Abstract
Aims
Lowering low-density lipoprotein cholesterol (LDL-C) reduces cardiovascular risk irrespective of age, but the evidence is less strong for older patients.
Methods and results
This prespecified analysis from ODYSSEY OUTCOMES compared the effect of alirocumab vs. placebo in 18 924 patients with recent acute coronary syndrome (ACS) according to age. We examined the effect of assigned treatment on occurrence of the primary study outcome, a composite of coronary heart disease death, myocardial infarction, ischaemic stroke, or unstable angina requiring hospitalization [major adverse cardiovascular event (MACE)] and all-cause death. Relative risk reductions were consistent for patients ≥65 vs. <65 years for MACE [hazard ratio (HR) 0.78, 95% confidence interval (CI) 0.68–0.91 vs. 0.89, 0.80–1.00; Pinteraction = 0.19] and all-cause death [HR 0.77, 0.62–0.95 vs. 0.94, 0.77–1.15; Pinteraction = 0.46], and consistent for MACE when dichotomizing at age 75 years (HR 0.85, 0.64–1.13 in ≥75 vs. 0.85, 0.78–0.93 in <75, Pinteraction = 0.19). When considering age as a continuous variable in regression models, advancing age increased risk of MACE, as well as the absolute reduction in MACE with alirocumab, with numbers-needed-to-treat for MACE at 3 years of 43 (25–186) at age 45 years, 26 (15–97) at age 75 years, and 12 (6–81) for those at age 85 years. Although adverse events were more frequent in older patients, there were no differences between alirocumab and placebo.
Conclusion
In patients with recent ACS, alirocumab improves outcomes irrespective of age. Increasing absolute benefit but not harm with advancing age suggests that LDL-C lowering is an important preventive intervention for older patients after ACS.
Keywords: Acute coronary syndrome, Alirocumab, Low-density lipoprotein cholesterol, Age
See page 2259 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz879)
Introduction
Cardiovascular disease is a major cause of death in older patients, and low-density lipoprotein cholesterol (LDL-C) remains a strong risk factor for cardiovascular events even at an advanced age.1 Statins reduce the risk of vascular mortality and major coronary events irrespective of age, although the evidence is less strong in patients above 75 years. A recent meta-analysis explored the effectiveness of statin therapy across many age categories.2 Overall, statin therapy compared with placebo or more intensive statin therapy compared with less intensive statin therapy reduced the risk of major coronary events, but there appeared to be a trend towards smaller proportional risk reductions with increasing age. Absolute risk reduction, however, increased with advancing age.
As older individuals were often underrepresented in the clinical trials included in that analysis, uncertainty remains regarding the balance between benefit and risk with intensive LDL-C lowering in the elderly, particularly after an acute coronary syndrome (ACS). This was identified as a specific knowledge gap in an American College of Cardiology/American Heart Association scientific statement.3 Older patients, who are often challenging to treat, are less likely to be prescribed lipid-lowering therapies, and when statins are used the dose is often lower.4 Reasons include fear of side-effects, perceived lack of benefit, concerns about polypharmacy, and potential drug–drug interactions. Older patients in secondary prevention also have lower adherence to statins, which has been associated with an increased risk of death.5 As a consequence, few elderly patients in secondary prevention appear to achieve the LDL-C levels recommended in guidelines.6 In view of these limitations, additional LDL-C lowering by non-statin therapies might, therefore, be particularly appealing for older patients with established coronary artery disease.7
Proprotein convertase subtilisin–kexin Type 9 (PCSK9) inhibition represents one option to substantially lower LDL-C levels in patients who do not achieve guideline-recommended goals. In the Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab (ODYSSEY OUTCOMES) trial, the PCSK9 inhibitor alirocumab, added to high-intensity or maximum-tolerated statin treatment, reduced the primary composite endpoint of death from coronary heart disease, non-fatal myocardial infarction, ischaemic stroke, or unstable angina requiring hospitalization compared with placebo in 18 924 patients with a recent ACS.8 In this trial, as well as in a large outcomes trial with evolocumab in patients with stable established atherothrombotic disease, there appeared to be no interaction between age and impact of treatment on the primary endpoint [65-year cut-off: 27% of the population in ODYSSEY OUTCOMES and 44% of those in Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER)].8,9 Recommendations for PCSK9 inhibitor therapy in guidelines are in general cautious and not age-specific, reflecting the limited evidence available in older patients.10,11
In this prespecified analysis of the ODYSSEY OUTCOMES trial, we assessed the impact of age on recurrent ischaemic cardiovascular events and the impact of age on the effect of treatment with alirocumab on LDL-C levels and recurrent ischaemic cardiovascular events in post-ACS patients with elevated atherogenic lipoproteins despite maximally tolerated intensive statin therapy.
Methods
The study design, including inclusion and exclusion criteria, and primary results have been published.8,12 Patients were eligible for enrolment if they were ≥40 years of age, provided written consent, had been hospitalized with an ACS (myocardial infarction or unstable angina) 1–12 months before randomization, and had an LDL-C level ≥1.8 mmol/L (70 mg/dL), a non-high-density lipoprotein cholesterol level of ≥2.59 mmol/L (100 mg/dL), or an apolipoprotein B level of ≥80 mg/dL, measured after ≥2 weeks of stable treatment with atorvastatin 40–80 mg daily, rosuvastatin 20–40 mg daily, or the maximum-tolerated dose of one of these statins (including no statin in the case of documented unacceptable side-effects). Patients who met trial entry criteria were randomized to receive alirocumab at a dose of 75 mg or matching placebo; randomization was stratified by country. All doses of alirocumab or placebo were administered by subcutaneous injection every 2 weeks.
Treatment assignments and lipid levels during the trial were concealed from the patients and investigators. Low-density lipoprotein cholesterol levels were calculated with the use of the Friedewald formula unless the triglyceride level exceeded 4.52 mmol/L (400 mg/dL) or the calculated LDL-C level was <0.39 mmol/L (15 mg/dL), in which case values were determined by beta-quantification. Among patients assigned to the alirocumab group, protocol-specified dose-adjustment algorithms were used to target an LDL-C level of 0.65–1.30 mmol/L (25–50 mg/dL) and to avoid sustained levels <0.39 mmol/L (15 mg/dL). Dose adjustments were performed under blinded conditions, without either the patient or the investigator being aware of the adjustment, including substitution of placebo for alirocumab in the case of sustained levels of LDL-C <0.39 mmol/L.
