [PubMed] [Google Scholar]
- 36. Kwak SM, Myung SK, Lee YJ, Seo HG.. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta-analysis of randomized, double-blind, placebo-controlled trials. Arch Intern Med 2012;172:686–694. [DOI] [PubMed] [Google Scholar]
- 37. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K.. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–1098. [DOI] [PubMed] [Google Scholar]
- 38. Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K.. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis 2008;200:135–140. [DOI] [PubMed] [Google Scholar]
- 39. Nosaka K, Miyoshi T, Iwamoto M, Kajiya M, Okawa K, Tsukuda S, Yokohama F, Sogo M, Nishibe T, Matsuo N, Hirohata S, Ito H, Doi M.. Early initiation of eicosapentaenoic acid and statin treatment is associated with better clinical outcomes than statin alone in patients with acute coronary syndromes: 1-year outcomes of a randomized controlled study. Int J Cardiol 2017;228:173–179. [DOI] [PubMed] [Google Scholar]
- 40. Bhatt DL, Steg G, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM.. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 41. Bhatt DL. REDUCE-IT: Residual Cardiovascular Risk in Statin-Treated Patients with Elevated Triglycerides: Now We Can REDUCE-IT!. Eur Heart J 2019;40:1174–1175.30982067 [Google Scholar]
- 42. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Jiao L, Tardif JC, Gregson J, Pocock SJ, Ballantyne CM; REDUCE-IT Investigators. Reduction in first and total ischemic events with icosapent ethyl across baseline triglyceride tertiles. J Am Coll Cardiol 2019;74:1159–1161. [DOI] [PubMed] [Google Scholar]
- 43. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Gregson J, Pocock SJ, Ballantyne CM; REDUCE-IT Investigators. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol 2019;73:2791–2802. [DOI] [PubMed] [Google Scholar]
- 44. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM.. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 45. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De LP, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM.. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 46. Picard F, Bhatt DL, Ducrocq G, Elbez Y, Ferrari R, Ford I, Tardif JC, Tendera M, Fox KM, Steg PG.. Generalizability of the REDUCE-IT trial in patients with stable coronary artery disease. J Am Coll Cardiol 2019;73:1362–1364. [DOI] [PubMed] [Google Scholar]
- 47. Kastelein JJP, Stroes E.. FISHing for the miracle of eicosapentaenoic acid. N Engl J Med 2019;380:89–90. [DOI] [PubMed] [Google Scholar]
- 48. Nicholls SJ, Lincoff AM, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, Pedersen TR, Ridker PM, Ray K, Karlson BW, Lundstrom T, Wolski K, Nissen SE.. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: rationale and design of the STRENGTH trial. Clin Cardiol 2018;41:1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Preston Mason R. New insights into mechanisms of action for omega-3 fatty acids in atherothrombotic cardiovascular disease. Curr Atheroscler Rep 2019;21:2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, Chew EY, Bosch J, Collins R, Lewington S, Armitage J, Clarke R.. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol 2018;3:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Collaborative Group AS, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J.. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018;379:1540–1550. [DOI] [PubMed] [Google Scholar]
- 52. Manson JE, Cook NR, Lee I-M, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE.. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O.. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2019; doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 54. Orringer CE, Jacobson TA, Maki KC.. National Lipid Association position on the use of icosapent ethyl in high and very high risk patients. Clin Lipid Update 2019;2019. [DOI] [PubMed] [Google Scholar]