Study endpoints
The primary endpoint was a composite of death from coronary heart disease, non-fatal myocardial infarction, fatal or non-fatal ischaemic stroke, or unstable angina requiring hospitalization. Key secondary endpoints include all-cause death, and the combined secondary endpoint of all-cause death, myocardial infarction, or ischaemic stroke.8 All endpoints were adjudicated by physicians who were unaware of the trial-group assignments.
Statistical analysis
Baseline characteristics, including patient demographics, medical history before the index ACS, type of index ACS and treatment, renal function, and type of lipid-lowering therapies are presented by age group, using the prespecified cut-off threshold of 65 years and the non-prespecified threshold of 75 years. Categorical variables are presented as counts (%), and differences among age groups were assessed using the chi-square tests. Continuous variables are presented as means and standard deviations (SDs), and differences among age groups were assessed using t-tests. The linearity of the relationship between age and the log-hazard of endpoints was tested using restricted cubic splines. Kaplan–Meier curves are presented by randomized treatment and age group (≥65/<65 years and ≥75/<75 years). A Cox regression model with age group, randomized treatment and their interaction was used to estimate age group-specific hazard ratios (HRs) comparing alirocumab vs. placebo. A separate Cox regression model with age as a continuous variable, randomized treatment and their interaction was used to estimate the event rate at 3 years and HRs comparing alirocumab vs. placebo at specific ages and to test the interaction between age as and randomized treatment. Additionally, using the same model, absolute risk reductions were estimated as a function of age. In cases where the absolute risk reduction was statistically significant, the number-needed-to-treat (NNT) with confidence interval (CI) is reported.
Sensitivity analyses were performed in the subgroup of patients medically managed and adjusting the randomized treatment comparison by statin dose at baseline. All analyses were performed separately from the sponsor by an independent academic statistician (D.M.W.) using SAS System version 9.4 (TS1M5).
Results
Patient characteristics according to age
The mean age of patients in ODYSSEY OUTCOMES was 59 (SD 9 years), with 5084 (26.9%) aged 65 years or older, 1007 (5.3%) aged 75 years or older, and 42 (0.2%) 85 years or older. Because of the small number of patients in the latter two categories, comparisons were made according to prespecified categories dichotomized at the prespecified age of 65 years.
Patients aged 65 years or older were more often female, and more likely to have a history of hypertension, diabetes mellitus, myocardial infarction, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), stroke, peripheral artery disease, and congestive heart failure (Table 1). Older patients were also more likely to have presented with non-ST-segment elevation myocardial infarction (54.2% vs. 46.4% in patients <65 years) (Table 1), while younger patients more often had ST-segment elevation myocardial infarction. Older patients were also less likely to have undergone revascularization (PCI or CABG) for their index ACS event (68.4% vs. 73.7% for patients <65 years). Comparable baseline differences were observed when dichotomizing patients at age 75 years (Supplementary material online, Table S1).
Table 1.
Baseline characteristics per age group
| Characteristics | Age at enrolment |
P-value | |
|---|---|---|---|
| <65 years (n = 13 840) | ≥65 years (n = 5084) | ||
| Age (years), mean (SD) | 54.2 (6.3) | 70.5 (4.7) | <0.0001 |
| Women, n (%) | 2948 (21.3) | 1814 (35.7) | <0.0001 |
| Race, n (%) | <0.0001 | ||
| White | 10 886 (78.7) | 4138 (81.4) | |
| Asian | 384 (2.8) | 89 (1.8) | |
| Black | 1894 (13.7) | 604 (11.9) | |
| Other | 673 (4.9) | 253 (5.0) | |
| Region of enrolment, n (%) | <0.0001 | ||
| Central and Eastern Europe | 3909 (28.2) | 1528 (30.1) | |
| Western Europe | 3073 (22.2) | 1102 (21.7) | |
| Canada or USA | 2099 (15.2) | 772 (15.2) | |
| Latin America | 1813 (13.1) | 775 (15.2) | |
| Asia | 1733 (12.5) | 560 (11.0) | |
| Rest of World | 1213 (8.8) | 347 (6.8) | |
| Medical history before index ACS, n (%) | |||
| Hypertension | 8390 (60.6) | 3859 (75.9) | <0.0001 |
| Diabetes mellitus | 3787 (27.4) | 1657 (32.6) | <0.0001 |
| Current tobacco smoker | 3973 (28.7) | 587 (11.5) | <0.0001 |
| Family history of premature coronary heart disease | 5248 (37.9) | 1525 (30.0) | <0.0001 |
| Myocardial infarction | 2499 (18.1) | 1134 (22.3) | <0.0001 |
| Percutaneous coronary intervention | 2212 (16.0) | 1029 (20.2) | <0.0001 |
| CABG | 582 (4.2) | 465 (9.1) | <0.0001 |
| Stroke | 337 (2.4) | 274 (5.4) | <0.0001 |
| Peripheral artery disease | 428 (3.1) | 331 (6.5) | <0.0001 |
| Congestive heart failure | 1800 (13.0) | 1014 (19.9) | <0.0001 |
| Index ACS, n (%) | n = 13 818 | n = 5075 | <0.0001 |
| STEMI | 5039 (36.4) | 1497 (29.4) | |
| Non-STEMI | 6422 (46.4) | 2753 (54.2) | |
| Unstable angina | 2357 (17.0) | 825 (16.2) | |
| PCI or CABG for index ACS, n (%) | 10 201 (73.7) | 3475 (68.4) | <0.0001 |
| Time from index ACS to randomization (months), mean (SD) | 3.6 (2.8) | 3.8 (2.8) | 0.0003 |
| Body mass index (kg/m2), mean (SD) | 28.8 (4.9) | 27.8 (4.6) | <0.0001 |
| Renal function | |||
| eGFR (mL/min), mean (SD) | 83.1 (18.5) | 70.2 (18.2) | <0.0001 |
| eGFR <60 mL/min, n (%) | 1163 (8.4) | 1377 (27.1) | <0.0001 |
| Lipid-lowering drugs at randomization, n (%) | <0.0001 | ||
| High-intensity atorvastatin/rosuvastatin | 12 565 (90.8) | 4246 (83.5) | |
| Atorvastatin 80 mg or rosuvastatin 40 mg | 4058 (29.3) | 1121 (22.0) | |
| Atorvastatin 40 mg or rosuvastatin 20 mg | 8507 (61.5) | 3125 (61.5) | |
| Low- or moderate-intensity atorvastatin/rosuvastatin | 1027 (7.4) | 580 (11.4) | |
| Other statin | 26 (0.2) | 20 (0.4) | |
| No statin | 222 (1.6) | 238 (4.7) | |
| Ezetimibe | 408 (2.9) | 142 (2.8) | 0.57 |
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Lipid-lowering therapies and low-density lipoprotein cholesterol levels according to age
At randomization, statin therapy was less intensive in older patients. Fewer patients ≥65 years were on atorvastatin 80 mg or rosuvastatin 40 mg, while the percentage of patients on atorvastatin 40 mg or rosuvastatin 20 mg was similar in both age categories (Table 1). More patients above the age of 65 years were on low- or moderate-intensity doses of these statins (11.4% vs. 7.4% for <65 years) or were not receiving any statin at baseline (4.7% vs. 1.6% for those <65 years). Ezetimibe was rarely used across both age categories. The mean baseline LDL-C levels were 2.38 mmol/L in patients <65 years of age in both treatment arms, and 2.43 (alirocumab) or 2.41 (placebo) mmol/L in patients aged 65 years and older.
Over the course of the study, the intensity of statin therapies changed, and this occurred largely to the same extent in both age categories and irrespective of treatment allocation (Supplementary material online, Table S2). At 2 years from randomization, statin intensity had decreased in 13.0% of patients (14.0% in ≥65 years, 12.6% in <65 years), and had increased in only 1.2% of patients (1.4% in ≥65 years, 1.1% in <65 years). At 3 years, LDL-C levels in the alirocumab arm were 62.5 mg/dL for <65 years and 56.9 mg/dL for ≥65 years, vs. 102.1 mg/dL and 97.0 mg/dL, respectively, in the placebo arm. Overall, the age-related trends in statin regimen changes over time were similar in patients on alirocumab or placebo. Similarly, persistence of assigned study treatment diminished over time in both age categories. Among patients ≥65 years, discontinuation of assigned study alirocumab or placebo occurred in 8.9% and 8.1%, respectively, at 1 year and in 13.9% and 13.6% at 2 years. In patients <65 years, discontinuation of alirocumab or placebo occurred in 7.0% and 7.1%, respectively, at 1 year and 11.5% and 11.9% at 2 years. The 75 mg alirocumab dose comprised 79.7% of the prescribed treatment for patients ≥65 years compared with 80.0% for patients <65 years. Blinded substitution of placebo for alirocumab for sustained very low LDL-C occurred in 7.9% of patients ≥65 years compared with 7.7% of patients <65 years.
Effect of alirocumab on low-density lipoprotein cholesterol levels according to age
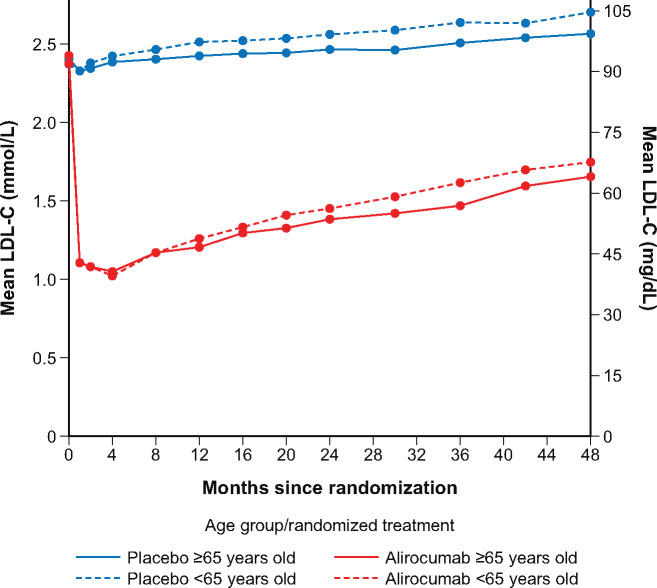
The extent of the initial LDL-C-lowering response to alirocumab was pronounced irrespective of age (Figure 1). Low-density lipoprotein cholesterol levels with alirocumab at 1 (both 1.11 mmol/L), 4 (1.02 vs. 1.05 mmol/L), and 8 (both 1.17 mmol/L) months after randomization were similar in patients <65 vs. ≥65 years. Subsequent LDL-C levels among patients assigned to the alirocumab group tended to rise irrespective of age. This effect was less pronounced in patients ≥65 years: LDL-C levels at 24 and 36 months were 1.39 and 1.47 mmol/L, respectively, in older patients vs. 1.45 and 1.62 mmol/L in younger patients. The more pronounced gradual increase in LDL-C levels over the course of the trial in younger vs. older patients appeared to be similar in the placebo arm.
Figure 1.
Low-density lipoprotein cholesterol levels during the trial per age category (intention-to-treat analysis).
Clinical outcomes according to age
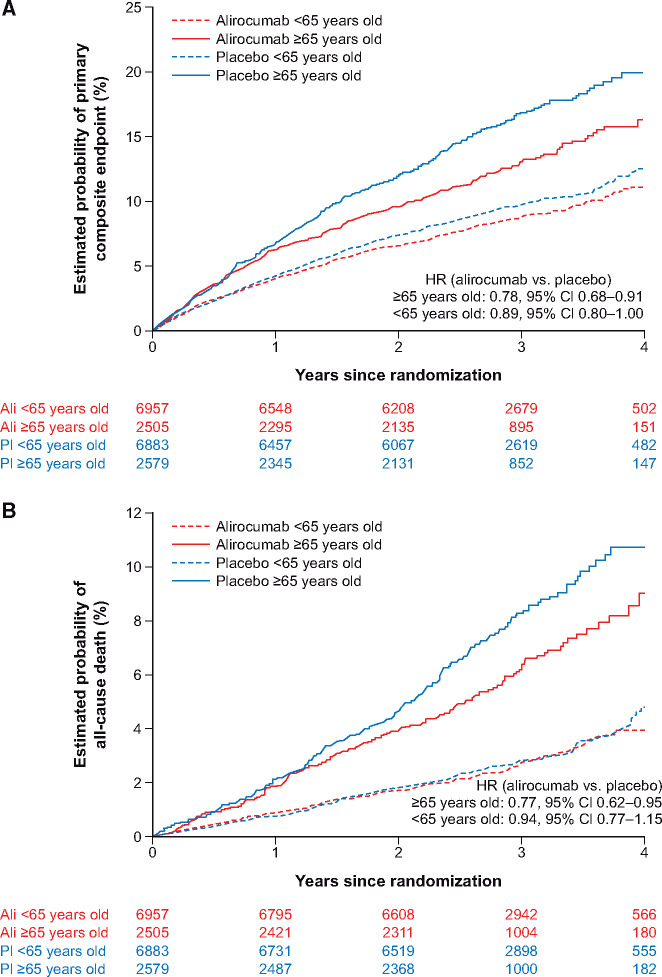
Alirocumab reduced the risk of the primary composite endpoint in patients ≥65 years (Kaplan–Meier at 3 years: 12.9% vs. 16.8%; HR 0.78, 95% CI 0.68–0.91) and in those <65 years (Kaplan–Meier at 3 years: 8.8% vs. 9.7%; HR 0.89, 0.80–1.00, Pinteraction = 0.19) (Supplementary material online, Table S3). Similarly, there was no significant difference by age category in the relative benefit of alirocumab over placebo for the secondary combined endpoint of all-cause death, myocardial infarction, or ischaemic stroke (HR 0.78, 95% CI 0.68–0.90 for ≥65 years vs. 0.91, 0.82–1.02; Pinteraction = 0.19), or for all-cause death (HR 0.77, 0.62–0.95 vs. 0.94, 0.77–1.15; Pinteraction = 0.46). The absolute risk of each of these endpoint events in the placebo group was considerably greater among patients ≥65 years compared with those <65 years. Accordingly, the absolute reduction in the primary endpoint with alirocumab was significantly greater among those ≥65 years of age than those <65 years of age (P = 0.015, Figure 2A). Reduction in death with alirocumab was also greater among those ≥65 years of age than among younger patients (P = 0.024, Figure 2B). Three-year Kaplan–Meier estimates for absolute reduction in the primary endpoint with alirocumab were 3.9% (95% CI 1.7–6.0) among those ≥65 years of age and 0.9% (95% CI −0.1 to 2.0) for those <65 years of age. Similarly, 3-year Kaplan–Meier estimates for absolute reduction in death with alirocumab were 2.1% (95% CI 0.5–3.7) among those ≥65 years of age compared with 0.1% (95% CI −0.5 to 0.7) for those <65 years of age. Adjusting for baseline statin therapy did not significantly alter these results (Supplementary material online, Table S4).
Figure 2.
Treatment effect by age group (≥65 vs. <65 years) for (A) the primary composite endpoint and (P = 0.015a) (B) all-cause death (P = 0.024a). aTest of heterogeneity of the randomized treatment effect in the absolute risk scale.
When using the non-prespecified 75-year cut-off, alirocumab reduced the risk of the primary endpoint in patients ≥75 years (Kaplan–Meier at 3 years: 18.8% vs. 21.4%; HR 0.85, 95% CI 0.64–1.13) and in those <75 years (Kaplan–Meier at 3 years: 9.4% vs. 11.1%; HR 0.85, 0.78–0.93, Pinteraction = 0.19) (Supplementary material online, Table S5 and Figures S1). There was no significant difference by age category set at 75 in the relative effect of alirocumab on all-cause death (HR 0.80, 0.55–1.17 in ≥75 years vs. 0.86, 0.74–1.01 in <75 years; Pinteraction = 0.46).
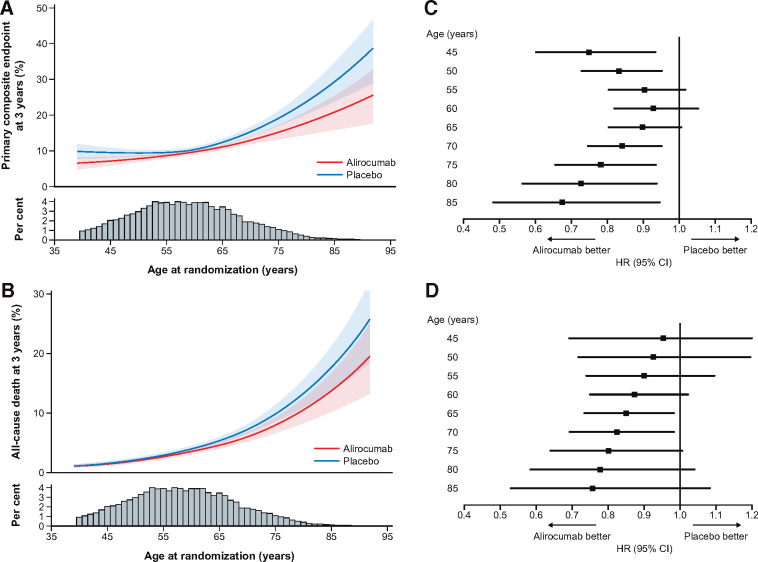
Using age as a continuous variable, the rates of the primary composite endpoint and all-cause death increased gradually with advancing age, with a notably steeper slope ≥65 years (Take home figureA and B). The relative benefit of alirocumab over placebo on the primary endpoint was consistent across the whole age range. The HR for the primary composite endpoint was 0.75 (95% CI 0.60–0.94) for a 45-year-old patient vs. 0.78 (0.65–0.94) for a 75-year-old patient, and 0.68 (0.48–0.95) for an 85-year-old patient (Pinteraction = 0.19) (Take home figureC). Similarly, the HR for a major coronary heart disease event was 0.75 (0.59–0.95) in a 45-year-old patient, 0.80 (0.66–0.98) for a 75-year-old patient, and 0.68 (0.47–0.98) for a patient at age 85 years (Pinteraction = 0.14) HRs for all-cause death are shown in Take home figureD).
Take home figure.
Treatment effect by the level of age as a continuous variable, shown in a continuous event curve and at landmark ages per 5-year interval for the primary composite endpoint (A and C, Pinteraction = 0.19) and all-cause death (B and D, Pinteraction = 0.46). The confidence intervals are for (A) the estimated probability of the primary composite endpoint at 3 years by randomized treatment and (B) the estimated probability of all-cause death at 3 years by randomized treatment.
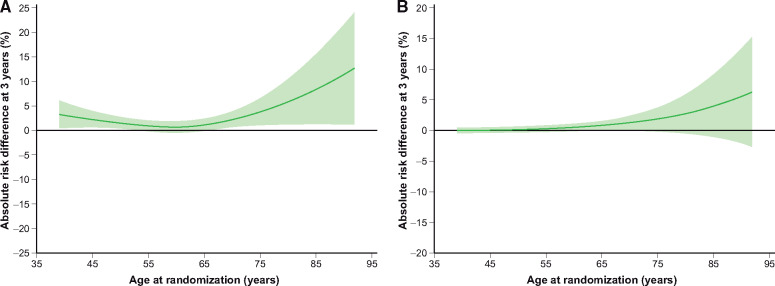
Because of the higher absolute risk at older age, the absolute benefits of alirocumab increased with advancing age (Figure 3). As a consequence, the NNT for 3 years to prevent one primary composite endpoint decreased with increasing age: 43 (25–186) at age 45; 26 (15–97) at age 75; and 12 (6–81) at age 85 years. For major coronary heart disease events, the NNT decreased from 49 (27–259) at age 45 to 34 (18–297) at age 75, and to 14 (7–269) at age 85. Similar results were observed for the relative benefit of alirocumab treatment among patients who were managed medically (i.e. without a coronary revascularization procedure for the index ACS): the HR for the primary composite endpoint at 3 years was 0.86 (95% CI 0.59–1.28) for a 45-year-old patient vs. 0.73 (0.56–0.96) or 0.56 (0.33–0.94) for a 75-year or 85-year-old patient (Pinteraction = 0.17). Among medically managed patients, the NNT for the primary endpoint was 17 (9–131) and 7 (4–58), respectively, for 75- and 85-year-old patients.
Figure 3.
Absolute risk reductions according to age for (A) the primary composite endpoint and (B) all-cause death. The confidence intervals are for absolute risk reduction at 3 years. Confidence intervals including the value 0 indicate no difference in the absolute risk scale.
Safety according to age
Adverse events by randomized treatment and age are listed in Table 2. Although adverse events were observed more frequently in patients above the age of 65 years, there were no differences between alirocumab and placebo in patients aged ≥65 or patients <65 years. Similarly, serious adverse events or adverse events that led to discontinuation of the trial regimen were more frequent in older patients but no differences between the two treatment arms were observed. Neurocognitive disorders were infrequent overall, more common among older than younger participants, but numerically less common in the alirocumab group compared to the placebo group in both age groups.
Table 2.
Adverse events per age group
| Adverse event | Randomized treatment |
Relative risk (95% CI) | |
|---|---|---|---|
| Alirocumab | Placebo | Alirocumab vs. placebo | |
| Any adverse event, n/N (%) | |||
| ≥65 years | 1974/2499 (79.0) | 2042/2574 (79.3) | 1.00 (0.97–1.02) |
| <65 years | 5191/6952 (74.7) | 5240/6869 (76.3) | 0.98 (0.96–1.00) |
| Serious adverse event, n/N (%) | |||
| ≥65 years | 703/2499 (28.1) | 781/2574 (30.3) | 0.93 (0.85–1.01) |
| <65 years | 1499/6952 (21.6) | 1569/6869 (22.8) | 0.94 (0.89–1.00) |
| Adverse event that led to discontinuation of the trial regimen, n/N (%) | |||
| ≥65 years | 121/2499 (4.8) | 128/2574 (5.0) | 0.97 (0.76–1.24) |
| <65 years | 222/6952 (3.2) | 196/6869 (2.9) | 1.12 (0.93–1.35) |
| Neurocognitive disorder, n/N (%) | |||
| ≥65 years | 52/2499 (2.1) | 65/2574 (2.5) | 0.82 (0.57–1.18) |
| <65 years | 91/6952 (1.3) | 102/6869 (1.5) | 0.88 (0.67–1.17) |
| New-onset diabetes among patients without diabetes at baseline, n/N (%) | |||
| ≥65 years | 156/1693 (9.2) | 167/1728 (9.7) | 0.95 (0.77–1.17) |
| <65 years | 492/5070 (9.7) | 509/4968 (10.2) | 0.95 (0.84–1.07) |
| Haemorrhagic stroke—adjudicated, n/N (%) | |||
| ≥65 years | 1/2499 (0.0) | 6/2574 (0.2) | 0.17 (0.02–1.42) |
| <65 years | 8/6952 (0.1) | 10/6869 (0.1) | 0.79 (0.31–2.00) |
| Alanine aminotransferase >3 times the upper limit of normal, n/N (%) | |||
| ≥65 years | 64/2475 (2.6) | 70/2539 (2.8) | 0.94 (0.67–1.31) |
| <65 years | 148/6894 (2.1) | 158/6802 (2.3) | 0.92 (0.74–1.15) |
| Aspartate aminotransferase >3 times the upper limit of normal, n/N (%) | |||
| ≥65 years | 52/2475 (2.1) | 48/2539 (1.9) | 1.11 (0.75–1.64) |
| <65 years | 108/6892 (1.6) | 118/6799 (1.7) | 0.90 (0.70–1.17) |
CI, confidence interval.
Discussion
Elevated LDL-C is a risk factor for recurrent ischaemic cardiovascular events after an ACS, including at an advanced age.1 Although statins reduce the risk of recurrent events irrespective of age, the evidence is less convincing in patients above the age of 65 years, in part due to the low number of older patients randomized in clinical trials. Nevertheless, guidelines recommend statin therapy for older patients in secondary prevention, but weaker evidence, as well as doubts about the safety of statins, is reflected in less stringent recommendations.
The present analysis from the ODYSSEY OUTCOMES trial demonstrates in patients with recent ACS and on maximum-tolerated statin therapy that alirocumab safely reduced the relative risk of recurrent ischaemic events and all-cause death across age categories or the continuous range of age. Importantly, because the absolute risk of these events increases with advancing age, the absolute treatment benefit of alirocumab also increased with advancing age. Consequently, the NNT to prevent one primary endpoint is relatively low in older patients and is even lower in the very old: 26 for 75-year-old patients and 12 for 85-year-old patients. Taken together, these findings illustrate that intensive LDL-C lowering therapy with PCSK9 inhibition added to statin may be an important secondary preventive intervention for older patients after an ACS, and in fact is a highly efficient intervention in those patients.
The current findings are analogous to previous studies that have indicated a consistent benefit of statin treatment in the elderly, particularly for secondary prevention of cardiovascular events. In contrast with older studies, a recent meta-analysis suggested that statins reduce the risk of vascular death and major coronary events irrespective of age.2 Although there appeared to be diminishing benefit with age in primary prevention, there was no trend towards smaller proportional reductions in death with increasing age in patients with established vascular disease.2 Likewise, in a recent French cohort study, statin use for secondary prevention in patients aged ≥75 years was associated with a 25% lower annual rate of ACS or death (HR 0.75, 95% CI 0.63–0.90), but the effectiveness was lower with use in high-risk primary prevention patients and even absent in older patients without modifiable risk factors.13 In addition, intensive lipid-lowering therapies vs. either placebo or moderate statin therapy after an ACS yielded at least similar benefits in older patients as in younger patients.7,14,15 Taken together, because of their high risk of recurrent ischaemic events, older patients benefit from lipid-lowering therapies.
Similarly, the present analysis demonstrates greater absolute risk of recurrent ischaemic events and greater absolute reduction in that risk with alirocumab with advancing age. There was a highly significant interaction of treatment and age (dichotomized at 65 years) on the absolute reduction of the composite primary outcome and all-cause death. Importantly, there appears to be no age limit as to the effectiveness of alirocumab, although inference is limited by small numbers of patients at very advanced ages.
The NNT with intensified lipid-lowering therapy with alirocumab in this analysis (26 at age 75 and 12 at age 85 years) is similar to that observed in the Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) that compared atorvastatin 80 mg with pravastatin 40 mg daily after ACS. In that trial, one ischaemic event (death, myocardial infarction, or unstable angina) was prevented in 13 patients ≥70 years old vs. 44 patients <70 years old treated to achieve LDL-C levels below 1.81 mmol/L.14 In addition, a low NNT (11) for the combined endpoint of cardiovascular death and major cardiac adverse events was observed for ACS patients ≥75 years of age in the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT), when adding ezetimibe to simvastatin over a 7-year period.7
Advancing age does not appear to affect the extent to which alirocumab lowers LDL-C levels. An analysis from alirocumab studies in patients with heterogenous familial hypercholesterolaemia previously suggested that LDL-C reductions were similar across all age groups studied, although only 90 patients ≥65 years of age were included.16 In the current study, alirocumab further lowered LDL-C levels in older patients to the same extent as in younger patients, despite a somewhat less intensive statin regimen at baseline in patients ≥65 years of age. Low-density lipoprotein cholesterol lowering became attenuated over the course of the trial, but to a lesser extent in older patients. However, the difference in LDL-C levels between older vs. younger patients observed from the end of the first year onward appeared to occur to the same extent in the placebo arm. This suggests that the less pronounced attenuation of LDL-C levels in older patients is not due to significant age-specific differences in alirocumab treatment or dose.
Treatment with alirocumab was found to be as safe in patients above the age of 65 as in younger patients, confirming previous findings.16,17 Although adverse events, in general, were more frequent in older patients in ODYSSEY OUTCOMES, there was no excess in any of the safety endpoints. Importantly, neurocognitive adverse events were uncommon, and no difference was observed between alirocumab and placebo in patients ≥65 years. A comprehensive analysis of neurocognitive adverse events from 14 Phase 2 and 3 trials with alirocumab, and up to 104 weeks of follow-up (but excluding ODYSSEY OUTCOMES) confirms that alirocumab does not appear to increase the risk of neurocognitive adverse events, including in older patients.17 In addition, although the present analysis represents the longest follow-up (4 years, median of 2.8 years) with alirocumab across a wide range of ages to date, longer follow-up is required to assess the long-term safety of reaching lower LDL-C levels with PCSK9 inhibition in patients above the age of 65 years.
Limitations
A minimum age of 40 years to qualify for the trial and analysis according to age dichotomized at age 65 years were prespecified in the protocol and statistical analysis plan. The number of very elderly patients was limited, leading to less precision in the estimates of efficacy and insufficient data to define safety in octogenarians. In addition, per design of the trial, patients needed to be on stable maximally tolerated high-intensity statin therapy after their ACS. As a consequence, older patients represented in these analyses were largely tolerant to intensive statin therapy at baseline and were unlikely to be frail. It remains unclear whether our findings are also applicable to less healthy older patients in the community. Since patients were followed for a median of 2.8 years and life expectancy for most patients is likely to be longer than that, it is difficult to extrapolate efficacy beyond the time horizon of the trial and calculate the number of life-years gained. Furthermore, as the results from the IMPROVE-IT trial did not become available until after the trial was well underway, and LDL-C levels were kept blinded, few patients were treated with ezetimibe over the course of the trial.
Conclusions
In the ODYSSEY OUTCOMES trial, adding alirocumab to maximally tolerated high-intensity statins significantly improves outcomes in patients after an ACS irrespective of age and without significant safety issues in older patients. Importantly, the absolute treatment benefit of alirocumab increased with advancing age, indicating that lipid-lowering therapy beyond statins may be an important secondary preventive intervention after ACS in older patients. These findings might be considered in future guideline recommendations for lipid-lowering therapies in older patients.
Supplementary Material
Acknowledgements
The authors thank the patients, study co-ordinators, and investigators who participated in this trial. Sophie Rushton-Smith (MedLink Healthcare Communications, London) provided editorial assistance in the preparation of the manuscript (limited to editing for style, referencing, figure, and table editing).
Funding
This work was supported by the Sanofi; Regeneron Pharmaceuticals, Inc. and the Fondation Assistance Publique-Hôpitaux de Paris.
Conflict of interest: P.R.S. reports personal fees from Sanofi, Abbott, Boehringer-Ingelheim, BMS, Pfizer, and Amgen; personal fees and non-financial support from AstraZeneca and Merck Sharp Dohme; and grants and personal fees from Daiichi Sankyo and Bayer. G.G.S. reports research grants to the University of Colorado from Resverlogix, Sanofi, The Medicines Company, and Roche; and is coinventor of pending US patent 14/657192 (‘Methods of Reducing Cardiovascular Risk’) assigned in full to the University of Colorado. D.M.W. reports receiving grant support from Sanofi (paid to the institution). M.A. reports personal fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Milestone, and Pfizer; and research funding from Sanofi. D.L.B. discloses the following relationships: Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, and Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute), for the PORTICO IDE trial (Portico Re-sheathable Transcatheter Aortic Valve System US IDE Trial), funded by St Jude Medical, now Abbott), Cleveland Clinic [including for the ExCEED trial (CENTERA THV System in Intermediate Risk Patients Who Have Symptomatic, Severe, Calcific, Aortic Stenosis Requiring Aortic Valve Replacement), funded by Edwards], Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine [for the ENVISAGE-TAVI AF trial (Edoxaban Compared to Standard Care After Heart Valve Replacement Using a Catheter in Patients With Atrial Fibrillation), funded by Daiichi Sankyo], Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC. org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute); REDUAL-PCI clinical trial (Evaluation of Dual Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting) steering committee funded by Boehringer Ingelheim; AEGIS-II trial (Study to Investigate CSL112 in Subjects With Acute Coronary Syndrome executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute [for the COMPASS trial (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) operations committee, publications committee, steering committee, and USA national coleader, funded by Bayer], Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi-Aventis, Synaptic, and The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, Takeda. V.A.B. reports research grants from Amgen, DalCor, Esperion, Sanofi, AstraZeneca, Bayer Healthcare, and The Medicines Company; honoraria from the American College of Cardiology, American Heart Association, and National Lipid Association; and serving as a consultant on advisory boards for Sanofi. C.-E.C. reports receiving personal fees from Sanofi, Pfizer, Novartis, Merck Sharp Dohme, AstraZeneca, Daiichi Sankyo, Bayer, and Boehringer Ingelheim. R.D. reports research grants from Sanofi, DalCor Pharmaceuticals, Population Health Research Institute, Duke Clinical Research Institute, the TIMI group, Amgen, Cirius, Montreal Health Innovations Coordinating Center, and Lepetit and personal fees, as a member of the Executive Steering Committee, from Amgen and Cirius. S.G.G. reports research grants from Daiichi-Sankyo, Luitpold Pharmaceuticals, Merck, Novartis, Servier, Regeneron Pharmaceuticals, Sanofi, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Eli Lilly, Pfizer, and Tenax Therapeutics; honoraria from Bristol-Myers Squibb, Eli Lilly, Esperion, Fenix Group International, Ferring Pharmaceuticals, Merck, Novartis, Pfizer, Servier, Regeneron Pharmaceuticals, Sanofi, Amgen, AstraZeneca, Bayer, and Boehringer Ingelheim; and serving as a consultant or on advisory boards (or both) for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, HLS Therapeutics, Pfizer, Servier, Tenax Therapeutics, Sanofi, Amgen, and Bayer. J.W.J. reports research grants from the Netherlands Heart Foundation, the Interuniversity Cardiology Institute of the Netherlands, and the European Commission Seventh Framework Programme; and research support from Amgen, Astellas, AstraZeneca, Daiichi- Sankyo, Lilly, Merck-Schering-Plough, Pfizer, Roche, and Sanofi. Y.-U.K. is an employee of and holds shares in Sanofi. R.P. is an employee of Regeneron Pharmaceuticals, Inc. M.T.R. reports research grant funding from Sanofi-Aventis, Astra Zeneca, Patient-Centered Outcomes Research Institute, Ferring Pharmaceuticals, Myokardia, Familial Hypercholesterolemia Foundation, and Bayer; and consulting or honoraria from Astra Zeneca, Amgen, Cytokinetics, Eli Lilly, Roche-Genentech, Janssen Pharmaceuticals, Regeneron, Novo Nordisk, Pfizer, Sanofi-Aventis, Signal Path, and Elsevier Publishers. All conflicts of interest are listed at https://www.dcri.org/about-us/conflict-of-interest. R.G.S. reports receiving personal fees from Sanofi, Amgen, Merck Sharp Dohme, and Pfizer. M.S. reports serving as a consultant or on advisory boards (or both) for CiVi, Resverlogix, Baxter, Esperion, and Regeneron Pharmaceuticals. H.D.W. reports receiving grant support paid to the institution and fees for serving on a steering committee for the ODYSSEY OUTCOMES trial (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) from Sanofi-Aventis and Regeneron Pharmaceuticals, for the ACCELERATE study (A Study of Evacetrapib in High-Risk Vascular Disease) from Eli Lilly, for the STRENGTH trial (Outcomes Study to Assess Statin Residual Risk Reduction With EpaNova in High CV Risk Patients With Hypertriglyceridemia) from Omthera Pharmaceuticals, for the SPIRE trial [The Evaluation of Bococizumab (PF-04950615; RN 316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects] from Pfizer USA, for the HEART-FID study (Randomized Placebo-Controlled Trial of FCM as Treatment for Heart Failure With Iron Deficiency) from American Regent; for the CAMELLIA-TIMI study [A Study to Evaluate the Effect of Long-term Treatment With BELVIQ (Lorcaserin HC) on the Incidence of Major Adverse Cardiovascular Events and Conversion to Type 2 Diabetes Mellitus in Obese and Overweight Subjects With Cardiovascular Disease or Multiple Cardiovascular Risk Factors] from Eisai Inc., for the dal-GenE study (Effect of Dalcetrapib vs. Placebo on CV Risk in a Genetically Defined Population With a Recent ACS) from DalCor Pharma UK Inc., for the AEGIS-II study from CSL Behring, for the SCORED trial (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) and the SOLOIST-WHF trial (Effect of Sotagliflozin on Cardiovascular Events in Patients With Type2 Diabetes Post Worsening Heart Failure) from Sanofi-Aventis Australia Pty Ltd, and for the CLEAR Outcomes Study [Evaluation of Major Cardiovascular Events in Patients With, or at High Risk for, Cardiovascular Disease Who Are Statin Intolerant Treated With Bempedoic Acid (ETC-1002) or Placebo] from Esperion Therapeutics Inc. H.D.W. was on the Advisory Boards for Acetelion, Sirtex and Genentech, Inc. (an affiliate of F. Hoffmann-La Roche Ltd, ‘Roche’; Lytics Post-PCI Advisory Board at European Society of Cardiology), and received lecture fees from AstraZeneca. A.M.Z. reports receiving fees for serving on a steering committee for the ODYSSEY OUTCOMES trial from Sanofi, and advisory board and speaker fees from Sanofi, Amgen, Boehringer Ingelheim, Bayer, Novartis, Pfizer, AstraZeneca, and Vifor. P.G.S. reports grants and non-financial support (cochair of the ODYSSEY OUTCOMES trial; as such he received no personal fees, but his institution has received funding for the time he has devoted to trial co-ordination, and he has received support for some travel related to trial meetings) from Sanofi; research grants and personal fees from Bayer (Steering Committee MARINER, grant for epidemiological study), Merck (speaker fees, grant for epidemiological studies), Sanofi (cochair of the ODYSSEY OUTCOMES trial; cochair of the SCORED trial; consulting, speaking), Servier (Chair of the CLARIFY registry; grant for epidemiological research), and Amarin [executive steering committee the REDUCE-IT trial (Disease Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial); consulting]; and personal fees from Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer, Novartis, Regeneron Pharmaceuticals, Lilly, and AstraZeneca. P.G.S. also has a European application number/patent number, issued on 26 October 2016 (No. 15712241.7), for a method for reducing cardiovascular risk. R.M.C.F. and M.D. have no conflict of interest to declare.
References
- 1. Lind L, Sundström J, Ärnlöv J, Lampa E.. Impact of aging on the strength of cardiovascular risk factors: a longitudinal study over 40 years. J Am Heart Assoc 2018;7:e007061.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cholesterol Treatment Trialists' Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019;393:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rich MW, Chyun DA, Skolnick AH, Alexander KP, Forman DE, Kitzman DW, Maurer MS, McClurken JB, Resnick BM, Shen WK, Tirschwell DL.. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. J Am Coll Cardiol 2016;67:2419–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ofori-Asenso R, Ilomaki J, Tacey M, Zomer E, Curtis AJ, Si S, Zullo AR, Korhonen MJ, Bell JS, Zoungas S, Liew D.. Switching, discontinuation, and reinitiation of statins among older adults. J Am Coll Cardiol 2018;72:2675–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA.. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol 2019;4:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wagner M, Gelbrich G, Kircher J, Kotseva K, Wood D, Morbach C, Leyh R, Ertl G, Karmann W, Stork S, Heuschmann PU.. Secondary prevention in younger vs. older coronary heart disease patients-insights from the German Subset of the EUROASPIRE IV survey. Int J Behav Med 2018;25:283–293. [DOI] [PubMed] [Google Scholar]
- 7. Bach RG, Cannon CP, Giugliano RP, White JA, Lokhnygina Y, Bohula EA, Califf RM, Braunwald E, Blazing MA. Effect of simvastatin-ezetimibe compared with simvastatin monotherapy after acute coronary syndrome among patients 75 years or older: a secondary analysis of a randomized clinical trial. JAMA Cardiol 2019;doi: 10.1001/jamacardio.2019.2306 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 8. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM.. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 9. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR.. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 10. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J.. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 12. Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Mahaffey KW, Moryusef A, Pordy R, Roe MT, Rorick T, Sasiela WJ, Shirodaria C, Szarek M, Tamby JF, Tricoci P, White H, Zeiher A, Steg PG.. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J 2014;168:682–689. [DOI] [PubMed] [Google Scholar]
- 13. Bezin J, Moore N, Mansiaux Y, Steg PG, Pariente A.. Real-life benefits of statins for cardiovascular prevention in elderly subjects: a population-based cohort study. Am J Med 2019;132:740–748.e7. [DOI] [PubMed] [Google Scholar]
- 14. Ray KK, Bach RG, Cannon CP, Cairns R, Kirtane AJ, Wiviott SD, McCabe CH, Braunwald E, Gibson CM.. Benefits of achieving the NCEP optional LDL-C goal among elderly patients with ACS. Eur Heart J 2006;27:2310–2316. [DOI] [PubMed] [Google Scholar]
- 15. Olsson AG, Schwartz GG, Szarek M, Luo D, Jamieson MJ.. Effects of high-dose atorvastatin in patients > or =65 years of age with acute coronary syndrome (from the myocardial ischemia reduction with aggressive cholesterol lowering [MIRACL] study). Am J Cardiol 2007;99:632–635. [DOI] [PubMed] [Google Scholar]
- 16. Ginsberg HN, Tuomilehto J, Hovingh GK, Cariou B, Santos RD, Brown AS, Sanganalmath SK, Koren A, Thompson D, Raal FJ.. Impact of age on the efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia. Cardiovasc Drugs Ther 2019;33:69.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harvey PD, Sabbagh MN, Harrison JE, Ginsberg HN, Chapman MJ, Manvelian G, Moryusef A, Mandel J, Farnier M.. No evidence of neurocognitive adverse events associated with alirocumab treatment in 3340 patients from 14 randomized Phase 2 and 3 controlled trials: a meta-analysis of individual patient data. Eur Heart J 2018;39:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.